Abstract
Recent metabolic profiles of human prostate cancer tissues showed a significant increase in cysteine (Cys) and a significant decrease in reduced glutathione (GSH) during cancer progression from low to high grade Gleason scores. Cys is primarily localized extracellularly, while GSH is present mostly inside the cell. We hypothesized that extra- or intracellular redox state alterations differentially regulate cell invasion in PC3 prostate carcinoma cells versus PrEC normal prostate epithelial cells. Cells were exposed to media with calculated Cys/CySS redox potentials (EhCySS) ranging from −60 to −180 mV. After 3 hr exposure to a reducing extracellular redox state (EhCySS −180 mV), matrix metalloprotease (MMP), gelatinase, and NADPH oxidase activities increased, correlating with increases in cell invasion, cell migration, and extracellular hydrogen peroxide levels in PC3 cells but not PrEC cells. Knockdown of NADPH oxidase or MMP with silencing RNAs during cultivation with EhCySS −180 mV media significantly decreased PC3 cell invasion. Modulation of extra- and intracellular redox states by exposure of PC3 cells to Cys/CySS-free media (approx. EhCySS −87 mV) containing 500 μM N-acetylcysteine resulted in a more reducing intracellular redox state and a significant decrease in cell invasive ability. The decrease in PC3 cell invasion induced by these conditions correlated with a decrease in MMP activity. Our studies demonstrated that an extracellular redox state that was more reducing than a physiologic microenvironment redox state increased PC3 cancer cell invasive ability, while an intracellular redox environmental that was more reducing than an intracellular physiologic redox state inhibited PC3 cell invasive ability.
Keywords: Cysteine, cystine, prostate cancer, invasion, growth
Introduction
Intracellular and extracellular redox states are the result of the net balance of reducing and oxidizing equivalents inside and outside the cell, respectively. Additionally, each subcellular compartment of each distinct cell type has a unique redox state designed to allow optimal physiological functioning. Significant changes in redox state in specific subcellular compartments and/or extracellular spaces result in cell adaptation and/or cell dysfunction. It has been demonstrated by several investigators that intracellular redox state is altered during cancer progression in certain cell types [1–3], but little is known about possible changes in extracellular redox state. We hypothesize that cancer cells develop specific alterations in both intra- and extracellular redox states as cancer progresses to a more aggressive state, with these changes postulated to promote abnormal behavior of cancer cells.
Extracellular/microenvironmental redox state is determined at least in part by the following factors [4]: 1) redox modulating proteins located in the plasma membrane such as NADPH oxidase (NOX1), 2) redox modulating proteins located outside of cells such as extracellular superoxide dismutase, 3) thiol/disulfide couples such as cysteine (Cys)/cystine (CySS), 4) reactive oxygen species (ROS)/reactive nitrogen species (RNS) that are capable of traveling across cell membranes, such as hydrogen peroxide (H2O2), 5) extracellular free radical damage products, including protein carbonyls, and 6) extracellular repair systems, such as protein disulfide isomerase. These molecules are the machinery that maintains redox homeostasis in the extracellular space/microenvironment.
The major aim of this study was to examine the possible role of the microenvironmental redox state in regulation of biology and biochemistry associated with prostate cancer cell behavior. We were particularly interested in the Cys/CySS redox couple due to its abundance in the extracellular space. Recent metabolic profiles of human prostate cancer tissues demonstrated a significant increase in Cys and a significant decrease in reduced glutathione (GSH) as cancer progresses in pathologic grade from low to high grade Gleason scores [5]. Moreover, it has been demonstrated that modifications of extracellular Cys/CySS could directly regulate cell proliferation by acting as an oxidant-reductant redox switch [6]. A reducing extracellular redox state modulated by Cys/CySS has been demonstrated to increase cell proliferation through a growth factor-signaling pathway in colon carcinoma cells [7]. A study in lung fibroblasts showed that an oxidizing extracellular redox state modulated by Cys/CySS stimulated cell proliferation and extracellular matrix expression [8]. These combined results suggest that extracellular Cys/CySS redox-dependent proliferation may be cell type specific.
Nevertheless, the relationship between extracellular redox state and prostate cancer progression has not been fully established. In the present study, we modulated Cys/CySS levels in the culture media of DU145 and PC3 prostate cancer cell lines and normal PrEC prostate epithelial cells. We were able to demonstrate that a reducing microenvironment with calculated redox potential of Cys/CySS (EhCySS) −180 mV favored prostate cancer invasive ability, at least partially through enhancing matrix metalloproteinase 9 (MMP9) activity and NADPH mediated-extracellular H2O2 production. In contrast, a reducing intracellular redox state induced by N-acetyl cysteine (NAC) resulted in a significant decrease in cell invasion and MMP activity in PC3 cells. Media in which EhCySS was altered (towards either oxidation or reduction) did not affect in vitro invasive ability of PrEC cells. Thus, the invasive abilities of prostate cancer cells were relatively more susceptible to changes of microenvironmental or intracellular redox states than normal prostate epithelial cells. Our results indicate the possibility of innovative chemotherapy targeting both extracellular and intracellular redox states.
Materials and Methods
Chemicals and reagents
All chemicals and reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO), unless otherwise specified. Tissue culture supplies were from Falcon Becton-Dickinson Labware (Franklin Lakes, NJ). All tissue culture reagents were obtained from Invitrogen Life Technologies (Carlsbad, CA) except prostate epithelial growth medium (PrEGM), which was obtained from Lonza Walkersville, Inc. (Walkersville, MD), Cys/CySS-free RPMI 1640 medium, which was obtained from Sigma-Aldrich Co., and fetal bovine serum (FBS), which was obtained from Tissue Culture Biologicals (Tulare, CA). Amplex Red H2O2/Peroxidase Assay kit and Orange Green 488 maleimide were obtained from Invitrogen Life Technologies. Polycarbonated (PCF) inserts and Amicon filter units were purchased from Millipore Co. (Billerica, MA). Pro-MMP2, pro-MMP9, and calcein-AM fluorescence dye were purchased from EMD Chemical Co. (Gibbstown, NJ). Microspin G-25 columns were obtained from Amersham Biosciences, (Little Chalfront, Bucks, U.K.). All silencing RNA (siRNA) and reagents were purchased from Dharmacon Inc. (Lafayette, CO). Fluorescein isothiocyanate (FITC)-conjugated gelatin was obtained from Elastin Products Co. (Owensville, MO). All antibodies were obtained from Santacruz Biotechnology Inc. (Santa Cruz, CA), except Trx1 antibody, which was purchased from AbFrontier Co. Ltd. (Geumcheon-gu, Seoul, Korea), and IRDye 800CW goat anti-rabbit IgG, which was purchased from Li-COR Biosciences (Lincoln, NE).
Preparation of Cys/CySS-supplemented media
The Cys/CySS-supplemented media were prepared fresh daily. Media with varying EhCySS were prepared by adding various concentrations of Cys-CySS (4 μM/300 μM or 450 μM/6 μM) to Cys-CySS-free RPMI 1640 medium containing 0.015 g/L methionine and 100 mg/L kanamycin sulfate (final pH 7.4). Stock solutions of Cys and CySS (10 mM, pH 7.4) were prepared fresh for every experiment and filtered through a 0.2 μM syringe filter before addition to Cys-CySS-free media. Before and after incubation with various cells, Cys and CySS concentrations were determined by HPLC with fluorescence detection.
