Abstract
After considerable debate and key experimental evidence, the importance of the arterial baroreflex in contributing to and maintaining the appropriate neural cardiovascular adjustments to exercise is now well accepted. Indeed, the arterial baroreflex resets during exercise in an intensity-dependent manner to continue to regulate blood pressure as effectively as at rest. Studies have indicated that the exercise resetting of the arterial baroreflex is mediated by both the feed-forward mechanism of central command and the feed-back mechanism associated with skeletal muscle afferents (the exercise pressor reflex). Another perhaps less appreciated neural mechanism involved in evoking and maintaining neural cardiovascular responses to exercise is the cardiopulmonary baroreflex. The limited information available regarding the cardiopulmonary baroreflex during exercise provides evidence for a role in mediating sympathetic nerve activity and blood pressure responses. In addition, recent investigations have demonstrated an interaction between cardiopulmonary baroreceptors and the arterial baroreflex during dynamic exercise, which contributes to the magnitude of exercise-induced increases in blood pressure as well as the resetting of the arterial baroreflex. Furthermore, neural inputs from the cardiopulmonary baroreceptors appear to play an important role in establishing the operating point of the arterial baroreflex. This symposium review will highlight recent studies in these important areas indicating that the interactions of four neural mechanisms (central command, the exercise pressor reflex, the arterial baroreflex and cardiopulmonary baroreflex) are integral in mediating the neural cardiovascular adjustments to exercise.
Keywords: arterial blood pressure, baroreceptors, central blood volume, baroreflex resetting, muscle sympathetic nerve activity
I. INTRODUCTION
The cardiovascular and hemodynamic adjustments to exercise are necessary to meet the metabolic demands of working skeletal muscle. These demands are met, in part, by precise alterations in sympathetic and parasympathetic nerve activity of the autonomic nervous system. Several neural mechanisms working in concert are responsible for these reflex adjustments and through complex interactions control the cardiovascular and hemodynamic changes in an intensity-dependent manner. Indeed, it is well accepted that central command, the exercise pressor reflex and the arterial baroreflex are all involved in mediating the characteristic cardiovascular and hemodynamic adjustments to physical activity. Less appreciated may be the role of the cardiopulmonary baroreflex. The focus of this symposium review will be on the importance of and mechanism(s) for resetting of the arterial baroreflex with exercise. In addition, studies demonstrating that inputs from the cardiopulmonary baroreceptors can influence the neural cardiovascular adjustments to dynamic exercise as well as arterial baroreflex resetting will be highlighted. The literature examining these mechanisms of neural cardiovascular control during exercise is vast. As a result, it is impossible to include all of the research within the field in a brief review. However, in an effort to be more inclusive, we have cited a number of reviews to direct the reader to additional research studies in these important areas. It should also be noted that the review focuses primarily on human studies and on more recently published work.
II. THE ARTERIAL BAROREFLEX
Arterial baroreceptors originating in the carotid artery and aorta play a pivotal role in the rapid reflex adjustments that accompany acute cardiovascular stressors. Marey (Marey, 1863) first described the inverse relationship that exists between heart rate (HR) and arterial blood pressure (BP) establishing the fundamental tenet of arterial baroreflex control. Subsequently, numerous investigations have been performed in an effort to identify the components of the baroreceptor reflex arc establishing the basis for our current understanding of arterial baroreceptor anatomy, neural processing and overall function (Sheehan et al., 1941; Heymans & Neil, 1958; Mancia & Mark, 1983; Sagawa, 1983). The carotid and aortic baroreflexes are comprised of unencapsulated free nerve endings located at the medial-adventitial border of blood vessels in the carotid sinus bifurcation and aortic arch, respectively (Sheehan et al., 1941; Sagawa, 1983). These mechanoreceptors function as the sensors in a negative feedback control system that responds to beat-to-beat changes in BP by reflexively altering autonomic neural outflow to adjust cardiac output and total vascular conductance. Increases or decreases in BP cause a conformational change in the baroreceptors leading to changes in afferent neuronal firing. A branch of the glossopharyngeal nerve, the Hering nerve, carries impulses from the carotid baroreceptors, while small vagal branches carry impulses from the aortic baroreceptors. These afferent signals converge centrally within the nucleus tractus solitarius of the medulla oblongata (Aicher & Randich, 1990). When BP is elevated, the baroreceptors are stretched and this deformation causes an increase in afferent neuronal firing which results in a reflex-mediated increase in parasympathetic nerve activity and decrease in sympathetic nerve activity. Conversely, when BP is lowered, afferent firing is reduced, resulting in a decrease in parasympathetic nerve activity and an increase in sympathetic nerve activity. In both cases, the neural adjustments will affect both the heart and the blood vessels in an effort to return BP to its original set point pressure.
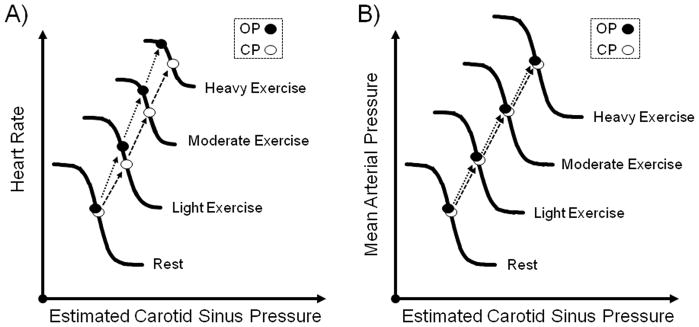
Since both BP and HR increase in a parallel fashion after the onset of dynamic exercise, the functional role of the arterial baroreflex during exercise was originally questioned (Raven et al., 1997; Joyner, 2006; Raven et al., 2006). If the baroreflex functions as a negative feedback control system, then directionally analogous changes in BP and HR should not occur unless the reflex has been altered or inhibited. In other words, the elevation in BP during exercise should be sensed by the arterial baroreceptors eliciting a reflex-mediated decrease in HR, but instead an increase in HR occurs. Thus, several early studies reported that the arterial baroreflex was “switched-off” and not necessary for the neural cardiovascular responses to exercise, or the sensitivity of the reflex had been significantly decreased by exercise (Pickering et al., 1972; Mancia et al., 1978). However, others provided clear evidence that the baroreflex functions normally during exercise. As early as 1966, Bevegard and Shepherd (Bevegard & Shepherd, 1966) reported that carotid baroreflex regulation of BP was unaltered during exercise. Subsequently, in an attempt to more completely define carotid baroreflex function during exercise, Melcher and Donald (Melcher & Donald, 1981) constructed full stimulus-response curves of the isolated carotid baroreceptors in chronically instrumented exercising dogs. These investigators were the first to demonstrate that the baroreflex function curve was reset by exercise without any change in sensitivity. Potts et al. (Potts et al., 1993) later confirmed these findings in humans by demonstrating that the carotid baroreflex is reset during leg cycling to functionally operate around the exercise-induced increase in BP. The upward and rightward shift of the stimulus-response curve to the higher BP and HR, along with preservation of reflex gain, allows the baroreflex to operate at the prevailing BP during exercise as effectively as at rest (Raven et al., 1997; Fadel et al., 2003; Raven et al., 2006). Additional studies have shown that this resetting occurs in direct relation to the intensity of exercise from rest to maximum, without a change in sensitivity Figure 1 (Papelier et al., 1994; Norton et al., 1999; Ogoh et al., 2003). Having established exercise-induced resetting of the baroreflex, the focus moved to understanding what contributed to this resetting during exercise.
