Abstract
Rationale
B cells are abundant in the adventitia of normal and diseased vessels. Yet, the molecular and cellular mechanisms mediating homing of B cells to the vessel wall and B cell effects on atherosclerosis are poorly understood. Inhibitor of Differentiation-3 (Id3), is important for atheroprotection in mice and polymorphism in the human ID3 gene has been implicated as a potential risk marker of atherosclerosis in humans. Yet the role of Id3 in B cell regulation of atherosclerosis is unknown.
Objective
To determine if Id3 regulates B cell homing to the aorta and atheroprotection, and identify molecular and cellular mechanisms mediating this effect.
Methods and Results
Loss of Id3 in Apoe−/− mice resulted in early and increased atherosclerosis. Flow cytometry revealed a defect in Id3−/− Apoe−/− mice in the number of B cells in the aorta, but not the spleen, lymph nodes and circulation. Similarly, B cells transferred from Id3−/− Apoe−/− mice into B cell deficient micereconstituted spleen, lymph node and blood similarly to B cells from Id3+/+ Apoe−/− mice, but aortic reconstitution and B cell-mediated inhibition of diet-induced atherosclerosis was significantly impaired. In addition to retarding initiation of atherosclerosis, B cells homed to regions of existing atherosclerosis, reduced macrophage content in plaque and attenuated progression of disease. The chemokine receptor, CCR6, was identified as an important Id3 target mediating aortic homing and atheroprotection.
Conclusions
Together, these results are the first to identify the Id3-CCR6 pathway in B cells and demonstrate its role in aortic B cell homing and B cell mediated protection from early atherosclerosis.
Keywords: Atherosclerosis, B lymphocytes, Transcription factors, Helix-loop-helix, Homing
INTRODUCTION
Atherosclerosis is a progressive, chronic inflammatory disease resulting in plaque formation in large arteries1, 2 that can lead to heart attack, stroke and death. Targeting the immune cells that participate in atherogenesis is a promising novel approaches for atherosclerosis treatment and prevention3. Much is known about the recruitment of macrophages and T cells to the vessel wall and their role in plaque progression4, however B cells in atherosclerosis are incompletely understood. As early as the 1960s, studies have identified B cells, plasma cells and immunoglobulins in association with atherosclerotic plaques of mice and humans5–14. More recent studies in mice identified B cell-containing aortic tertiary lymphoid organs (ATLO) in the adventitia adjacent to atherosclerotic plaques, raising the possibility that adventitial B cells may regulate a local immune response within the vessel wall5, 6, 14. In addition, while ATLOs are not present in the normal/non-inflamed aorta prior to Western feeding5, a significant population of leukocytes, including B cells, are present in the aortic adventitia of normal vessels14. Global B cell deficiency established prior to Western diet feeding15, 16 results in increased atherosclerosis in mice, suggesting B cells function in early atheroprotection. Yet, the molecular mechanisms that regulate homeostatic trafficking of B cells to the aorta and their impact on the development of atherosclerosis are unknown.
Inhibitor of Differentiation-3 (Id3), a member of the helix-loop-helix (HLH) family of transcription factors, is a dominant negative inhibitor of bHLH protein-DNA binding and gene expression in B cells17. The Id3−/− mouse has normal numbers and maturity of B cells, but develops a Sjögren’s-like syndrome with lachrymal and salivary glands lymphocytic infiltrates18, 19, raising the interesting possibility that Id3 may regulate B cell homing to sites of disease. That hyperlipemia increased Id3 expression in vitro and in the vessel wall in a porcine model suggested a link between Id3 and atherosclerosis20. This link was recently confirmed by studies demonstrating that aged and Western diet-fed Apoe−/− mice null for Id3 had significantly increased atherosclerosis compared with Apoe−/− mice wildtype for Id321. Moreover, Id3 may be involved in atheroprotection in humans as the human ID3 gene contains a single nucleotide polymorphism (SNP) that alters Id3 protein function and is associated with increased carotid intima-media thickness (cIMT) in humans21.
The present study demonstrates a new function for Id3, as a critical regulator of B cell aortic trafficking and B cell-mediated atheroprotection and identifies CCR6 as an Id3 target gene mediating these effects. In addition, we provide the first evidence that B cells home to regions prone to and with existing atherosclerosis leading to reduced macrophage accumulation and attenuation of lesion progression.
METHODS
Detailed experimental procedures and associated references are in Supplemental Material available at http://circres.ahajournals.org. All procedures using animals were carried out according to protocols approved by the Animal Care and Use Committee at the University of Virginia. For B cell adoptive transfer studies, spleens were harvested from 10–12 week old mice and B cells were isolated by negative selection using MACS anti-CD43 microbeads or a combination of MACS anti-CD43, anti-CD4, and anti-CD11b microbeads (Miltenyi Biotec). Serum cholesterol levels were determined using an Archtect 8000 series analyzer. Antibody titers were determined as detailed in the Supplemental Material.
Analysis of Atherosclerosis
After euthanizing the mice, the aorta was harvested from the heart to the iliac bifurcation. Both en face Sudan IV staining of the aorta and cross-sectional analysis of the root was utilized in this study to quantify atherosclerosis. Fluorescent imaging at the UVA Advanced Microscopy Core enabled identification of CFDA-SE-labeled B cells within the aorta. Immunohistochemical analysis of atherosclerosis was achieved through staining for MCP-1 (Santa Cruz Biotechnology Inc) and macrophage content was determined with Mac-2 staining (Cedarlane Laboratories). These protocols are detailed in the Supplemental Material.
Bone Marrow Transplantation
Id3+/+Apoe−/− and Id3−/− Apoe−/− mice were subjected to a sub-lethal dose of radiation (500 rads × 2 irradiations) and subsequently reconstituted with 5 × 106 bone marrow cells harvested from the femurs and tibias of Id3+/+Apoe−/− and Id3−/− Apoe−/− donor mice as detailed in the Supplemental Material.
Flow Cytometry
Lymph nodes, spleens, blood and aortas, including the adventitia, were harvested under a dissection microscope and processed for flow cytometry as previously described14 and detailed in the Supplemental Material.
Ex Vivo Imaging of Radiolabeled B cells
B cells were radiolabeled by incubation in indium-111 oxine solution. Following adoptive transfer of radiolabeled B cells, aortas were harvested and exposed to a high sensitivity, medium resolution phosphor imaging screen (PerkinElmer) overnight. The phosphor imaging screen was scanned using a PerkinElmer Cyclone Plus Phosphor Imaging System. This protocol is detailed in the Supplemental Material.
Optical Imaging of Aortic B Cells
Ex vivo fluorescence-mediated tomography (FMT) quantitative imaging (FMT 2500, VisEn Medical) was performed following incubation of aortas with Cy5.5-labeled anti-B220 antibodies (eBiosciences and Rockland Immunochemicals). This protocol is detailed in the Supplemental Material.
Real-time PCR
Total cellular RNA was collected from B cells using an RNeasy kit (Qiagen) as per the manufacturer’s instructions, cDNA was then synthesized using an iScript cDNA synthesis kit (BioRad), and real-time PCR reaction using a Bio-Rad iCycler and iQ SYBR Green Supermix (BioRad) was performed as detailed in the Supplemental Material.
Cellular Migration Studies
Splenic B cells were purified from Id3+/+ Apoe−/− and Id3−/− Apoe−/− mice and placed in the upper chamber of 5 μm pore size transwells containing either 1000 ng/ml of CXCL13 or 500 ng/ml of CCL20 in the bottom of the transwell. After incubating for 6 hours, the number of cells which had migrated through the transwell were counted using a flow cytometer as detailed in the Supplemental Material.
