Abstract
Leading hypotheses to explain helminth-mediated protection against autoimmunity postulate that type 2 or regulatory immune responses induced by helminth infections in the host limit pathogenic Th1-driven autoimmune responses. We tested these hypotheses by investigating whether infection with the filarial nematode Litomosoides sigmodontis prevents diabetes onset in IL-4-deficient nonobese diabetic (NOD) mice and whether depletion or absence of regulatory T cells, IL-10, or TGFβ alters helminth-mediated protection.
In contrast to IL-4-competent NOD mice, IL-4-deficient NOD mice failed to develop a type 2 shift in either cytokine or antibody production during L. sigmodontis infection. Despite the absence of a type 2 immune shift, infection of IL-4-deficient NOD mice with L. sigmodontis prevented diabetes onset in all mice studied. Infections in immunocompetent and IL-4-deficient NOD mice were accompanied by increases in CD4+CD25+FoxP3+ regulatory T cell frequencies and numbers, respectively, and helminth infection increased proliferation of CD4+FoxP3+ cells. However, depletion of CD25+ cells in NOD mice or FoxP3+ T cells from splenocytes transferred into NOD.scid mice did not decrease helminth-mediated protection against diabetes onset. Continuous depletion of the anti-inflammatory cytokine TGFβ, but not blockade of IL-10 signaling, prevented the beneficial effect of helminth infection on diabetes. Changes in Th17 responses did not seem to play an important role in helminth-mediated protection against autoimmunity as helminth infection was not associated with a decreased Th17 immune response.
This study demonstrates that L. sigmodontis-mediated protection against diabetes in NOD mice is not dependent on the induction of a type 2 immune shift but does require TGFβ.
Keywords: Type I diabetes, nonobese diabetic (NOD), filaria, helminth, Litomosoides sigmodontis, interleukin 4 (IL-4), autoimmune, IL-10, TGFβ, regulatory T cells, FoxP3, IL-17
Introduction
Helminth infections have been shown to ameliorate or prevent the onset of autoimmune diseases in a multitude of animal and human studies (1–9). In this study, we sought to determine whether type 2 or regulatory immune responses induced by helminths were required for protection against autoimmunity.
Unlike other infectious agents, helminths induce type 2 immune responses characterized by eosinophilia, elevated levels of IgE, and increases in T-cell production of IL-4, IL-5, and IL-13. As interleukin 4 (IL-4) counterregulates Th1 responses by inhibiting Th1 differentiation (10, 11) a type 2 shift might protect against Th1-driven autoimmune diseases by reducing autoimmune Th1-driven pathology. Indeed, several lines of evidence suggest that IL-4 can directly attenuate ongoing Th1-driven autoimmune inflammation. Injection of IL-4 improves proteoglycan-induced arthritis in mice (12) and local delivery of IL-4 by retrovirus-transduced lymphocytes improves both experimental autoimmune encephalomyelitis (13) and collagen-induced arthritis (14). Further, expression of IL-4 by pancreatic beta cells prevents the onset of autoimmune diabetes in nonobese diabetic (NOD) mice (15). In addition to direct effects of IL-4, since signaling through the IL-4R alpha chain can induce differentiation of CD4+CD25− cells into FoxP3+ T-regulatory cells (16), it is possible that some of the regulatory immune responses induced by chronic helminth infections are dependent on initial type 2 responses. For these reasons, several investigators have hypothesized that helminth-mediated protection against autoimmunity may be due in part to helminth-driven type 2 immune responses (17–22). Since active work is being conducted to develop helminth-based therapies for autoimmune diseases, and as type 2 responses can drive allergic diseases, in this study we evaluated whether helminth-mediated type 2 responses were necessary for protection against autoimmunity.
Another leading hypothesis states that helminth infections protect against Th1 associated autoimmune diseases by inducing immune regulatory networks driven by regulatory T cells, IL-10, and TGFβ (17–19, 22). In regard to Type I diabetes several studies have demonstrated that regulatory T cells are crucial in maintaining peripheral tolerance and suggest that a loss of regulatory T cells or their suppressive function may contribute to diabetes development (23–25), whereas the transfer of antigen-specific regulatory T cells can delay diabetes and even reverse diabetes development (26, 27). Induction of a large regulatory T-cell population by helminths may therefore be sufficient to prevent diabetes (28). Helminth-induced production of the anti-inflammatory cytokines IL-10 and TGFβ may further contribute to the protective effect against diabetes onset. Whereas two studies have shown IL-10 to have a paradoxical effect of accelerating Type 1 diabetes development (29, 30), IL-10 has also been shown to be protective (31). Experiments utilizing transgenic expression of TGFβ in islets or DCs have clearly shown TGFβ to be protective in autoimmune diabetes (32–34).
Recently, we demonstrated that infection with Litomosoides sigmodontis, a filarial roundworm parasite of rodents in which adult worms reside in the pleural space and first stage larvae (microfilariae) circulate in the blood, fully prevents the onset of diabetes in NOD mice (1). This protection was associated with elevated levels of IL-4 production, a type 2 shift in autoantigen antibody isotype profiles, and an increased total number of splenic CD4+CD25+FoxP3+ regulatory T cells (1).
In the current study, we tested the hypothesis that helminth infections protect against Th1-driven autoimmune diseases through induction of a type 2 shift by infecting IL-4-deficient NOD mice with L. sigmodontis. Subsequent experiments analyzed the role of helminth-induced regulatory immune responses including regulatory T cells, IL-10 and TGFβ.
Materials and Methods
Mice and parasites
Female NOD/LtJ, IL-4-deficient NOD.129P2(B6)-Il4tm1Cgn/DvsJ, NOD/ShiLt-Tg(Foxp3-EGFP/cre)1Jbs/J, and NOD.CB17-Prkdcscid/J mice (The Jackson Laboratory) were maintained at the Uniformed Services University (USU) animal facility with free access to food and water. All experiments were performed under protocols approved by the USU Institutional Animal Care and Use Committee. A subset of experiments were performed at the Institute of Medical Microbiology, Immunology and Parasitology according to the animal welfare guidelines and approved by the German ethical board for animal experiments.
Infectious-stage L3 larvae from Litomosoides sigmodontis were isolated by lavage from the pleural cavity of four-day infected jirds (Meriones unguiculatus, obtained from TRS Laboratory Inc.) as previously described (35). At six weeks of age mice were infected by subcutaneous injection of 40 infective-stage L3 larvae in 100 μl of RPMI media (Mediatetech, Herndon, VA) in the dorsal neck region.
Assessment of diabetes
Glucose levels of mice were determined from blood taken by orbital bleeds every other week using a standard blood glucose meter (Accu-Check Advantage, Roche Diagnostics GmbH, Mannheim, Germany). NOD mice with glucose levels greater than 230 mg/dl in two consecutive measurements were considered diabetic. In the experiments regarding the role of CD25+ or FoxP3+ regulatory T cells, mice with blood glucose levels greater than 250 mg/dl in two consecutive measurements were considered diabetic. Immunological studies were performed one to two weeks after uninfected controls developed diabetes (range: 16–25 weeks of age) and compared to age-matched L. sigmodontis infected animals.
Assessment of pancreas inflammation
Pancreases were isolated 1–2 weeks after the controls developed diabetes (16–23 weeks of age, mean 19,8 weeks of age for uninfected controls and 20,1 weeks of age for infected animals) and fixed in 10% formalin (Protocol, Fisher Scientific Company, Kalamazoo, MI). Haematoxylin-eosin stained slices were assessed for inflammation by a pathologist (J.T.S.) blinded to the intervention group. Total numbers of islets of two longitudinal sections 400 μm apart of each pancreas were assessed. The severity of insulitis was scored as non-infiltrated, periinsulitis (lymphocytes at the periphery of islets), or intrainsulitis (lymphocyte infiltration into the interior of the islets lesser or greater than 50%).
