Abstract
In vivo anti-polysaccharide (PS) Ig responses to isolated PS are T cell-independent, rapid, and fail to generate memory. However, little is known regarding PS-specific Ig responses to intact Gram-positive (GP) and Gram-negative (GN) extracellular bacteria. We previously demonstrated that intact heat-killed Streptococcus pneumoniae (Pn), a GP bacterium, elicited a rapid primary IgG anti-capsular PS (PPS) response in mice that was dependent on CD4+ T cells, B7-dependent costimulation, and CD40-CD40L interactions. However, this response was ICOS-independent and failed to generate a boosted PPS-specific secondary IgG response. In the present study we analyzed the murine anti-capsular PS (MCPS) Ig response to i.p.-injected intact, heat-killed Neisseria meningitidis serogroup C (MenC), a GN bacterium. In contrast to Pn, the IgG anti-MCPS response to MenC exhibited delayed primary kinetics and was highly boosted after secondary immunization, whereas the IgG anti-MCPS response to isolated MCPS was rapid, without secondary boosting, and consisted of only IgG1 and IgG3, as opposed to all four IgG isotypes in response to intact MenC. The secondary, but not primary, IgG anti-MCPS response to MenC was dependent on CD4+ T cells, CD40L, CD28 and ICOS. The primary and secondary IgG anti-MCPS responses were lower in TLR4-defective (C3H/HeJ), although not TLR2−/− or MyD88−/− mice, but secondary boosting was still observed. Of interest, co-immunization of Pn and MenC, resulted in a boosted secondary IgG anti-PPS response to Pn. Our data demonstrate that the nature of the in vivo anti-PS response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain.
Keywords: Rodent, B cells, T cells, bacterial, antibodies, antigens, vaccination, memory, costimulation, transgenic/knockout mice
Introduction
Polysaccharide (PS)-encapsulated extracellular bacteria like Streptococcus pneumoniae (Pn) [Gram-positive (GP)], and Neisseria meningitidis (Men) [Gram-negative (GN)] are major sources of global morbidity and mortality among infants, the elderly and the immunosuppressed (1). Adaptive immunity to extracellular bacteria is mediated largely by antibodies specific for both protein and PS Ags (2). Protein and PS Ags are biochemically distinct and are processed differently by cells of the immune system. Unlike proteins, non-zwitterionic PS fail to associate with MHC class-II molecules (3, 4) and are unable to recruit cognate CD4+ T cell help for induction of anti-PS responses (5). However, PS in contrast to proteins can deliver strong and sustained signals to specific B cells through multivalent membrane Ig crosslinking via repeating, identical structural units (6), which critically impacts on the nature of the B cell response to various second signals (7). Thus, protein and PS Ags are classified as T cell-dependent (TD) and T cell-independent (TI) Ags, respectively.
This central dogma is derived mostly from studies using purified protein and PS (5). However, covalent linkage of protein and PS to create a soluble conjugate vaccine converts the PS into a TD Ag, including the ability to generate PS-specific memory (8). Intact bacteria are complex particulate immunogens in which multiple protein and PS Ags and bacterial adjuvants are co-expressed. This raises the question as to whether the PS expressed by intact bacteria also behave like TD Ags, similar to those in conjugate vaccines. We previously demonstrated that the IgG anti-PS (PPS14) response to intact, heat-killed Pn, capsular type 14 (Pn14) a GP extracellular bacteria, is dependent on CD4+ T cells, B7-dependent costimulation and CD40/CD40L interactions and comprise all four isotypes of IgG (as opposed to predominantly IgG3 and some IgG1 for isolated PS Ags) (9, 10), similar to that observed for the IgG anti-protein response. In contrast to the anti-protein response, the IgG anti-PPS14 response to intact Pn14 exhibits a rapid primary IgG response, dependent upon a shorter period of T cell help and B7-dependent costimulation, and fails to generate a boosted secondary response (11). Furthermore, the IgG anti-PPS14, in contrast to the IgG anti-protein response to Pn is ICOS-independent, extra-follicular (10) and more apoptosis prone (12). Thus, PPS14 in the context of intact Pn14 combines certain features of both an isolated PS Ag and a PS-protein conjugate vaccine (11).
Studies on the anti-PPS14 response to intact Pn14 indicate that the bacterium can markedly influence the immunobiology of the expressed PS Ag. These studies, however, left unresolved whether the nature of the PPS14-specific Ig response to intact Pn14 was characteristic of intact PS-expressing extracellular bacteria in general, or perhaps represented a characteristic feature of PPS14, the underlying structure and/or composition of intact Pn, or perhaps a more general dichotomy between GP and GN bacteria. Thus PPS14, among several other pathogen-derived substances, can bind to SIGN-R1, a scavenger receptor present on marginal zone macrophages (13). Capsular PS may additionally vary based on molecular weight (14), charge characteristics (15), sialic acid content (16), or unique immunomodulatory properties (17), which may influence the nature of the associated immune response. Further, bacteria may express components within the cell wall, such as phosphorylcholine (PC), expressed by Pn as well as other pathogens, which may inhibit immunity (18).
In addition to the above considerations, the structure of intact GP and GN extracellular bacteria are significantly different, and these differences may influence the nature of the anti-PS response to the intact bacteria. Thus, capsular PS expressed by GP bacteria are covalently linked to a thick, underlying cell wall peptidoglycan to which a number of proteins are also covalently attached (19, 20). Capsular PS expressed by GN bacteria, which express a thin peptidoglycan cell wall, is attached through a labile covalent linkage to the acyl glycerol moiety of the outer membrane. The outer membrane is known to have multiple immunomodulatory properties, in part due to the presence of porin proteins [TLR2 ligand] and LPS [TLR4 ligand] (21, 22). Notably, immunization of mice with either (i) crude outer membrane complexes from Neisseria meningitidis serogroup C [MenC] (containing both LPS and capsular PS [MCPS]), (ii) purified complexes (lacking LPS), and (iii) outer membrane complexes (lacking MCPS) all resulted in significant boosting of the IgG anti-MCPS response following secondary immunization (23). In addition, mice primed systemically with live MenC exhibited a highly boosted IgG anti-MCPS response following secondary infection (24). Collectively, these data suggest that the nature of the PS-specific Ig response to intact Pn and Men may differ.
In this report, we utilized intact, heat-killed MenC in order to determine how the biochemically complex bacterial particle influences the nature of the anti-MCPS response, relative to that elicited by isolated, soluble MCPS. In addition, these experiments were performed in a manner analogous to those previously conducted using intact heat-killed Pn14, in order to determine whether bacteria comprising different structures may influence the Ig anti-PS responses in distinct ways. We demonstrate that the anti-MCPS response to intact MenC is strikingly different from that elicited by isolated MCPS, as well as to the anti-PPS14 response to intact Pn14. We conclude that the nature of the in vivo anti-PS response is markedly influenced by the composition and/or architecture of the subcapsular domain.
Materials and methods
Mice
CD28−/− mice (C57BL/6 background; B6.129S2-Cd28tm1Mak/J, catalog no. 002666), ICOS−/− mice (C57BL/6 background; B6129P2-Icostm1Mak/J, catalog no. 004859), C3H/HeJ (catalog no. 000659) and C3H/HeOuJ mice (catalog no. 000635), TLR2−/− mice (C57BL/6 background; B6.129-Tlr2tm1Kir/J, catalog no. 004650), and MyD88−/− mice (C57BL/6 background; B6.129P2(SJL)-Myd88tm1.1Defr/J, catalog no. 009088) were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6, BALB/c, and athymic nude (BALB/c background) mice were purchased from the National Cancer Institute (Frederick, MD). Female mice were used between 7 and 10 week of age. These studies were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, revised 1996), and were approved by the Uniformed Services University of the Health Sciences Institutional Animal Care and Use Committee.
Bacterial strains
Neisseria meningitidis serogroup C (MenC) (strain M1883] was obtained from American Type Culture Collection [Manassas, VA] (ATCC 53414). The encapsulated MenC strain FAM18 C+ and its isogenic unencapsulated variant strain FAM18 C-was a kind gift of Dr. Mustafa Akkoyunlu (FDA, Bethesda, MD) (25). Lyophilized or frozen stocks of bacteria were grown overnight on BBL blood agar plates (VWR International, Bridgeport, NJ). Isolated colonies on blood agar were grown in Brain Heart Infusion media (BD Biosciences, San Jose, CA) to mid-log phase, collected, and heat-killed by incubation at 65°C for 2 h or inactivated by overnight UV irradiation. Sterility was confirmed by subculture on blood agar plates. After extensive washings, the bacterial suspension was adjusted with PBS to give an absorbance reading at 650 nm of 0.6 which corresponded to 109 CFU/ml. Bacteria were then aliquoted at 1010 CFU/ml and frozen at −20°C until their use as immunogen for mouse immunizations. Streptococcus pneumoniae, capsular type 14 (strain R614) was prepared as described previously (26).