Statistical Analysis
A p-value <0.05 was considered statistically significant. All statistical analyses were performed using NCSS 2001 (Number Crunching Statistical Software, Kaysville, Utah) and GraphPad Prism5 (La Jolla, California). Statistical analysis is detailed in the Supplemental Material.
RESULTS
Id3 is Necessary for Early Atheroprotection
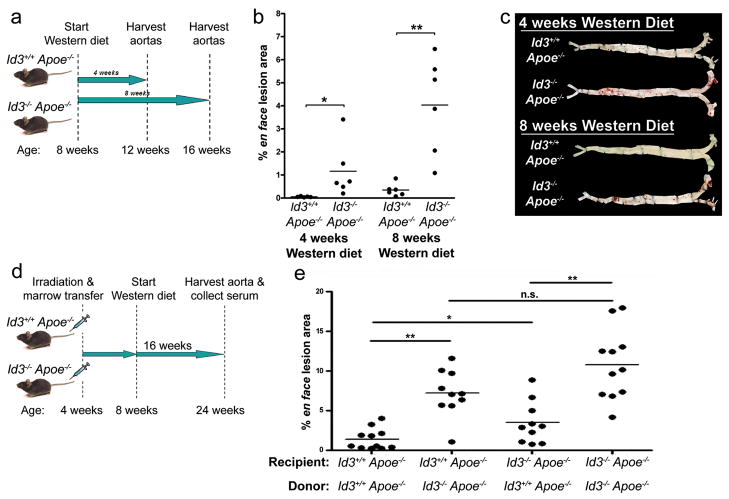
To determine if Id3−/− Apoe−/− mice developed premature atherosclerosis, Id3+/+ Apoe−/− and Id3−/− Apoe−/− mice were fed Western diet for four or eight weeks, then harvested for en face analysis of lesion area (Figure 1a). After four weeks of Western feeding, Id3−/− Apoe−/− but not Id3+/+ Apoe−/− mice developed a discernable amount of lesion (0.053% ± 0.009 vs 1.16% ± 0.483; p=0.004). After eight weeks of Western feeding, both genotypes developed detectable atherosclerosis, with significantly more lesion seen in the Id3−/− Apoe−/− mouse (0.347% ± 0.111 vs 4.03% ± 0.859; p=0.002) (Figure 1b). There were no differences in body weight, serum lipids, or the content of immunochemically detected oxidized phospholipids (OxPL) or malondialdehyde (MDA) epitopes on apoB lipoproteins (oxPL/ApoB or MDA/apoB), glucose or insulin in Id3−/− Apoe−/− compared with Id3+/+ Apoe−/− mice (Supplementary Table I).
Figure 1. Loss of Id3 accelerates atherosclerosis development in Apoe−/− mice fed Western diet – an effect mediated by a bone marrow-derived cell type.
a, Schematic of the experiment. Starting at 8 weeks of age, Id3+/+ Apoe−/− or Id3−/− Apoe−/− mice were placed on a Western diet for 4 or 8 weeks duration. Aortas were perfused with paraformaldehyde, harvested, opened longitudinally and stained with Sudan IV. b, En face lesion area was quantitated using Image-Pro 5.0 software. *:p=0.004, **:p=0.002. c, Representative en face images are shown d, Schematic of the experiment. At 4 weeks of age, Id3+/+ Apoe−/− or Id3−/− Apoe−/− mice were irradiated and transplanted with donor bone marrow, allowed to recover for four weeks, and then placed on a Western diet for 16 weeks. e, Aortas were then harvested and en face lesion area was quantitated as above. Each point represents a single animal. *:p=0.01, **:p=0.0005.
Id3 Atheroprotection is Predominantly Mediated by a Bone Marrow-Derived Cell
Bone marrow chimeras fed a Western diet for 16 weeks confirmed that Id3 deletion increased atherosclerosis and revealed that Id3−/− Apoe−/− mice reconstituted with bone marrow from Id3+/+ Apoe−/− mice had a significant attenuation of atherosclerosis compared to those reconstituted with Id3−/− Apoe−/− marrow (3.1-fold reduction). Similarly, Id3+/+ Apoe−/− mice developed significantly less atherosclerosis when reconstituted with bone marrow from Id3+/+ Apoe−/− compared with Id3−/− Apoe−/− mice (5.2-fold reduction) (Figure 1e). There was a significant increase in atherosclerosis when Id3−/− Apoe−/− compared to control Id3+/+ Apoe−/− recipient mice were reconstituted with bone marrow from Id3+/+ Apoe−/− mice, suggesting some contribution of loss of Id3 in cells not derived from bone marrow, although this effect was much less marked than when the bone marrow cells lacked Id3. No differences in weight, lipid parameters, glucose or insulin were noted in the bone marrow chimeras (Supplementary Table II).
Id3−/− Apoe−/− Mice Have Significantly Fewer B Cells in the Aortas Compared with Id3+/+ Apoe−/− Mice
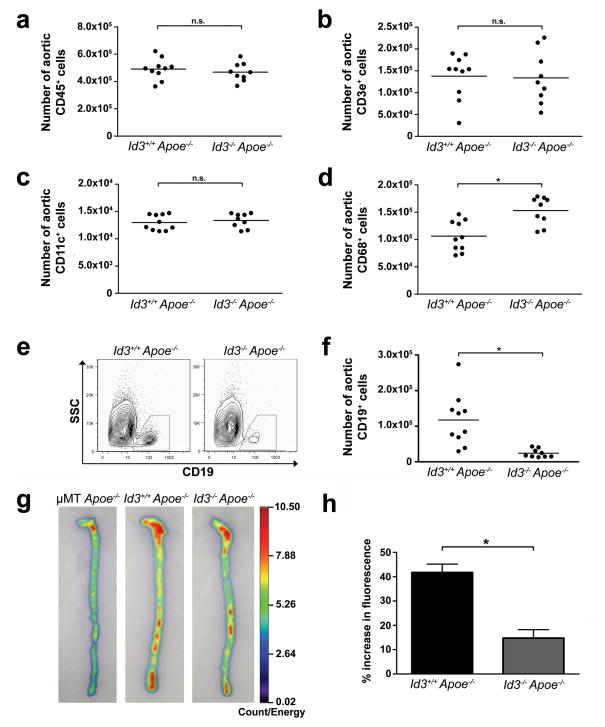
The early onset atherosclerosis in the Id3−/− Apoe−/− mice raised the interesting hypothesis that Id3 may regulate immune cells resident in the aorta prior to lesion development. Flow cytometry of aortas from chow-fed Id3−/− Apoe−/− and Id3+/+ Apoe−/− mice demonstrated equivalent numbers of total leukocytes (CD45+) (Figure 2a), T cells (Figure 2b) and dendritic cells (Figure 2c). The number of macrophages was higher in the aortas of Id3−/− Apoe−/− mice (Figure 2d). In contrast, the number of B cells in the aorta was significantly lower in Id3−/− Apoe−/− mice (Figure 2e and f). This finding cannot be explained by a global reduction in the number of B cells in the Id3−/− Apoe−/− mouse, as consistent with previous findings in Id3−/− mice18, the number of B cells in the spleen, periaortic lymph nodes and whole blood were similar between Id3+/+ Apoe−/− and Id3−/− Apoe−/− mice (Table 1).
Figure 2. B cell number in the aortas of Id3−/− Apoe−/− mice is significantly decreased compared with the aortas of Id3+/+ Apoe−/− mice.