Spleen and pancreatic lymph node cell culture
Spleen and pancreatic lymph node cells from uninfected controls were prepared one to two weeks after uninfected controls developed diabetes (range: 16–25 weeks of age) and compared to age-matched infected mice (10–19 weeks post L. sigmodontis infection) and cultured as previously reported (1). In brief, single cell suspensions were obtained, red blood lysis performed for spleen cells (ACK Lysing Buffer, Quality Biological, Inc., Gaithersburg, MD), and cells were plated at a concentration of 2×106 cells/ml in enriched media (Iscove’s Dulbecco modified medium (Mediatech) including 10% fetal calf serum (Valley Biomedical, Winchester, VA), 1% L-glutamine (Mediatech), 1% insulin-transferrin-selenium medium (Invitrogen Inc., Carlsbad, CA) and 80 μg/ml gentamicin (Quality Biological, Inc.)), stimulated with 5 μg/ml anti-CD3 and 2 μg/ml anti-CD28 (eBioscience, San Diego, CA), and cultured at 37°C, 5% CO2.
Flow cytometric analysis of regulatory T cells and intracellular cytokine production by T cells
Spleen and pancreatic lymph node cells were prepared for flow cytometric analysis as previously reported (1). In brief, after two hours of incubation, BD GolgiStop was added (BD Biosciences, San Jose, CA) and cells were incubated for an additional four hours. Collected cells were fixed and permeabilized (eBioscience) over night. For analysis cells were washed once with phosphate-buffered saline (PBS)/1%BSA (bovine serum albumine, Sigma, St. Louis, MO), followed by a blocking step with PBS/1%BSA. Cells were stained for five- or four-color-flow using CD4 PerCP, IL-4 APC (BD Biosciences), CD8a Pacific Blue, gamma interferon (IFN-γ) FITC, and IL-17 PE (eBioscience) or CD4 PerCP (BD Biosciences), FoxP3 FITC, CD25 APC-Alexa Fluor 750, and IL-10 PE or CTLA-4 PE (eBioscience). Measurement of proliferation was done by staining of fixed cells that were treated as described above with Ki67 PE, CD4 PerCP (BD Biosciences), CD25 APC AF 750 and FoxP3 FITC (eBioscience). For identification of regulatory CD8 T-cells and regulatory B cells, fixed and cryopreserved (PBS/10% DMSO) spleen cells were washed once, blocked with CD16/CD32 (BD Biosciences) and stained with CD4 Qdot 605 (Invitrogen), CD8 PE Cy5, FoxP3 FITC, (all eBioscience) CD25 APC-Alexa Fluor 750, or B220 PerCP, CD1d FITC, CD5 APC (all BD Bioscience).
Flow cytometry was performed using a BD LSRII system and subsequently analyzed with FACSDiVa 6.1 software (BD Biosciences). During analysis, cut-offs for cytokine and CD25-positivity were set using the fluorescence minus one approach and the cut-off for Ki67 determined using an isotype control.
Measurement of cytokines and antibodies by ELISA
Cytokine enzyme-linked immunosorbent assays (ELISAs) were performed from spleen and pancreatic lymph node cells as previously described (1). In brief, culture supernatants from cells that were cultured as described above and stimulated with anti-CD3 and anti-CD28 were collected after 72h of incubation. IFN-γ, IL-5, IL-10, IL-13, IL-17, and TGFβ were quantified according to the manufacturer’s instructions (IFN-γ, IL-5, IL-10: BD Biosciences; IL-13, TGFβ: R&D Systems, Minneapolis, MN; IL-17: eBioscience). To measure bioactive TGFβ from plasma, samples were not activated by acidification and bioactive TGFβ was measured using the TGFβ ELISA from eBioscience.
Plasma derived insulin-specific IgG1 and IgG2c as well as total IgE were analyzed by sandwich ELISA as previously described (1). All samples were analyzed as duplicates at the same time on the same plate to allow accurate comparison between groups by OD.
Depletion of TGFβ, CD25+ cells, and blocking of IL-10R
Starting at 8 weeks of age, two weeks after a subset of mice was infected with L. sigmodontis, NOD mice were treated i.p. every 5 days with 500 μg anti-IL-10R (Clone 1B1.3A, Bio X Cell, West Lebanon, NH), 500 μg rat IgG1 isotype (Clone HRPN, Bio X Cell), or three times a week with 100 μg anti-TGFβ (Clone 1D11.16.8, Bio X Cell) until 16 weeks of age.
CD25+ cells were depleted in NOD mice that were 9 weeks of age, 4 weeks after a subset of mice were infected with L. sigmodontis. Mice were injected weekly i.p. with 1 mg of anti-CD25 (PC61 5.3, American Type Culture Collection (ATCC), Manassas, VA) or 1 mg rat IgG1 isotype until 20 weeks of age. Anti-CD25 antibody was obtained from PC61 rat B-cell hybridoma cells that were cultured as recommended by ATCC. Culture supernatant was collected every 2–4 days and stored at 4°C. PC61 antibodies were purified from the culture supernatant via a protein G column and subsequently desalted using FPLC. The antibodies were sterile filtrated using a 0.22 μm filter (Millipore) and concentration was measured using the BCA assay (Thermo Fisher Scientific Inc., Rockford, IL).
Transfer experiments into NOD.scid mice
A subset of female NOD mice that express eGFP on FoxP3+ cells were infected with L. sigmodontis at 6 weeks of age and at 14 weeks of age spleen cells were isolated as described above. A subset of the spleen cells was resuspended in HBSS/enriched media (4:1) immediately before cell sorting. 7-AAD negative, FoxP3+ and FoxP3− cells were sorted using a BD FACSAria. Immediately before the injection into NOD.scid mice, cells were washed twice with RPMI 1640. 8×106 spleen cells from diabetic NOD mice (glucose >500 mg/dl) were injected into the tail vein of 6-week old female NOD.scid mice together with 1×107 whole spleen cells or 1×107 spleen cells depleted of FoxP3+ cells from L. sigmodontis-infected animals and controls. In a separate experiment, NOD.scid mice were intravenously administered with 1×107 whole spleen cells or 1×107 spleen cells depleted of FoxP3+ cells from 14 week old mice that had been infected with L. sigmodontis for 8 weeks and from age-matched control mice in the absence of spleen cells from diabetic mice.
Statistics
Statistical analyses were performed with GraphPad Prism software (GraphPad Software, San Diego, CA). Differences between multiple groups were tested for significance using the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons. Differences between paired groups for in vitro studies were analyzed using one-tailed paired T-test and differences between two unpaired groups for in vivo studies were tested for significance with the Mann-Whitney-U-test. P-values <0.05 were considered significant.
Results
No type 2 cytokine shift in L. sigmodontis-infected IL-4-deficient NOD mice
To determine whether IL-4-deficiency in NOD mice abrogates the type 2 immune shift induced by L. sigmodontis infection, we compared cytokine production from splenocytes and pancreatic lymph node cells of infected or uninfected IL-4 competent mice with those from infected or uninfected IL-4-deficient mice 1–2 weeks after development of diabetes in uninfected mice (range 16–25 weeks, mean 21 weeks). Importantly, both immunocompetent and IL-4-deficient NOD mice were infected with L. sigmodontis, with each group harboring an average of 2–3 adult worms per mouse in the pleural space at study endpoint (data not shown).