Cell culture and treatment
PC3 and DU145 cells were obtained from ATCC, whereas normal prostate epithelial cells (PrEC) were obtained from Lonza Walkersville Inc. PC3 and DU145 cells were tested and confirmed for authenticity using short tandem repeats DNA typing by Biosynthesis Cell Inc. (Lewisville, TX). PC3 and DU145 cells were cultured in RPMI 1640 supplemented with 5% FBS, whereas PrEC cells were cultured in PrEGM for routine maintenance. PrEGM components are listed in Supplementary Table. 1. Cells were grown at 37°C in a humidified atmosphere of 95% air and 5% CO2. Trypsin (0.05%)/0.53 mM EDTA and soybean trypsin inhibitor were used for routine subculture. To determine extracellular redox potential during steady state, PC3 cells were cultured in regular RPMI 1640 medium while PrEC cells were cultured in PrEGM for 0 hr and 24 hr. To modulate extra- or intracellular redox states, DU145, PC3, and PrEC cells were cultured in RPMI 1640 serum-free medium for 24 hr and washed with PBS pH 7.4 three times before addition of Cys/CySS-free medium, Cys/CySS supplemented media, or the low molecular weight redox modulating compound, NAC, respectively.
Analysis of Cys, CySS, GSH, GSSG, and CyS-GSH disulfide (CySGSH)
Concentrations of Cys, CySS, GSH, GSSG, and CySGSH in cell culture media were determined by HPLC with fluorescence detection as described elsewhere [9]. Briefly, media were added (1:1) to ice-cold 10% perchloric acid solution containing 0.2 M boric acid. Samples were derivatized with iodoacetic acid (IAA) and dansyl chloride. Derivatized samples were centrifuged and the aqueous layer was applied to a Supelcosil LC-NH2 column. Thiols and disulfides were separated and quantified by integration of their HPLC peaks relative to the internal standard (gamma-glutamylglutamate). Extracellular redox potential of the redox couples Cys/CySS (EhCySS) and GSH/GSSG (EhGSSG) were calculated using the Nernst equation, EhCySS = −250 + 30 log [(CySS)/(Cys)2] and EhGSSG = −264 + 30 log [(GSSG)/(GSH)2], respectively.
In vitro invasion assay
Cells (5 × 105) were cultured in Cys/CySS-free medium or Cys/CySS supplemented media for 3 hr prior to seeding in the upper chamber of an in vitro cell invasion assay. Conditioned media and cells were placed together in the upper chambers whereas RPMI 1640 with 5% FBS was placed in the lower chambers. Invasion assays were performed as previously described [9].
Measurement of extracellular H2O2
Extracellular H2O2 levels were detected using the Amplex Red H2O2/Peroxidase Assay Kit based on the reaction of Amplex Red reagent and H2O2 to produce resorufin [1].
Matrix metalloproteinase activity and zymography assays
Conditioned media were concentrated using an Amicon Filter unit. Samples of concentrated conditioned media samples were analyzed by using MMP activity assay kit from Invitrogen Life Technologies or by electrophoresis (12% SDS-PAGE copolymerized with 1% gelatin as substrate) as previously described [9]; gelatinolytic activities were detected as white bands against a blue background.
siRNA transfection
Two different siRNAs for each protein were designed and synthesized by Dharmacon Inc. as follows: for NOX1 (cat. no. D-010193), GCACACCUGUUUAACUUUG (I) and UGAGAAGGCCGACAAAUAC (III); for MMP9 (cat. no. D-005970), GGAACCAGCUGUAUUUGUU (I) and GAAUACCUGUACCGCUAUG (III). Non-specific siRNA, which does not have specificity to any known cellular mRNAs, was used as control. Cells were seeded at 1 × 105 cells/well in 24 well-plates and allowed to grow to 60% confluence. Cells were transfected with 2 μM NOX1 or MMP9 siRNA with 2 μl DharmaFECT2 Transfection reagent in 1 mL serum-free medium for 48 hr, and then 1 mL fresh RPMI 1640 medium with 5% FBS was added to each well for 72 hr before treatment with Cys/CySS supplemented media.
In vitro wounding assay
Cells were seeded at 1 × 106 cells/well in 6 well-plates and allowed to grow to 80% confluence. Cells were incubated with Cys/CySS-free or Cys/CySS supplemented media for 3 hr; at the end of 3 hr, cells were wounded with a P200 pipette tip to create a straight line and washed with RPMI 1640 medium to remove cell debris and smooth the edge of the scratch [10]. Cells were incubated with 2 mL of RPMI 1640 medium containing 10% FBS and were assessed at 6, 12, 18, and 24 hr to measure scratch distances using a Nikon Eclipse Ti-U microscope with phase contrast filters. Migration distances were analyzed with NIS-Elements D 3.0 software.
Fluorescent gelatin degradation assay
FITC-conjugated gelatin-coated coverslips were prepared as previously described [11]. Gelatin-coated coverslips were quenched with RPMI 1640 medium containing 5% FBS at 37°C for 30 min prior to plating the cells. Cells were incubated with Cys/CySS-free or Cys/CySS supplemented media for 3 hr, and then cultured on FITC-gelatin-coated coverslips for 18 hr. Cells were fixed and stained for actin with rhodamine phalloidin. Degraded gelatin areas (pixels unit) were photographed using a Nikon Eclipse Ti-U microscope and subsequently analyzed with NIS-Elements D 3.0 software. Data were expressed as gelatin degraded area.
NADPH oxidase activity assay
PC3 cells were cultured with various Cys/CySS supplemented media for 3 hr. Cell homogenates were then collected as previously described [2]. Photoemission generated by the reaction of superoxide radical with lucigenin was monitored every minute for a total of 15 min.
Protein concentration, western blotting, and redox western blotting
Protein concentrations of conditioned media and cell lysates were determined by the Bradford assay according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA, USA). The protocols of western blot analysis were detailed elsewhere [1]. Twenty micrograms of protein from cell lysates or concentrated conditioned media were placed in each well. Anti-NOX1, -NOX2, -NOX4, -MMP2, -MMP9, -Trx1, or -GAPDH antibodies were added. The data images were analyzed using Image Quant software.
Redox western blotting was performed as previously described with slight modifications [8]. Briefly, cells were washed with ice-cold PBS and then were immediately lysed in 6 M guanidinium choride, 50 mM Tris/HCl, 3 mM EDTA, 0.5% Triton-X-100 containing 50 mM IAA, pH 8.3. After 30 min incubation at 37°C, excess IAA was removed using microspin G-25 columns. Trx1 redox isoforms were separated by native polyacrylamide gels and IRDye 800CW-conjugated goat anti-rabbit secondary antibody was used. Bands were visualized using an Odyssey scanner and analyzed using Odyssey analysis software (Li-Cor, Lincoln, NE, USA). The data of Trx1 redox western blotting were presented as a percentage of each Trx1 form [fully reduced (R), mixture of reduced and oxidized (Oxy1, one disulfide), and fully oxidized (Oxy2, two disulfides) forms] to total Trx1. Dithiothreitol (DTT, 100 mM) and H2O2 (10 mM) were incubated with PC3 cells for 30 min and were used as reduced Trx1 and oxidized Trx1 controls, respectively.