Aortas, spleens, lymph nodes and whole blood were harvested from chow-fed Id3+/+ Apoe−/− or Id3−/− Apoe−/− mice at eight weeks of age. Each point represents an aorta from a single animal. Quantitation of aortic a, leukocytes (CD45+). b, T cells (CD45+ CD3e+). c, dendritic cells (CD45+ CD11c+) and d, macrophages (CD45+ CD68+). *p=0.003 e, Representative plots and f, quantitation of aortic B cells (CD45+ CD19+). g, Representative 2-dimensional fluorescent reflectance images of aortas after incubation with Cy5.5-labeled anti-B220 antibody. h, Quantitation of mean fluorescence of Id3+/+Apoe−/− and Id3−/− Apoe−/− mice. Data are represented as increase above mean fluorescence in the μMT Apoe−/− control group. Error bars reflect the SEM. *: p<0.0006.
Table 1.
Number of B cells in the spleen, peri-aortic lymph nodes and whole blood.
| Id3+/+ Apoe−/− | Id3−/− Apoe−/− | p-value | |
|---|---|---|---|
| Peripheral B lymphocytes (×106 CD19+ CD45+ cells) | |||
| Spleen | 56.5 ± 10.4 | 56.3 ± 9.31 | n.s. |
| Lymph nodes | 0.89 ± 0.11 | 0.88 ± 0.14 | n.s. |
| Blood (per ml) | 1.96 ± 0.21 | 1.91 ± 0.29 | n.s. |
Values are the mean ± standard deviation. n.s.: not significant.
Optical Imaging Confirms Fewer B Cells in Aorta of Id3−/− Apoe−/− mice and Reveals that the B cells present predominantly localize to Atheroprone Regions of the Aorta
Optical imaging using a Cy5.5-labeled anti-B220 antibody provided a second method to demonstrate B cells in the aorta of young chow-fed Id3−/− Apoe−/− and Id3+/+ Apoe−/− mice. As a control, μMT mice, which lack peripheral B cells due to deletion of genomic DNA sequences that encode the transmembrane domain of the B cell receptor μ heavy chain22, were bred to Apoe−/− mice to generate B cell-deficient Apoe−/− mice (μMT Apoe−/−). B cell-deficient μMT Apoe−/− aortas were found to have a minimal amount of background signal, which likely represents the non-specific retention of the Cy5.5-labeled anti-B220 antibody. While a significant increase in fluorescence over background was observed in both Id3+/+ Apoe−/− and Id3−/− Apoe−/− aortas (Figure 2g), consistent with our previous flow cytometric data, the increase in fluorescence over controls in the aortas of Id3−/− Apoe−/− mice was significantly lower than that in the aortas of Id3+/+ Apoe−/− mice (15% vs 42%; p<0.0006) (Figure 2g and h). Notably, peak fluorescence was greatest within the aortic arch and the abdominal aorta (Figure 2g), regions that are particularly prone to the development of atherosclerosis in this animal model23.
Id3 is Necessary for B Cell Homing to the Aorta
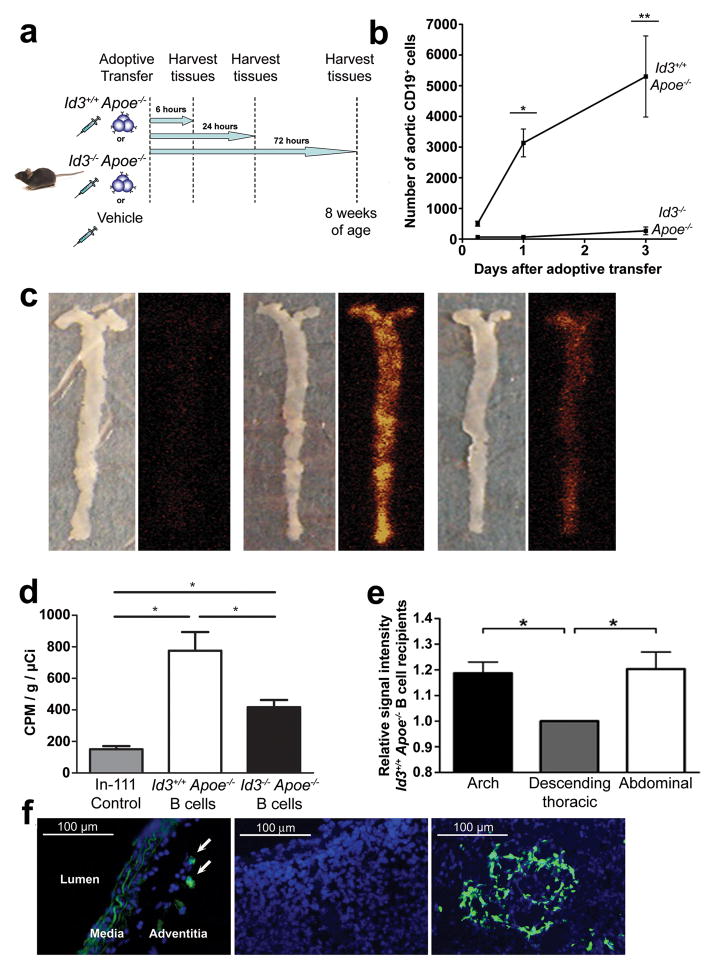
To determine if Id3 regulated homeostatic trafficking of B cells to the aorta, adoptive transfer studies were conducted. Splenic B cells from Id3+/+ Apoe−/− or Id3−/−Apoe−/− mice were adoptively transferred to μMT Apoe−/− mice. Control animals received an equal volume of vehicle. Six, 24 or 72 hours after the transfer of purified B cells, the aorta, spleen, lymph nodes and whole blood were harvested (Figure 3a) and analyzed by flow cytometry as described above. Apoe−/− B cells wild-type for Id3 appear in the aorta six hours after tail vein injection and continue to accumulate in the aorta up to 72 hours later. In contrast, no appreciable numbers of Id3−/− Apoe−/− B cells appear in the aorta at any of the time points tested (Figure 3b). Analysis of the spleens, lymph nodes and whole blood of the same animals revealed that B cell reconstitution of these compartments was similar between the two genotypes at each time point, providing evidence that reduced aortic B cell number was not due to a reduction in the total B cell pool (Table 2).
Figure 3. Id3 is necessary for B cell homing to the aorta.
a, Schematic of the experiment. b, Quantitation of aortic B cells after adoptive transfer. Plotted values indicate the average B cell number obtained from six animals ± SEM for each time point. *:p=0.03, **:p=0.002. c, Representative en face images of aortas and corresponding ex vivo phosphor images of chow-fed μMT Apoe−/− recipient mice 20 hours following tail vein injection of In-111 control or 1 × 107 In-111 radiolabeled B cells from Id3+/+ Apoe−/−,or Id3−/− Apoe−/− mice. d, Quantitation of aortic radioactivity by gamma well counting (counts per minute/grams of aortic tissue/injected radioactive dose in μCi) Error bars represent SEM. *:p<0.05. e, Regional signal intensity analysis comparing the aortic arch and the abdominal aorta relative to the descending thoracic aorta for ex vivo phosphor images of Id3+/+ Apoe−/− B cell recipient aortas. Error bar represents SEM. *:p<0.05. f, Left panel: Representative immunofluorescent images showing the location of CFDA-SE labeled Id3+/+ Apoe−/− B cells within the adventitia 72 hours after adoptive transfer to chow-fed μMT Apoe−/− mice. Center panel: spleen negative control from μMT Apoe−/− mice that received PBS vehicle. Right panel: spleen positive control, demonstrating CFDA-SE labeled B cells
Table 2.