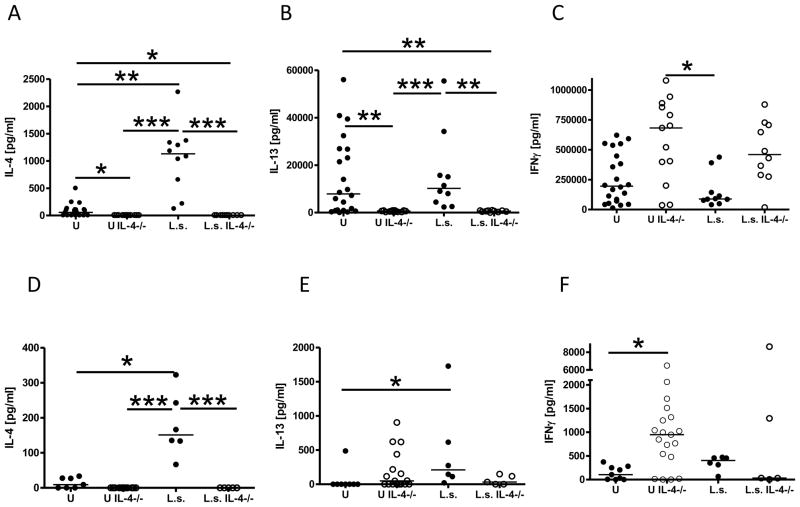
Cytokine analysis from immunocompetent NOD mice revealed that helminth infection increased the production of the type 2 cytokines IL-4 (Fig. 1A) and IL-5 (data not shown), but not IL-13 (Fig. 1B), from anti-CD3/anti-CD28 stimulated spleen cells as well as release of IL-4 (p>0.05) and IL-13 from pancreatic lymph node cells compared to uninfected controls (Fig. 1D, E). As IFN-γ production from spleen cells (Fig. 1C) or pancreatic lymph node cells (Fig. 1F) between infected and uninfected NOD mice was not significantly different, these results demonstrate that L. sigmodontis infection induces a general type 2 shift in cytokine production in NOD mice. Of note, this type 2 shift was also observed in comparison to age matched uninfected non-diabetic controls. Similar to the comparisons to uninfected diabetic controls, L. sigmodontis infected NOD mice had increased production of splenic IL-4, no differences in IL-13 concentrations, and decreased IFNγ levels compared to uninfected non-diabetic controls in response to polyclonal activation (Suppl. 1A–C). The Th2 shift in cytokine production was already present at 12 weeks of age, 6 weeks post L. sigmodontis infection, as anti-CD3/anti-CD28 stimulated spleen cells of L. sigmodontis infected NOD mice produced more IL-4, but less IFNγ, compared to uninfected controls (Suppl. 1D, E).
FIGURE 1. IL-4-deficient NOD mice fail to develop type 2 cytokine responses during L. sigmodontis infection.
Type 1 and type 2 cytokine production from splenocytes and pancreatic lymph node cells of uninfected (U) and L. sigmodontis-infected (L.s.) immunocompetent and IL-4-deficient NOD mice 1–2 weeks after the controls developed diabetes. Splenic production of (A) IL-4, (B) IL-13, (C) IFNγ, and production of (D) IL-4, (E) IL-13, (F) IFNγ from pancreatic lymph node cells that were stimulated with anti-CD3/anti-CD28. Each dot represents one mouse. Data is joined from 2–4 independent experiments. Statistical significance was assessed using the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons. *p<0.05; **p<0.01; ***p<0.001
In contrast, IL-4-deficient NOD mice were not able to mount a type 2 shift in cytokine production when infected with L. sigmodontis. Concentrations of IL-5 (data not shown) and IL-13 (Fig. 1B) produced from splenocytes and IL-13 production from pancreatic lymph node cells (Fig. 1E) of infected IL-4-deficient NOD mice were equivalent to those of uninfected IL-4-deficient NOD mice and lower than those of helminth-infected immunocompetent NOD mice (p>0.05 for IL-13 in the pancreatic lymph node). As expected, IL-4 was not detected in IL-4-deficient mice (Fig. 1A). Additionally, there were no statistically significant differences in splenic (Fig. 1C) and pancreatic lymph node IFN-γ production (Fig. 1F) between infected and uninfected IL-4-deficient NOD mice, though frequencies of splenic CD4+IFN-γ+ but not CD8+IFN-γ+ T cells (data not shown) were elevated in infected IL-4-deficient NOD mice compared to uninfected controls. Of note, splenic anti-CD3/anti-CD28 stimulated IL-13 and IFNγ cytokine production from IL-4 deficient NOD mice was similar between age matched non-diabetic and diabetic controls (Suppl. 1F, G). When compared to immunocompetent NOD mice, IL-4-deficient NOD mice had increased IFN-γ levels from spleen (Fig. 1C) and pancreatic lymph node cells (Fig. 1F), although in splenocytes this difference did not reach statistical significance. Taken together, these results demonstrate that unlike immunocompetent NOD mice, IL-4-deficient NOD mice do not develop a type 2 shift in cytokine production when infected with L. sigmodontis.
No type 2 shift in antibody production in L. sigmodontis-infected IL-4-deficient NOD mice
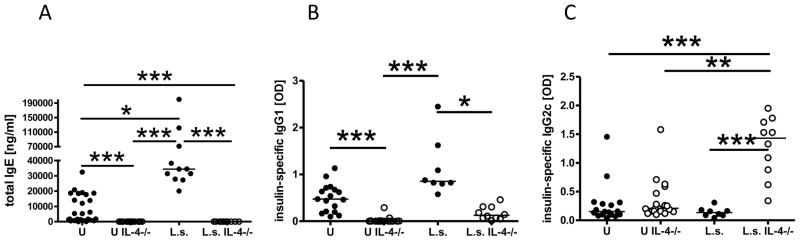
The impaired type 2 shift observed in cytokine production in IL-4-deficient NOD mice was also present in antibody production. In mice, type 2 immune responses drive the production of IgE and IgG1, whereas type 1 responses induce production of IgG2 antibodies. As expected, helminth-infected immunocompetent NOD mice had significantly increased levels of total IgE as well as elevated insulin-specific IgG1 levels compared to uninfected controls (Fig. 2A, B). In contrast, infected IL-4-deficient NOD mice did not develop measurable amounts of total IgE (detection limit 188 ng/ml) or significantly increased levels of insulin-specific IgG1 compared to uninfected IL-4-deficient NOD mice (Fig. 2A, B). Total IgE and insulin-specific IgG1 levels in infected IL-4-deficient NOD mice were significantly lower compared to infected immunocompetent NOD mice, whereas insulin-specific IgG2c levels (Fig. 2C) were significantly increased only in infected IL-4-deficient NOD mice. These results demonstrate that in the absence of IL-4 L. sigmodontis infection of NOD mice induces a type 1 rather than a type 2 shift of antibody isotypes against insulin, one of the main autoantigens in diabetes
FIGURE 2. Helminth-infected IL-4 deficient NOD mice develop a predominant type 1 shift in antibody isotype profiles.
A, plasma levels of total IgE (ng/ml) and (B) insulin-specific IgG1 as well as (C) insulin-specific IgG2c (OD) of uninfected (U) and L. sigmodontis-infected (L.s.) immunocompetent and IL-4-deficient NOD mice 1–2 weeks after the controls developed diabetes. Each dot represents one mouse. Data is joined from 2–4 independent experiments. Statistical significance was assessed using the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons. *p<0.05; **p<0.01; ***p<0.001
Infection of IL-4-deficient NOD mice with L. sigmodontis prevents the onset of diabetes
To determine whether type 2 responses were required for L. sigmodontis-mediated protection against type 1 diabetes in NOD mice, we measured serial blood glucose levels of infected and uninfected IL-4-deficient NOD mice and conducted histology on their pancreases at study endpoint.
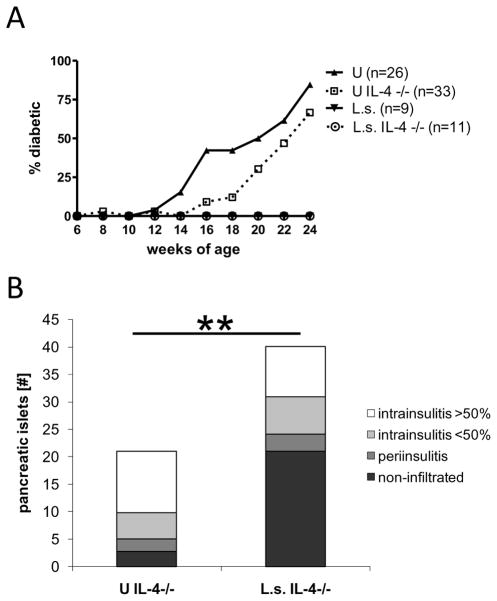
As seen in Fig. 3, NOD mice that were deficient for IL-4 were still protected against diabetes by infection with L. sigmodontis. Whereas none of the infected IL-4-deficient NOD mice developed diabetes, 67% of uninfected IL-4-deficient controls developed diabetes (Fig. 3A). These results are similar to disease development in immunocompetent NOD mice, where none of the infected NOD mice, but 85% of the uninfected controls, developed diabetes (Fig. 3A).