Statistics
All experiments were repeated at least three times. Statistical analysis was performed with student's t test or one way ANOVA followed by multiple comparison test using SPSS10 software. Mean differences were considered significant at p-value ≤ 0.05. All data are presented as Mean ± SEM.
Results
Correlation of extracellular redox state with prostate cancer cell growth and invasion
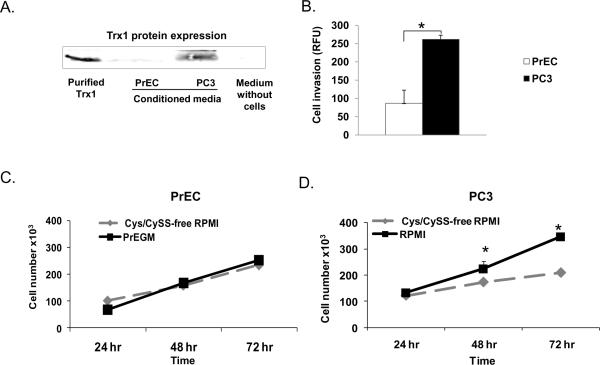
To establish correlations between extracellular redox state and prostate cancer cell growth and invasion, PC3 or PrEC cells were grown in their preferred media (RPMI 1640 or PrEGM, respectively). Media were collected after 0 hr or 24 hr for measurement of Cys, CySS, GSH, GSSG, and CySGSH concentrations. The redox potential values (defining redox status of the media, EhCySS or EhGSSG) were calculated, greater negative values indicating a more reducing environment. At 0 hr, PC3 cells demonstrated a significantly lower level of EhCySS (−19 mV) compared to PrEC cells (−46 mV). After culture for 24 hr, PC3 cells demonstrated significantly higher levels of extracellular Cys, CySS, GSH, GSSG, CySGSH, calculated EhCySS (−141 mV) and EhGSSG (−153 mV) compared to PrEC cells (EhCySS −135 mV and EhGSSG −147 mV) (Table. 1), indicating a more reducing environment of PC3 cells. Additionally, the level of Trx1 protein expression in the conditioned media of PC3 cells was significantly higher than PrEC cells (Fig. 1A). Cell invasion assays demonstrated that PC3 cells invaded through Matrigel matrix membranes at a significantly higher level than PrEC cells (Fig. 1B). These results suggest the possibility that a reducing environment may play an important role in PC3 cell invasion.
Table 1.
Extracellular CySS, Cys, GSSG, GSH, and CySGSH levels and calculated EhCySS and EhGSSG in PC3 and PrEC cells after 0 hr and 24 hr culture with regular RPMI 1640 or PrEGM.
| PC3+RPMI | PrEC+PrEGM | |||
|---|---|---|---|---|
| 0 hr | 24 hr | 0 hr | 24 hr | |
| EhCySS (mV) | −19 ± 2** | −141 ± 1.6*,** | −46 ± 2 | −135 ± 0.5* |
| CySS (μM) | 295 ± 5** | 77.9 ± 7*,** | 153 ± 4 | 26.5 ± 1.5* |
| Cys (μM) | 2.4 ± 0.2** | 134.9 ± 5.7*,** | 4.9 ± 0.2 | 62.4 ± 2* |
| EhGSSG (mV) | −67 ± 15 | −153 ± 1.3*,** | −58 ± 7 | −147 ± 0.8* |
| GSSG (μM) | 0.008 ± 0.005 | 1.5 ± 0.2*,** | 0.008 .± 002 | 0.4 ± 0.03* |
| GSH (μM) | 0.04 ± 0.01 | 17.5 ± 2.2*,** | 0.03 ± 0.01 | 0.9 ± 0.1* |
| CySGSH (μM) | 5.6 ± 0.2** | 24.9 ± 1.3*,** | 0.2 ± 0.005 | 7.6 ± 0.2* |
Data represents average of three individual experiments.
p-value ≤ 0.05 when compared with 0 hr for each cell type,
p-value ≤ 0.05 when comparing PC3 cells with PrEC cells.
Figure 1. Comparison of PrEC vs. PC3 cells: Trx1 in culture media, cell invasive ability, and cell growth in response to Cys/CySS.
(A) Trx1 protein expression in media cultured with PrEC or PC3 cells for 24 hr; control is RPMI 1640 medium without cultured cells. (B) Comparison of cell invasive ability through Matrigel matrix membranes at 24 hr between PrEC and PC3 cells. (C–D) Effect of 450 μM Cys/6 μM CySS supplemented medium on growth of PrEC cells or PC3 cells. *p-value ≤ 0.05, #p-value = 0.09
Absence of Cys/CySS resulted in reduction of cell growth in PC3 but not PrEC cells (Fig. 1B–C). After addition of Cys/CySS back into Cys/CySS-free medium, PC3 cell growth was increased (Supplementary Fig. 1), whereas PrEC cell growth remained the same (data not shown). These data indicated essential role(s) of extracellular thiols on PC3 cell growth.
Calculated extracellular EhCySS of Cys/CySS supplemented media
Redox media with varying redox potential values were prepared by adding various concentrations of Cys and CySS to Cys/CySS-free RPMI 1640 medium. Extracellular levels of Cys, CySS, and EhCySS were measured and calculated. EhCySS were not significantly different between media without the cells and media after culture with cells at 0 hr (data not shown). EhCySS of media after culture with cells at 0 hr are indicated in Table. 2. EhCySS of Cys/CySS-free RPMI 1640 medium was approx. −59 mV, Cys/CySS-free RPMI 1640 medium containing 4 μM Cys/300 μM CySS was approx. −37 mV, and Cys/CySS-free RPMI 1640 medium containing 450 μM Cys/6 μM CySS was approx. −182 mV.
Table 2.
Concentrations of Cys/CySS and calculated redox potentials (EhCySS) in Cys/CySS-free RPMI 1640 and Cys/CySS supplemented media at 0 hr or 3 hr after culture with PC3 or PrEC cells.
| 0 hr | 3 hr | ||||||
|---|---|---|---|---|---|---|---|
| Cell | Media | Cys (μM) | CySS (μM) | Eh (mV) | Cys (μM) | CySS (μM) | Eh (mV) |
| PC3 | Cys/CySS-free RPMI | 1 ± 1.5 | 0.6 ± 0.5 | −59 | 1.4 ± 0.2 | 0.5 ± 0.2 | −88 |
| PC3 | RPMI+4 μM Cys/300 μM CySS | 6 ± 0.9* | 412 ± 12* | −37* | 16 ± 2.3* | 341 ± 18* | −65* |
| PC3 | RPMI+ 450 μM Cys/6 μM CySS | 408 ± 13* | 31 ± 1.6* | −182* | 350 ± 5* | 26 ± 1.6* | −180* |
| PrEC | Cys/CySS-free RPMI | 1 ± 1.5 | 0.6 ± 0.5 | −59 | 0.9 ± 0.4 | 0.2 ± 0.04 | −87 |
| PrEC | RPMI+4 μM Cys/300 μM CySS | 6 ± 0.9* | 412 ± 12* | −37* | 12 ± 0.5* | 338 ± 4.7* | −60* |
| PrEC | RPMI+ 450 μM Cys/6 μM CySS | 408 ± 13* | 31 ± 1.6* | −182* | 331 ± 1.4* | 25 ± 0.5* | −180* |
Data represent average of three individual experiments.
p-value ≤ 0.05 when compared with Cys/CySS-free RPMI for each cell type.