Reagents
A covalent conjugate of MCPS and tetanus toxoid (TT) [MCPS-TT] was prepared as previously described (9). Purified MCPS was obtained from the Serum Institute of India (Pune, India). Purified S. pneumoniae capsular polysaccharide type 14 (PPS14) was purchased from ATCC. Rat IgG2b anti-mouse CD4 mAb (clone GK1.5) was purified from ascites by ammonium sulfate precipitation and passage over a protein G column. Purified polyclonal rat IgG was purchased from Sigma (St. Louis, MO). Hamster IgG mouse anti-CD40L mAb (clone MR1), polyclonal hamster IgG, rat IgG2a anti-mouse CD275 mAb (HK5.3), and rat IgG2a isotype control (clone 2A3) were purchased from BioXcell (West Lebanon, NH). Alum (Allhydrogel 2%) was obtained from Brenntag Biosector (Denmark). A stimulatory 30 mer CpG-containing oligodeoxynucleotide (CpG-ODN) was synthesized as previously described (27).
Purification of native PorB protein from Neisseria meningitidis
N. meningitidis serogroup B [strain H44/76] (28) was grown on GC-agar plates containing 1% (v/v) Isovitalex overnight at 37°C in a humidified incubator with 5% (v/v) CO2. The next day, colonies were inoculated in liquid GC medium and grown to exponential phase as previously described (29). After overnight expansion of the cultures, the bacterial suspension was centrifuged at 3900 × g for 15 min at 4°C and the total protein content was extracted with the CaCl2-Zwittergent method. PorB is purified using an AktaPrime protein chromatography machine as previously described (29). Briefly, the protein suspension was first subject to ion-exchange chromatography on two DEAE/carboxymethyl columns in tandem, followed by a gel filtration chromatography on a Sephacryl S-300 column and finally a Matrex Cellufine Sulfate column for removal of endotoxin traces.
Preparation of MenC whole protein extract
Unencapsulated FAM18 C− bacteria were centrifuged at 3000 rpm for 20 min and the supernatant was discarded. The B-PER bacterial protein extraction reagent from Pierce (Rockford, IL) was added to the bacterial pellet (1:10 ratio), mixed well by shaking and incubated at RT for 15 min and again centrifuged at 3000 rpm for 10 min. The supernatant containing the soluble proteins was obtained and the concentration was determined by the BCA assay (Pierce, Rockford, IL).
Production and purification of recombinant PspA
Recombinant pneumococcal surface protein A (PspA) was expressed in Saccharomyces cerevisiae BJ3505. The supernatant was then passaged through a Q FF column (Amersham Pharmacia, Piscataway, NJ) and eluted with 0.2 M NaCl solution containing 20 mM Tris pH 9.0. Eluted PspA was then added to a Phenyl HP column (Amersham Pharmacia) equilibrated with 40 mM phosphate buffer containing 1.3 M ammonium sulfate, pH 7.0. PspA was then eluted from this column using a 1.3 M to 0.4 M ammonium sulfate gradient. Pooled PspA was dialyzed against 20 mM sodium acetate, pH 4.7 solution, and loaded into an S15 column (Amersham Pharmacia). PspA was then eluted with a 0.3 M NaCl solution containing 20 mM sodium acetate. Eluted PspA was dialyzed and concentrated and found to be >95% pure by Coomassie blue staining.
Immunization
Depending on the experiment, groups of 7 mice were immunized with 2 × 108 CFU heat-killed or UV-inactivated MenC in saline (i.p. or i.v.), 5 × 106 CFU live MenC (i.p.), 10 µg of purified MCPS in saline (i.p), or 1 µg of TT-MCPS adsorbed on 13 µg of alum mixed with 25 µg of CpG-ODN (i.p.). Serum samples were prepared, at different time points, from blood obtained through the tail vein.
ELISA
For measurement of serum titers of MCPS-specific Ig, Immulon 4 ELISA plates were pre-coated with poly-L-lysine [Sigma] (5 µg/ml, 100 µl/well) in PBS for 1 h at 37°C. The plates were then washed 3× with PBS + 0.1% Tween 20 and then coated overnight at 4°C with purified MCPS (10 µg/ml, 100 µl/well) in PBS. To measure the serum titers of PorB- or whole protein-specific Ig, the plates were directly coated with PorB or whole protein extract (2 µg/ml, 50 µl/well). Plates were then washed 3× with PBS + 0.1% Tween 20 and were blocked with PBS + 1.0% BSA for 1 h at 37°C. Three-fold dilutions of serum samples, starting at a 1/50 serum dilution, in PBS + 1.0% BSA were incubated overnight at 4°C and plates were then washed 3× with PBS + 0.1% Tween 20. Alkaline phosphatase-conjugated polyclonal goat anti-mouse IgM, IgG, IgG3, IgG1, IgG2b, IgG2a, or IgG2c Abs (200 ng/ml) in PBS + 1.0% BSA were then added, and plates were incubated at 37°C for 1 h. Plates were washed 3× with PBS + 0.1% Tween 20. Substrate (p-nitrophenyl phosphate, disodium; Sigma) at 1 mg/ml in 1 M Tris + 0.3 mM MgCl2 (pH 9.8) was then added for color development. Color was read at an absorbance of 405 nm on a Multiskan Ascent ELISA reader (Labsystems, Finland). Serum titers of PPS14- and PspA-specific Ig were measured as described previously (10).
Statistics
Serum Ig isotype titer was expressed as the geometric means ± SEM of the individual serum Ig isotype titer. Significance was determined by the Student t test. p-values of ≤ 0.05 were considered statistically significant. All experiments were performed at least two times.
Results
The primary and secondary IgG anti-MCPS responses to intact MenC are similar to that elicited by a meningococcal conjugate vaccine
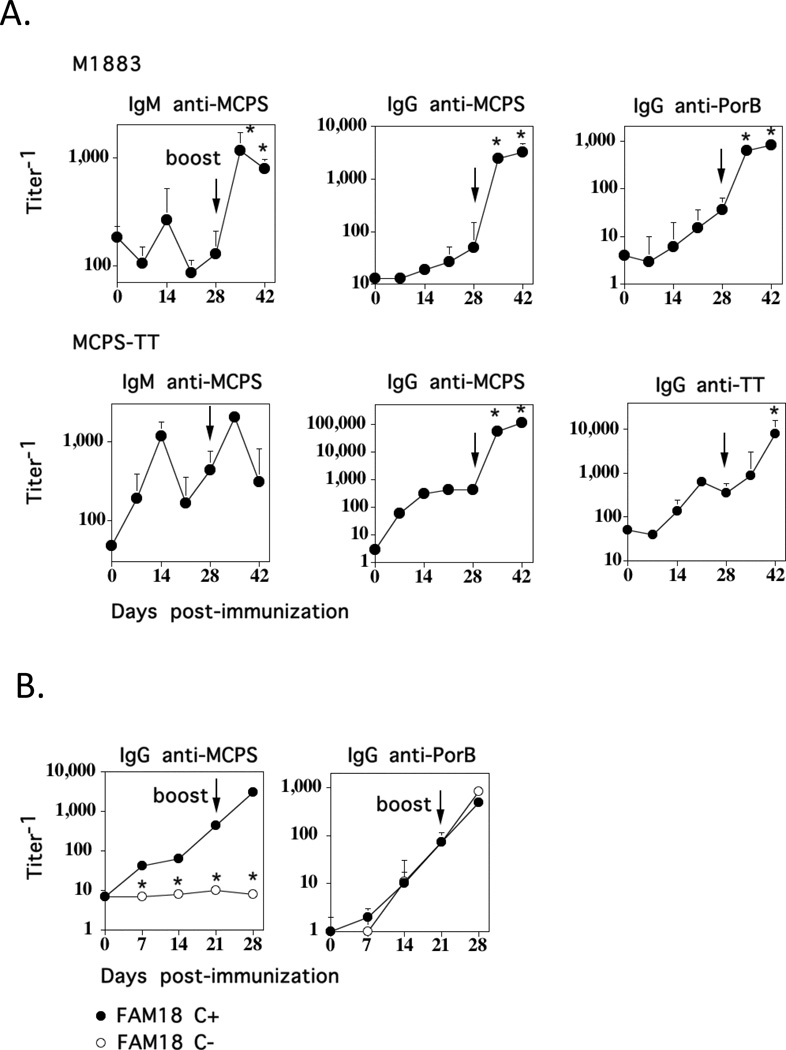
We previously demonstrated that the anti-PPS14 response to intact heat-killed Pn14, a GP bacterium, is distinct from that elicited by a soluble covalent conjugate of PPS14 and pneumococcal surface protein A (PPS14-PspA), in that the former exhibited rapid primary kinetics (peak day 6) with no secondary boosting, despite the CD4+ T cell-dependence of the IgG anti-PPS14 responses to both Pn14 and PPS14-PspA (11). Based on the distinct subcapsular composition and/or structure of GP and GN bacteria, we hypothesized that the PPS14-specific Ig response to Pn14 might be immunologically different from a PS-specific Ig response induced by a GN bacterium. In this regard, we chose to study the nature of the anti-MCPS response to intact MenC, a GN bacterium, in comparison to that elicited by a meningococcal conjugate vaccine. We immunized BALB/c mice i.p. with either UV-inactivated MenC [strain M1883] (2 × 108 CFU/mouse) in saline or MCPS-TT conjugate (1 µg/mouse) in alum + CpG-ODN, and boosted the mice 28 days later in a similar fashion. As illustrated in Fig. 1A, there was minimal induction of serum IgM anti-MCPS in the primary response to M1883, with a ~4-fold significant boost in serum titers following secondary immunization. In contrast, MCPS-TT elicited a significant, though transient primary induction of IgM anti-MCPS, and no boosting following secondary immunization. The primary IgG anti-MCPS responses to both M1883 and MCPS-TT were relatively modest and peaked on day 21 (M1883) or day 14 (MCPS-TT) (Fig. 1A). In contrast to IgM, the secondary IgG anti-MCPS responses to both M1883 and MCPS-TT were highly and rapidly boosted. The two MCPS-specific IgG responses were similar in kinetics and boosting to the associated anti-protein responses (PorB for M1883, TT for MCPS-TT). Similar primary and secondary IgM and IgG anti-MCPS responses were observed in response to 5 × 106 CFU/mouse of live M1883 (injected i.p.) or 2 × 108 CFU/mouse of heat-killed M1883 (injected i.v.) [data not shown] or i.p. (all experiments below). Immunization i.p. with heat-killed M1883 at 2 × 108 CFU/mouse versus 1 × 109 CFU/mouse gave similar results (data not shown).