FIGURE 3. Helminth-infection prevents the development of diabetes in NOD mice even in the absence of IL-4.
A, percentages of diabetic mice (glucose >230 mg/dl) over time. Shown are immunocompetent and IL-4-deficient NOD mice infected with L. sigmodontis (L.s.) or corresponding uninfected controls (U). B, mean total numbers of pancreatic islets from IL-4-deficient L. sigmodontis infected NOD mice and uninfected controls (n=10 per group) 1–2 weeks after the controls developed diabetes. Pancreatic islets were classified as non-infiltrated, periinsulitis, and intrainsulitis with less than or greater than 50% infiltrated lymphocytes. Data is joined from 4 independent experiments. Significant differences between groups were analyzed by the Mann-Whitney test (** p<0.01).
Histology performed 1–2 weeks after the uninfected controls developed diabetes revealed that the total number of pancreatic islets was significantly increased in age-matched helminth-infected IL-4-deficient NOD mice compared to uninfected IL-4-deficient controls (Fig. 3B). Furthermore, greater than 50% of the islets from helminth-infected IL-4-deficient NOD mice were healthy islets, whereas uninfected controls had on average less than 10% of remaining islets classified as healthy and more than 50% of remaining islets documented as heavily infiltrated. While helminth infection prevented the onset of diabetes, it is important to note that infection did not reverse or completely stop the inflammatory response in the pancreas, as we observed on average 23% of islets in helminth-infected mice with more than 50% of infiltrated lymphocytes. These histological results of the pancreases were similar to results we reported previously in immunocompetent NOD mice (1).
Natural Regulatory T cells do not appear necessary for L. sigmodontis-mediated protection against Type I diabetes
Due to the fact that helminths are potent inducers of regulatory immunological networks that may prevent autoimmune diabetes, we investigated L. sigmodontis-induced regulatory responses. Natural regulatory T cells were identified by flow cytometry as CD4+CD25+FoxP3+ T cells (Fig. 4A). Analysis performed 1–2 weeks after the controls developed diabetes revealed significantly higher frequencies of natural regulatory T cells in the spleens of infected NOD mice compared to uninfected controls (Fig. 4B). Infection of IL-4-deficient NOD mice caused an increase in total numbers of splenic CD4+CD25+FoxP3+ cells in comparison to uninfected IL-4-deficient NOD mice, (Fig. 4C), though the difference in regulatory T cell frequencies between those groups was not statistically significant. Frequencies and total numbers of regulatory T cells in the pancreases of infected and uninfected animals were not significantly different (data not shown). Analysis of age-matched diabetic and non-diabetic uninfected mice revealed that diabetes status did not alter frequencies or numbers of CD4+CD25+FoxP3+ cells in uninfected controls (Suppl. 2A–B).
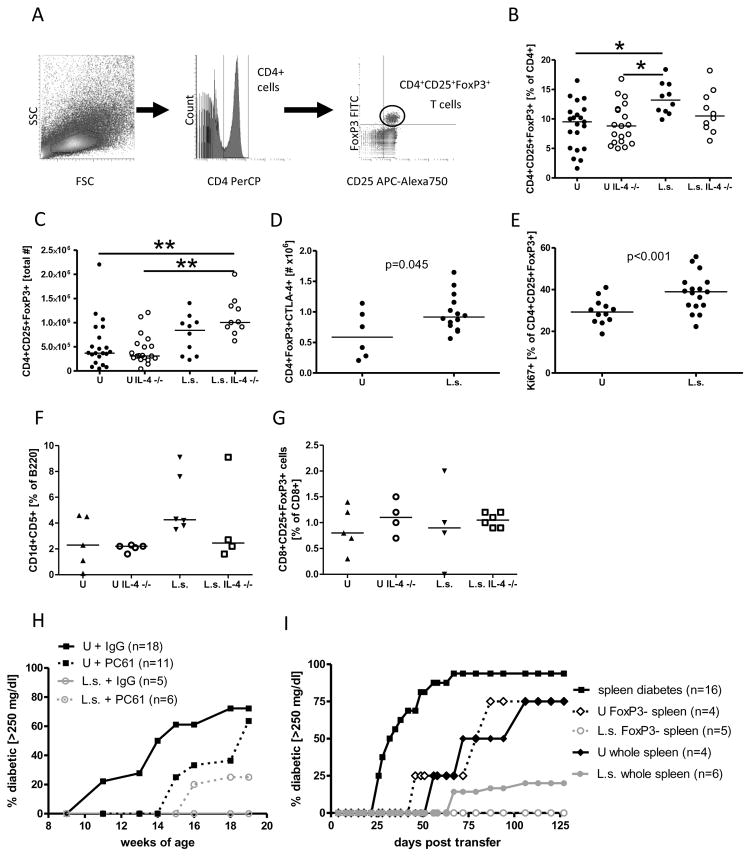
FIGURE 4. Regulatory T cells are not required for L. sigmodontis-mediated protection against Type 1 diabetes.
A, gating strategy for flow cytometric identification of regulatory T cells. Lymphocytes were gated by forward- (FSC) and sidescatter (SSC) characteristics (left panel). CD4 positive lymphocytes were gated (middle panel) and analyzed for CD25 and FoxP3 positivity (right panel). B, frequency and C, total number of splenic CD4+CD25+FoxP3+ T cells obtained from L. sigmodontis-infected (L.s.) and uninfected (U) IL-4-competent and IL-4-deficient NOD mice 1–2 weeks after the controls developed diabetes. D, total number of CD4+FoxP3+ spleen cells that express CTLA-4 in response to anti-CD3/anti-CD28. E, frequency of CD4+CD25+FoxP3+ spleen cells that spontaneously express the proliferation marker Ki67. F, frequency of splenic CD1dCD5+B220+ regulatory B cells and G, CD8+CD25+FoxP3+ regulatory T cells obtained from L. sigmodontis-infected (L.s.) and uninfected (U) IL-4-competent and IL-4-deficient NOD mice 1–2 weeks after the controls developed diabetes. H, Percentages of L. sigmodontis (L.s.) infected and uninfected (U) NOD mice that developed diabetes (glucose >250 mg/dl) while receiving anti-CD25 (PC61) or isotype control (IgG) weekly from 9 weeks of age to 20 weeks of age. I, Percentages of NOD.scid mice that developed diabetes after spleen cell transfer. NOD.scid mice received either splenocytes from diabetic NOD mice (squares), splenocytes from 14-week-old uninfected NOD mice (closed diamond), splenocytes depleted of FoxP3+ cells from 14-week-old uninfected NOD mice, splenocytes from L. sigmodontis-infected 14-week-old mice (open circles), or splenocytes depleted of FoxP3+ cells from 14-week-old L. sigmodontis-infected NOD mice.
Each dot represents one mouse. Data for Fig. 4 A–E is joined from 2–4 independent experiments, F, G represents data from a single experiment, H and I, are the joined results from two and three independent experiments, respectively. Statistical significance was assessed using the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons. Differences between two unpaired groups were tested for significance with the one-tailed Mann-Whitney test. *p<0.05; **p<0.01; ***p<0.001
The regulatory T cells were further characterized by their expression of CTLA-4, a molecule that transmits an inhibitory signal to T cells, and Ki67, a marker for proliferation. The total number of CD4+FoxP3+ spleen cells that expressed CTLA-4 after stimulation with anti-CD3/anti-CD28 was increased in L. sigmodontis-infected animals compared to controls (Fig. 4D) and spontaneous expression of the proliferation marker Ki67 was significantly increased in CD4+CD25+FoxP3+ spleen cells from helminth-infected animals compared to uninfected controls (Fig. 4E) as well as after stimulation with anti-CD3/anti-CD28 (data not shown). These results demonstrate that L. sigmodontis infection upregulates CD4+CD25+FoxP3+ cells and increases their basal proliferation and activation-induced CTLA-4 expression.