Figure 1. A schematic illustration of the intensity-dependent resetting of the carotid baroreflex during dynamic exercise.
The centring point (CP) is the point at which there is an equal depressor and pressor response to a given change in blood pressure (BP), the operating point (OP) is the pre-stimulus blood pressure. Both the carotid baroreflex-heart rate (HR; panel A) and mean arterial pressure (MAP; panel B) stimulus-response curves progressively reset during exercise in an intensity-dependent manner without significant changes in maximal gain (sensitivity). A consistent observation for the baroreflex control of HR is the relocation of the OP away from the CP and closer to the threshold of the stimulus-response curve along with a reduction in the response range as exercise intensity increases (panel A). The relocation of the operating point for heart rate control positions the baroreflex in a more optimal position to counter hypertensive stimuli with increasing exercise intensity. In contrast, for the carotid baroreflex-MAP stimulus-response curve the OP does not relocate away from the centring point and the response range remains the same as at rest with an upward and rightward shift in parallel with an increase in the intensity of exercise (panel B).
III. ARTERIAL BAROREFLEX RESETTING
In 1990, Rowell and O’Leary (Rowell & O’Leary, 1990) presented a hypothetical scheme suggesting that central command (feed-forward signals from higher brain centers) and the exercise pressor reflex (feed-back from contracting skeletal muscles) were primarily involved in the resetting of the carotid baroreflex during exercise. Subsequently, a series of experiments developed by Raven and colleagues was performed to test the original hypothesis by Rowell and O’Leary discerning the roles of central command and the exercise pressor reflex in the resetting of the baroreflex during exercise. The following section will present evidence from the Raven laboratory and others supporting a role for both of these neural mechanisms in contributing to arterial baroreflex resetting with exercise.
a) THE ARTERIAL BAROREFLEX AND CENTRAL COMMAND
Direct activation of central command pathways by electrical stimulation of the mesencephalic locomotor region in paralyzed cats has been shown to shift the linear relationship between carotid sinus pressure, and BP or HR upward providing evidence that central command is capable of resetting the carotid baroreflex in animals (McIlveen et al., 2001). In humans, Iellamo and colleagues attempted to minimize the influence of central command on baroreflex resetting using involuntary muscle contraction (Iellamo et al., 1997). The sequence technique was used to assess the relationship between spontaneous fluctuations in systolic arterial pressure and R-R interval to provide an estimate of cardiac baroreflex sensitivity. During electrically-induced exercise, the sensitivity of the relationship between systolic arterial pressure and R-R interval was reduced suggesting that central command was necessary to preserve baroreflex sensitivity. Carrington and White (Carrington & White, 2002) also using the sequence technique reported resetting of the baroreflex in older subjects during voluntary isometric calf exercise that appeared to largely be due to central command. Although these studies reported a role for central command in modulating the arterial baroreflex, the validity of spontaneous measures to assess baroreflex sensitivity during exercise has been questioned (Fisher et al., 2009). Indeed, such methods do not allow the full stimulus-response curve to be determined and have been reported to only track the operating point gain of the carotid-HR baroreflex curve as the intensity of exercise and vagal withdrawal increases (Ogoh et al., 2005).
With this in mind, Querry et al. (Querry et al., 2001) constructed full stimulus-response curves using the variable pressure neck chamber to apply neck pressure and neck suction during static and dynamic handgrip exercise. To manipulate central command input, these investigators used the technique of partial axillary blockade (2% lidocaine) to block motor fibers and increase the central requirements necessary to perform work. Basically, the muscle weakness induced by axillary blockade would require greater motor recruitment and thus, the central effort required to maintain a given workload. Under blockade conditions, dynamic and static exercise relocated the carotid-cardiac and carotid-mean arterial pressure stimulus-response curves further upward on the response arm and rightward to higher arterial pressures compared to exercise without blockade. Further, the sensitivity of the reflex was unaltered during exercise in any of the conditions studied. However, it should be noted that a potential limitation of this protocol was that partial axillary blockade may not selectively augment central command. Indeed, in addition to causing muscle weakness, lidocaine would also be expected to attenuate afferent feedback from the working muscle, which may have influenced the results of the study by decreasing input from the exercise pressor reflex. Thus, in an attempt to more specifically modulate central command without affecting feedback from exercising muscle, Gallagher et al. (Gallagher et al., 2001b) used a curare-derivative (Norcuron) to induce partial neuromuscular blockade and muscle weakness. Systemic administration of Norcuron, reducing muscle strength ~50%, requires increased central input to recruit additional muscle fibers needed to complete a given work load. During dynamic and static exercise, augmentation of central command with Norcuron caused a resetting of the carotid-cardiac and carotid-mean arterial pressure baroreflex curves upward on the response arm and rightward to higher arterial pressures to a greater degree than exercise prior to blockade. Again, this exercise resetting was not accompanied by a change in the sensitivity of the reflex under any condition. Interestingly, the finding that central command affected the resetting of the carotid-mean arterial pressure baroreflex curve during low intensity exercise (20% maximal voluntary contraction) suggested that central command was capable of modulating sympathetic nerve activity (SNA) at work loads much less than previously thought (Victor et al., 1989; Victor et al., 1995).
Subsequently, Ogoh et al. (Ogoh et al., 2002) used the patellar tendon vibration technique to manipulate central command during static exercise. This innovative approach, adapted from Mitchell and colleagues (Goodwin et al., 1972), allows for both an enhancement and attenuation of central command input. Reducing central command influence by vibration of the patellar tendon during knee extension resulted in a shifting of the carotid baroreflex function curve downward on the response arm and leftward to lower arterial pressures in comparison to the location of the curve during control exercise (i.e., without vibration). Conversely, enhancing central command by vibration of the patellar tendon during flexion further reset the carotid baroreflex upward and rightward as compared to control exercise. Collectively, these studies indicated that central command has a primary role in the resetting of the carotid baroreflex during dynamic and static exercise in humans.
b) THE ARTERIAL BAROREFLEX AND THE EXERCISE PRESSOR REFLEX
In addition to central command, several investigations have also established a primary role of the exercise pressor reflex in the resetting of the arterial baroreflex during exercise. Indeed. Papelier et al. (Papelier et al., 1997) reported that the selective activation of the metabolic component of the exercise pressor reflex with post-exercise ischemia shifted the carotid-mean arterial pressure stimulus-response curve above rest and exercise, whereas the carotid-cardiac stimulus-response curve was minimally affected. In contrast to the latter, others have reported that activation of the exercise pressor reflex with lower body positive pressure, potentially activating both the mechanical and metabolic components of the reflex, reset the cardiac component of the carotid baroreflex and increased its sensitivity (Eiken et al., 1992). However, this same technique has also been reported to decrease the sensitivity of the carotid baroreflex (Shi et al., 1993b). More recently, in an attempt to better understand the influence of the exercise pressure reflex on the resetting of the carotid-cardiac and the carotid-mean arterial pressure stimulus-response curves, Gallagher et al. (Gallagher et al., 2001a) used medical anti-shock (MAS) trousers during dynamic and static exercise to augment exercise pressor reflex activity. Notably, MAS trousers stimulate both the mechanoreceptors and metaboreceptors. Enhanced activation of the exercise pressor reflex with the MAS trousers relocated the carotid-mean arterial pressure stimulus-response curve upward on the response arm and rightward to higher arterial pressures as compared to control exercise (i.e., no MAS trousers). Interestingly, the carotid-cardiac response curve was only relocated further rightward with MAS trousers. The sensitivity of the carotid-mean arterial pressure and carotid-cardiac response curves was unaltered under any condition.