Figure 1. The primary and secondary IgG anti-MCPS responses to intact MenC are similar to that elicited by a meningococcal conjugate vaccine.
(A). BALB/c mice (7 per group) were immunized i.p. with 2 × 108 CFU of U.V.-inactivated intact MenC (strain M1883) in saline or 1µg of MCPS-TT in alum + CpG-ODN, and boosted i.p. with the same dose on day 28. Serum titers of Ag-specific IgM and IgG were measured by ELISA. Significance (*) p≤0.05 between secondary titers relative to peak primary titers (B). BALB/c mice (7 per group) were immunized i.p. with 2 × 108 CFU of heat-inactivated intact FAM18 C+ (encapsulated MenC) or FAM18 C− (unencapsulated MenC), and boosted i.p. with the same dose on day 21. Serum titers of Ag-specific IgG were determined by ELISA. Significance (*) p≤0.05 between FAM18 C+ and FAM18 C−.
The specificity of the MCPS-specific ELISA was established using a different strain of encapsulated MenC (FAM18 C+) versus its unencapsulated isogenic mutant (FAM18 C−) (25) (Fig. 1B). Thus, FAM18 C− failed to elicit a detectable IgG anti-MCPS response, in contrast to FAM18 C+, the latter exhibiting detectable primary and highly boosted secondary IgG anti-MCPS responses, whereas both strains induced comparable IgG anti-PorB responses. Collectively, these data demonstrate that the kinetics and boosting characteristics of the IgG anti-MCPS response to intact MenC is similar to that of a corresponding conjugate vaccine, but distinct from the previously reported IgG anti-PPS14 response intact Pn14. In addition, the weak IgM anti-MCPS response to M1883 contrasts with the previously reported high-titer IgM anti-PPS14 response to Pn14.
The MCPS-specific IgM and IgG isotype responses to isolated MCPS and intact M1883 are distinct
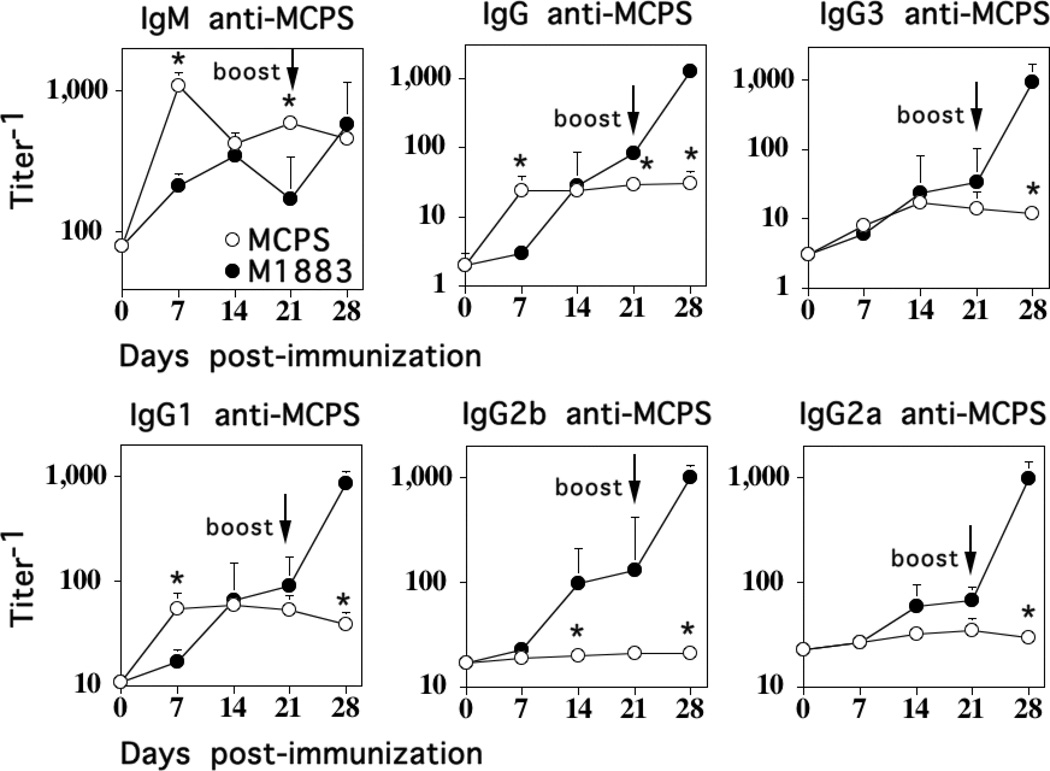
Isolated PS are poorly immunogenic, elicit Ig responses largely of the IgM isotype, with smaller amounts of IgG3, and sometimes IgG1, and fail to show boosted IgG anti-PS responses following secondary immunization (5). To determine the effect of M1883 on the associated anti-MCPS response, we directly compared the MCPS-specific IgM and IgG isotype responses to isolated, soluble MCPS (10 µg/mouse in saline) versus heat-killed, intact M1883 (2 × 108 CFU/mouse) both injected i.p., followed by a similar secondary immunization on day 21. As illustrated in Fig. 2, the primary IgM anti-MCPS response to purified MCPS peaked by day 7 and failed to show a boosted secondary response, whereas the primary IgM anti-MCPS response to intact M1883 peaked by day 14 at somewhat lower peak titers than that observed for isolated MCPS, and also failed to show a boosted secondary response. The primary peak titers of MCPS-specific IgG were somewhat higher for mice immunized with M1883 versus MCPS (3-fold, p<0.05), although the latter response peaked earlier (Fig. 2). In contrast, M1883 induced a 15-fold boost in IgG anti-MCPS serum titers following secondary immunization relative to the primary, whereas MCPS failed to induce any significant boost. Thus, M1883 overall elicited 42-fold higher secondary serum titers of IgG anti-MCPS relative to secondary titers following MCPS immunization. The IgG isotypes elicited by isolated MCPS were IgG3 and IgG1, with no detectable IgG2b or IgG2a, whereas intact M1883 induced all 4 IgG isotypes (Fig. 2). Collectively, these data demonstrate that the intact bacterium markedly changes the nature of the associated MCPS-specific Ig response relative to that observed with isolated MCPS.
Figure 2. The MCPS-specific IgM and IgG isotype responses to isolated MCPS and intact M1883 are distinct.
BALB/c mice (7 per group) were immunized i.p. with 2 × 108 CFU of heat- inactivated intact M1883 or purified MCPS in saline, and boosted i.p. with the same dose on day 21. Serum titers of MCPS-specific IgM, IgG, and IgG subclasses (IgG3, IgG1, IgG2b and IgG2a) were determined. Significance (*) p≤0.05 between MenC and MCPS.