Additional flow cytometric analyses were conducted in a single experiment to evaluate other regulatory cell populations. As seen in Figure 4F, L. sigmodontis infection increased the frequency of splenic CD1d+CD5+B220+ regulatory B cells compared to uninfected controls in wildtype, but not IL-4 deficient NOD mice, though this difference did not reach statistical significance. The percentages of splenic CD8+ T-cells which exhibited a regulatory CD25+FoxP3+ phenotype were similar between L. sigmodontis infected NOD mice and controls, independent of their ability to produce IL-4 (Fig. 4G).
To analyze whether regulatory T cells are necessary for L. sigmodontis-mediated protection against Type I diabetes, we first monitored development of diabetes in NOD mice administered repeated injections of anti-CD25 antibody (clone PC61) in two independent experiments. PC61 efficiently depletes CD25+ cells and has been previously used by several investigators to evaluate the role of regulatory T cells. As seen in Figure 4H, 72% (13 of 18) of uninfected mice given isotype injections developed diabetes whereas infection of isotype treated NOD mice prevented the onset of diabetes in all mice studied. Depletion of CD25+ cells did not alter diabetes development in L. sigmodontis-infected NOD mice as only one out of six (17%) L. sigmodontis-infected mice that were treated with PC61 developed diabetes (Fig. 4H). However, treatment of uninfected controls with PC61 delayed the onset of diabetes, too, with only 4 out of 11 animals (36%) developing diabetes by 18 weeks of age. The observed partial protective effect of PC61 antibody administration in uninfected NOD mice may be due to depletion of effector T cells, which can express CD25 when activated. Therefore, it is not possible to definitively conclude from this experiment using PC61 antibody whether regulatory T cells are required for helminth-mediated protection against diabetes in NOD mice.
To clarify the role of regulatory T cells in helminth-mediated protection against diabetes onset, we next conducted experiments in which different splenocyte populations sorted from eGFP FoxP3+ mice were transferred into NOD.scid mice. Transfer of spleen cells from a diabetic donor into NOD.scid mice is commonly used as an inducible type 1 diabetes model as NOD.scid mice do not spontaneously develop diabetes. As evident in figure 4I, intravenous transfer of 8×106 spleen cells from diabetic NOD mice into NOD.scid mice induced diabetes starting at 26 days post transfer, with diabetes developing in 50% of animals at 32 days post transfer. Transfer of 1×107 spleen cells from uninfected NOD mice that were 14 weeks of age, a timepoint at which insulitis is typically present but overt diabetes is not yet manifest, resulted in diabetes onset in NOD.scid recipients, albeit at substantially later timepoints than observed after transfer of cells from diabetic mice (50% diabetes onset: non-diabetic splenocytes day 72, diabetic splenocytes day 32, Fig. 4I). Transfer of the same number of splenocytes from 14 week old uninfected animals after depletion of FoxP3 cells by FACS sorting of eGFP FoxP3+ cells did not substantially alter diabetes development (initial diabetes onset: FoxP3-depleted day 40, FoxP3-containing day 50; 50% diabetes onset day 79 vs. day 72). In marked contrast to transfer of non-diabetic splenocytes from uninfected mice, splenocytes of 14 week old NOD mice infected with L. sigmodontis for 8 weeks exhibited almost no capacity to induce diabetes. Whereas 75% of the NOD.scid mice that received spleen cells from non-diabetic uninfected NOD mice developed diabetes, only one out of six mice that received whole splenocytes from infected mice and none of the mice that received splenocytes depleted of FoxP3 cells from L. sigmodontis infected animals developed diabetes up to 125 days post transfer (Fig. 4I). These results demonstrate that splenocytes of NOD mice infected with L. sigmodontis for 8 weeks have little ability to induce autoimmune diabetes and suggest that FoxP3+ regulatory T cells, at least at that point of transfer, are not necessary to actively suppress autoimmunity.
To more directly test whether delayed diabetes onset after transfer of splenocytes from infected mice was due in part to active suppression of effector cells at time of transfer, we next conducted a series of experiments in which we transferred mixed splenocyte populations. Each of these mixed transfer experiments was conducted three times. Addition of 1×107 splenocytes from 14 week old mice infected with L. sigmodontis to 8×106 splenocytes obtained from uninfected diabetic mice did not delay diabetes onset, with 50% of NOD.scid mice developing diabetes at day 28 after transfer of the combined splenocyte population as compared to day 32 after transfer of just 8×106 splenocytes from diabetic mice (data not shown). Depletion of FoxP3+ cells from the splenocytes of non-diabetic infected and uninfected donors resulted in the onset of diabetes at 28 days post transfer in 50% of the animals of both groups when these cells were combined with splenocytes of a diabetic mouse during transfer (data not shown). These experiments suggest that splenocytes of L. sigmodontis-infected mice do not have the ability to actively downregulate autoimmunity induced by splenocytes of diabetic mice when transferred into NOD.scid mice.
TGFβ, but not IL-10, is required for helminth-mediated protection
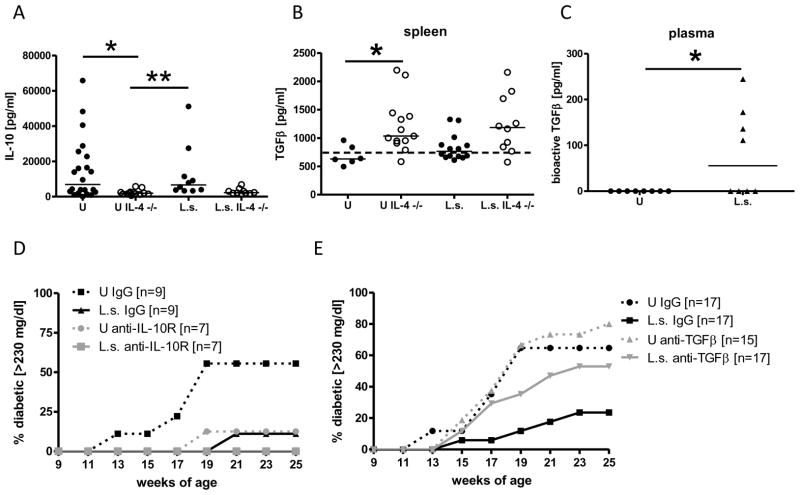
As the administration or expression of the anti-inflammatory cytokines IL-10 and TGFβ have been shown to prevent the onset of diabetes in NOD mice, and as both of these cytokines have been shown to play important roles in the immunoregulation that takes place in chronic filariasis (36–38), we tested whether L. sigmodontis-mediated protection against diabetes onset is dependent on TGFβ or IL-10. Infection with L. sigmodontis for 10–19 weeks did not increase splenic IL-10 production compared to uninfected mice in either IL-4-deficient or immunocompetent NOD mice (Fig. 5A). As with T-regulatory cells, no differences were seen in IL-10 production from splenocytes of age matched diabetic and non-diabetic uninfected wild type and IL-4-deficient NOD mice (Suppl. 2C). IL-4-deficient NOD mice produced less IL-10 compared to immunocompetent NOD mice, although this difference did not reach statistical significance in helminth-infected mice. Splenic production of TGFβ was slightly increased in L. sigmodontis-infected immunocompetent and IL-4-deficient NOD mice compared to uninfected controls, but these differences did not reach statistical significance (Fig. 5B). IL-4-deficient NOD mice produced significantly more splenic TGFβ than immunocompetent NOD mice in the absence of infection (Fig. 5B). Bioactive TGFβ levels were detectable in the plasma of half of the L. sigmodontis infected NOD mice at 10 (Fig. 5C) and 15 weeks of age (data not shown), whereas bioactive TGFβ concentrations were below the detection limit in all uninfected controls.