Number of B cells in the spleen, peri-aortic lymph nodes and whole blood after adoptive transfer to μMT Apoe−/− mice
| Vehicle | Id3+/+ Apoe−/− | Id3−/− Apoe−/− | p-value | |
|---|---|---|---|---|
| Peripheral B lymphocytes (×105 CD19+ CD45+ cells) | ||||
| 6 hours post-transfer | ||||
| Spleen | 39.9 ± 0.59 | 37.6 ± 0.22 | n.s. | |
| Lymph node | 1.37 ± 0.64 | 1.02 ± 0.41 | n.s | |
| Blood (per ml) | 1.50 ± 0.40 | 1.60 ± 0.30 | n.s | |
| 24 hours post-transfer | ||||
| Spleen | 0.006 ± 0.001 | 72.1 ± 1.42 | 76.7 ± 1.37 | n.s. |
| Lymph node | 0.005 ± 0.001 | 1.11 ± 0.12 | 1.22 ± 0.19 | n.s. |
| Blood (per ml) | 0.006 ± 0.001 | 1.20 ± 0.30 | 1.00 ± 0.20 | n.s. |
| 72 hours post-transfer | ||||
| Spleen | 94.5 ± 0.50 | 93.4 ± 0.58 | n.s. | |
| Lymph node | 1.01 ± 0.10 | 1.12 ± 0.08 | n.s. | |
| Blood (per ml) | 1.40 ± 0.70 | 1.50 ± 0.40 | n.s. | |
Values are the mean ± standard error of the mean. n.s.: not significant.
To confirm Id3-dependent trafficking of B cells to the aorta, imaging of radiolabeled B cells injected intoμMT Apoe−/− mice was performed. Splenic B cells were isolated from Id3+/+ Apoe−/− and Id3−/− Apoe−/− mice and radiolabeled with indium-111 (In-111) oxine. Recipient μMT Apoe−/− mice were injected with 1 × 107 radiolabeled B cells or with In-111 oxine in normal saline as control. Aortas were harvested 20 hours later, opened en face and phosphor imaging was performed. Significantly more radioactive signal was present in the aortas of mice receiving Id3+/+ Apoe−/− compared with Id3−/−Apoe−/− B cells (Figure 3c and d). Consistent with optical imaging data (Figure 2g), radiolabelled B cells traffic to regions of the aorta that are prone to atherosclerosis (Figure 3c and e). To determine the layer of the vessel wall to which B cells traffic in these experiments, splenic B cells were purified from Id3+/+ Apoe−/− mice, incubated with CFDA-SE and adoptively transferred to μMT Apoe−/− mice. Seventy-two hours after transfer, aortas were harvested from mice, sectioned and analyzed for the presence of these labeled B cells. While the media and scattered adventitial cells revealed autofluorescence, CFDA-SE labeled B cells were only detected within the adventitia (Figure 3f). B cells in the spleen served as a positive control and spleen from mice receiving vehicle injection served as negative control.
Reconstitution of μMT Apoe−/− Mice with Id3+/+ Apoe−/− but not Id3−/− Apoe−/− B Cells Inhibited Western diet-induced Atherosclerosis
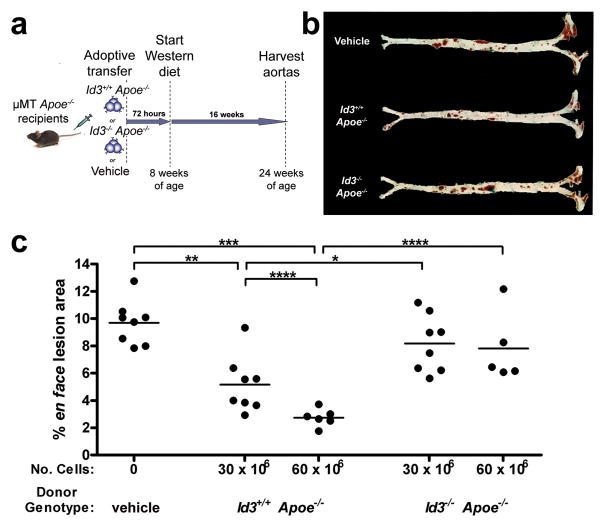
To determine whether Id3 is essential for B cell-mediated attenuation of atherosclerosis, adoptive transfer studies in μMT Apoe−/− mice were performed. Splenic B cells from Id3+/+ Apoe−/− or Id3−/− Apoe−/− mice were transferred via tail vein injection to B cell-deficient μMT Apoe−/− mice. Control mice received an equal volume of PBS vehicle. All recipient mice were then fed a Western diet for 16 weeks after which atherosclerosis was assessed by en face analysis (Figure 4a). Consistent with previous studies of B cell-deficient mice15, 16, μMT Apoe−/− mice fed 16 weeks of a Western diet developed significant atherosclerosis. A single injection of 30 × 106 Id3+/+Apoe−/− B cells to μMT Apoe−/−mice significantly reduced atherosclerotic lesion area (9.69% ± 0.568 vs 5.10% ± 0.836; p<0.002). B cell inhibition of atherosclerosis was dose-dependent, as there was an even greater reduction in atherosclerosis with the delivery of 60 × 106 Id3+/+Apoe−/− B cells (9.69% ± 0.568 vs 2.74% ± 0.263; p<0.001). In contrast, lesion area was not significantly changed in animals receiving either 30 or 60 × 106 Id3−/− Apoe−/− B cells (9.69% ± 0.568 vs 8.18% ± 0.735 or 7.82% ± 1.16) (Figure 4b and c). Flow cytometry confirmed a dose dependent increase in aortic B cell number with increasing numbers of Id3+/+Apoe−/− B cells injected. Injection of 30 × 106 Id3+/+Apoe−/− B cells resulted in an average of 2034 aortic B cells (data not shown) and 60 × 106 Id3+/+Apoe−/− B cells resulted in >5000 aortic B cells (Figure 3b), measured 72 hours after tail vein injection. For B cells from Id3−/− Apoe−/− mice, there were < 200 B cells in the aorta regardless of the number of B cells injected (data not shown and Figure 3b). There were no differences in the numbers of peripheral B cells (Table 3). These data demonstrate that B cell delivery prior to Western diet feeding protects μMT Apoe−/− mice from diet-induced atherosclerosis and that Id3 is essential for this B cell-mediated attenuation of atherosclerosis.
Figure 4. Id3+/+ Apoe−/− but not Id3−/− Apoe−/− B cells confer atheroprotection to B cell-deficient μMT ApoE−/− mice.
a, Schematic of the experiment. At eight weeks of age, 30 × 106 or 60 × 106 splenic B cells from either Id3+/+ Apoe−/− or Id3−/− Apoe−/− donors were adoptively transferred into μMT Apoe−/− recipients by tail vein injection. Control animals received an equal volume of PBS vehicle. Animals were allowed to recover for 72 hours, then fed a Western diet for 16 weeks. Aortas were harvested and analyzed by en face as described in Figure 1. b, Representative photos of aortic Sudan IV staining. c, Quantitation of aortic lesion size. Each point represents a single animal. *:p<0.05, **:p<0.002, ***:p<0.001, ****:p<0.005.
Table 3.
Number of B cells in the spleen, peri-aortic lymph nodes and whole blood after adoptive transfer to μMT Apoe−/− mice and 16 weeks of Western Diet feeding
| Id3+/+ Apoe−/− | Id3−/− Apoe−/− | p-value | |
|---|---|---|---|
| Peripheral B lymphocytes (×106 CD19+ CD45+ cells) | |||
| 30 × 106 B cells transferred | |||
| Spleen | 11.1 ± 1.31 | 10.6 ± 1.23 | n.s. |
| Lymph nodes | 1.86 ± 0.12 | 1.75 ± 0.17 | n.s. |
| Blood (per ml) | 0.29 ± 0.10 | 0.20 ± 0.10 | n.s. |
| 60 × 106 B cells transferred | |||
| Spleen | 16.9 ± 1.15 | 16.3 ± 0.81 | n.s. |
| Lymph nodes | 2.70 ± 0.16 | 2.60 ± 0.10 | n.s. |
| Blood (per ml) | 0.44 ± 0.20 | 0.29 ± 0.10 | n.s. |
Values are the mean ± standard error of the mean. n.s.: not significant.