CD4+ T cells are required for the induction of the secondary, but not primary IgG anti-MCPS response to intact M1883
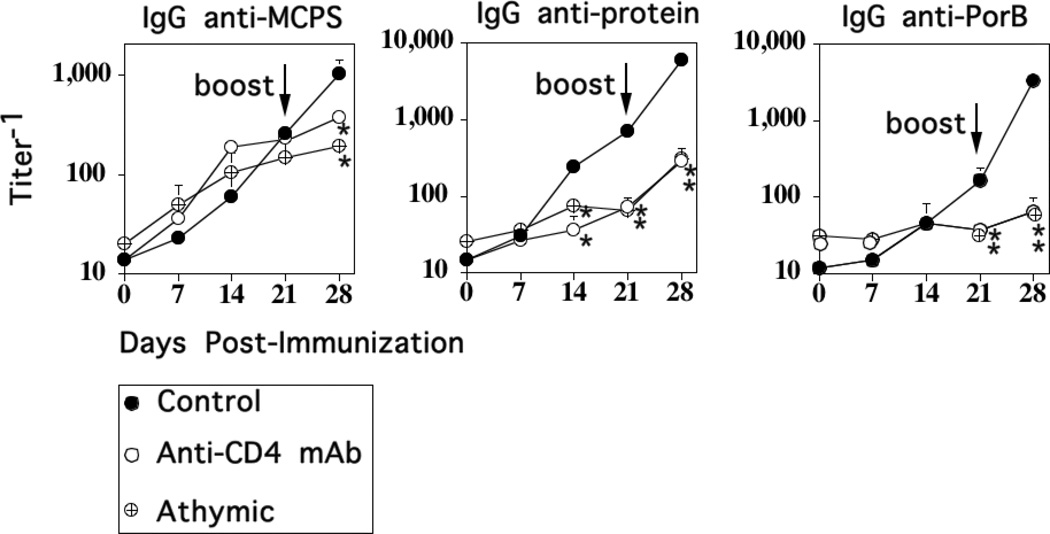
Whereas the Ig responses to isolated PS are largely TI, our previous studies using intact Pn14 demonstrated that the rapid primary IgG anti-PPS14 response was dependent on CD4+ T cells (11). In light of the slower primary, and highly boosted secondary IgG anti-MCPS response to MenC (both M1883 and FAM18 C+), we were interested in determining their relative CD4+ T cell-dependence. To determine this, we injected M1883 into T cell-deficient athymic nude mice or BALB/c mice acutely depleted of CD4+ T cells by a single injection of a depleting rat IgG anti-mouse CD4 mAb (clone GK1.5) given 24h prior to primary immunization with M1883. M1883-immunized mice injected 24h earlier with polyclonal rat IgG were used as controls. Injection of anti-CD4 mAb depleted CD4+ T cells by >95%, as determined 24h later by flow cytometry, whereas rat IgG had no effect (data not shown). Mice were boosted with M1883 on day 21 in the absence of anti-CD4 mAb or polyclonal rat IgG. As illustrated in Fig. 3, the primary IgG anti-MCPS IgG response to intact M1883 in athymic nude or anti-CD4 mAb-injected mice was similar to that observed in control mice, whereas the primary IgG response specific for either PorB or a total protein extract derived from unencapsulated FAM18 C−, was largely abrogated. In contrast, little or no boosting of serum titers of IgG anti-MCPS, anti-protein, or anti-PorB following secondary immunization was observed in the absence of T cells (Fig. 3). Collectively, these data demonstrate that the primary and boosted secondary IgG anti-MCPS responses to M1883 are TI and TD respectively, in contrast to previous observations that the primary IgG anti-PPS14 response to Pn is TD, with no secondary boosting, even in the presence of T cells. However, both the primary and secondary anti-protein responses to either Pn14 or M1883 are TD.
Figure 3. CD4+ T cells are required for the induction of the secondary, but not primary IgG anti-MCPS response to intact M1883.
BALB/c mice (7 per group) were injected i.p. either with a depleting anti-CD4+ mAb (clone GK.1.5) or polyclonal rat IgG (“Control”) [0.5 mg per mouse]. Both Ab-injected mice and athymic nude mice (BALB/c background) were then immunized, 1 day later, i.p. with 2 × 108 CFU of heat-inactivated intact M1883 in saline, and boosted 21 days later in the absence of Ab. Serum titers of Ag-specific IgG were measured by ELISA. Significance (*) p≤0.05 between “Control” versus and-CD4 mAb-injected mice or athymic nude mice.
CD28- ICOS-, and CD40L-dependent costimulation is critical for the induction of the secondary, but not primary, IgG anti-MCPS response to M1883
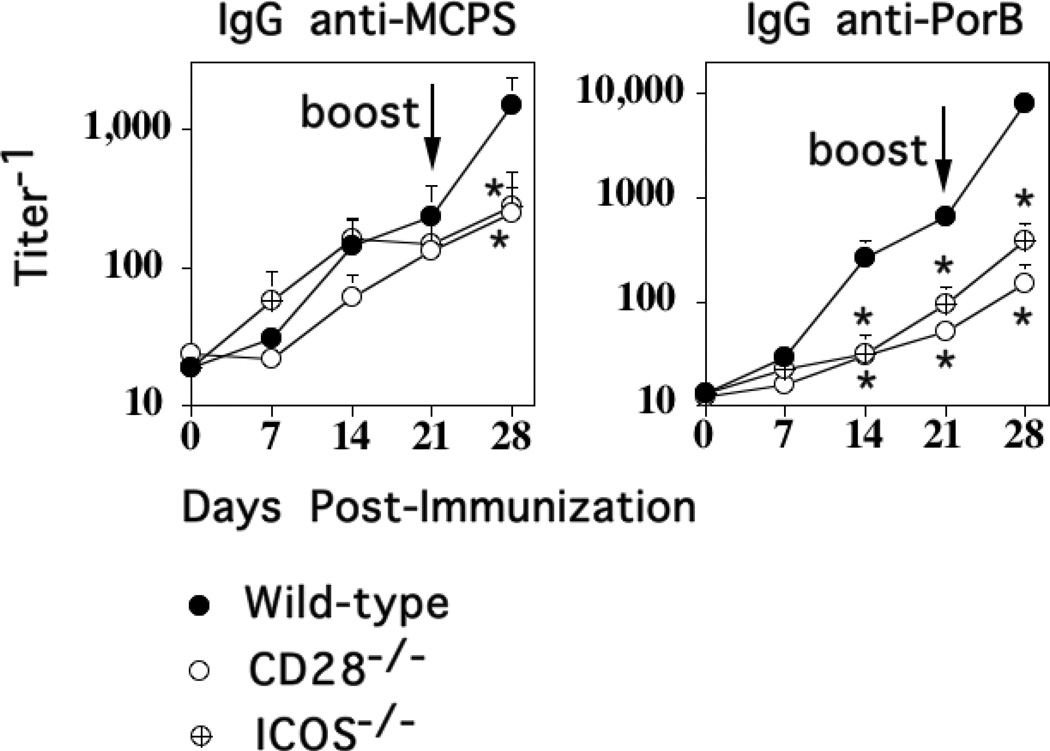
Inducible Costimulator (ICOS) is a member of the CD28 family that is induced on CD4+ T cells upon T cell receptor cross-linking and CD28-mediated signaling (30, 31). CD28, which is expressed constitutively on CD4+ T cells, is critical for initiation of CD4+ T cell activation, whereas ICOS plays a role in the subsequent T cell effector response such as the germinal center reaction and the generation of memory (32, 33). We previously demonstrated that the CD4+ T cell-dependent primary IgG anti-PPS14 response to Pn14 is CD28/B7-2-dependent, but ICOS-independent (10). In light of the CD4+ T cell dependence of the boosted secondary IgG anti-MCPS response to M1883, we wished to determine whether or not it was CD28− and/or ICOS-dependent. Thus, mice genetically-deficient in CD28 (CD28−/−), ICOS (ICOS−/−) and strain-matched C57BL/6 WT mice were immunized i.p. with M1883 and boosted 3 weeks later. As illustrated in Fig. 4, M1883-immunized WT mice elicited a detectable primary IgG anti-MCPS and anti-PorB response, and following secondary immunization, significant boosting in both MCPS- and PorB-specific IgG serum titers. The primary IgG anti-MCPS responses in CD28−/− and ICOS−/− mice were similar to that observed in WT control mice, consistent with the TI nature of the response (Fig. 3), whereas the CD4+ T cell-dependent secondary IgG anti-MCPS responses were essentially abrogated in both mutant mouse strains (Fig. 4). In contrast, both the primary and secondary IgG anti-PorB responses were markedly inhibited in both CD28−/− and ICOS−/− mice. Similar data were obtained using neutralizing anti-ICOSL mAb (HK5.3) versus control polyclonal hamster IgG, injected into mice one day before immunization with M1883 (data not shown).
Figure 4. CD28- and ICOS-dependent costimulation is critical for the induction of the secondary, but not primary, IgG anti-MCPS response to M1883.
C57BL/6 (“Wild-type”), CD28−/− (C57BL/6 background), and ICOS−/− (C57BL/6 background) mice (7 per group) were immunized i.p. with 2 × 108 CFU of heat-inactivated intact M1883 in saline and boosted on day 21. Serum titers of Ag-specific IgG were determined by ELISA. Significance (*) p≤0.05 between “Wild-type” versus CD28−/− or ICOS−/− mice.