In a subsequent study, Smith et al. (Smith et al., 2003) attempted to reduce exercise pressor reflex activation by partially blocking skeletal muscle afferent input using epidural anesthesia. Decreasing exercise pressor reflex input during dynamic and static exercise caused a bi-directional relocation of the carotid-mean arterial pressure stimulus response curve downward on the response arm and leftward to lower operating pressures as compared to control exercise. These changes occurred without alterations in carotid baroreflex sensitivity. Others have attempted to isolate the metabolic and mechanical components of the exercise pressor reflex to better understand their selective influences on the arterial baroreflex as well as the potential for sensitization of the mechanoreflex by metabolites to further modulate baroreflex function (Drew et al., 2008a; Drew et al., 2008b; Fisher et al., 2008). These studies provide additional support for an interaction between the exercise pressor reflex and the arterial baroreflex during exercise. In addition to these human studies, Potts & Mitchell (Potts & Mitchell, 1998) have demonstrated in anesthetized cats that activation of skeletal muscle afferents relocates the threshold of the carotid baroreflex to a higher operating range. Collectively, these studies demonstrate that the exercise pressor reflex is capable of actively resetting the carotid baroreflex during exercise.
c) INTERACTIONS BETWEEN CENTRAL COMMAND, THE EXERCISE PRESSOR REFLEX, AND THE ARTERIAL BAROREFLEX
As described in detail above, it is now apparent that central command and the exercise pressor reflex are both involved in baroreflex resetting during exercise. Indeed, selective augmentation of central command relocates the carotid baroreflex stimulus-response curve for both mean arterial pressure and HR rightward to higher arterial pressures and upward on the response arm. In agreement, reductions in central command activity have been shown to attenuate carotid-mean arterial pressure and HR baroreflex resetting. Additionally, activation of the exercise pressor reflex also results in the resetting of the carotid-mean arterial pressure curve rightward and upward. However, the exercise pressor reflex only resets the carotid-cardiac stimulus-response curve rightward to operating at higher arterial pressures with no upward resetting. Diminishing exercise pressor reflex input during exercise produces similar results but in the opposite direction. Thus, depending upon the variable of interest, the exercise pressor reflex can evoke an upward and rightward relocation of the curve (i.e. mean arterial pressure) or a relocation of the curve in a rightward direction only (i.e. HR). These studies suggest that the exercise pressor reflex is capable of actively resetting the carotid-mean arterial pressure stimulus-response curve during exercise but it appears to only modulate the carotid-cardiac stimulus-response curve. Collectively, from these findings it can be concluded that both central command and the exercise pressor reflex are actively involved in baroreflex resetting during exercise.
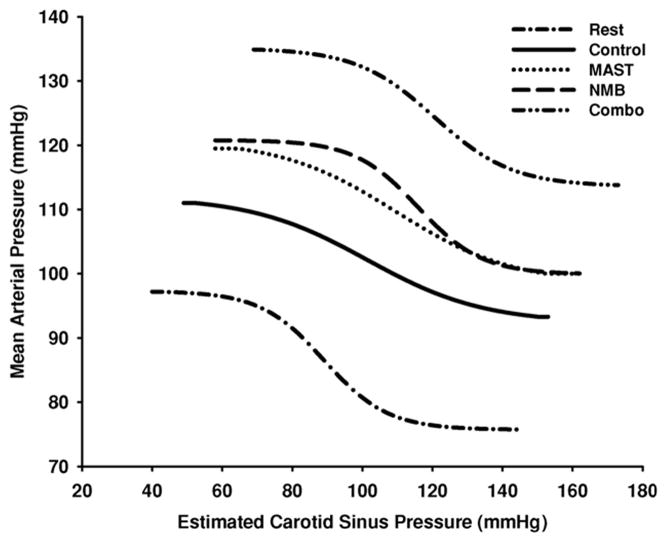
Given the clear evidence that central command and the exercise pressor reflex can independently reset the carotid baroreflex during exercise, Gallagher et al. (Gallagher et al., 2006) next sought to assess the interactive relationship between central command and the exercise pressor reflex in mediating exercise resetting of the baroreflex. These investigators used neuromuscular blockade (Norcuron) to enhance central command activation and MAS trousers to augment exercise pressor reflex activation, as discussed above. These strategies were used alone and in combination during static handgrip to compare responses of the enhancement of each input by itself with the simultaneous augmentation of both inputs together. For both the carotid baroreflex regulation of HR and mean arterial pressure, the extent of baroreflex resetting was greater during the combined enhanced activation of central command and the exercise pressor reflex than during overactivation of either input alone (Figure 2). This finding suggests that central command and the exercise pressor reflex interact such that signals from one input facilitate signals from the other, resulting in an accentuated resetting of the baroreflex during exercise. Thus, central command and the exercise pressor reflex play both independent and interactive roles in the resetting of the arterial baroreflex with exercise. In summary, as central command is characterized as a feed-forward mechanism it is likely the primary regulator of baroreflex resetting with exercise whereas the exercise pressor reflex, a feedback mechanism, plays more of a modulatory role in exercise resetting. Further, it appears that both inputs interact and are important for the complete resetting of the baroreflex during exercise.
Figure 2. Influence of central command and the exercise pressor reflex on the resetting of the carotid baroreflex-mean arterial pressure stimulus-response curve during exercise.
Carotid baroreflex-mean arterial pressure stimulus-response curves are presented at rest and during static exercise under control, neuromuscular blockade (NMB; enhanced central command activation), medical anti-shock trousers (MAST; enhanced exercise pressor reflex activation) and the combination of NMB and MAST (Combo) conditions. Lines represent fitted logistic functions developed from the mean baroreflex curve parameters for all subjects. As discussed in detail in the text, central command and the exercise pressor reflex play both independent and interactive roles in the resetting of the arterial baroreflex with exercise. (Reproduced with permission from Gallagher et al. 2006.)
d) DYNAMIC RESETTING OF THE ARTERIAL BAROREFLEX
The studies discussed above examining arterial baroreflex regulation during exercise and its interactions with central command and the exercise pressor reflex have primarily focused on steady-state exercise conditions. However, studies have also indicated that the functional characteristics of the baroreflex dynamically change throughout a given bout of exercise (Komine et al., 2003; Ichinose et al., 2006). Thus, arterial baroreflex characteristics derived during steady-state exercise may not be representative of the entire exercise period. Indeed, there is accumulating evidence from animal and human studies to support progressive temporal changes in arterial baroreflex control throughout a given bout of exercise. The dynamics and mechanisms contributing to these time related changes in baroreflex control appear dependent on whether HR, BP, or muscle sympathetic nerve activity (MSNA) is the effector variable of interest. The following section will briefly review these studies; however, readers are directed to a companion review by Matsukawa for a more detailed description of this work.