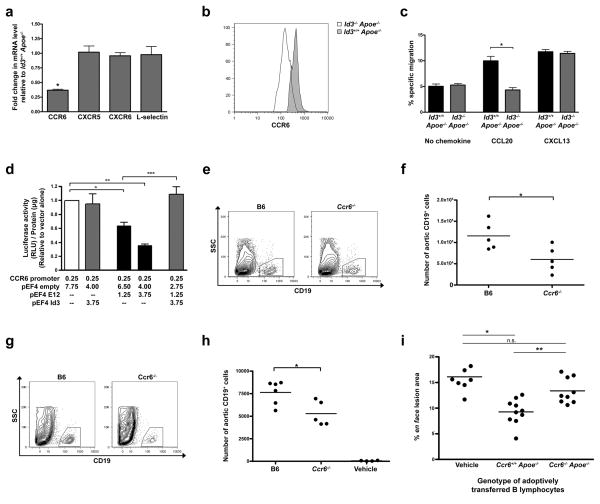
CCR6 is a novel Id3 target regulating B cell homing to the aorta and atheroprotection
To determine the chemokine receptors on B cells that may be regulating homing to the aorta in an Id3-dependent manner, we performed a murine PCR chemokines and receptor array (SA Biosciences) using RNA from B cells derived from Id3+/+ Apoe−/− or Id3−/− Apoe−/− mice. Notably, the chemokine receptor, CCR6, implicated in cell homing to sites of disease was significantly reduced. Real time PCR confirmed the array results, identifying a three-fold reduction in CCR6 mRNA expression in Id3−/− Apoe−/− as compared with Id3+/+ Apoe−/− B cells (Figure 5a). No change was noted in the levels of CXCR5, CXCR6 and L-selectin, which have previously been demonstrated to play a role in T cell homing to the aorta14, 24. Differences in surface expression of CCR6 protein was confirmed by flow cytometry (Figure 5b) and impaired migration of Id3−/− Apoe−/− B cells in response to the cognate ligand for CCR6 (CCL20) was demonstrated using transwell assays (Figure 5c). Id3 regulates target gene expression through dimerization with E2A gene products, like E12, antagonizing E12 DNA binding and transcription regulatory effects. Transient co-transfection studies in BJAB cells (a human B cell lymphoma line), demonstrated that E12 inhibits CCR6 promoter activation, an effect antagonized by co-transfection with an expression plasmid encoding Id3 (Figure 5d). Flow cytometry of aorta from C57BL/6 (B6) and CCR6−/− mice revealed fewer aortic B cells in CCR6−/− mice than those of age-matched littermate B6 control mice (Figure 5e and f). Adoptive transfer of CCR6−/− B cells to μMT Apoe−/− mice yielded significantly fewer B cells within the aortas of mice receiving B cells from CCR6−/− compared with B6 mice (Figure 5g and h). Moreover, adoptive transfer of 60 × 106 Ccr6+/+Apoe−/− B cells to μMT Apoe−/−mice significantly reduced atherosclerotic lesion area in response to 16 weeks of Western diet feeding (16.1% ± 0.992 vs 9.28% ± 0.783; p=0.0002). In contrast, lesion area was not significantly changed in animals receiving Ccr6−/− Apoe−/− B cells (16.1% ± 0.992 vs 13.4% ± 0.823; p=n.s.) (Figure 5i). These data identify CCR6 as an Id3 target involved in the aortic ‘address’ for B cell homing and B cell-mediated atheroprotection.
Figure 5. Id3−/− Apoe−/− B cells have decreased expression of CCR6 and expression of CCR6 on B cells mediates homing to the aorta and atheroprotection.
a, Real time PCR (see Methods for sequences) of B cell mRNA from Id3−/− Apoe−/− mice expressed relative to Id3+/+ Apoe−/− values. b, CCR6 staining of B cells from Id3+/+ Apoe−/−or Id3−/− Apoe−/− mice. c, Percent specific migration of B cells in response to 500 ng/ml of CCL20 or 1000 ng/ml of CXCL13. *:p=0.006. d, Luciferase activity from BJAB cells co-transfected with 2.3 kb human CCR6 promoter-luciferase construct and a pEF4 E12, empty pEF4 or pEF4 Id3 expression vectors. Experiments performed in triplicate were repeated 3 times. e, Representative flow plot and f, quantitation of B cell staining within aortas harvested from 10 to 12 week-old C57BL/6 (B6) or Ccr6−/− mice (n=5) *p=0.032. g, Representative flow plot and h, quantitation of B cell staining within aortas of μMT Apoe−/− mice 24 hours after adoptive transfer of 60 × 106 splenic B cells from B6 or Ccr6−/− mice or vehicle control. Each point represents a single animal. *p=0.009. i, Quantitation of en face lesion area of μMT Apoe−/− mice after adoptive transfer of 60 × 106 B cells from either Ccr6+/+ Apoe−/− or Ccr6−/− Apoe−/− mice or vehicle control. Animals were fed a Western diet for 16 weeks starting 72 hours after adoptive transfer. Each point represents a single animal. *:p=0.0002, **:p=0.002, n.s. = not significant.
B Cells traffic to Regions of the Aorta with Existing Atherosclerosis
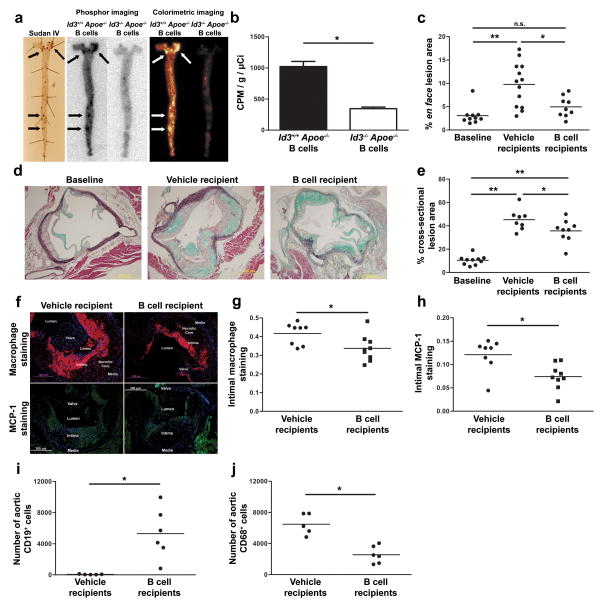
Optical and phosphor imaging demonstrated that B cells traffic to regions of the aorta known to be prone to atherosclerosis (Figure 2 and 3). To directly determine if B cells traffic to regions of atherosclerosis, μMT Apoe−/− mice were fed 8 weeks of Western diet to develop atherosclerosis. Animals were then injected with 2 × 107 radiolabeled Apoe−/− B cells or vehicle control. En face staining with Sudan IV demonstrated lipid deposition predominantly in the arch and abdominal aorta. Ex vivo phosphor imaging of aortas revealed the highest signal intensity in the regions of the aorta positive for Sudan IV staining (Figure 6a). Injection of an equal number of radiolabeled Id3−/− Apoe−/− B cells served as a control as we previously demonstrated an aortic homing defect in B cells null for Id3 (Figure 6a and b).