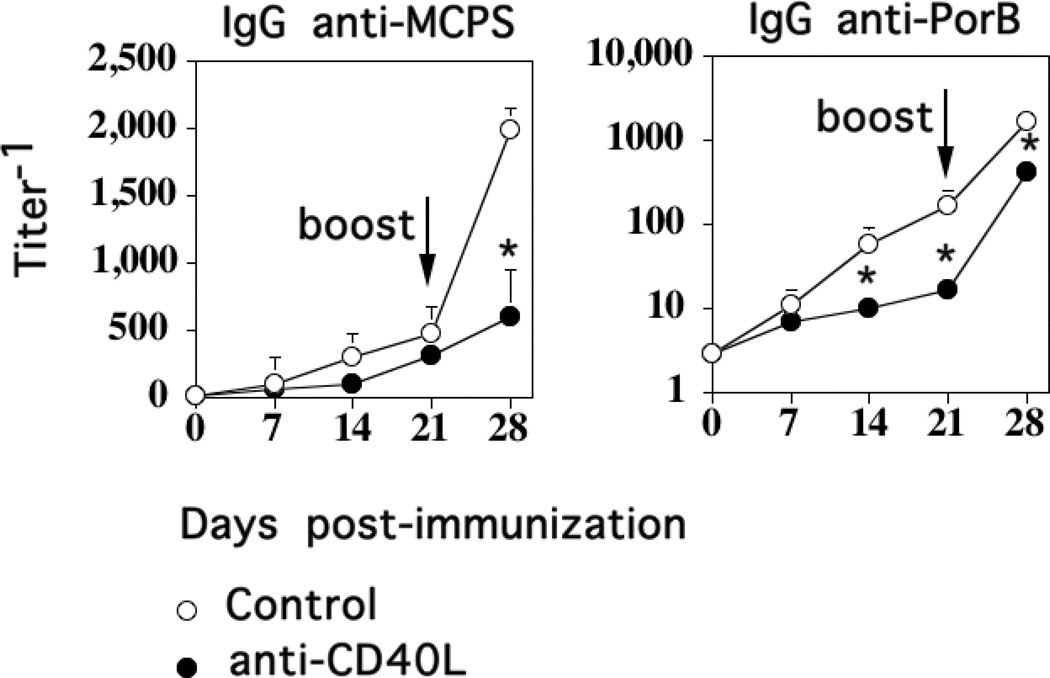
The interaction of CD40 on APC with CD40L, induced on CD4+ T cells following TCR/CD28-mediated activation, is critical for the induction of the TD primary Ig response, germinal center reaction, and the development of memory in response to immunization with protein Ags, or for the anti-PS response to conjugate vaccines (34, 35). A role for endogenous CD40L has also been reported in stimulating Ig responses to some, but not all PS Ags (36–39). We previously demonstrated that the primary CD4+ T cell-dependent IgG anti-PPS14 response to Pn was CD40L−, as well as CD28-dependent (9, 10). We thus wished to determine whether CD40L-dependent costimulation was critical for the primary TI and/or TD IgG anti-MCPS response to M1883. Thus, we injected BALB/c mice i.p. with a single dose of blocking hamster IgG anti-mouse CD40L mAb (MR1) one day before M1883 immunization, using polyclonal hamster IgG as a control, with a secondary immunization, 21 days later, using M1883 alone. As shown in Fig. 5, there was no difference in the primary anti-MCPS IgG response between the control and the MR1-injected group, whereas the secondary IgG anti-MCPS response in the MR1-injected group was markedly inhibited compared to control mice. In contrast, both the primary and secondary IgG anti-PorB responses were significantly reduced in the MR1-treated mice compared to controls. Collectively, these data demonstrate that the TD secondary, though not TI primary, IgG anti-MCPS response to intact M1883 is dependent on CD28−, ICOS−, and CD40L-dependent costimulation, in contrast to the TD primary IgG anti-PPS14 response to Pn14 which is CD28− and CD40L-dependent, but ICOS-independent.
Figure 5. CD40 ligand-dependent costimulation is critical for the induction of the secondary, but not primary, IgG anti-MCPS response to M1883.
BALB/c mice (7 per group) were injected i.p. with either anti-CD40L mAb (clone MR1) or polyclonal hamster IgG (“Control”) [0.3 mg per mouse]. Mice were then immunized, 1 day later, i.p. with 2 × 108 CFU of heat- inactivated intact M1883 in saline and boosted 21 days later. Serum titers of Ag-specific IgG were determined by ELISA. Significance (*) p≤0.05 between “Control” versus anti-CD40L-injected mice.
Endogenous TLR4, but not TLR2 or MyD88, signaling is critical for induction of peak primary and secondary serum titers of MCPS-specific IgG in response to M1883, but is not critical for secondary boosting
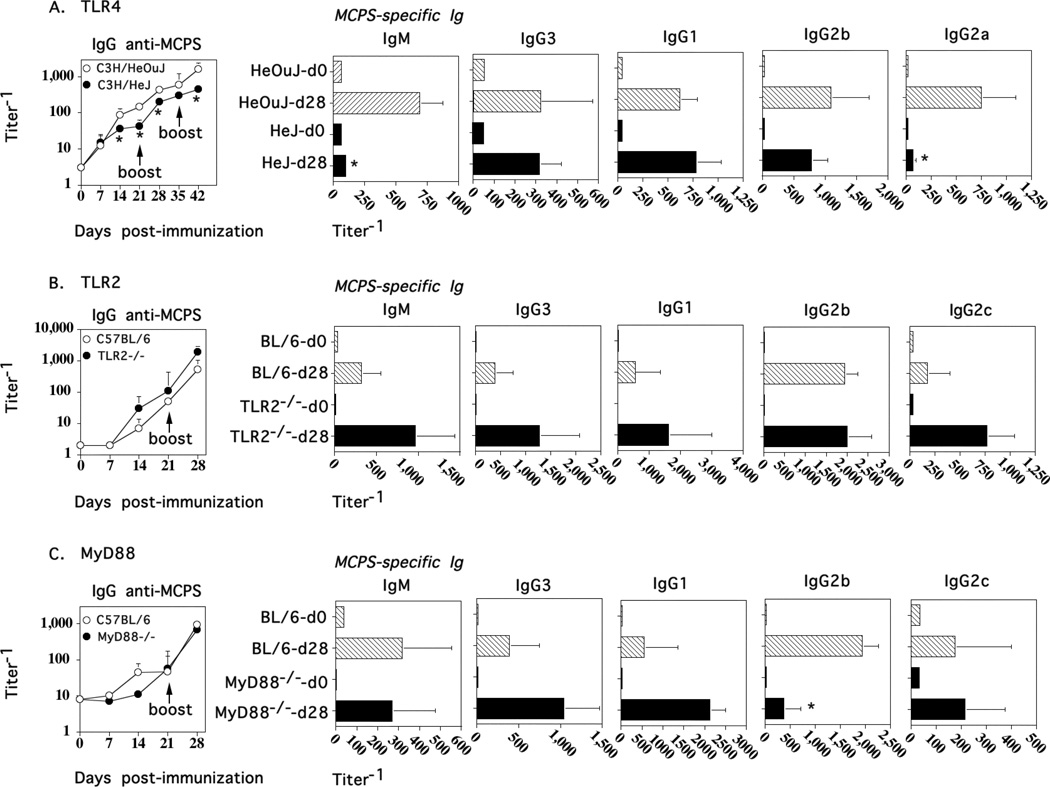
Lipopolysaccharide (LPS), a component of the outer membranes of GN bacteria is a ligand for TLR4, and a potent activator of the innate immune system (40). We thus wished to determine whether endogenous TLR4 signaling affected the humoral immune response to M1883 and might account for the differences observed with the IgG anti-PPS14 response to Pn14. We previously observed no role for TLR4 in the IgG anti-PPS14 response to Pn14 (data not shown), although genetic deficiency of TLR2 resulted in a significant reduction in IgG3, IgG2b, and IgG2a, but not IgG1 PPS14-specific serum titers (41). C3H/HeJ (Tlr4Lps-d) mice that carry a spontaneous mutation in the TLR4 gene and C3H/HeOuJ control mice were immunized i.p. with M1883 and boosted on days 21 and 35. As illustrated in Fig. 6A, both the primary, secondary, and tertiary IgG anti-MCPS responses in C3H/HeJ mice were significantly reduced, though not eliminated relative to control mice. Of note, the absence of TLR4 signaling did not prevent a secondary boost in the IgG anti-MCPS response. In contrast, there were no significant differences in the primary, secondary, or tertiary protein-specific IgG serum titers between the two groups (data not shown). Further analysis of serum titers of IgM and IgG isotypes following tertiary immunization indicated that TLR4 deficiency significantly reduced MCPS-specific IgM and IgG2a (Fig. 6A). Thus, endogenous TLR4 signaling plays a significant role in the magnitude of both the TI and TD components of the IgG anti-MCPS response, but is not critical for the generation of memory. Of note, in additional experiments utilizing TLR2−/− (Fig. 6B) and MyD88−/− (Fig. 6C) mice, no significant reductions in serum titers of MCPS-specific IgM or IgG were observed, although a significant 4-fold reduction in the IgG2b anti-MCPS titer was noted in MyD88−/− relative to control C57BL/6 mice.