Several animal studies have indicated that baroreflex-mediated cardiac responses are transiently attenuated at the immediate onset of exercise and restored during the latter or steady-state period of exercise (Komine et al., 2003; Murata et al., 2004; Matsukawa et al., 2006). Initial studies using a decerebrate cat model demonstrated a blunting of cardiac baroreflex responsiveness at the onset of static exercise suggesting a rapid modulation of the baroreflex by skeletal muscle afferents (McWilliam et al., 1991). More recent work by Matsukawa and colleagues (Komine et al., 2003; Murata et al., 2004; Matsukawa et al., 2006) has also reported an attenuation of baroreflex cardiac responses at the onset of voluntary isometric exercise. However, by using a trained conscious cat model, these investigators demonstrated a role for central command in the immediate blunting of the baroreflex-mediated HR responses at the onset of exercise. Moreover, experimental maneuvers to selectively activate the exercise pressor reflex had no effect. Interestingly, the baroreflex-mediated BP response at exercise onset was unaltered in these studies indicating a differential modulation of baroreflex control of HR and blood pressure at the onset of exercise. Recent work in humans has also demonstrated a transient blunting of baroreflex-mediated control of HR at the onset of high-intensity isometric exercise, while the baroreflex control of BP was well maintained (Fisher et al., 2007). Importantly, in both the human and animal studies, the attenuated cardiac responses observed at exercise onset were restored during the latter period of exercise (> 15 s from start). It has been suggested that the selective and transient blunting of baroreflex-mediated HR responses at the onset of exercise provides a mechanism by which voluntary exercise rapidly increases HR at exercise onset. Collectively, these studies suggest that the cardiac component of the baroreflex is attenuated at the onset of exercise via inputs from skeletal muscle afferents and/or central command, whereas baroreflex-mediated BP responses at exercise onset are preserved.
Recent studies have also indicated dynamic modulation of the arterial baroreflex control of MSNA throughout a bout of exercise. Indeed, Ichinose and colleagues (Ichinose et al., 2006) have indicated that along with a progressive resetting of the arterial baroreflex control of MSNA during 3 min of handgrip exercise, a time-dependent increase in MSNA baroreflex sensitivity was observed. Because the increases in the baroreflex control of MSNA were also present during isolation of the muscle metaboreflex using a period of post exercise ischemia, it has been suggested that the muscle metaboreflex was the primary mediator of the time-dependent changes in MSNA baroreflex control during static handgrip. Indeed, Cui et al (Cui et al., 2001) have demonstrated that the sensitivity of baroreflex control of MSNA is increased during the selective activation of the muscle metaboreflex. Interestingly, these findings of temporal changes in arterial baroreflex control have also been reported during dynamic exercise (Ogoh et al., 2007b; Ichinose et al., 2008). However, the intensity of exercise may influence the degree of the change in baroreflex sensitivity for MSNA control (Ogoh et al., 2007b). In this regard, in humans the carotid baroreflex control of MSNA appears preserved during moderate-intensity one-legged kicking and arm cycling exercise (Fadel et al., 2001; Keller et al., 2004; Ogoh et al., 2007b), whereas during moderate to high intensity leg cycling an increase in arterial baroreflex-MSNA gain has been reported (Ichinose et al., 2008). The sensitivity of the arterial baroreflex control of renal SNA has also been shown to be increased during high-intensity treadmill exercise in rats (Miki et al., 2003). Taken together, these studies indicate an immediate and progressive resetting of the baroreflex control of SNA to operate around the exercise-induced elevations in BP with a maintained or increased sensitivity. Furthermore, the functional characteristics of the baroreflex dynamically change throughout a given bout of exercise.
e) SIGNIFICANCE OF ARTERIAL BAROREFLEX RESETTING
The resetting of the arterial baroreflex with exercise is essential for evoking and maintaining an appropriate neural cardiovascular response to physical activity. In exercising dogs, acute baro-denervation has been shown to lead to an exaggerated increase in BP (Walgenbach & Donald, 1983b). Likewise, in humans who have surgically denervated carotid baroreceptors, the BP response to exercise is greater (Smit et al., 2002; Timmers et al., 2004). An emerging concept is that the baroreflex acts to partially restrain the BP response to exercise by buffering increases in SNA produced by activation of central command and the exercise pressor reflex. This concept is substantiated by the 3-fold greater increase in MSNA observed during handgrip in healthy subjects when baroreflex activation was prevented by pharmacologically clamping BP at resting values and negating the exercise-induced rise in BP (Scherrer et al., 1990). Thus, an impaired arterial baroreflex function can lead to an insufficient buffering of increases in SNA during exercise. This unrestrained sympathoexcitation would lead to greater sympathetically-mediated vasoconstriction and contribute to a larger increase in BP. At the same time, greater vasoconstriction within the exercising skeletal muscle would limit the increases in perfusion necessary to meet the metabolic demands of the active tissue. As such, a diminished baroreflex function would not only lead to an augmented BP response to exercise but may also contribute to reductions in muscle blood flow and a functional muscle ischemia. Indeed, it has been suggested that impairments in BP regulation during exercise in disease states may lead to inappropriately high BP elevations and decreases in skeletal muscle perfusion with the latter potentially contributing to reductions in exercise tolerance (Joyner, 2006).
IV. THE CARDIOPULMONARY BAROREFLEX
Although not as well studied or recognized, there is accumulating data demonstrating that the cardiopulmonary baroreflex is intimately involved in evoking and maintaining neural cardiovascular responses to exercise. These low pressure mechanically-sensitive stretch receptors are located in the heart, great veins and blood vessels of the lungs and sense changes in central blood volume and pressure (Mark & Mancia, 1983; Hainsworth, 1991; Ray & Saito, 1999). Elevations in central volume (pressure) increase vagal afferent nerve firing reflexively decreasing SNA, while reductions in central volume (pressure) evoke profound increases in SNA. Although the majority of research on these low pressure receptors has focused on the cardiovascular adjustments to orthostatic stress, the limited information available regarding exercise suggests that the cardiopulmonary baroreflex plays a role in modulating MSNA and BP responses as well as arterial baroreflex resetting during dynamic exercise.
a) CARDIOPULMONARY BAROREFLEX AND EXERCISE
Initial observations in humans examining the cardiopulmonary baroreflex during exercise reported a threefold greater increase in forearm resistance when lower body negative pressure (to unload cardiopulmonary baroreceptors) was added to handgrip (Walker et al., 1980; Arrowood et al., 1993). These data indicated a facilitative interaction between lower body negative pressure and static handgrip such that the removal of the inhibitory influence of the cardiopulmonary baroreceptors by causing blood pooling in the lower limbs augmented the vasoconstrictor and pressor responses to exercise. However, this may be dependent on the magnitude of cardiopulmonary baroreflex unloading. In contrast to forearm vascular resistance; other investigators have observed little effect of lower body negative pressure on the MSNA responses to static handgrip (Sanders & Ferguson, 1988; Scherrer et al., 1988; Seals, 1988). These findings in humans were in contrast to results from studies in rabbits (DiCarlo & Bishop, 1990; O’Hagan et al., 1994) and rats (Collins & DiCarlo, 1993) suggesting that cardiac afferents modulate the increase in renal SNA during dynamic exercise. Considering the effects of the muscle pump and the large increases in venous return and preload that occur during dynamic exercise, the finding that sympathetic responses can be influenced by cardiopulmonary baroreceptors was not surprising. However, it was not until the work of Ray et al. (Ray et al., 1993) and Saito et al. (Saito et al., 1993) that a role for the cardiopulmonary baroreflex in modulating the MSNA responses to dynamic exercise was clearly recognized in humans. Instead of lower body negative pressure, Ray et al. (Ray et al., 1993) used postural changes to modulate cardiopulmonary load during one legged kicking. These investigators reported a reduction in MSNA below baseline values when exercise was performed in the upright position whereas when the subject was in the supine position no changes in MSNA were observed from rest to exercise. Importantly, central venous pressure tracked the changes in MSNA such that during leg kicking in the upright position central venous pressure was increased due to enhanced venous return while no change in central venous pressure was found during supine exercise. In agreement, Saito et al. (Saito et al., 1993) reported a decrease in MSNA from rest during light intensity leg cycling in the upright position. These findings indicated that the increases in central blood volume associated with the muscle pump activate the cardiopulmonary baroreceptors and inhibit MSNA. Importantly, this effect would be overcome by stimulation of skeletal muscle afferents during higher intensity exercise contributing to increases in MSNA. Taken together, these studies demonstrate that the cardiopulmonary baroreflex can modulate the MSNA response to dynamic exercise.