Figure 6. Adoptively transferred B cells traffic to regions of atherosclerosis, decrease plaque macrophage content and attenuate atherosclerosis progression.
a, Sudan IV staining and ex vivo phosphor imaging of aorta from μMT Apoe−/− mice fed eight weeks of Western diet and injected with 2 × 107 In-111 radiolabeled Apoe−/− or Id3−/−Apoe−/−B cells. b, Quantification (n=4) of counts adjusted for injected radioactivity and aortic weight. *:p=0.0003. c, Quantification of en face lesion area of μMT Apoe−/− mice fed 8 weeks of a Western diet (baseline, n=10) or injected with vehicle control (n=13) or 45 × 106 Apoe−/− B cells (n=9) followed by an additional 8 weeks of Western diet *:p=0.011, **:p=0.0005, n.s. = not significant. d, Representative images and e, quantitation of MOVAT stained cross-sections at the aortic cusp *:p<0.05, **:p<0.001. f, Representative images and quantitation of g, macrophage and h, MCP-1 content in μMT Apoe−/− mice with 8 weeks of prior Western diet feeding that received injection of vehicle control or Apoe−/− B cells and an additional 8 weeks of Western diet feeding. Aortic cross sections (4 per animal at 60 μm intervals) were stained using DAPI (blue) and either a Mac-2 monoclonal antibody (red) or a MCP-1 polyclonal antibody (green) *:p=0.024 and 0.007 respectively. Quantitation of aortic i, B cell (CD19+ CD45+) and j, macrophage (CD68+ CD45+) content determined by flow cytometry 72 hours following adoptive transfer of either PBS vehicle injection or 60 × 106 Id3+/+ Apoe−/− B cells to chow-fed μMT Apoe−/− mice. *:p=0.006 and 0.0005 respectively.
Delivery of Id3+/+ Apoe−/− B Cells Inhibited Progression of Western diet-induced Atherosclerosis: An effect attenuated by loss of Id3
B cell injection significantly inhibited atherosclerosis development when the B cells were delivered prior to Western diet feeding15, 16 (Figure 4). To determine whether B cells impact the progression of existing atherosclerosis, Apoe−/− B cells were adoptively transferred into μMT Apoe−/− mice with existing atherosclerosis. The μMT Apoe−/− mice fed 8 weeks of a Western diet underwent baseline atherosclerosis quantification (n=10) or received 45 × 106 Apoe−/− B cells (n=9) or vehicle control (n=13). Injected animals received an additional 8 weeks of Western diet and were then assessed by en face staining of the descending aorta and cross-sectional analysis of the aortic arch. Movat staining of cross sections confirm that en face lipid staining was accompanied by the histomorphologic components of atherosclerotic plaque (necrotic core and cellular infiltration). Consistent with whole aorta en face findings (Figures 4 and 6), there was significant atherosclerosis in the abdominal aorta by en face staining and in the arch by cross-sectional analysis in the vehicle treated group. In contrast, adoptive transfer of B cells from Apoe−/− mice significantly attenuated the progression of atherosclerosis when compared with vehicle recipients as measured by en face (4.9 ± 0.7% vs 9.7 ± 1.3% respectively, p=0.011) (Figure 6c) and cross-sectional analysis (35.6 ± 3.2% vs 45.3 ± 3.1%, p=0.046) (Figure 6d and e). Serum collected at the time of aorta harvest was analyzed for lipids. There were no significant differences in plasma cholesterol, triglycerides, or OxPL/apoB levels between the groups (data not shown).
B Cells Attenuate Aortic macrophage content
The mechanisms whereby aortic B cells attenuate plaque development are unknown. As Id3−/− mice have increased total IgM in serum25, we investigated the serum levels of antibodies known to be correlated with atherosclerosis in Id3+/+ Apoe−/− and Id3−/−Apoe−/− chow-fed mice. Paradoxically, IgM MDA-LDL and IgM Cu-OxLDL, which are associated with lower levels of atherosclerosis26, were found to be increased in Id3−/−Apoe−/− mice compared with Id3+/+ Apoe−/− mice. Levels of IgG MDA-LDL and IgG Cu-OxLDL were not significantly different between groups (Supplement Table I). Macrophages are present in the aorta prior to Western diet feeding14 (Figure 2d) and infiltrate the vessel wall early in the disease process3. To determine if B cell delivery to μMT Apoe−/− mice alters plaque macrophage content, immunohistochemical staining of plaques from vehicle and B cell injected μMT Apoe−/− mice was performed. Compared to control, adoptive transfer of B cells resulted in a significant reduction in Mac2 (Figure 6f and g, 0.34 ± 0.02 vs. 0.42 ± 0.02 respectively, p=0.024), and MCP-1 staining (Figure 6f and h, 0.074 ± 0.009 vs. 0.121 ± 0.012, p=0.007). Flow cytometry analysis of μMT Apoe−/− mice injected with Apoe−/− B cells to prior to Western diet feeding also revealed a significant decrease in aortic macrophage number (Figure 6i and j).
DISCUSSION
The present study clearly demonstrates that B cells can attenuate atherosclerosis and provides the first evidence linking resident aortic B cells with this atheroprotection. Previous studies utilizing transplantation of bone marrow from B cell-deficient μMT to Ldlr−/− mice16 or splenectomy of Apoe−/− mice15 had suggested an atheroprotective role for B cells. However, more recent studies using a strategy of reducing circulating B cells with CD20 monoclonal antibody treatment resulted in attenuation of atherosclerosis27, 28, suggesting that the impact of B cells on atherosclerosis may be subset and context-dependent. Indeed, recent studies have addressed the subset question and provide evidence that B2 cells promote27, 28 while B1a cells attenuate Western diet-induced atherosclerosis29. Results herein, address the question of context, providing the first evidence that mice with B cells resident in the aorta at baseline have less atherosclerosis in response to Western diet feeding compared to those with few aortic B cells. Id3−/− Apoe−/− mice, which had preserved numbers of circulating B cells but lacked sufficient aortic B cells (Figure 2f and g), developed significantly more atherosclerosis than Id3+/+ Apoe−/− mice (Figure 1). While it is possible that, in addition to B cells, other cell types could contribute to the increased atherosclerosis observed in the Id3−/− Apoe−/− mouse, the use of the μMT Apoe−/− recipient mouse in B cell adoptive transfer studies demonstrates a clear role for Id3 in mediating B cell homing and atheroprotection, as in the μMT Apoe−/− model Id3 is present in all cell types. Adoptive transfer of splenic B cells from Id3−/− Apoe−/− mice led to equal reconstitution of peripheral lymph tissue (spleen, lymph node and blood) as B cells from Id3+/+ Apoe−/− mice (Table 2), but aortic reconstitution (Figure 3b) and attenuation of Western diet-induced atherosclerosis (Figure 4c) were significantly impaired. That the adoptively transferred B cells come from a hyperlipemic mouse on an atherogenic background (Apoe−/−) appears to be important as neither 528 or 6030 million B cells from B6 mice were atheroprotective when transferred into μMT Apoe−/− recipient mice. Consistent with these findings, Western diet feeding induced Id3 expression in B cells (data not shown). Further support for aortic B cell-mediated atheroprotection is provided by the finding that B cells from Apoe−/− mice null for CCR6 but wildtype for Id3 also had reduced aortic B cell homing and B cell-mediated atheroprotection (Figure 5). Taken together, results provide evidence for a model of atheroprotection whereby resident aortic adventitial B cells serve as early responders to atherogenic signals in the vessel wall to limit atherosclerosis.