PC3 and PrEC cells were incubated with Cys/CySS supplemented media for 3 hr. At this time, EhCySS of Cys/CySS-free RPMI 1640 media were approx. −87 mV for PrEC cells and −88 mV for PC3 cells, EhCySS of Cys/CySS-free RPMI 1640 media containing 4 μM Cys/300 μM CySS were approx. −60 mV for PrEC cells and −65 mV for PC3 cells, and EhCySS of Cys/CySS-free RPMI 1640 media containing 450 μM Cys/6 μM CySS were approx. −180 mV for both PrEC and PC3 cells. Media with EhCySS −87 to −88 mV approximates a normal extracellular redox state (physiological EhCySS in human plasma is around −80 ± 9 mV [7]), media with EhCySS −60 to −65 mV result in an oxidizing extracellular redox state (p-value ≤ 0.05 when compared with normal microenvironment), and media with EhCySS −180 mV result in a reducing extracellular redox state (p-value ≤ 0.05 when compared with normal extracellular redox state). The EhCySS of Cys/CySS conditioned supplemented media were not statistically significantly different between PC3 vs. PrEC cells. These selected EhCySS were chosen for our study since preliminary studies demonstrated significant biological changes in PC3 cells upon exposure to these varying redox potential values.
Regulation of aggressive prostate cancer but not normal prostate epithelial cell invasion by a reducing extracellular redox state
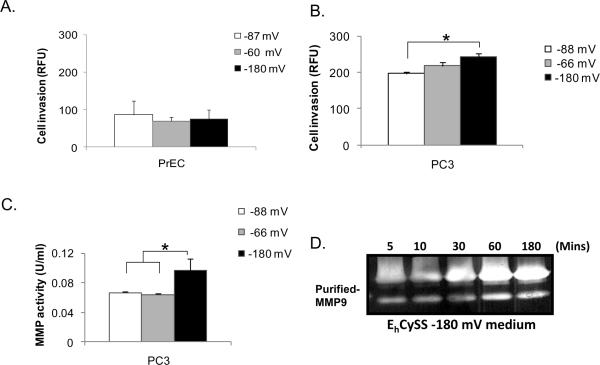
Cells were cultured in EhCySS −87, −88, −60, −65, or −180 mV media for 3 hr and were collected for in vitro invasion assays. Cys/CySS supplemented media (EhCySS −60 or −180 mV) did not affect cell invasive ability of PrEC cells after 3 hr (Fig. 2A) or 24 hr (data not shown) of culture when compared to EhCySS −87 mV medium. In contrast, PC3 cells had an increase in cell invasive abilities after culture with EhCySS −180 mV medium as early as 3 hr, whereas culture with EhCySS −65 mV medium only slightly increased cell invasive ability (Fig. 2B). These biological changes continued for at least 24 hr (data not shown). Additionally, the MMP activity was significantly increased in PC3 cells that were cultured with EhCySS −180 mV medium (Fig. 2C). Direct incubation of purified pro-MMP9 with EhCySS −180 mV medium demonstrated an increase in MMP9 activity, with activity increasing with time (Fig. 2D). The changes in invasive ability may be specific to prostate cancer cells, since aggressive prostate cancer DU145 cells demonstrated similar results as in PC3 cells after culture with Cys/CySS supplemented media (Supplementary Fig. 2) and these changes were not observed in PrEC cells.
Figure 2. Increase in invasive ability and MMP enzymatic activity of PC3 cells but not PrEC cells by incubation with a reducing extracellular redox state.
Cells were cultured with Cys/CySS supplemented media for 3 hr. Cell invasive ability through Matrigel matrix membrane and MMP activity were analyzed. (A) PrEC cell invasive ability. (B) PC3 cell invasive ability. (C) Total MMP activity in the media. (D) Zymography assay of MMP9. Purified pro-MMP9 proteins were directly incubated with EhCySS −180 mV medium and zymography enzyme activity was analyzed at different time points. *p-value ≤ 0.05.
Effect of reducing extracellular redox state on aggressive prostate cancer cell migration and gelatinase/matrix metalloprotease activities
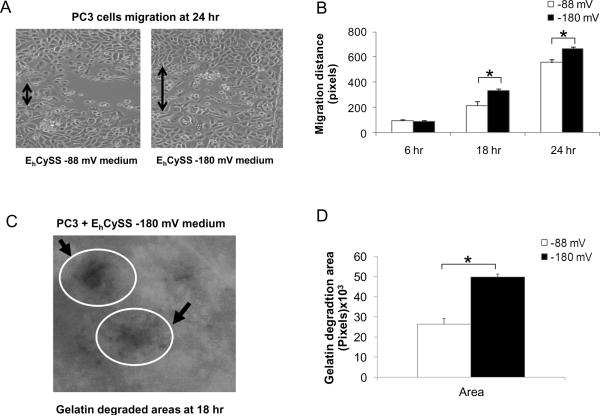
PC3 cells were cultured with EhCySS −88 or −180 mV media for 3 hr and an in vitro wounding assay was performed. PC3 cells that were cultured with EhCySS −180 mV medium had a faster migration (~ 1.6 fold increase), an effect first detectable at 18 hr (Fig. 3B). Additionally, fluorescent gelatin degradation assay demonstrated a significant increase in gelatin digestive area in PC3 cells that were cultured with EhCySS −180 mV medium (Fig. 3D). These data support a possible role of a reducing extracellular redox state in PC3 cell migration/invasion abilities.
Figure 3. Induction of PC3 cell migration ability and gelatinase activity in media with a reducing extracellular redox state.
PC3 cells were cultured with either EhCySS −88 or −180 mV media. After culture for 3 hr, in vitro wounding assays were performed at 6 hr, 18 hr, and 24 hr and fluorescent gelatin degradation assays were performed at 18 hr after wounding. (A) Phase-contrast photographs of migration of PC3 cells at 24 hr. (B) Quantitative analysis of migration distance. (C) Photograph of FITC-conjugated gelatin areas at 18 hr that were degraded by gelatinase enzymes (arrows) of PC3 cells. (D) Quantitative analysis of gelatin degradation areas. *p-value ≤ 0.05.