FIGURE 5. Helminth-mediated protection against diabetes onset requires TGFβ.
A, Production of IL-10 and (B) TGFβ in response to anti-CD3/anti-CD28 by splenocytes from uninfected (U) and L. sigmodontis-infected (L.s.) immunocompetent and IL-4-deficient NOD mice 1–2 weeks after the controls developed diabetes. The dashed line shows the baseline TGFβ concentration of the culture media. C, bioactive TGFβ levels in the plasma of L. sigmodontis-infected (L.s.) and uninfected NOD mice at 10 weeks of age. Development of diabetes (glucose >230 mg/dl) in mice that were either treated repeatedly with anti-IL-10R (D), anti-TGFβ (E), or isotype control. Shown are NOD mice infected with L. sigmodontis (L.s.) or corresponding uninfected controls (U). Each dot represents one mouse. Data for Fig. 5 A and B is joined from 2–4 independent experiments, C and D show results from one experiment, E shows joined results from two independent experiments. Statistical significance was assessed using the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons. *p<0.05; **p<0.01
We tested the requirement of IL-10 and TGFβ for the helminth-mediated protective effect against diabetes onset in NOD mice by depleting TGFβ in two independent experiments or blocking the IL-10 receptor (IL-10R) by repeated antibody treatment in a single experiment. Neither depletion of TGFβ nor blocking of IL-10R prevented L. sigmodontis development as all NOD mice that were euthanized at 17 weeks of age had 2–3 living adult worms present. Whereas 55% of isotype-treated uninfected animals developed diabetes by 19 weeks of age, none of the animals that were infected with L. sigmodontis and received isotype control antibody developed diabetes by that time point (Fig. 5D). Treatment with anti-IL-10R substantially delayed the onset of diabetes in uninfected animals. Only one out of 8 mice (12.5%) and none of the L. sigmodontis-infected anti-IL-10R treated NOD mice developed diabetes by 19 weeks of age (Fig. 5D). In contrast, treatment with anti-TGFβ reduced helminth-mediated protection against diabetes onset (17 weeks of age: 29% vs. 6% in L. sigmodontis infected controls treated with isotype antibody, 53% vs. 24% at 25 weeks of age, Fig. 5E). Treatment with anti-TGFβ did not alter diabetes onset in uninfected mice (38% vs. 35% for isotype treated uninfected NOD mice at 17 weeks of age).
L. sigmodontis infection does not decrease the Th17 immune response
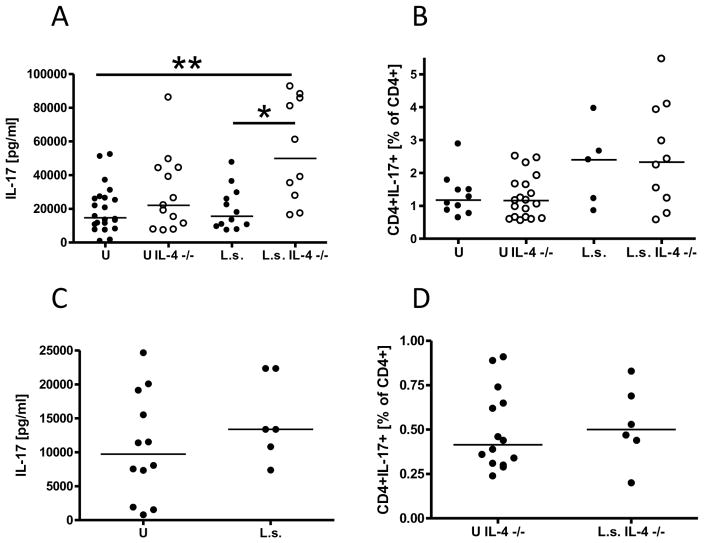
To determine whether helminth infections may ameliorate autoimmune diseases by reducing potentially pathological Th17 immune responses, we analyzed IL-17 release and the frequency of CD4+IL-17+ cells from spleen and pancreatic lymph nodes of immunocompetent and IL-4 deficient NOD mice.
Analysis of the Th17 immune response occurred 1–2 weeks after diabetes development in uninfected mice. L. sigmodontis infection of IL-4-competent or IL-4-deficient NOD mice did not significantly reduce splenocyte production of IL-17 (Fig. 6A) or frequencies of splenic CD4+IL-17+ T cells (Fig. 6B) in comparison to uninfected control mice. Indeed, IL-17 production from splenocytes of L. sigmodontis-infected IL-4-deficient NOD mice, which are protected against diabetes, was significantly greater than IL-17 production from uninfected control NOD mice. Similarly, there were also no significant differences in IL-17 production (Fig. 6C, tested with immunocompetent NOD mice) or frequencies of CD4+IL17+ cells (Fig. 6D, tested with IL-4 deficient NOD mice) in pancreatic lymph nodes of L. sigmodontis-infected mice.
FIGURE 6. Helminth infection of NOD mice does not reduce the Th17 immune response.
A, splenocyte production of IL-17 and (B) frequency of CD4+IL-17+ cells from spleens of uninfected (U) and L. sigmodontis-infected (L.s.) immunocompetent and IL-4-deficient NOD mice after activation with anti-CD3/anti-CD28. C, IL-17 release after anti-CD3/anti-CD28 stimulation of pancreatic lymph node cells of immunocompetent NOD mice. D, frequencies of CD4+CD17+ pancreatic lymph node cells from IL-4-deficient NOD mice. Each dot represents one mouse. Data is joined from 2–4 independent experiments. Statistical significance was assessed using the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons. *p<0.05; **p<0.01
Discussion
Studies in both animal models and humans have demonstrated that helminth infections and helminth antigens can protect against Th1-driven autoimmune diseases (1, 2, 7–9, 39–41). One of the leading hypotheses has been that helminth-induced type 2 responses may play an important role by downregulating autoimmune Th1 responses (17–22). The results of this study, however, demonstrate that chronic helminth infection can protect against type 1-driven autoimmune disease even in the absence of a Th2 response. While L. sigmodontis induced strong type 2 immune responses in immunocompetent NOD mice, infected IL-4-deficient NOD mice showed no evidence of a type 2 immune shift. Specifically, in contrast to infected immunocompetent NOD mice, L. sigmodontis infected IL-4-deficient mice exhibited no significant increases in production of IL-5 or IL-13, failed to make any detectable IgE, and did not develop a type 2 shift in autoantigen-specific IgG antibody isotypes. Although IL-13 signals through IL-4Rα, we believe these results convincingly demonstrate that in this study IL-13 did not assume the role of IL-4 in inducing a type 2 immune shift. Despite this lack of a type 2 immune shift, all L. sigmodontis-infected IL-4-deficient NOD mice were protected from the onset of type 1 diabetes. While the immune system of IL-4 deficient NOD mice develops in the absence of IL-4, and thus one might speculate that the protective immune responses exerted by helminths may be different in these mice than in wild type mice, both our results and those of another study show that IL-4 deficiency does not substantially alter development of type 1 diabetes in NOD mice (42). Consequently, we believe our results convincingly demonstrate that helminth infection can protect against autoimmunity in the absence of type 2 immune responses.
It is important to note that our finding that helminth-mediated autoimmune protection is not dependent on type 2 immune responses is in contrast to studies in experimental autoimmune encephalitis and trinitrobenzene sulfonic acid (TNBS) induced colitis which found that Schistosoma egg administration failed to protect against autoimmunity in mice deficient in STAT6 (21, 39) or depleted of IL-4 (39). While this discrepancy may be due to intrinsic differences in the mechanisms by which Schistosoma eggs and live L. sigmodontis filarial worms protect against autoimmunity, we suspect it is more likely due to differences in the time course of disease progression in the various models of autoimmunity. Whereas disease development in the NOD model occurs over a period of months, TNBS-induced colitis was maximal 3 days after TNBS challenge (39) and experimental autoimmune encephalitis 15–17 days after disease induction (21). Thus, it is possible that helminth-induced immunoprotective responses that are not dependent on type 2 immunity are either not strong enough to counteract rapid autoimmune inflammation or require several weeks to develop. As decreases in cellular proliferation and induction of regulatory T cells and downregulatory cytokines become most apparent in BALB/c mice months after infection with L. sigmodontis (43), we suspect the latter explanation is more likely.