Chemokine receptors provide the ‘address’ for leukocyte homing to tissues31 and results of the present study provide the first evidence that CCR6 is important for B cell trafficking to the aorta and attenuation of Western diet-induced atherosclerosis. Yet, mice globally deficient in CCR6 have attenuated atherosclerosis32. CCR6 is expressed on various cell types involved in atherosclerosis33, and mice globally deficient in CCR6 have reduced circulating monocytes due to an reduced ability of these cells to leave the bone marrow32, providing evidence that CCR6 may have opposing effects on atherosclerosis development dependent on the cell type in which it is modulated. CCR6 has been identified as an important component of B cell trafficking to form isolated lymphoid follicle formation in the gut in inflammatory states34 suggesting that CCR6 may be a common chemokine receptor mediating B cell homing to tissue sites to modulate local inflammation5, 6. Results of the present study demonstrate that CCR6 is an Id3 target and that CCR6 regulates B cell homing to the aorta and B cell-mediated atheroprotection. Of note, the magnitude of the aortic homing defect in B cells from Id3−/−Apoe−/− mice is greater than that seen in CCR6−/− mice, suggesting that additional chemokine receptors may participate with CCR6 in the aortic ‘address’. Previous reports have identified CXCR6 and L-selectin as important regulators of T cell homing to the aorta14, 24, however our results suggest that the ‘address’ for B cell homing to the aorta is independent of these factors. Grabner, et al. have implicated vessel wall expression of CXCL13 and CCL21 in the formation of B cell rich ATLOs adjacent to advanced plaques in aged Apoe−/− mice5. Yet, in our studies of younger mice, B cells from Id3+/+ Apoe−/− and Id3−/− Apoe−/− mice had similar expression of CXCR5 (receptor for CXCL13) and CCR7 (receptor for CCL21) and transmigration in response to CXCL13 compared with controls (Figure 5 and data not shown), suggesting that unique chemokine signals may recruit B cells to the vessel wall prior to lesion development and in the early stages of atherosclerosis as compared with advanced lesions. It is also possible that Id3 may regulate other downstream target genes not involved in B cell homing, but involved in B cell-mediated atheroprotection.
Id3 dimerizes with E-proteins such as the E2A gene products, E12 and E47, which have critical functions in B cells35. As such, B cells from Id3 null mice may have other functional defects that promote atherogenesis. B cells produce antibodies and cytokine that modulate innate and adaptive immune responses. Evidence suggests that antibodies directed at oxidized phospholipid epitopes, such as OxLDL and MDA-LDL, may modulate atherosclerosis. In particular, IgM specific for OxLDL and MDA-LDL have been suggested to attenuate the development of atherosclerosis26, 36–39 while IgG specific for these epitopes may be atherogenic38, 40. Given that the major site of LDL oxidation is in the artery wall, it seems plausible that resident aortic B cells may be stimulated with vessel wall antigens to produce protective autoantibodies in the spleen or local tissue. We therefore explored circulating levels of several of these antibodies known to be associated with atherosclerosis. Paradoxically, serum levels of IgM to MDA-LDL and OxLDL were both increased in Id3−/− Apoe−/− mice compared with control. Consistent with previous literature18 examining antibody levels in young Id3−/− mice, the loss of Id3 in Apoe−/− mice was also associated with an increase in total serum IgM levels. This raises the possibility that the increased IgM MDA-LDL and OxLDL observed in these mice may be secondary to a nonspecific global increase in all IgMs. Moreover, the increased, not decreased, levels of the putatively protective IgM MDA-LDL and OxLDL in the Id3−/−Apoe−/− mice make it unlikely that production of these specific antibodies is the mechanism for the increased atherosclerosis.
B cells have long been known to reside in the adventitia of diseased arteries of mice and humans5–14, yet the specific location of these adventitial B cells in relation to atheroprone regions of the vascular tree has not been known. We demonstrate that B cells traffic to (Figure 3d) and reside (Figure 2g) in atheroprone regions of the aorta (the aortic arch and the descending abdominal aorta). Moreover, we demonstrate that B cells traffic to regions with existing lipid deposition (Figure 6a), reduce macrophage content within lesions (Figure 6f, g, and h) and retard the progression of these early lesions (Figure 6c). Interestingly, our results suggest that B cells regulate macrophage content of the aorta in response to Western diet feeding and even prior to feeding. Although μMT Apoe−/− mice do not have B cells in their aortas, they do have abundant macrophages. Reconstitution of the aorta of μMT Apoe−/− mice with Id3+/+ Apoe−/− B cells resulted in a significant reduction in aortic wall-associated macrophages (Figure 6i and j). This significant alteration of the aortic cellular milieu did not occur when Id3−/− Apoe−/−B cells were used (data not shown), suggesting that the effect is dependent on aortic B cell number. Notably, eight week old chow-fed Id3−/− Apoe−/− mice, which have fewer aortic B cells, were also found to have more aortic macrophages (Figure 2). The number of cells in the aortas of Id3−/− Apoe−/− and adoptively transferred μMT Apoe−/− mice cannot be directly compared due to the fact that these are distinct animal models and there is a significant difference in the degree of aortic B cell deficiency as well as in the chronicity of the B cell deficiency. However, both models independently provide evidence that B cells regulate aortic macrophage content. Given the rapid rate at which these changes occur, it is appealing to hypothesize that this effect is cytokine-mediated. Functional conduits connecting B cell-containing ATLOs in the adventitia and the vessel wall have recently been identified,5 suggesting that adventitial B cells may pass signals into the vessel wall. Immunoglobulins are too large to pass through these conduits5, however these conduits do allow passage of low molecular weight molecules such as cytokines from the ATLO to the media. Thus, adventitial B cells could limit macrophage accumulation in the intima through local production of anti-inflammatory cytokines that can pass into the vessel wall via these conduits. Future studies will be necessary to confirm these hypotheses and identify the cytokines involved.
The Id3−/− Apoe−/− mouse provides a unique model with which to explore the cellular and molecular atheroprotective mechanisms of leukocytes resident in the aortic adventitia of non-diseased vessels. Using this model, our findings provide evidence for a model whereby resident adventitial B cells within this microenvironment are poised to react to atherogenic stimuli from the vessel wall leading to production of factors that limit macrophage accumulation in plaque and progression of lesion development. Given the previously identified association between a functionally significant SNP in the human ID3 gene and carotid intima medial thickness (cIMT), our findings in mice may also have implications for human disease. Thus, identification of Id3 as a critical regulator of aortic B cell homing and atheroprotection is not only important for our understanding of these mechanisms, but may also lead to novel strategies to attenuate atherosclerosis in humans.
Supplementary Material
Novelty and Significance.
What is known?
B cells and immunoglobulins are in associated with atherosclerotic plaques in mice and humans, yet the factors regulating trafficking of B cells to the aorta and their impact on atherosclerosis are poorly understood.
Inhibitor of Differentiation-3 (Id3) regulates gene expression in B cells and deletion of Id3 in Apoe −/− mice null increases atherosclerosis.
Humans with a functionally significant polymorphism in the Id3 gene have increased carotid intima-media thickness compared with those homozygous for the ancestral allele.
What new information does this article contribute?
Id3 is necessary for early atheroprotection.
Loss of Id3 reduces B cell CCR6 expression and deficiency of both ID3 and CCR6 reduces B cell aortic homing and B cell-mediated atheroprotection.
B cells home to sites in the aorta prone to atherosclerosis and regions with early lipid deposition, reduce the macrophage content of the lesion and attenuate atherosclerosis progression.
B cells have been shown to both aggravate and attenuate atherogenesis, suggesting that the effects of B cells may be subset- or context- dependent. Here, we provide evidence that the effects of B cell on atherosclerosis are context-dependent based on the following findings: 1) Apoe−/− mice with reduced number of aortic, but normal number of peripheral B cells have increased atherosclerosis. 2) B cells home to, and attenuate initiation and progression of atherosclerosis in, regions of the aorta prone to atherosclerosis and attenuate initiation and progression of atherosclerosis. Our results identify the helix-loop-helix factor, ID3 as a critical regulator of B cell aortic homing and B cell mediated atheroprotection and define CCR6 as one downstream target of ID3 that promotes aortic B cell homing and B cell mediated atheroprotection. Taken together with previous findings of a functionally significant SNP in the human Id3 gene associated with carotid thickening in humans, these results suggest that strategies to enhance Id3 expression or function may provide immune protection against atherosclerosis.