b) CARDIOPULMONARY BAROREFLEX AND ARTERIAL BAROREFLEX RESETTING
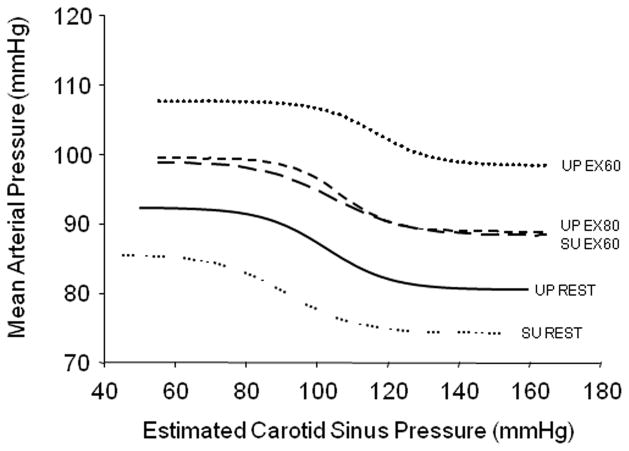
A functional role of the cardiopulmonary baroreflex in modulating BP during exercise was first recognized in the classic dog studies of Donald and colleagues (Walgenbach & Donald, 1983a; Daskalopoulos et al., 1984). Similarly, several studies in humans have identified an important role of the cardiopulmonary baroreflex in maintaining the exercise-induced increase in BP (Mack et al., 1988; Sprangers et al., 1991). More recent data suggests that alterations in cardiopulmonary baroreceptor load during dynamic exercise not only influences the prevailing exercise-induced BP but also, the resetting of the arterial baroreflex (Volianitis et al., 2004; Ogoh et al., 2006b; Ogoh et al., 2007a). Indeed, when leg cycling was added to arm cranking exercise, BP was reduced below that of arm exercise alone and resulted in the operating point of the carotid baroreflex-mean arterial pressure curve to relocate to a lower BP (Volianitis et al., 2004). Thus, even though a larger muscle mass was engaged and more work was being performed with arm plus leg exercise, BP and the operating point of the carotid-mean arterial pressure baroreflex curve were reduced. These data are in disagreement with the concept that greater activation of central command and the exercise pressor reflex elicited by increasing the exercise intensity or exercising muscle mass leads to an intensity-dependent resetting of the baroreflex function curve. Rather these findings suggest that increases in central blood volume during leg exercise, due to muscle-pump induced elevations in venous return, reduces the BP response to exercise and the locus of the operating point of the baroreflex-mean arterial pressure curve. Thus, inputs from cardiopulmonary baroreceptors appear to influence arterial baroreflex control during exercise. In agreement, increases in cardiopulmonary baroreceptor load, by changing subject posture from the upright to supine position and by increasing pedal frequency to enhance the muscle pump at the same amount of central command, has been shown to reduce the magnitude of exercise-induced increases in BP and carotid baroreflex resetting during dynamic exercise (Figure 3) (Ogoh et al., 2007a). Collectively, these findings suggest that changes in central blood volume, produced by the muscle pumps enhancement of venous return during leg exercise, influences the exercise-induced BP response and the locus of the operating point of the carotid baroreflex-mean arterial pressure curve (Volianitis et al., 2004; Ogoh et al., 2006b; Ogoh et al., 2007a).
Figure 3. Influence of increasing central blood volume on carotid baroreflex resetting during dynamic exercise in humans.
Carotid baroreflex-mean arterial pressure stimulus-response curves are presented at rest in the upright (UP) and supine (SU) positions as well as during cycling exercise under control (upright cycling at 60 revolutions per minute (rpm); UP EX60), supine cycling at 60 rpm (SU EX60), and upright cycling exercise at 80 rpm (UP EX80). All bouts of exercise were performed at the same oxygen consumption in an attempt to maintain similar central command and exercise pressor reflex activation. As discussed in detail in the text, increasing central blood volume, by changing subject posture from the upright to supine position and by increasing pedal frequency from 60 to 80 rpm to enhance the muscle pump, reduced the magnitude of exercise-induced increases in blood pressure and carotid baroreflex resetting during cycling exercise. (Adapted from Ogoh et al. 2007a.)
Recent data indicate that the cardiopulmonary baroreflex also resets during physical activity to operate around the exercise-induced increase in central blood volume without a change in reflex sensitivity (Ogoh et al., 2006a). Also, although not universal (Takeshita et al., 1979; Eiken et al., 1994), several studies have indicated that unloading the cardiopulmonary baroreceptors may enhance carotid baroreflex gain at rest and during exercise (Koike et al., 1975; Bevegard et al., 1977a; Bevegard et al., 1977b; Pawelczyk & Raven, 1989; Shi et al., 1993a; Potts et al., 1995). Overall, there is ample evidence demonstrating that inputs from the cardiopulmonary baroreceptors can influence arterial baroreflex control during dynamic exercise. Thus, along with central command and the exercise pressor reflex, the cardiopulmonary baroreflex plays an important role in baroreflex resetting during exercise.
c) SIGNIFICANCE OF CARDIOPULMONARY BAROREFLEX DURING EXERCISE
The available data demonstrates that the cardiopulmonary baroreflex may play an important modulatory role in evoking and maintaining neural cardiovascular responses to dynamic exercise. Indeed, the cardiopulmonary baroreceptors have been shown to provide a strong inhibitory influence on the MSNA response to dynamic exercise. In addition, inputs from the cardiopulmonary baroreceptors can influence arterial baroreflex resetting and function during dynamic exercise. Thus, an impaired cardiopulmonary baroreflex can lead to exaggerated increases in SNA directly via a diminished input to central sympathetic control centers as well as indirectly via its neural interaction with the arterial baroreflex. Overall, these impairments would lead to an insufficient buffering of SNA and contribute to unrestrained sympatho-excitation and larger increases in BP with exercise. Although the exact contribution of an altered cardiopulmonary baroreflex in the setting of exercise in disease populations is unclear, studies demonstrating an inability of the cardiopulmonary baroreceptors to alter resting sympathetic outflow in heart failure patients lend credence to such impairments during exercise (Mohanty et al., 1989). Thus, it is plausible that the abnormal haemodynamic and sympathoexcitatory responses to exercise observed in many cardiovascular diseases are mediated, at least in part, by diminished cardiopulmonary baroreflex function. This is an area that warrants further investigation.