Type 2-independent mechanisms responsible for helminth-mediated protection against autoimmunity are generally thought to be due to the induction of immunoregulatory networks. Some of the mechanisms by which filariae have been shown to suppress immune responses during chronic infection include induction of regulatory T cells, production of downregulatory cytokines such as TGFβ and IL-10, direct immunomodulation by parasite-produced molecules such as protease inhibitors and cytokine homologs, and alterations in T cell activation by alternatively activated macrophages (43–45). Regulatory T cells are implicated in control of autoimmune diabetes and recent reports suggest that loss of the suppressive function of regulatory T cells contributes to diabetes onset (23, 24, 46). This concept is supported by the finding that transfer of antigen-specific regulatory T cells or increase of endogenous regulatory T cell populations by IL-2 administration prevents the onset of diabetes in NOD mice (26, 27, 47). Accordingly, the protective effect of Schistosome egg antigen against diabetes onset is associated with increased numbers of CD4+CD25+FoxP3+ T cells in NOD mice and depletion of CD25+ regulatory T cells reverses this protective effect (48). Similarly, an increased total number of regulatory T cells in IL-4-deficient and immunocompetent L. sigmodontis infected NOD mice was observed in our study. However, transfer experiments in this study suggest that the L. sigmodontis-mediated protective effect against diabetes onset is independent of FoxP3+ regulatory T cells. Unlike splenocytes of uninfected mice, transfer of spleen cells from L. sigmodontis-infected NOD mice into NOD.scid mice did not induce diabetes. This did not appear to be due to active immune regulatory factors during the time of transfer, since splenocytes of infected NOD mice did not suppress diabetes induction when mixed with splenocytes of uninfected NOD mice. Further, depletion of FoxP3+ cells from splenocytes of L. sigmodontis-infected mice did not increase diabetes incidence, suggesting that L. sigmodontis-induced protection is likely not dependent on continuous immune downmodulation by FoxP3+ regulatory T cells. Consistent with our results Heligmosoides polygyrus-mediated protection against autoimmune diabetes in the absence of CD25+ regulatory T cells was recently reported (49), although our own results show that depletion of CD25+ T cells by PC61 administration delays diabetes onset even in control animals, probably by depletion of effector T cells. Future studies may investigate whether L. sigmodontis infection is able to protect against cyclophosphamide-induced diabetes in NOD mice, a model where the induction of diabetes is associated with the reduction of CD4+CD25+FoxP3+ regulatory T-cells (50). Of note, because we did not conduct T-regulatory cell depletion studies on IL-4 deficient mice, we cannot exclude the possibility that these cells may play an important role in L. sigmodontis-mediated protection against autoimmunity in the absence of type 2 immune responses.
IL-10 signaling was not required for the L. sigmodontis mediated protective effect. Treatment with anti-IL-10R did not abolish the helminth-mediated protective effect, but rather delayed the onset of diabetes in IL-10R treated control animals, suggesting a disease promoting role of IL-10. Such a diabetes inducing role of IL-10 was reported earlier in studies that used genetic expression of IL-10 on pancreatic islet cells (29, 30), whereas other studies showed a protective role for IL-10 (31, 51). Similar to our study, absence of IL-10 signaling did not prevent Heligmosoides polygyrus-mediated protection of experimental colitis or of autoimmune diabetes in NOD mice (4, 49). This suggests that IL-10 signaling is not required for helminth-mediated protection against autoimmune diseases and may even contribute to autoimmune diabetes onset.
In contrast to IL-4, CD25+ cells, Foxp3+ cells, and IL-10, TGFβ was shown to be essential for L. sigmodontis-mediated protection against Type 1 diabetes in this study as administration of neutralizing antibody to TGFβ completely reversed the beneficial effects of infection. Interestingly, TGFβ has also been shown to be necessary for Type I diabetes prevention in NOD mice mediated by glutamic acid decarboxylase (GAD) and zymosan administration (24, 52). Given that chronic helminth infections upregulate TGFβ production, and that TGFβ induces regulatory T cell populations and directly limits differentiation and activation of effector CD4 and CD8 T cells, (reviewed in (53)), it seems reasonable to conclude that TGFβ may indeed be one of the key regulatory molecules responsible for helminth-mediated protection against autoimmunity. It is important to note that while our transfer experiments did not reveal a necessary role for FoxP3+ regulatory T cells 8 weeks into L. sigmodontis infection, we cannot exclude the possibility that FoxP3+ regulatory T cells play an important role in helminth-mediated protection against autoimmunity at an earlier time point. Future work will focus on determining the cell types which release TGFβ during L. sigmodontis infection and the mechanisms by which TGFβ blocks diabetes development in helminth-infected NOD mice. Specifically, future studies will evaluate the effects helminth infection has on frequencies of autoantigen-specific CD4 and CD8 T cells and the role TGFβ plays in the development of both those cell types as well as regulatory T cells. As Burton et al. described that TGFβ+PD-L1+ macrophages prevented diabetes onset in NOD mice by infiltrating the pancreas of zymosan treated NOD mice and expanding FoxP3+ regulatory T-cells (52), it will be interesting to investigate whether L. sigmodontis infection induces similar anti-inflammatory macrophage populations in the pancreas and whether L. sigmodontis mediated protection is dependent on PD-L1. Further, we will analyze in future studies the role of L. sigmodontis induced NKT cells. NOD mice have a deficiency for NKT cells (54) and protection by soluble Schistosome worm or egg antigens was associated with the induction of Vα 14i NKT cells (55). Interestingly, TGFβ was shown to induce FoxP3+ regulatory NKT cells, a cell population that prevented experimental autoimmune encephalitis (56). As NKT cells can induce TGFβ production from myeloid cells and regulatory T-cells, future studies will investigate whether L. sigmodontis infection protects NOD mice via the induction of NKT cells.
Further, given that some filariae secrete a TGFβ homolog that binds and signals through TGF receptors (38, 57), future studies will evaluate whether L. sigmodontis produces a TGFβ homolog that is able to bind host TGF receptors and determine the relative contributions of helminth- and host-derived TGFβ. Of note, in this study we also evaluated IL-17 responses in NOD mice during chronic L. sigmodontis infection. Recent studies suggest that Th17 responses may have a role in the induction of Type I diabetes (58–60), although this may be due to the plasticity of Th17 cells and their conversion to pathogenic Th1 cells (61). Our finding of equivalent levels of IL-17 production by CD4+ T cells in helminth-infected and uninfected mice suggests that L sigmodontis mediated protection against diabetes does not occur by downregulation of Th17 responses.
In summary, this study demonstrates that L. sigmodontis-mediated protection against the onset of diabetes in the NOD model is very robust and does not depend on a Th2 shift, IL-10, or, after 8 weeks of infection, FoxP3+ regulatory T cells. In contrast, TGFβ was found to be crucial for autoimmune protection by L. sigmodontis. Given that type 2 immune responses drive allergic disease, and that these responses are not necessary for helminth-mediated protection against autoimmunity, these findings suggest that it may be possible to develop helminth-derived therapies for autoimmunity which induce protective regulatory mechanisms without upregulating potentially harmful pro-allergic Th2 immune responses.
Supplementary Material
Acknowledgments
We thank Drs. David Harlan and Klaus Pechhold from the University of Massachusetts’ Diabetes Center of Excellence for helpful discussions on working with the NOD model of diabetes. We also thank Karen Wolcott and Kateryna Lund at the USU Biomedical Instrumentation Center and Susanne Pechhold from the University of Massachusetts Medical School for their valuable assistance with flow cytometry.