Figure 6. Endogenous TLR4, but not TLR2 or MyD88, signaling is critical for induction of peak primary and secondary serum titers of MCPS-specific IgG in response to M1883, but is not critical for secondary boosting.
(A) C3H/HeJ(Tlr4Lps-d) and C3H/HeOuJ (control mice), or (B) TLR2−/− or (C) MyD88−/− mice (same group of control C57BL//6 mice used for both “B” and “C”) [7 per group] were immunized i.p. with 2 × 108 CFU of heat-inactivated intact M1883 in saline and boosted on day 21 and 35. Serum titers of MCPS-specific IgM, IgG, and IgG isotypes were determined by ELISA from sera obtained on the indicated days.
Co-immunization of MenC and Pn14 promotes secondary boosting of the IgG anti-PPS14 response to Pn14
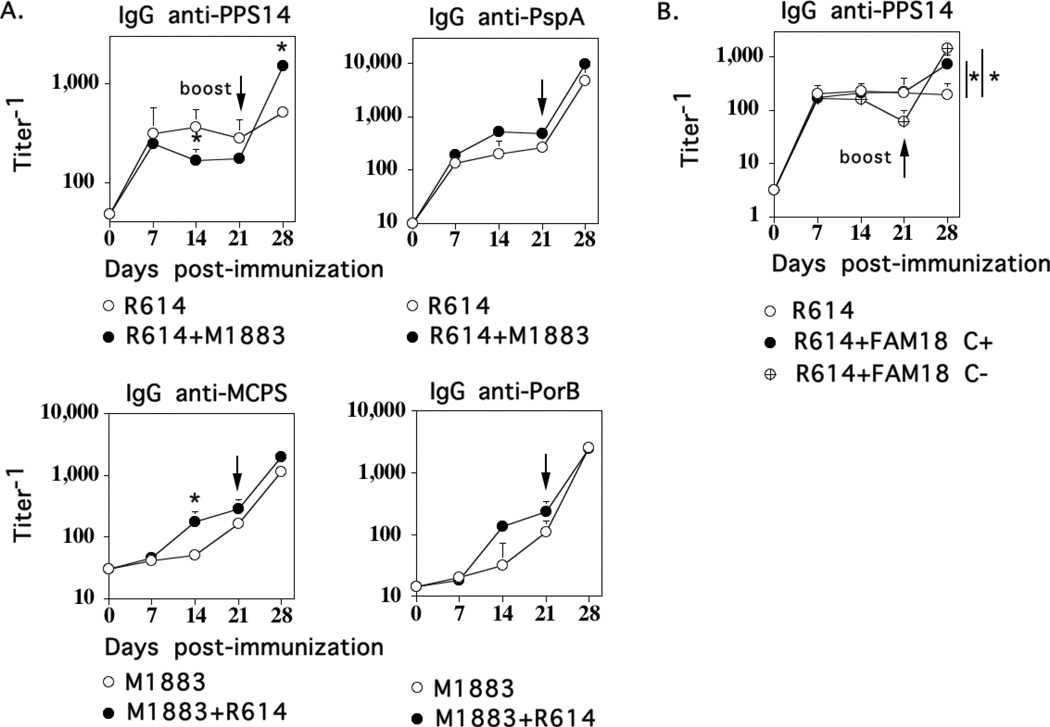
The inability of Pn14 to induce a boosted secondary IgG anti-PPS14 response remains unexplained. One possibility is that Pn14 fails to provide adequate innate signaling and/or inhibits the generation and/or elicitation of PPS14-specific IgG memory following secondary immunization. We therefore wished to determine whether signals provided by MenC could promote secondary boosting to the IgG anti-PPS response to Pn. Therefore, we immunized mice i.p. with Pn14 (strain R614) or M1883 alone or the two together. In all groups, 2 × 108 CFU/mouse of each bacterial strain was used. Mice were similarly boosted on day 21. As shown in Fig. 7A, the primary IgG anti-PPS14 response to R614 alone peaked on day 7, with no boosting in titers following secondary immunization. In the presence of M1883, the primary IgG anti-PPS14 response to R614 was modestly reduced at day 14, but more importantly, showed significant secondary boosting (3-fold increase). Of note, priming and secondary immunization of mice with as high as 1 × 109 CFU R614/mouse failed to elicit a boosted secondary IgG anti-PPS response (data not shown). M1883 had no effect on the IgG anti-pneumococcal surface protein A (PspA) response to R614 (Fig. 7). Conversely, with the exception of a modest increase in the primary IgG anti-MCPS response to M1883 on day 14, co-immunization with R614 had no other significant effects on either the IgG anti-MCPS or IgG anti-PorB response. Isolated MCPS has been reported to increase expression of CD86 and MHC-II on murine B cells (17), suggesting a possible role in augmenting adaptive immunity. In this regard, in an additional experiment, co-immunization of R614 with either FAM18 C+ or FAM18 C− similarly boosted the secondary IgG anti-PPS14 response (Fig. 7B) indicating that the boosting effect of MenC is not secondary to the presence of the MCPS capsule. Thus, these data suggest that the failure of Pn to induce a boosted secondary IgG response may reflect either sub-critical innate signaling by Pn or an inhibitory effect that can be overcome by MenC.
Figure 7. Co-immunization of MenC and Pn14 promotes secondary boosting of the IgG anti-PPS14 response to Pn14.
BALB/c mice (7 per group) were immunized i.p. with 2 × 108 CFU of heat-inactivated intact MenC ([A] strain M1883 or [B] strains FAM18 C+ or FAM18 C−]) or 2 × 108 CFU of heat-inactivated intact Pn14 (strain R614) or a combination of MenC and R614 (2 × 108 CFU each), and boosted in a similar fashion on day 21. Serum titers of Ag-specific IgG were determined by ELISA. Significance (*) p≤0.05 between mice immunized with single bacteria versus combination.
Discussion
The inability of non-zwitterionic capsular PS to associate with MHC-II molecules for presentation to CD4+ T cells (3, 4), results in a limited capacity of these Ags to elicit PS-specific IgG isotypes, GC formation, and IgG-specific memory (5). However, isolated PS have been reported to induce TI IgM-specific memory that is down-regulated by PS-specific IgG (42). Covalent attachment of an immunogenic protein to an isolated PS, results in the induction of a TD PS-specific IgG memory response (43). This likely occurs through specific uptake of PS-protein conjugate by PS-specific B cells with subsequent presentation of protein peptide/MHC-II to CD4+ T cells (35), although CD4+ T cells specific for the PS and combined PS-protein components are also elicited (44). Capsular PS expressed by extracellular bacteria is associated indirectly with immunogenic proteins, through covalent attachment to cell wall peptidoglycan (GP bacteria) (19, 20) or the acyl glycerol moiety of the outer membrane (45). This suggests the possibility that PS-specific Ig responses to intact bacteria might behave in a manner similar to that elicited by conjugate vaccines. Indeed, somatically mutated PS-specific IgG is elicited in response to encapsulated bacteria, suggesting a role for T cells in this process (46, 47). However, BCR-mediated, Ag uptake by B cells involves endocytic vesicles that are only 50–150 nm in diameter, and thus would be predicted to exclude intact bacteria (48). Nevertheless, a single study demonstrated BCR-mediated uptake of intact Salmonella typhimurium by human B cells with subsequent display of MHC-II/peptide complexes and primary CD4+ T cell activation (49).
Our previous studies on the in vivo IgG anti-PPS14 response to intact, heat-killed Pn14 indicated that the bacteria significantly altered the nature of the associated PPS14-specific IgG response relative to that elicited by isolated PPS14. The IgG anti-PPS14 response to intact Pn14 was similar to that elicited by a TD conjugate vaccine, in that both responses were dependent on CD4+ T cells, B7-dependent costimulation, and CD40/CD40L interactions, with elicitation of all 4 IgG isotypes (9, 10). However, in contrast to conjugate vaccines, the IgG anti-PPS14 response to Pn14 was relatively rapid, failed to induce a boosted secondary response, and was ICOS-independent, similar to that elicited by TI isolated PS Ags. Nevertheless, Pn14 elicited a protein-specific (i.e. PspA) IgG response that was classically TD (9, 10). The basis for this unique immunologic behavior of intact Pn14 is a matter of ongoing investigation (26).