Correlation of extracellular H2O2 levels with prostate cancer cell invasion in a reducing extracellular redox state
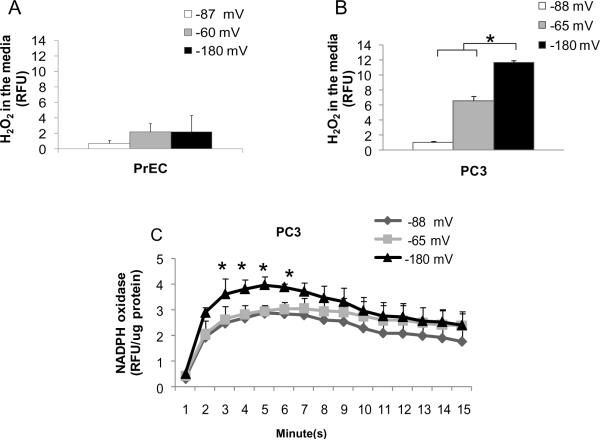
We measured extracellular ROS/RNS levels in cells that were cultured with EhCySS −87 or −88 mV media compared to EhCySS −60, −65, or −180 mV media. There was no increase in extracellular H2O2 (Fig. 4A) and nitrite levels (data not shown) in EhCySS −60 or −180 mV media of PrEC cells, whereas, extracellular H2O2 levels were increased in PC3 cells that were cultured with EhCySS −65 mV or −180 mV media, especially with EhCySS −180 mV medium (Fig. 4B). There was no significant increase in extracellular nitrite levels of PC3 cells cultured with either EhCySS −65 mV or −180 mV media (Fig. 4C). We further investigated the source of extracellular H2O2 and found that NADPH oxidase activity was significantly increased in PC3 cells that were cultured with EhCySS −180 mV medium (~ 1.6 fold increase); the increase in NADPH oxidase activity correlated with extracellular H2O2 levels.
Figure 4. Induction of extracellular H2O2 levels and NADPH oxidase activity after culture in media with a reducing extracellular redox state.
Cells were incubated with Cys/CySS supplemented media for 3 hr. Media or cell homogenates were collected for analysis. (A) H2O2 levels in media of PrEC cells. (B) H2O2 levels in media of PC3 cells. (C) NADPH oxidase activity of PC3 cells. *p-value ≤ 0.05.
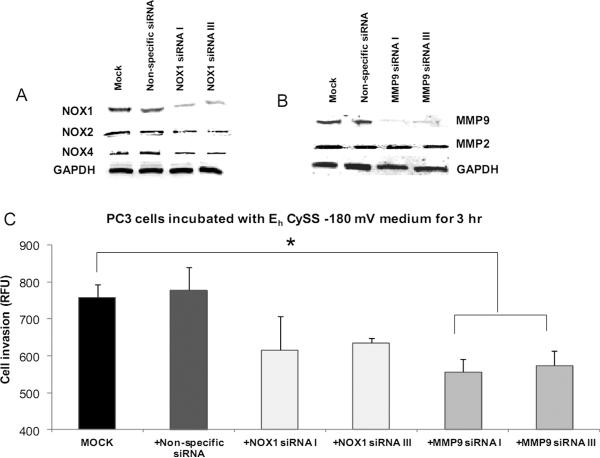
Effect of NADPH oxidase or MMP9 on aggressive prostate cancer cell invasion in a reducing extracellular redox state
To further establish whether NADPH oxidase (NOX1) or MMP (MMP9) play role(s) in prostate cancer cell invasion ) in a reducing extracellular redox state, PC3 cells were transfected with NOX1 (sequence I or III) or MMP9 (sequence I or III) siRNA or non-specific siRNA and simultaneously placed in EhCySS −180 mV medium for 3 hr. As shown in Fig. 5A–B, NOX1 or MMP9 protein levels were decreased in NOX1 or MMP9 siRNA transfected PC3 cells (~ 70% decrease for NOX1 and MMP9, supplementary Fig. 3A–B). There were no significant changes of NOX2, NOX4, or MMP2 protein expression levels (Fig. 5A). MMP9 siRNA transfected PC3 cells cultured in EhCySS −180 mV medium showed a significant decrease in invasive ability (~ 1.5 fold decrease), whereas NOX1 siRNA transfected PC3 cells showed a decrease that did not achieve statistical significance (~ 1.3 fold decrease, Fig. 5C) when compared with mock cells. There were no changes in cell invasive ability of PC3 cells transfected with non-specific siRNA. These data strongly supported role(s) of MMP9 and H2O2 in enhancement of cell invasion observed in PC3 cells exposed to a reducing extracellular redox state.
Figure 5. Effects of decreased NADPH oxidase or MMP9 protein levels on PC3 cell invasion after culture in media with a reducing extracellular redox state.
PC3 cells were transfected with NOX1, MMP9, or non-specific siRNAs for 48 hr and continuously cultured for an additional 72 hr. siRNA transfected-PC3 cells were cultured with EhCySS −180 mV medium for 3 hr. Cells were collected for western blot (A and B) and in vitro invasion analysis (C). Mock= PC3 cells that were cultured with EhCySS −180 mV medium for 3 hr. *p-value ≤ 0.05.
Effect of a combined imbalance of intra- and extracellular redox states on PC3 invasive ability
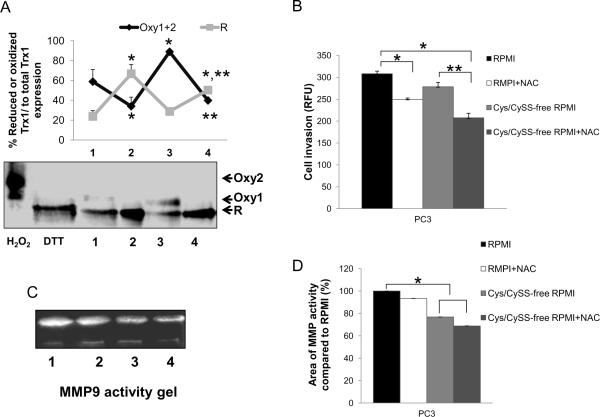
We further analyzed the role of extracellular redox state on cell invasion by incubation of PC3 cells with Cys/CySS-free RPMI 1640 medium for 3 hr to analyze effects of the absence of the Cys/CySS couple on cancer cell biochemistry and cancer cell invasion; Trx1 redox western blots, MMP activity, and cell invasive ability were analyzed. Redox western analysis was used to determine the relative amounts of reduced and oxidized Trx1 forms. As demonstrated in Fig. 6A, PC3 cells that were cultured in Cys/CySS-free RPMI 1640 medium demonstrated higher levels of the oxidized form (mostly Oxy1) of Trx1 when compared to PC3 cells that were cultured with regular RPMI 1640 media. Incubation of PC3 cells with 500 μM NAC significantly increased the reduced form (R) of Trx1 in PC3 cells. Cell viability assay was performed to ensure that 500 μM NAC did not induced cell proliferation or cell death at 3 hr (data not shown). More importantly, cell invasive ability was significantly decreased in PC3 cells that were treated with 500 μM NAC either in regular or in Cys/CySS-free RPMI 1640 media (Fig. 6B). The decrease in PC3 cell invasive ability correlated with a decrease in MMP9 activity (Fig. 6C). Thus, imbalance of either intra- or extracellular redox states affected PC3 cell invasion.
Figure 6. Regulation of PC3 cell invasion and MMP activity by modulation of extra- and intracellular redox states.