Grant support: This work was supported by grant 1DP2DK083131 from NIH/NIDDK and part of this project by the European Community’s Marie Curie International Reintegration grant C-051.0150.
References
- 1.Hübner MP, Stocker JT, Mitre E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology. 2009;127:512–522. doi: 10.1111/j.1365-2567.2008.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 3.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 4.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 5.Fleming JO, Cook TD. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67:2085–2086. doi: 10.1212/01.wnl.0000247663.40297.2d. [DOI] [PubMed] [Google Scholar]
- 6.Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, Collins SM. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002;70:5931–5937. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summers RW, Elliott DE, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Maggi E, Parronchi P, Manetti R, Simonelli C, Piccinni MP, Rugiu FS, De Carli M, Ricci M, Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148:2142–2147. [PubMed] [Google Scholar]
- 11.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 12.Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383–5390. [PubMed] [Google Scholar]
- 13.Shaw MK, Lorens JB, Dhawan A, DalCanto R, Tse HY, Tran AB, Bonpane C, Eswaran SL, Brocke S, Sarvetnick N, Steinman L, Nolan GP, Fathman CG. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:1711–1714. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, Mule JJ, McDonagh KT, Fox DA. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J Clin Invest. 2001;107:1275–1284. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184:1093–1099. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25−CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 17.Cooke A. Review series on helminths, immune modulation and the hygiene hypothesis: how might infection modulate the onset of type 1 diabetes? Immunology. 2009;126:12–17. doi: 10.1111/j.1365-2567.2008.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol. 2007;37:457–464. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Harnett W, Harnett MM. Therapeutic immunomodulators from nematode parasites. Expert Rev Mol Med. 2008;10:e18. doi: 10.1017/S1462399408000720. [DOI] [PubMed] [Google Scholar]
- 20.Maizels RM, Yazdanbakhsh M. T-cell regulation in helminth parasite infections: implications for inflammatory diseases. Chem Immunol Allergy. 2008;94:112–123. doi: 10.1159/000154944. [DOI] [PubMed] [Google Scholar]
- 21.Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 22.Wilson MS, Maizels RM. Regulation of allergy and autoimmunity in helminth infection. Clin Rev Allergy Immunol. 2004;26:35–50. doi: 10.1385/CRIAI:26:1:35. [DOI] [PubMed] [Google Scholar]
- 23.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 24.Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, Yu P, Zaghouani H. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–7316. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 25.Tritt M, Sgouroudis E, d’Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 26.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonkin DR, He J, Barbour G, Haskins K. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. J Immunol. 2008;181:4516–4522. doi: 10.4049/jimmunol.181.7.4516. [DOI] [PubMed] [Google Scholar]
- 29.Moritani M, Yoshimoto K, Tashiro F, Hashimoto C, Miyazaki J, Ii S, Kudo E, Iwahana H, Hayashi Y, Sano T, et al. Transgenic expression of IL-10 in pancreatic islet A cells accelerates autoimmune insulitis and diabetes in non-obese diabetic mice. Int Immunol. 1994;6:1927–1936. doi: 10.1093/intimm/6.12.1927. [DOI] [PubMed] [Google Scholar]
- 30.Wogensen L, Lee MS, Sarvetnick N. Production of interleukin 10 by islet cells accelerates immune-mediated destruction of beta cells in nonobese diabetic mice. J Exp Med. 1994;179:1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennline KJ, Roque-Gaffney E, Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994;71:169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- 32.Grewal IS, Grewal KD, Wong FS, Wang H, Picarella DE, Janeway CA, Jr, Flavell RA. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun. 2002;19:9–22. doi: 10.1006/jaut.2002.0599. [DOI] [PubMed] [Google Scholar]
- 33.Moritani M, Yoshimoto K, Wong SF, Tanaka C, Yamaoka T, Sano T, Komagata Y, Miyazaki J, Kikutani H, Itakura M. Abrogation of autoimmune diabetes in nonobese diabetic mice and protection against effector lymphocytes by transgenic paracrine TGF-beta1. J Clin Invest. 1998;102:499–506. doi: 10.1172/JCI2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piccirillo CA, Chang Y, Prud’homme GJ. TGF-beta1 somatic gene therapy prevents autoimmune disease in nonobese diabetic mice. J Immunol. 1998;161:3950–3956. [PubMed] [Google Scholar]
- 35.Hübner MP, Torrero MN, McCall JW, Mitre E. Litomosoides sigmodontis: A simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus) Exp Parasitol. 2009;123:95–98. doi: 10.1016/j.exppara.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-{beta} pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Specht S, Volkmann L, Wynn T, Hoerauf A. Interleukin-10 (IL-10) counterregulates IL-4-dependent effector mechanisms in Murine Filariasis. Infect Immun. 2004;72:6287–6293. doi: 10.1128/IAI.72.11.6287-6293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korten S, Buttner DW, Schmetz C, Hoerauf A, Mand S, Brattig N. The nematode parasite Onchocerca volvulus generates the transforming growth factor-beta (TGF-beta) Parasitol Res. 2009;105:731–741. doi: 10.1007/s00436-009-1450-9. [DOI] [PubMed] [Google Scholar]
- 39.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385–391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 40.El-Wakil HS, Aboushousha TS, El Haddad O, Gamil NB, Mansour T, El-Said H. Effect of schistosoma mansoni egg deposition on multiple low doses streptozotocin induced insulin dependent diabetes. J Egypt Soc Parasitol. 2002;32:987–1002. [PubMed] [Google Scholar]
- 41.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang B, Gonzalez A, Hoglund P, Katz JD, Benoist C, Mathis D. Interleukin-4 deficiency does not exacerbate disease in NOD mice. Diabetes. 1998;47:1207–1211. doi: 10.2337/diab.47.8.1207. [DOI] [PubMed] [Google Scholar]
- 43.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites--masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 44.Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol. 2005;27:417–429. doi: 10.1111/j.1365-3024.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 45.Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol. 2006;176:6918–6927. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- 46.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121:15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39:1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 49.Liu Q, Sundar K, Mishra PK, Mousavi G, Liu Z, Gaydo A, Alem F, Lagunoff D, Bleich D, Gause WC. Helminth infection can reduce insulitis and type 1 diabetes through CD25− and IL-10-independent mechanisms. Infect Immun. 2009;77:5347–5358. doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6603–6612. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 51.Phillips JM, Parish NM, Drage M, Cooke A. Cutting edge: interactions through the IL-10 receptor regulate autoimmune diabetes. J Immunol. 2001;167:6087–6091. doi: 10.4049/jimmunol.167.11.6087. [DOI] [PubMed] [Google Scholar]
- 52.Burton OT, Zaccone P, Phillips JM, De La Pena H, Fehervari Z, Azuma M, Gibbs S, Stockinger B, Cooke A. Roles for TGF-beta and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in nonobese diabetic mice. J Immunol. 2010;185:2754–2762. doi: 10.4049/jimmunol.1001365. [DOI] [PubMed] [Google Scholar]
- 53.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. A defect in interleukin 12-induced activation and interferon gamma secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus. J Exp Med. 1999;190:963–972. doi: 10.1084/jem.190.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–1449. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 56.Monteiro M, Almeida CF, Caridade M, Ribot JC, Duarte J, Agua-Doce A, Wollenberg I, Silva-Santos B, Graca L. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-beta. J Immunol. 2010;185:2157–2163. doi: 10.4049/jimmunol.1000359. [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Escobar N, Gregory WF, Maizels RM. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta, expressed in microfilarial and adult stages of Brugia malayi. Infect Immun. 2000;68:6402–6410. doi: 10.1128/iai.68.11.6402-6410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooke A. Th17 cells in inflammatory conditions. Rev Diabet Stud. 2006;3:72–75. doi: 10.1900/RDS.2006.3.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.