V. SUMMARY
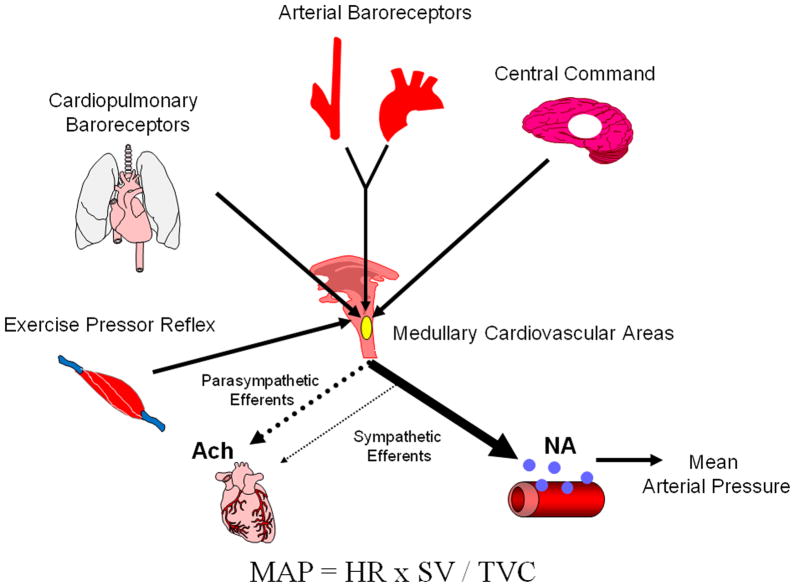
The arterial baroreflex is reset from rest to exercise in an intensity-dependent manner to continue to regulate BP as effectively as at rest. Studies have demonstrated that activation of central command and the exercise pressor reflex play both independent and interactive roles in the resetting of the arterial baroreflex with exercise. Since central command is characterized as a feed-forward mechanism, it is likely the primary regulator of baroreflex resetting with the exercise pressor reflex, a feedback mechanism, subserving in a modulatory role. Inputs from the cardiopulmonary baroreceptors also appear to be playing an important role in exercise resetting and modulating the locus of the operating point of the baroreflex-mean arterial pressure curve. Thus, the interactions of four neural mechanisms (central command, the exercise pressor reflex, the arterial baroreflex and cardiopulmonary baroreflex) contribute importantly to the neural cardiovascular adjustments to exercise (Figure 4).
Figure 4. A schematic representation of the neural mechanisms mediating the neural cardiovascular adjustments to exercise.
Neural signals originating from the brain (central command) and afferent feedback from the aortic and carotid arteries (arterial baroreflex), the heart and lungs (cardiopulmonary baroreflex) and skeletal muscle (exercise pressor reflex) contribute to the intensity-dependent modulation of sympathetic and parasympathetic nerve activity during exercise. These signals converge centrally within cardiovascular control areas in the medulla oblongata. The ensuing alterations in autonomic outflow mediate changes in heart rate (HR) and contractility, as well as the diameter of resistance and capacitance vessels within various tissue beds to modulate cardiac output (HR × stroke volume (SV)) and total vascular conductance (TVC), respectively. These changes then lead to alterations in mean arterial pressure (MAP) appropriate for the intensity and modality of the exercise. Ach, acetylcholine; NA, noradrenaline.
Acknowledgments
This manuscript was prepared as part of the forty year celebration symposium of Professor Jere H. Mitchell’s fundamental studies with Goodwin and McCloskey into the “Neural Control of the Circulation during Exercise” sponsored by the journal of Experimental Physiology. We both wish to acknowledge and thank Dr Mitchell for the advice and collaboration that he has afforded us and continues to provide us over some 40 years for Dr Raven and 15 years for Dr Fadel. P.J.F. is supported by NIH Grant no. HL-093167 and P.B.R. is supported by HL-106431.
References
- Aicher SA, Randich A. Antinociception and cardiovascular responses produced by electrical stimulation in the nucleus tractus solitarius, nucleus reticularis ventralis, and the caudal medulla. Pain. 1990;42:103–119. doi: 10.1016/0304-3959(90)91096-2. [DOI] [PubMed] [Google Scholar]
- Arrowood JA, Mohanty PK, McNamara C, Thames MD. Cardiopulmonary reflexes do not modulate exercise pressor reflexes during isometric exercise in humans. J Appl Physiol. 1993;74:2559–2565. doi: 10.1152/jappl.1993.74.5.2559. [DOI] [PubMed] [Google Scholar]
- Bevegard BS, Shepherd JT. Circulatory effects of stimulating the carotid arterial stretch receptors in man at rest and during exercise. J Clin Invest. 1966;45:132–142. doi: 10.1172/JCI105317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevegard S, Castenfors J, Lindblad LE. Effect of carotid sinus stimulation on cardiac output and peripheral vascular resistance during changes in blood volume distribution in man. Acta Physiol Scand. 1977a;101:50–57. doi: 10.1111/j.1748-1716.1977.tb05982.x. [DOI] [PubMed] [Google Scholar]
- Bevegard S, Castenfors J, Lindblad LE, Tranesjo J. Blood pressure and heart rate regulating capacity of the carotid sinus during changes in blood volume distribution in man. Acta Physiol Scand. 1977b;99:300–312. doi: 10.1111/j.1748-1716.1977.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Carrington C, White M. Spontaneous baroreflex sensitivity in young and older people during voluntary and electrically evoked isometric exercise. Age and Ageing. 2002;31:359–364. doi: 10.1093/ageing/31.5.359. [DOI] [PubMed] [Google Scholar]
- Collins HL, DiCarlo SE. Cardiac afferents attenuate the muscle metaboreflex in the rat. J Appl Physiol. 1993;75:114–120. doi: 10.1152/jappl.1993.75.1.114. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Daskalopoulos DA, Shepherd JT, Walgenbach SC. Cardiopulmonary reflexes and blood pressure in exercising sinoaortic-denervated dogs. J Appl Physiol. 1984;57:1417–1421. doi: 10.1152/jappl.1984.57.5.1417. [DOI] [PubMed] [Google Scholar]
- DiCarlo SE, Bishop VS. Regional vascular resistance during exercise: Role of cardiac afferents and exercise training. Am J Physiol. 1990;258:H842–847. doi: 10.1152/ajpheart.1990.258.3.H842. [DOI] [PubMed] [Google Scholar]
- Drew RC, Bell MP, White MJ. Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol. 2008a;104:716–723. doi: 10.1152/japplphysiol.00956.2007. [DOI] [PubMed] [Google Scholar]
- Drew RC, McIntyre DB, Ring C, White MJ. Local metabolite accumulation augments passive muscle stretch-induced modulation of carotid-cardiac but not carotid-vasomotor baroreflex sensitivity in man. Exp Physiol. 2008b;93:1044–1057. doi: 10.1113/expphysiol.2008.042234. [DOI] [PubMed] [Google Scholar]
- Eiken O, Convertino VA, Doerr DF, Morariu GA, Mekjavic I. Characteristics of the carotid baroreflex in man during normal and flow-restricted exercise. Acta Physiol Scand. 1992;144:325–331. doi: 10.1111/j.1748-1716.1992.tb09301.x. [DOI] [PubMed] [Google Scholar]
- Eiken O, Sun JC, Mekjavic IB. Effects of blood-volume distribution on the characteristics of the carotid baroreflex in humans at rest and during exercise. Acta Physiol Scand. 1994;150:89–94. doi: 10.1111/j.1748-1716.1994.tb09663.x. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol. 2003;88:671–680. doi: 10.1113/eph8802650. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2001;280:H1383–1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Junor C, Khaja A, Northrup M, Fadel PJ. Spontaneous baroreflex measures are unable to detect age-related impairments in cardiac baroreflex function during dynamic exercise in humans. Exp Physiol. 2009;94:447–458. doi: 10.1113/expphysiol.2008.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Young CN, Keller DM, Fadel PJ. Exercise intensity influences cardiac baroreflex function at the onset of isometric exercise in humans. J Appl Physiol. 2007;103:941–947. doi: 10.1152/japplphysiol.00412.2007. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Effect of muscle metaboreflex activation on carotid-cardiac baroreflex function in humans. Am J Physiol Heart Circ Physiol. 2008;294:H2296–2304. doi: 10.1152/ajpheart.91497.2007. [DOI] [PubMed] [Google Scholar]