In this report, we wished to determine whether the nature of the PPS14-specific Ig response to intact Pn14 was characteristic of intact PS-expressing extracellular bacteria in general, or perhaps represented either a characteristic feature of PPS14, the underlying structure and/or composition of intact Pn, or perhaps a more general dichotomy between GP and GN bacteria. To begin to address this question, we studied the nature of the MCPS-specific Ig response to intact MenC, a GN bacterium, and compared this response to that elicited by an MCPS-TT conjugate or isolated MCPS, as well as to our previously published data on Pn14. In this report we demonstrate the following: 1) Intact MenC elicits an IgG anti-MCPS response that, similar to the MCPS-TT conjugate, shows prolonged primary kinetics of development and significant boosting upon secondary immunization. This is in distinct contrast to the IgG anti-PPS14 response to intact Pn14 that showed rapid primary kinetics of induction and no secondary boosting. The IgG anti-PorB and total IgG anti-MenC protein response to MenC shows similar kinetics and boosting to that of the IgG anti-MCPS response. 2) In contrast to MenC, the IgG anti-MCPS response to isolated MCPS shows rapid primary kinetics of induction, no boosting upon secondary immunization, and expression of IgG3 and IgG1 in contrast to all 4 IgG isotypes elicited by MenC, indicating a marked effect of the intact bacterium on the associated MCPS-specific IgG response. The absence of secondary boosting observed using isolated MCPS is consistent with early studies using other soluble PS Ags (50, 51). 3) Surprisingly, the primary IgG anti-MCPS response to intact MenC is TI, although the boosted secondary response, similar to both the primary and secondary IgG anti-PorB response, is dependent on CD4+ T cells, and requires CD28, ICOS, and CD40L. This is in contrast to the primary IgG anti-PPS14 response to Pn14 that was dependent on CD4+ T cells, CD28, and CD40L, although not on ICOS. 4) Both the TI and TD IgG anti-MCPS response to MenC is significantly reduced in TLR4-defective (C3H/HeJ), but not TLR2−/− or MyD88−/− mice, although boosting of the IgG anti-MCPS response is still observed after secondary MenC immunization. 5) Co-immunization of MenC and Pn14 results in a boosting of the IgG anti-PPS14 response to Pn14, independent of expression by MenC of MCPS.
These data, and our previously published reports, demonstrate distinct differences in the nature of the PS-specific Ig responses to intact Pn14 versus MenC. Although it is clear from these data that the intact bacterium significantly alters the nature of the PS-specific Ig response to the associated PS Ag, it remains unresolved whether the differences between intact Pn14 and MenC reflect a more general dichotomy between GP and GN bacteria, the biochemical nature of the specific expressed PS Ag and its interaction with the immune system, or unique features in the composition and/or structure of one or both of these bacteria, not manifested by other bacterial species. In this regard, isolated MCPS and type V capsular PS from Streptococcus agalactiae, but not NP-Ficoll were shown to inhibit a number of B cell functions mediated by BAFF and APRIL (17). Additionally, MCPS enhanced the expression of CD86 and MHC-II on B cells. Other bacterial PS, such as C-polysaccharide and PPS1 of Pn, Staphylococcus aureus capsular polysaccharides types 5 and 8, and PS-A of Bacteroides fragilis, are zwitterionic on the basis of expression of both positive and negative charges, and can stimulate CD4+ T cells through association with MHC-II (15). Additional differences between capsular PS such as the ability to bind to SIGN-R1, a scavenger receptor on splenic marginal zone macrophages (13, 52), capacity to fix complement (53), or expression of terminal sialic acid residues that interact with the inhibitory CD22 receptor on B cells (16), may also have potential effects on PS-specific Ig responses to intact bacteria. Thus, a determination of how differences in bacterial composition and structure may potentially impact the specific Ig response to an expressed capsular PS, will require study of distinct bacteria with biochemically identical PS capsules.
The boosting of the IgG anti-MCPS response following secondary immunization with intact MenC and its dependence on CD4+ T cells, CD40L, CD28 and especially ICOS (30) strongly suggests that MCPS-specific B cells enter into a germinal center reaction in which MCPS-specific memory B cells are generated. This further suggests that MCPS-specific B cells engage in cognate interactions with CD4+ T cells that are specific for bacterial protein, and that association, although not direct covalent attachment, of capsular PS and protein within the bacterial particulate is critical. It remains to be determined whether PS-specific B cells can directly internalize intact bacteria for presentation of bacterial protein to CD4+ T cells or perhaps obtain bacterial protein indirectly following uptake, transport and/or processing of intact bacteria by other immune cells, including dendritic cells and macrophages (54). Of note, the primary TI IgG anti-MCPS response to MenC was independent of CD40L. Previous reports have indicated a role for CD40L-CD40 interactions in the PPS-specific IgM and IgG response to isolated PPS (36), although not to TNP-Ficoll (39). The reasons for these differences remain unexplained.
Previous studies from our laboratory using an in vitro polyclonal model for multivalent membrane (m)Ig crosslinking in response to PS Ags (i.e. multiple anti-IgD antibodies conjugated to dextran [αδ-dex]) indicated that multivalent mIg crosslinking alone, or in concert with CD40-mediated activation, induced vigorous proliferation but no Ig secretion or isotype switching by highly purified B cells (7). However, addition of a number of cytokines or various TLR ligands such as bacterial lipoproteins (TLR2), Neisserial porins (TLR2), unmethylated CpG-containing oligodeoxynucleotides (TLR9), or LPS (TLR4) to αδ-dex-activated B cell cultures induced substantial Ig secretion and isotype switching (7). On this basis we proposed that during natural infections with PS-encapsulated bacteria, the associated bacterial TLR ligands will play an especially critical role in eliciting the TI component of the anti-PS response, either through direct activation of B cells and/or via cytokine induction through innate cell stimulation (7). Our data (Fig 6) demonstrating an important role for TLR4 in enhancing the magnitude of the primary TI anti-MCPS response to intact MenC supports this hypothesis. TLR4 signaling in response to MenC is likely mediated by lipooligosaccharide (LOS) (55). In this regard, the capacity of at least some isolated PS Ags to induce Ig secretory responses in vivo may reflect the presence of contaminating TLR ligands remaining after PS purification from the intact bacteria (56). Whether this is true for the IgM and IgG anti-MCPS to isolated MCPS remains to be determined.
TLR4-defective mice also elicited a reduced secondary TD MCPS-specific IgG response to MenC, with the reduction in IgG due to selective abrogation of the IgG2a response. The capacity of LPS to potently induce IL-12-dependent IFN-γ (57), a switch factor for the IgG2a subclass (58), likely accounts for this effect. In addition to LPS, IFN-γ has also been shown to stimulate maturation of B cells into IgM-secreting cells (59), perhaps accounting for the reduction in the IgM anti-MCPS as well. In this regard, a recent report demonstrated the ability of isolated MCPS to inhibit IFN-γ-mediated DC release of BAFF (17), a mediator of B cell maturation and class switching to IgG. Perhaps the stimulating effects of intact MenC override this inhibitory property of isolated MCPS. Nevertheless, TLR4-defective mice exhibited a boosted MCPS-specific secondary IgG response to MenC, despite reduced serum titers relative to WT controls, and no changes in the more prolonged kinetics of primary MCPS-specific IgG induction. Thus, differences in TLR signaling between Pn14 and MenC per se are unlikely to account for the distinct PS-specific IgG responses between the two bacteria. In this regard, the demonstration that co-immunization of Pn14 with MenC results in a boosted secondary IgG anti-PPS14 response to Pn14, suggests the possibility that non-viable Pn14 exerts an inhibitory influence on the PPS14-specific secondary IgG response that is at least partially overcome by innate signaling in response to MenC. The comparable IgM and IgG anti-MCPS responses to MenC, observed in MyD88−/− versus WT mice, despite reduced responses in TLR4-defective mice, suggest a possible role for TLR4-dependent TRIF signaling (60). Finally, although MenC expresses porins that are TLR2 ligands and signal in a MyD88-dependent manner (22), no significant differences in Ig responses to MenC were observed in TLR2−/− versus WT mice.
Acknowledgments
Supported by: N.I.H. 2R01-AI49192 (CMS) and USUHS Dean’s Research and Education Endowment Fund (CMS).