Acknowledgments
The authors would like to thank Hong Pei and the UVA Mouse Surgery Transplantation and Physiology Core for excellent technical assistance, Jeremy Gatesman from the UVA Center for Comparative Medicine for excellent technical assistance, Dr. Borna Mehrad (UVA) for generously providing CCR6−/− mice, Dr. Yuan Zhuang (Duke University) for generously providing Id3−/− mice and guidance and Joanne Lannigan and Mike Solga from the UVA Flow Cytometry Core for excellent advice.
SOURCE OF FUNDING: This work was supported by NIH P01 HL55798 (C.A.M.), NIH RO1 HL62522 (C.A.M.), NIH R01 HL096447 (CAM), NIH R01 HL58108 (K.L.), R01 HL086559 (JLW and ST), P01 HL088093 (JLW and ST), NIH ARRA HL096447-01A1 (ST), Fondation Leducq (ST and JLW), NIH Training Grant 5-T32 HL007355-29 (M.J.L. and A.C.), American Heart Association Pre-Doctoral Fellowship (A.C.D.), and American Heart Association Mid-Atlantic Affiliate postdoctoral fellowship award 10POST3560000 (MJL).
Non-Standard Abbreviations and Acronyms
- Id3
Inhibitor of Differentiation-3
- ATLO
aortic tertiary lymphoid organs
- HLH
helix-loop-helix
- cIMT
carotid intima-media thickness
- OxPL
Oxidized phospholipids
- MDA
malondialdehyde
- ApoB
apolipoprotein B
- CFDA-SE
carboxyfluorescein diacetate succinimidyl ester
Footnotes
DISCLOSURES: None
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 4.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annual review of immunology. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, Hehlgans T, Mebius RE, van der Wall M, Kruspe D, Englert C, Lovas A, Hu D, Randolph GJ, Weih F, Habenicht AJ. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206(1):233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25(11):2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz CJ, Mitchell JR. Cellular infiltration of the human arterial adventitia associated with atheromatous plaques. Circulation. 1962;26:73–78. doi: 10.1161/01.cir.26.1.73. [DOI] [PubMed] [Google Scholar]
- 8.Aubry MC, Riehle DL, Edwards WD, Maradit-Kremers H, Roger VL, Sebo TJ, Gabriel SE. B-Lymphocytes in plaque and adventitia of coronary arteries in two patients with rheumatoid arthritis and coronary atherosclerosis: preliminary observations. Cardiovasc Pathol. 2004;13(4):233–236. doi: 10.1016/j.carpath.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Higuchi ML, Gutierrez PS, Bezerra HG, Palomino SA, Aiello VD, Silvestre JM, Libby P, Ramires JA. Comparison between adventitial and intimal inflammation of ruptured and nonruptured atherosclerotic plaques in human coronary arteries. Arq Bras Cardiol. 2002;79(1):20–24. doi: 10.1590/s0066-782x2002001000003. [DOI] [PubMed] [Google Scholar]
- 10.Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193(2):263–269. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH774>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Sangawa A, Sasaki Y, Yamashita M, Tanaka-Shintani M, Shintaku M, Ishikawa Y. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J Atheroscler Thromb. 2007;14(6):325–331. doi: 10.5551/jat.e489. [DOI] [PubMed] [Google Scholar]
- 12.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14(1):32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Hansson GK. Detection of B cells and proinflammatory cytokines in atherosclerotic plaques of hypercholesterolaemic apolipoprotein E knockout mice. Scand J Immunol. 1999;50(1):25–30. doi: 10.1046/j.1365-3083.1999.00559.x. [DOI] [PubMed] [Google Scholar]
- 14.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203(5):1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109(6):745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22(11):1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 17.Kee BL, Murre C. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J Exp Med. 1998;188(4):699–713. doi: 10.1084/jem.188.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol. 1999;19(9):5969–5980. doi: 10.1128/mcb.19.9.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren’s syndrome. Immunity. 2004;21(4):551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Taylor AM, Li F, Thimmalapura P, Gerrity RG, Sarembock IJ, Forrest S, Rutherford S, McNamara CA. Hyperlipemia and oxidation of LDL induce vascular smooth muscle cell growth: an effect mediated by the HLH factor Id3. J Vasc Res. 2006;43(2):123–130. doi: 10.1159/000090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doran AC, Lehtinen AB, Meller N, Lipinski MJ, Slayton RP, Oldham SN, Skaflen MD, Yeboah J, Rich SS, Bowden DW, McNamara CA. Id3 is a novel atheroprotective factor containing a functionally significant single-nucleotide polymorphism associated with intima-media thickness in humans. Circulation research. 2010;106(7):1303–1311. doi: 10.1161/CIRCRESAHA.109.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- 24.Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, Weber C, Ley K. CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation. 2007;116(16):1801–1811. doi: 10.1161/CIRCULATIONAHA.106.678474. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa I, Tedder TF, Zhuang Y. B-lymphocyte depletion ameliorates Sjogren’s syndrome in Id3 knockout mice. Immunology. 2007;122(1):73–79. doi: 10.1111/j.1365-2567.2007.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. JLipid Res. 2007;48(2):425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Ait-Oufella H, Herbin O, Bouaziz J-D, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, Sirvent Jrm, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. The Journal of Experimental Medicine. 2010;207(8):1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh B-H. Conventional B2 B Cell Depletion Ameliorates whereas Its Adoptive Transfer Aggravates Atherosclerosis. The Journal of Immunology. 2010;185(7):4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 29.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh B–H. B1a B Lymphocytes Are Atheroprotective by Secreting Natural IgM That Increases IgM Deposits and Reduces Necrotic Cores in Atherosclerotic Lesions. Circulation research. 2011 doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 30.Lipinski MJ, Perry HM, Doran AC, Oldham SN, McNamara CA. Comment on “Conventional B2 B Cell Depletion Ameliorates whereas Its Adoptive Transfer Aggravates Atherosclerosis”. The Journal of Immunology. 2011;186(1):4. doi: 10.4049/jimmunol.1090119. [DOI] [PubMed] [Google Scholar]
- 31.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12(3):336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 32.Wan W, Lim JK, Lionakis MS, Rivollier A, McDermott DH, Kelsall BL, Farber JM, Murphy PM. Genetic Deletion of Chemokine Receptor Ccr6 Decreases Atherogenesis in ApoE-Deficient Mice/Novelty and Significance. Circulation research. 109(4):374–381. doi: 10.1161/CIRCRESAHA.111.242578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine & Growth Factor Reviews. 2003;14(5):409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 34.McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170(4):1229–1240. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6(11):1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 36.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, Kohler HV, Kaveri SV, Nicoletti A. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol. 2007;50(6):540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 37.Nicoletti A, Kaveri S, Caligiuri G, Bariety J, Hansson GK. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J Clin Invest. 1998;102(5):910–918. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsimikas S, Bergmark C, Beyer RW, Patel R, Pattison J, Miller E, Juliano J, Witztum JL. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41(3):360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL Immunization induced T-cell-dependent antibody formation and protection aginst atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- 40.Fraley AE, Schwartz GG, Olsson AG, Kinlay S, Szarek M, Rifai N, Libby P, Ganz P, Witztum JL, Tsimikas S. Relationship of Oxidized Phospholipids and Biomarkers of Oxidized Low-Density Lipoprotein With Cardiovascular Risk Factors, Inflammatory Biomarkers, and Effect of Statin Therapy in Patients With Acute Coronary Syndromes: Results From the MIRACL (Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering) Trial. Journal of the American College of Cardiology. 2009;53(23):2186–2196. doi: 10.1016/j.jacc.2009.02.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.