PC3 cells were cultured with regular RPMI 1640 medium ± 500 μM NAC or Cys/CySS-free RPMI 1640 medium ± 500 μM NAC for 3 hr. Cells or conditioned media were collected for redox western blot, cell invasion, and MMP activity assays. (A) Redox western blot assay. Oxy2= fully oxidized (two disulfides) Trx1, Oxy1= mixture of reduced and oxidized Trx1, R= fully reduced Trx1. PC3 cells were treated with DTT or H2O2 for 30 min and were used to determine fully reduced Trx1 or fully oxidized Trx1. (B) Cell invasion assay. (C) MMP9 zymography activity gel. (D) Relative quantification of MMP9 zymography activity gels. Lane 1 = regular RPMI 1640 medium, lane 2 = regular RPMI 1640 medium + NAC, lane 3 = Cys/CySS-free RPMI 1640 medium, lane 4 = Cys/CySS-free RPMI 1640 medium + NAC. *p-value ≤ 0.05 when compared with regular RPMI 1640 medium. **p-value = 0.06 when compared with Cys/CySS-free RPMI 1640 medium. Data represent average of at least three individual experiments
Discussion
Redox state has been implicated in cancer initiation, progression, and metastasis. The production of ROS/RNS by cancer cells and/or surrounding stromal/inflammatory cells are important factors for modulation of redox state, with possible resultant effects on cancer cell behavior. The role of the tumor microenvironment in determination of the cancer phenotype and effects on cancer progression and metastasis has been recently addressed [12]. The Cys/CySS couple is the major low molecular weight redox couple present in the cellular microenvironment and is one of the central redox control couples in biological systems [6]. Most cysteine residues in extracellular proteins are present as disulfide bonds; the inside of the cell has a reducing environment in which most cysteines are present in sulfhydryl forms [13]. The major oxidized form, CySS, exists predominantly in plasma and the calculated Cys/CySS redox potential (EhCySS) is used as a measure of the extracellular redox state.
In the present study, we are the first to demonstrate significant increased levels of extracellular Cys, CySS, GSH, GSSG, CySGSH, thiols, and Trx1 in conditioned media of highly aggressive PC3 prostate cancer cells in comparison to normal prostate epithelial PrEC cells. These results are in agreement with previously published data by our laboratory, which demonstrated a significant increase of extracellular reduced GSH in highly aggressive WPE1-NB26 prostate cancer cells in comparison to immortalized RWPE1 prostate epithelial cells [9, 13]. Jonas et. al demonstrated that glutamine (4 mM) and growth factors such as insulin like growth factor-1 (IGF-1, 1 μg/mL) and epidermal growth factor (EGF, 100 ng/mL) stimulated a shift of extracellular redox state under extremes of oxidizing or reducing conditions toward a reducing condition [8]. Extracellular EhCySS of PrEC cells after culture for 24 hr are unlikely to be affected by growth factors since it is more oxidizing rather than reducing when compared to extracellular EhCySS of PC3 cells. In addition, EhCySS in the media of PrEC cells at 0 hr was more reduced than EhCySS in the media of PC3 cells (−46 mV vs. −19 mV, Table 1), whereas EhCySS in the media of PrEC cells at 24 hr was more oxidized than EhCySS in the media of PC3 cells (−135 mV vs. −141 mV, Table 1). The magnitude of changes in EhCySS due to the differences in growth factor concentrations was relatively small. Thus, we conclude that more invasive prostate cancer cell lines display extracellular redox states which contain more reduced SH groups. In fact, incubation of PC3 cells with relatively oxidized media (−59 and −37 mV, Table. 2) resulted in a more reducing extracellular EhCySS (−88 and −65 mV, Table 2), EhGSSG (Supplementary Table 2) and increased levels of thiols in the media (Supplementary Fig. 4). These results indicate that PC3 cells shifted extracellular redox to a more reducing state. The existences of differences in thiol/disulfide pools in conditioned media indicate that there must be a mechanism(s) that controls redox state precisely and efficiently so that extracellular proteins can properly function.
A recent study from metabolic profiles of human prostate cancer tissues showed a significant increase in Cys and a significant decrease in reduced GSH as cancer progresses in pathologic grade from low to high grade Gleason scores [5]. We hypothesized that redox imbalance, particularly involving thiols, outside prostate cancer cells may be responsible for altered protein conformation of key molecules involved in prostate cancer progression and metastasis. We demonstrated that modulation of the extracellular redox state by varying extracellular redox potentials EhCySS (−65 or −180 mV) regulated cell growth and invasive ability of aggressive prostate cancer cells but not normal prostate epithelial cells, suggesting a specific response of cancer cells to alteration of extracellular redox state.
In humans, the physiological EhCySS in plasma of healthy subjects is around −80 ± 9 mV [7], whereas in subjects with disease, including patients with cardiovascular disease, or in aging, redox state becomes more oxidized to between −62 to −20 mV [6, 14–20]. Modifications of extracellular EhCySS could directly regulate cell functions, including cell proliferation. Colon carcinoma Caco2 cells and normal human retinal pigment epithelial cells [15] demonstrated greater cell proliferation at more reducing EhCySS, whereas lung fibroblasts demonstrated greater cell proliferation at more oxidized EhCySS [7–8]. We also found that PC3 cell growth was affected by extracellular Cys/CySS but not PrEC cell growth. These data suggest that extracellular Cys/CySS may act as a redox switch in cell proliferation and the effect may be cell type specific. In our study, it appears to be specific to prostate cancer but not normal prostate epithelial cells. The mechanism of how extracellular Cys/CySS induced PC3 cell growth in our study is unclear, and further studies need to be performed. Nkabyo et. al demonstrated that extracellular Cys/CySS induced Caco-2 cell proliferation via cleavage of MMP and release of TGF-alpha [14]. We speculate that extracellular Cys/CySS stimulated PC3 cell proliferation through inhibition of ·NO production since nitrite levels in the media were decreased after 24 hr incubation; this possibility was supported by the reduction of PC3 cell viability following treatment with the ·NO donor, SNAP, for 48 hr (Supplementary Fig. 1D). The negative relationship between cancer cell growth and ·NO has been addressed by other [21–22].
Prostate cancer patient death usually results from the spread of cancer to distant organs rather than the primary growth itself; thus, inhibition of prostate cancer cell metastatic processes may be important in design of rational prostate cancer therapies. Herein, we demonstrated that exposure to EhCySS −180 mV medium enhanced the ability of PC3 prostate cancer cells to invade through Matrigel matrix membranes, at least partially through MMP activation and NADPH oxidase mediated-extracellular H2O2 production. It appears that major effects of redox state on metastasis are extracellular. This is an important consideration in the design of therapeutic agents with potential to regulate metastasis of prostate cancer.