- Gallagher K, Fadel P, Stromstad M, Ide K, Smith S, Querry R, Raven P, Secher N. Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. J Physiol. 2001a;533:871–880. doi: 10.1111/j.1469-7793.2001.t01-2-00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher K, Fadel P, Stromstad M, Ide K, Smith S, Querry R, Raven P, Secher N. Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol. 2001b;533:861–870. doi: 10.1111/j.1469-7793.2001.t01-1-00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Smith SA, Stromstad M, Ide K, Secher NH, Raven PB. The interaction of central command and the exercise pressor reflex in mediating baroreflex resetting during exercise in humans. Exp Physiol. 2006;91:79–87. doi: 10.1113/expphysiol.2005.032110. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiol Rev. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Heymans C, Neil E. Reflexogenic areas in the cardiovascular system. Churchill; London: 1958. [Google Scholar]
- Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol. 2008;586:2753–2766. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Kondo N, Nishiyasu T. Time-dependent modulation of arterial baroreflex control of muscle sympathetic nerve activity during isometric exercise in humans. Am J Physiol Heart Circ Physiol. 2006;290:H1419–1426. doi: 10.1152/ajpheart.00847.2005. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Legramante JM, Raimondi G, Peruzzi G. Baroreflex control of sinus node during dynamic exercise in humans: effects of central command and muscle reflexes. Am J Physiol. 1997;272:H1157–1164. doi: 10.1152/ajpheart.1997.272.3.H1157. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol. 2006;91:27–36. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- Keller DM, Fadel PJ, Ogoh S, Brothers RM, Hawkins M, Olivencia-Yurvati A, Raven PB. Carotid baroreflex control of leg vasculature in exercising and non-exercising skeletal muscle in humans. J Physiol. 2004;561:283–293. doi: 10.1113/jphysiol.2004.071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Mark AL, Heistad DD, Schmid PG. Influence of cardiopulmonary vagal afferent activity on carotid chemoreceptor and baroreceptor reflexes in the dog. Circ Res. 1975;37:422–429. doi: 10.1161/01.res.37.4.422. [DOI] [PubMed] [Google Scholar]
- Komine H, Matsukawa K, Tsuchimochi H, Murata J. Central command blunts the baroreflex bradycardia to aortic nerve stimulation at the onset of voluntary static exercise in cats. Am J Physiol Heart Circ Physiol. 2003;285:H516–526. doi: 10.1152/ajpheart.00013.2003. [DOI] [PubMed] [Google Scholar]
- Mack G, Nose H, Nadel ER. Role of cardiopulmonary baroreflexes during dynamic exercise. J Appl Physiol. 1988;65:1827–1832. doi: 10.1152/jappl.1988.65.4.1827. [DOI] [PubMed] [Google Scholar]
- Mancia G, Iannos J, Jamieson GG, Lawrence RH, Sharman PR, Ludbrook J. Effect of isometric hand-grip exercise on the carotid sinus baroreceptor reflex in man. Clin Sci Mol Med. 1978;54:33–37. doi: 10.1042/cs0540033. [DOI] [PubMed] [Google Scholar]
- Mancia G, Mark AL. Handbook of Physiology The Cardiovascular System Peripheral Circulation and Organ Blood Flow. Am. Physiol. Soc; Bethesda, MD: 1983. Arterial baroreflexes in humans; pp. 755–793. [Google Scholar]
- Marey EJ. Physiologie medicale de la circulation du sang. Paris: Delahaye; 1863. [Google Scholar]
- Mark AL, Mancia G. Cardiopulmonary baroreflexes in humans. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2: The cardiovascular system, peripheral circulation and organ blood flows. American Physiological Society; Bethesda, MD: 1983. pp. 795–813. [Google Scholar]
- Matsukawa K, Komine H, Nakamoto T, Murata J. Central command blunts sensitivity of arterial baroreceptor-heart rate reflex at onset of voluntary static exercise. Am J Physiol Heart Circ Physiol. 2006;290:H200–208. doi: 10.1152/ajpheart.00013.2005. [DOI] [PubMed] [Google Scholar]
- McIlveen SA, Hayes SG, Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol. 2001;280:H1454–1463. doi: 10.1152/ajpheart.2001.280.4.H1454. [DOI] [PubMed] [Google Scholar]
- McWilliam PN, Yang T, Chen LX. Changes in the baroreceptor reflex at the start of muscle contraction in the decerebrate cat. J Physiol (London) 1991;436:549–558. doi: 10.1113/jphysiol.1991.sp018566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher A, Donald DE. Maintained ability of carotid baroreflex to regulate arterial pressure during exercise. Am J Physiol. 1981;241:H838–849. doi: 10.1152/ajpheart.1981.241.6.H838. [DOI] [PubMed] [Google Scholar]
- Miki K, Yoshimoto M, Tanimizu M. Acute shifts of baroreflex control of renal sympathetic nerve activity induced by treadmill exercise in rats. J Physiol. 2003;548:313–322. doi: 10.1113/jphysiol.2002.033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty PK, Arrowood JA, Ellenbogen KA, Thames MD. Neurohumoral and hemodynamic effects of lower body negative pressure in patients with congestive heart failure. Am Heart J. 1989;118:78–85. doi: 10.1016/0002-8703(89)90075-6. [DOI] [PubMed] [Google Scholar]
- Murata J, Matsukawa K, Komine H, Tsuchimochi H, Nakamoto T. Central inhibition of the aortic baroreceptors-heart rate reflex at the onset of spontaneous muscle contraction. J Appl Physiol. 2004;97:1371–1378. doi: 10.1152/japplphysiol.00307.2004. [DOI] [PubMed] [Google Scholar]
- Norton KH, Boushel R, Strange S, Saltin B, Raven PB. Resetting of the carotid arterial baroreflex during dynamic exercise in humans. J Appl Physiol. 1999;87:332–338. doi: 10.1152/jappl.1999.87.1.332. [DOI] [PubMed] [Google Scholar]
- O’Hagan KP, Bell LB, Mittelstadt SW, Clifford PS. Cardiac receptors modulate the renal sympathetic response to dynamic exercise in rabbits. J Appl Physiol. 1994;76:507–515. doi: 10.1152/jappl.1994.76.2.507. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha SAOY, Raven PB. Cardiopulmonary baroreflex is reset during dynamic exercise. J Appl Physiol. 2006a;100:51–59. doi: 10.1152/japplphysiol.00804.2005. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, AOY, Raven PB. Effects of changes in central blood volume on carotid-vasomotor baroreflex sensitivity at rest and during exercise. J Appl Physiol. 2006b;101:68–75. doi: 10.1152/japplphysiol.01452.2005. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol. 2003;550:317–324. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol. 2005;566:599–611. doi: 10.1113/jphysiol.2005.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Fadel PJ, Raven PB. Increases in central blood volume modulate carotid baroreflex resetting during dynamic exercise in humans. J Physiol. 2007a;581:405–418. doi: 10.1113/jphysiol.2006.125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2007b;293:H2202–2209. doi: 10.1152/ajpheart.00708.2007. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Wasmund WL, Keller DM, AOY, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol. 2002;543:349–364. doi: 10.1113/jphysiol.2002.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Gauthier JP, Rowell LB. Carotid baroreflex control of blood pressure and heart rate in men during dynamic exercise. J Appl Physiol. 1994;77:502–506. doi: 10.1152/jappl.1994.77.2.502. [DOI] [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Helloco F, Rowell LB. Muscle chemoreflex alters carotid sinus baroreflex response in humans. J Appl Physiol. 1997;82:577–583. doi: 10.1152/jappl.1997.82.2.577. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Raven PB. Reductions in central venous pressure improve carotid baroreflex responses in conscious men. Am J Physiol. 1989;257 doi: 10.1152/ajpheart.1989.257.5.H1389. [DOI] [PubMed] [Google Scholar]; Heart Circ Physiol. 26:H1389–H1395. [Google Scholar]