Abbreviations
- MenC
intact Neisseria meningitidis, serogroup C
- MCPS
serogroup C polysaccharide from Neisseria meningitidis
- Pn14
Streptococcus pneumoniae, capsular type 14
- PPS14
capsular type 14 polysaccharide
- PS
polysaccharide
- GP
Gram-positive
- GN
Gram-negative
References
- 1.Klein Klouwenberg P, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin Dev Immunol 2008. 2008:628963. doi: 10.1155/2008/628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AlonsoDeVelasco E, Verheul AF, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding CV, Roof RW, Allen PM, Unanue ER. Effects of pH and polysaccharides on peptide binding to class II major histocompatiblity complex molecules. Proc Natl Acad Sci USA. 1991;88:2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishioka GY, Lamont AG, Thomson D, Bulbow N, Gaeta FC, Sette A, Grey HM. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol. 1992;148:2446–2451. [PubMed] [Google Scholar]
- 5.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 6.Brunswick M, Finkelman FD, Highet PF, Inman JK, Dintzis HM, Mond JJ. Picogram quantities of anti-Ig antibodies coupled to dextran induce B cell proliferation. J Immunol. 1988;140:3364–3372. [PubMed] [Google Scholar]
- 7.Snapper CM, Mond JJ. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996;157:2229–2233. [PubMed] [Google Scholar]
- 8.Guttormsen HK, Wetzler LM, Finberg RW, Kasper DL. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan AQ, Lees A, Snapper CM. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J Immunol. 2004;172:532–539. doi: 10.4049/jimmunol.172.1.532. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Cannons JL, Paton JC, Akiba H, Schwartzberg PL, Snapper CM. A novel ICOS-independent, but CD28- and SAP-dependent, pathway of T cell-dependent, polysaccharide-specific humoral immunity in response to intact Streptococcus pneumoniae versus pneumococcal conjugate vaccine. J Immunol. 2008;181:8258–8266. doi: 10.4049/jimmunol.181.12.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snapper CM. Differential regulation of protein- and polysaccharide-specific Ig isotype production in vivo in response to intact Streptococcus pneumoniae. Curr Protein Pept Sci. 2006;7:295–305. doi: 10.2174/138920306778017972. [DOI] [PubMed] [Google Scholar]
- 12.Chattopadhyay G, Khan AQ, Sen G, Colino J, Dubois W, Rubtsov A, Torres RM, Potter M, Snapper CM. Transgenic expression of Bcl-xL or Bcl-2 by murine B cells enhances the in vivo antipolysaccharide, but not antiprotein, response to intact Streptococcus pneumoniae. J Immunol. 2007;179:7523–7534. doi: 10.4049/jimmunol.179.11.7523. [DOI] [PubMed] [Google Scholar]
- 13.Lanoue A, Clatworthy MR, Smith P, Green S, Townsend MJ, Jolin HE, Smith KG, Fallon PG, McKenzie AN. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J Exp Med. 2004;200:1383–1393. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dintzis HM, Dintzis RZ, Vogelstein B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc Natl Acad Sci USA. 1976;73:3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–6153. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- 16.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci USA. 2009;106:2500–2505. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanswal S, Katsenelson N, Allman W, Uslu K, Blake MS, Akkoyunlu M. Suppressive effect of bacterial polysaccharides on BAFF system is responsible for their poor immunogenicity. J Immunol. 2011;186:2430–2443. doi: 10.4049/jimmunol.1002976. [DOI] [PubMed] [Google Scholar]
- 18.Grabitzki J, Lochnit G. Immunomodulation by phosphocholine--biosynthesis, structures and immunological implications of parasitic PC-epitopes. Mol Immunol. 2009;47:149–163. doi: 10.1016/j.molimm.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen UB, Henrichsen J, Chen HC, Szu SC. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 20.Jedrzejas MJ. Extracellular virulence factors of Streptococcus pneumoniae. Front Biosci. 2004;9:891–914. doi: 10.2741/1299. [DOI] [PubMed] [Google Scholar]
- 21.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 23.Beuvery EC, Miedema F, van Delft RW, Haverkamp J, Leussink AB, te Pas BJ, Teppema KS, Tiesjema RH. Preparation and physicochemical and immunological characterization of polysaccharide-outer membrane protein complexes of Neisseria meningitidis. Infect Immun. 1983;40:369–380. doi: 10.1128/iai.40.1.369-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colino J, Outschoorn I. Dynamics of the murine humoral immune respose to neisseria meningitidis group B capsular polysaccharide. Infect Immun. 1998;66:505–513. doi: 10.1128/iai.66.2.505-513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kocabas C, Katsenelson N, Kanswal S, Kennedy MN, Cui X, Blake MS, Segal DM, Akkoyunlu M. Neisseria meningitidis type C capsular polysaccharide inhibits lipooligosaccharide-induced cell activation by binding to CD14. Cell Microbiol. 2007;9:1297–1310. doi: 10.1111/j.1462-5822.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 26.Colino J, Chattopadhyay G, Sen G, Chen Q, Lees A, Canaday DH, Rubtsov A, Torres R, Snapper CM. Parameters underlying distinct T cell-dependent polysaccharide-specific IgG responses to an intact gram-positive bacterium versus a soluble conjugate vaccine. J Immunol. 2009;183:1551–1559. doi: 10.4049/jimmunol.0900238. [DOI] [PubMed] [Google Scholar]
- 27.Sen G, Flora M, Chattopadhyay G, Klinman DM, Lees A, Mond JJ, Snapper CM. The critical DNA flanking sequences of a CpG oligodeoxynucleotide, but not the 6 base CpG motif, can be replaced with RNA without quantitative or qualitative changes in Toll-like receptor 9-mediated activity. Cell Immunol. 2004;232:64–74. doi: 10.1016/j.cellimm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Tommassen J, Vermeij P, Struyve M, Benz R, Poolman JT. Isolation of Neisseria meningitidis mutants deficient in class 1 (porA) and class 3 (porB) outer membrane proteins. Infect Immun. 1990;58:1355–1359. doi: 10.1128/iai.58.5.1355-1359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massari P, King CA, MacLeod H, Wetzler LM. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr Purif. 2005;44:136–146. doi: 10.1016/j.pep.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 31.Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–390. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- 32.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 33.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 34.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guttormsen HK, Sharpe AH, Chandraker AK, Brigtsen AK, Sayegh MH, Kasper DL. Cognate stimulatory B-cell-T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect Immun. 1999;67:6375–6384. doi: 10.1128/iai.67.12.6375-6384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeurissen A, Wuyts M, Kasran A, Ramdien-Murli S, Boon L, Ceuppens JL, Bossuyt X. Essential role for CD40 ligand interactions in T lymphocyte-mediated modulation of the murine immune response to pneumococcal capsular polysaccharides. J Immunol. 2002;168:2773–2781. doi: 10.4049/jimmunol.168.6.2773. [DOI] [PubMed] [Google Scholar]
- 37.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 38.Renshaw BR, Fanslow WC, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E, Noelle RJ. gp39-CD40 interaction are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 41.Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect Immun. 2005;73:298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203:305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthukkumar S, Stein KE. Immunization with meningococcal polysaccharide-tetanus toxoid conjugate induces polysaccharide-reactive T cells in mice. Vaccine. 2004;22:1290–1299. doi: 10.1016/j.vaccine.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 45.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 46.Lucas AH, Reason DC. Polysaccharide vaccines as probes of antibody repertoires in man. Immunol Rev. 1999;171:89–104. doi: 10.1111/j.1600-065x.1999.tb01343.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhou J, Lottenbach KR, Barenkamp SJ, Reason DC. Somatic hypermutation and diverse immunoglobulin gene usage in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae Type 6B. Infect Immun. 2004;72:3505–3514. doi: 10.1128/IAI.72.6.3505-3514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sette A, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, Peters B, Rafii-El-Idrissi Benhnia M, Hoffmann J, Su HP, Singh K, Garboczi DN, Head S, Grey H, Felgner PL, Crotty S. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28:847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souwer Y, Griekspoor A, Jorritsma T, de Wit J, Janssen H, Neefjes J, van Ham SM. B cell receptor-mediated internalization of salmonella: a novel pathway for autonomous B cell activation and antibody production. J Immunol. 2009;182:7473–7481. doi: 10.4049/jimmunol.0802831. [DOI] [PubMed] [Google Scholar]
- 50.Klaus GG, Humphrey JH. The immunological properties of haptens coupled to thymus-independent carrier molecules. I. The characteristics of the immune response to dinitrophenyl-lysine-substituted pneumococcal polysaccharide (SIII) and levan. Eur J Immunol. 1974;4:370–377. doi: 10.1002/eji.1830040513. [DOI] [PubMed] [Google Scholar]
- 51.Baker PJ, Stashak PW, Amsbaugh DF, Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971;20:469–480. [PMC free article] [PubMed] [Google Scholar]
- 52.Koppel EA, Wieland CW, van den Berg VC, Litjens M, Florquin S, van Kooyk Y, van der Poll T, Geijtenbeek TB. Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcus pneumoniae infection. Eur J Immunol. 2005;35:2962–2969. doi: 10.1002/eji.200526216. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z, Koralov SB, Kelsoe G. Regulation of humoral immune responses by CD21/CD35. Immunol Rev. 2000;176:194–204. doi: 10.1034/j.1600-065x.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28:609–619. doi: 10.1016/j.immuni.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Pridmore AC, Wyllie DH, Abdillahi F, Steeghs L, van der Ley P, Dower SK, Read RC. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR4/MD2. J Infect Dis. 2001;183:89–96. doi: 10.1086/317647. [DOI] [PubMed] [Google Scholar]
- 56.Sen G, Khan AQ, Chen Q, Snapper CM. In Vivo Humoral Immune Responses to Isolated Pneumococcal Polysaccharides Are Dependent on the Presence of Associated TLR Ligands. J Immunol. 2005;175:3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]
- 57.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 58.Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 59.Snapper CM, Rosas F, Moorman MA, Jin L, Shanebeck K, Klinman DM, Kehry MR, Mond JJ, Maliszewski CR. IFN-γ is a potent inducer of Ig secretion by sort-purified murine B cells activated through the mIg, but not the CD40, signaling pathway. Int Immunol. 1996;8:877–885. doi: 10.1093/intimm/8.6.877. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]