Gelatinases and MMPs are extracellular matrix degrading enzymes that play critical roles in the metastatic process of prostate cancer. High levels of MMP9 in plasma have been demonstrated to correlate with prostate cancer metastasis [23–24]. MMPs are regulated transcriptionally and posttranslationally via proteolytic activation of precursor zymogens as well as by regulation of their inhibitors, tissue inhibitor of MMPs (TIMP) [25]. The activation of MMPs involves an alteration of the bond between the active Zn2+ site and a Cys residue [28]. This mechanism has been referred to as a Cys switch. We hypothesize that a reducing extracellular environment favors the activation of MMP9 by alteration and/or protection of the Zn2+-Cys bond, consequently leading to autoactivation [26–27]. This hypothesized mechanism is supported by our in vitro experiment in which direct addition of Cys/CySS to purified pro-MMP2 (data not shown) or MMP9 (Fig. 4D) resulted in increases in MMP2/9 activities in a time-dependent fashion. Thus, the increase in MMP9 activity observed in our studies was likely due to increased enzyme activity rather than increased protein expression. The reaction of pure pro-MMPs with Cys/CySS can result in dual effects, activation or inhibition (reduction of MMP9 activity at 180 min) depending on the concentration of MMP and Cys/CySS, incubation time, and steric conformation. Previous studies have demonstrated dual effects of Cys/CySS on MMP2 activity depending on the molar ratio of thiol to MMP2. By increasing the molar ratio from 0–10 to 100–10,000, MMP2 activity was changed from activation to inhibition [29].
As described in the results, increased cell invasive ability and MMP9 activity appeared as early as 3 hr (Fig. 4B) and continued through at least 24 hr after exposure to EhCySS −180 mV medium. However, at 24 hr, increased cell invasive ability and MMP9 activity could be due to the influx into or effects of Cys/CySS in cells rather than effects outside the cells. PC3 cells transport Cys/CySS mainly via the xc− cystine/ glutamate antiporter, a plasma membrane CySS transporter that cancer cells can express, in particular when they are more aggressive [28]. The EhCySS of RPMI 1640 media containing 450 μM Cys/6 μM CySS that were cultured with PC3 cells or PrEC cells were not significantly different at 0 hr (−182 mV) and 3 hr (−180 mV) (Table. 2), indicating that the induction of cell invasion at 3 hr was due to alteration of extracellular redox state rather than influx of Cys/CySS into cells.
We have demonstrated correlations of enhancement of cell invasion with NADPH oxidase activity, extracellular H2O2, and MMP activity. A reducing extracellular redox state increased NADPH oxidase activity which potentially resulted in extracellular H2O2 production. Intracellular GSH/GSSG ratio at 3 hr after exposure of PC3 cells to a reducing extracellular redox medium demonstrated no significant change, thus excluding the possibility that elevation of extracellular H2O2 levels at 3 hr was due to elevation of intracellular H2O2 levels (data not shown). Incubation of active antioxidant enzymes superoxide dismutase (SOD, 9.6 U/mL)/catalase (CAT, 2 U/mL) with EhCySS −180 mV medium for 3 hr prior to in vitro cell invasion assay decreased invasive ability of PC3 cells, but the results did not achieve statistical significance (~ 1.2 fold decrease) (Supplementary Fig. 5). These results are consistent with redox signaling being involved in induction of cell invasion by a reducing extracellular redox state.
H2O2 may act as a catalytic co-factor and increase MMP activity by modifying or protecting the Zn2+-CyS bond complex. Our in vitro experiments demonstrated a dose dependent increase in total MMP activity by H2O2 (Supplementary Fig. 6). H2O2 production was detectable as early as 3 hr after exposure of cells to media with reducing extracellular redox state, although the increase in H2O2 production was less after 24 hr (data not shown), possibly due to enzymatic catalysis of H2O2 by extracellular antioxidants [29]. Activation of MMPs activity is also regulated through TIMP. The critical residues that are involved in MMP inhibition are located around Cys1 and Cys70 [30]; thus, a reducing extracellular redox state and extracellular H2O2 may potentially directly inhibit TIMP activity, resulting in induction of MMP9 activation. We also demonstrated that a reducing extracellular redox state mediated PC3 cell migration. The stimulation of cell migration by reducing extracellular redox state and H2O2 may be due to direct interaction with specific plasma membrane receptors (e.g. protein thiols, ICAM, P-selectin), and reversible modification of sufhydryl groups on active Cys residues may affect protein conformation, ligands binding to receptors, or protein-protein interactions that subsequently stimulate cell migration [31].
We further confirmed the role of extracellular Cys/CySS in PC3 cell invasion by removal of Cys/CySS. We found that MMP activity and invasive ability of PC3 cells were slightly decreased, with further decreases augmented by co-incubation with NAC. Importantly, intracellular redox state became more oxidized after removal of Cys/CySS as indicated by an increase in fully oxidized Trx1. Total Trx1 protein expression levels both inside and outside the cells were not changed after removal of Cys/CySS or co-incubation with NAC (Supplementary Fig. 7). Since Cys is a precursor of GSH and is required to maintain GSH levels [9], the intracellular oxidized redox state induced by removal of Cys/CySS may be due to the reduction of the GSH/GSSG ratio. In fact, co-incubation with NAC (precursor of Cys and GSH) resulted in a more reducing intracellular redox state. Several studies indicated that NAC stimulates cell proliferation with an increase in intracellular GSH. Treatment of PC3 cells with 500 μM NAC for only 3 hr did not induce cell proliferation or cell death (data not shown), possibly due to the fact that 3 hr is an insufficient time period for NAC to penetrate inside cells and modulate intracellular redox state. Thus, the effect of NAC treatment at 3 hr is at the cell membrane level or outside the cells.
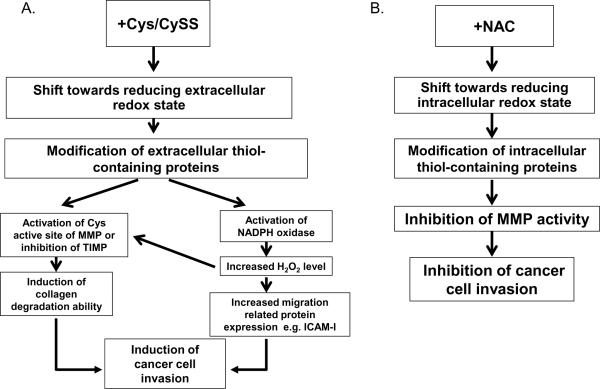
These data confirm the dual roles of intra- and extracellular redox states on PC3 cell invasion. The inherent alterations of intra- and extracellular redox states of PC3 cells may account for the observed responses of PC3 cells to extracellular Cys/CySS [1]. Proposed mechanism(s) of how intra- and extracellular redox states regulate prostate cancer cell invasion are illustrated in Fig. 7.
Figure 7. Proposed mechanisms by which extra- or intracellular redox state regulates prostate cancer cell invasion.
(A) Extracellular Cys/CySS shift medium redox state towards reducing, resulting in an alteration or protection of the bond between the active Zn2+ site and a Cys residue, and leading to activation of MMP. Alternatively, a reducing extracellular redox state may affect TIMP with resultant inhibition of TIMP protein function. A shift of extracellular redox state towards reducing may activate NADPH oxidase on the cell membrane, resulting in an increase in extracellular H2O2 production. H2O2 may act as a catalytic co-factor and increase MMP activity by altering the Zn2+-Cys bond complex, altering TIMP activity, or activating migration related proteins. (B) NAC and/or extracellular oxidizing redox state shift intracellular redox state towards reducing, which results in a decrease in MMP activity. Intracellular reducing redox state could possibly inhibit MMP secretion or modulate other redox signaling-related proteins involved in invasion processes.