- Pickering TG, Gribbin B, Petersen ES, Cunningham DJ, Sleight P. Effects of autonomic blockade on the baroreflex in man at rest and during exercise. Circ Res. 1972;30:177–185. doi: 10.1161/01.res.30.2.177. [DOI] [PubMed] [Google Scholar]
- Potts JT, Mitchell JH. Rapid resetting of carotid baroreceptor reflex by afferent input from skeletal muscle receptors. Am J Physiol. 1998;275:H2000–2008. doi: 10.1152/ajpheart.1998.275.6.H2000. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi X, Raven PB. Cardiopulmonary baroreceptors modulate carotid baroreflex control of heart rate during dynamic exercise in humans. Am J Physiol. 1995;268 doi: 10.1152/ajpheart.1995.268.4.H1567. [DOI] [PubMed] [Google Scholar]; Heart Circ Physiol. 37:H1567–H1576. doi: 10.1152/ajpheart.00564.2004. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol. 1993;265 doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]; Heart Circ Physiol. 34:H1928–H1938. [Google Scholar]
- Querry RG, Smith SA, Stromstad M, Ide K, Raven PB, Secher NH. Neural blockade during exercise augments central command’s contribution to carotid baroreflex resetting. Am J Physiol. 2001;280:H1635–H1644. doi: 10.1152/ajpheart.2001.280.4.H1635. [DOI] [PubMed] [Google Scholar]
- Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- Raven PB, Potts JT, Shi X. Baroreflex regulation of blood pressure during dynamic exercise. Exerc Sport Sci Rev. 1997;25:365–389. [PubMed] [Google Scholar]
- Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol. 1993;264:H1–7. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- Ray CA, Saito M. The Cardiopulmonary Baroreflex. In: Saltin B, Boushel R, Secher NH, Mitchell JH, editors. Exercise and Circulation in Health and Disease. 1999. pp. 43–51. [Google Scholar]
- Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Sagawa K. Handbook of Physiology The Cardiovascular System. Am. Physiol. Soc; Bethesda, MD: 1983. Baroreflex control of systemic arterial pressure and vascular bed; pp. 453–496. [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Sanders JS, Ferguson DW. Cardiopulmonary baroreflexes fail to modulate sympathetic responses during isometric exercise in humans: direct evidence from microneurographic studies. J Am Coll Cardiol. 1988;12:1241–1251. doi: 10.1016/0735-1097(88)92607-1. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Floistrup S, Vissing S, Victor RG. Effect of lower body negative pressure on sympathetic nerve responses to static exercise in humans. Circulation. 1988;78:49–59. doi: 10.1161/01.cir.78.1.49. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Pryor SL, Bertocci LA, Victor RG. Arterial baroreflex buffering of sympathetic activation during exercise-induced elevations in arterial pressure. J Clin Invest. 1990;86:1855–1861. doi: 10.1172/JCI114916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR. Cardiopulmonary baroreflexes do not modulate exercise-induced sympathoexcitation. J Appl Physiol. 1988;64:2197–2203. doi: 10.1152/jappl.1988.64.5.2197. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Mulholland JH, Safiroff B. Surgical anatomy of the carotid sinus nerve. Anat Rec. 1941;8o:431–442. [Google Scholar]
- Shi X, Crandall CG, Potts JT, Foresman BH, Raven PB. A diminished aortic-cardiac reflex during hypotension in aerobically fit young men. Med Sci Sports Exerc. 1993a;25 [PubMed] [Google Scholar]
- Shi X, Potts JT, Foresman BH, Raven PB. Carotid baroreflex responsiveness to lower body positive pressure-induced increases in central venous pressure. Am J Physiol. 1993b;265 doi: 10.1152/ajpheart.1993.265.3.H918. [DOI] [PubMed] [Google Scholar]; Heart Circ Physiol. 34:H918–H922. [Google Scholar]
- Smit AJ, Timmers HM, Wieling W, Wagenaar M, Marres HM, Lenders JM, Montfrans GAv, Karemaker JM. Long-term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation. 2002;105:1329–1335. doi: 10.1161/hc1102.105744. [DOI] [PubMed] [Google Scholar]
- Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol. 2003;551:1013–1021. doi: 10.1113/jphysiol.2003.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers RL, Wesseling KH, Imholz AL, Imholz BP, Wieling W. Initial blood pressure fall on stand up and exercise explained by changes in total peripheral resistance. J Appl Physiol. 1991;70:523–530. doi: 10.1152/jappl.1991.70.2.523. [DOI] [PubMed] [Google Scholar]
- Takeshita A, Mark AL, Eckberg DL, Abboud FM. Effect of central venous pressure on arterial baroreflex control of heart rate. Am J Physiol. 1979;236 doi: 10.1152/ajpheart.1979.236.1.H42. [DOI] [PubMed] [Google Scholar]; Heart Circ Physiol. 5:H42–H47. [Google Scholar]
- Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Cardiovascular responses to stress after carotid baroreceptor denervation in humans. Ann N Y Acad Sci. 2004;1018:515–519. doi: 10.1196/annals.1296.063. [DOI] [PubMed] [Google Scholar]
- Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res. 1989;65:468–476. doi: 10.1161/01.res.65.2.468. [DOI] [PubMed] [Google Scholar]
- Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res. 1995;76:127–131. doi: 10.1161/01.res.76.1.127. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Yoshiga CC, Vogelsang T, Secher NH. Arterial blood pressure and carotid baroreflex function during arm and combined arm and leg exercise in humans. Acta Physiol Scand. 2004;181:289–295. doi: 10.1111/j.1365-201X.2004.01292.x. [DOI] [PubMed] [Google Scholar]
- Walgenbach SC, Donald DE. Cardiopulmonary reflexes and arterial pressure during rest and exercise in dogs. Am J Physiol. 1983a;244:H362–369. doi: 10.1152/ajpheart.1983.244.3.H362. [DOI] [PubMed] [Google Scholar]
- Walgenbach SC, Donald DE. Inhibition by carotid baroreflex of exercise-induced increases in arterial pressure. Circ Res. 1983b;52:253–262. doi: 10.1161/01.res.52.3.253. [DOI] [PubMed] [Google Scholar]
- Walker JL, Abboud FM, Mark AL, Thames MD. Interaction of cardiopulmonary and somatic reflexes in humans. J Clin Invest. 1980;65:1491–1497. doi: 10.1172/JCI109814. [DOI] [PMC free article] [PubMed] [Google Scholar]