Our studies are the first to demonstrate that a reducing extracellular redox state favored prostate cancer invasive ability, at least partially through activation of migration, gelatinase, MMP9, and NADPH oxidase mediated-H2O2 production, whereas a reducing intracellular redox state decreased prostate cell invasion and MMP activity. The changes in invasive ability of prostate cancer cells due to alterations of extracellular redox state may be specific to prostate cancer cells, since they were not observed in normal prostate epithelial cells. Alteration of extracellular redox-modulating amino acids, peptides, and proteins may exert dual roles (anticancer or promotion of cancer functions) depending on the targeted proteins, the concentration, the distinct cancer cell type, and the stage of cancer. A recent study in melanoma cells demonstrated that drugs targeting both Cys/CySS and GSH were lethal for melanoma cells and increased cells sensitivity to arsenic treatment [32]. Based on our experiments, it is appropriate to say that combination treatment aimed at both extra- and intracellular redox states or modulation of multiple types of extracellular redox-related proteins may offer new strategies towards development of effective therapies against prostate cancer metastasis.
Supplementary Material
Acknowledgments
The contents do not represent the views of the U.S. Department of Veterans Affairs. This work was supported in part by funds from the University of Wisconsin, Department of Pathology Research and Development Committee, University of Wisconsin-Madison Graduate School, a 2010 mini-fellowship award from the Society for Free Radical Biology & Medicine and from the National Cancer Institute, UW Carbone Cancer Center (NIH grant P30 CA014520), NIH grants RO1 CA07359902 and RO1 CA09485301 (TDO; Co-I), and resources and facilities at the William S. Middleton Memorial Veterans Hospital (Madison, WI, USA). The authors would like to thank Dr. Young-Mi Go, Department of Medicine, Emory University, Atlanta, Georgia for technical training of Trx1 redox western blot technique.
List of abbreviations
- CAT
catalase
- Cys
cysteine
- CySS
cystine
- EhCySS
calculated Cys/CySS redox potential
- EhGSSG
calculated GSH/GSSG redox potential
- FITC
fluorescein isothiocyanate
- DTT
dithiothreitol
- GSH
glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- IAA
iodoacetic acid
- MMP
matrix metalloproteinase
- NAC
N-acetylcysteine
- •NO
nitric oxide
- NOX1
NADPH oxidase 1
- PDI
protein disulfide isomerase
- PrEC
normal prostate epithelial cell
- PrEGM
normal prostate epithelial growth medium
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- siRNA
silencing RNA
- SNAP
S-nitroso-N-acetyl-l,l-penicillamine
- SOD
superoxide dismutase
- Trx1
thioredoxin 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chaiswing L, Bourdeau-Heller JM, Zhong W, Oberley TD. Characterization of redox state of two human prostate carcinoma cell lines with different degrees of aggressiveness. Free Radic Biol Med. 2007;43:202–215. doi: 10.1016/j.freeradbiomed.2007.03.031. [DOI] [PubMed] [Google Scholar]
- [2].Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, Oberley LW, Yan T, Zhong W, Jiang X, Oberley TD. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer. 2000;89:123–134. [PubMed] [Google Scholar]
- [3].Oberley TD, Zhong W, Szweda LI, Oberley LW. Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate. 2000;44:144–155. doi: 10.1002/1097-0045(20000701)44:2<144::aid-pros7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [4].Chaiswing L, Oberley TD. Extracellular/Microenvironmental Redox State. Antioxid Redox Signal. 2009 [Google Scholar]
- [5].Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [6].Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- [7].Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33:1499–1506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- [8].Ramirez A, Ramadan B, Ritzenthaler JD, Rivera HN, Jones DP, Roman J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L972–981. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
- [9].Chaiswing L, Zhong W, Cullen JJ, Oberley LW, Oberley TD. Extracellular redox state regulates features associated with prostate cancer cell invasion. Cancer Res. 2008;68:5820–5826. doi: 10.1158/0008-5472.CAN-08-0162. [DOI] [PubMed] [Google Scholar]
- [10].Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- [11].Bowden ET, Coopman PJ, Mueller SC. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 2001;63:613–627. doi: 10.1016/s0091-679x(01)63033-4. [DOI] [PubMed] [Google Scholar]
- [12].Witz IP. Yin-yang activities and vicious cycles in the tumor microenvironment. Cancer Res. 2008;68:9–13. doi: 10.1158/0008-5472.CAN-07-2917. [DOI] [PubMed] [Google Scholar]
- [13].Adimora NJ, Jones DP, Kemp ML. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antioxid Redox Signal. 2010;13:731–743. doi: 10.1089/ars.2009.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1352–1359. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- [15].Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P, Jr., Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005;46:1054–1061. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- [16].Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- [17].Jones DP, Mody VC, Jr., Carlson JL, Lynn MJ, Sternberg P., Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- [18].Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Harrison DG, Quyyumi AA. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol. 2006;47:1005–1011. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- [19].Jonas CR, Puckett AB, Jones DP, Griffith DP, Szeszycki EE, Bergman GF, Furr CE, Tyre C, Carlson JL, Galloway JR, Blumberg JB, Ziegler TR. Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr. 2000;72:181–189. doi: 10.1093/ajcn/72.1.181. [DOI] [PubMed] [Google Scholar]
- [20].Iyer SS, Ramirez AM, Ritzenthaler JD, Torres-Gonzalez E, Roser-Page S, Mora AL, Brigham KL, Jones DP, Roman J, Rojas M. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296:L37–45. doi: 10.1152/ajplung.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- [22].Akaike T. Mechanisms of biological S-nitrosation and its measurement. Free Radic Res. 2000;33:461–469. doi: 10.1080/10715760000301001. [DOI] [PubMed] [Google Scholar]
- [23].Gohji K, Fujimoto N, Hara I, Fujii A, Gotoh A, Okada H, Arakawa S, Kitazawa S, Miyake H, Kamidono S, Nakajima M. Serum matrix metalloproteinase-2 and its density in men with prostate cancer as a new predictor of disease extension. Int J Cancer. 1998;79:96–101. doi: 10.1002/(sici)1097-0215(19980220)79:1<96::aid-ijc18>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [24].Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998;58:1395–1399. [PubMed] [Google Scholar]
- [25].Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38:73–83. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- [26].Fu X, Parks WC, Heinecke JW. Activation and silencing of matrix metalloproteinases. Semin Cell Dev Biol. 2008;19:2–13. doi: 10.1016/j.semcdb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- [27].Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- [28].Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- [29].Yu YP, Yu G, Tseng G, Cieply K, Nelson J, Defrances M, Zarnegar R, Michalopoulos G, Luo JH. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 2007;67:8043–8050. doi: 10.1158/0008-5472.CAN-07-0648. [DOI] [PubMed] [Google Scholar]
- [30].Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- [31].Jordan PA, Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxid Redox Signal. 2006;8:312–324. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- [32].Vene R, Castellani P, Delfino L, Lucibello M, Ciriolo MR, Rubartelli A. The Cystine/Cysteine Cycle and GSH Are Independent and Crucial Antioxidant Systems in Malignant Melanoma Cells and Represent Druggable Targets. Antioxid Redox Signal. 2011;15:2439–2453. doi: 10.1089/ars.2010.3830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.