Abstract
The mechanism of action of protein-bound polysaccharide K (PSK; KRESTIN®) involves the following actions: (1) recovery from immunosuppression induced by humoral factors such as transforming growth factor (TGF)-β or as a result of surgery and chemotherapy; (2) activation of antitumor immune responses including maturation of dendritic cells, correction of Th1/Th2 imbalance, and promotion of interleukin-15 production by monocytes; and (3) enhancement of the antitumor effect of chemotherapy by induction of apoptosis and inhibition of metastasis through direct actions on tumor cells. The clinical effectiveness of PSK has been demonstrated for various cancers. In patients with gastric or colorectal cancer, combined use of PSK with postoperative adjuvant chemotherapy prolongs survival, and this effect has been confirmed in multiple meta-analyses. For small-cell lung carcinoma, PSK in conjunction with chemotherapy prolongs the remission period. In addition, PSK has been shown to be effective against various other cancers, reduce the adverse effects of chemotherapy, and improve quality of life. Future studies should examine the effects of PSK under different host immune conditions and tumor properties, elucidate the mechanism of action exhibited in each situation, and identify biomarkers.
Keywords: PSK, Biological mechanism, Gastric cancer, Colorectal cancer, Biomarker
Introduction
Whether human immunity is effective against cancer, which originates from mutation of the host’s normal cells, has long been a subject of skepticism. In 1991, van der Bruggen et al. [1] identified the tumor antigen in human melanoma that is recognized by cytotoxic T lymphocytes (CTLs) and proved at the molecular level that host immunity acts also against cancer. Thereafter, immunotherapy was developed mainly along the lines of vaccine therapy and cell therapy, with the aim of boosting specific immunity [2]. Research began to show that antigen-nonspecific innate immunity and antigen-specific acquired immunity are closely associated via the dendritic cells (DCs) that possess the important function of antigen presentation [3]. Also, antigen-presenting cells (APCs), including DCs, are known to be activated by recognizing various foreign pathogens via the Toll-like receptors (TLRs) [4]. These developments indicate that activation of nonspecific immunity plays certain roles in augmenting antitumor immunity. However, the antitumor effect of biological response modifiers (BRMs) is not necessarily potent [5]. For this reason, they have been used in combination with chemotherapy. The effect of chemotherapy is known to be affected by the performance status (PS) and nutritional and immune status of the patient [6–8]. Recently, Apetoh et al. [9] have presented basic research evidence that tumor cells damaged by chemotherapy release high mobility group box 1, which interacts with TLR4 to stimulate DCs and activates antitumor immunity, and these activities contribute to the success of chemotherapy. Clinically, these investigators also have reported earlier relapse after combined anthracycline-based chemotherapy and local radiotherapy in breast cancer patients with a functionally deficient TLR4 polymorphism, compared to breast cancer patients with normal TLR4. These findings show the importance of the host’s immune capacity during chemotherapy, and suggest the importance of nonspecific immune activation in cancer patients whose immune functions are compromised.
Protein-bound polysaccharide K (PSK; KRESTIN®) is isolated and purified from the cultured mycelium of the Basidiomycete Coriolus versicolor, has an average molecular weight of approximately 100,000, and contains 18–38% protein. PSK exhibits antitumor activity against various experimental tumors, and nonspecific immunomodulatory activity is considered the principal mechanism of action of this agent [10]. PSK has been shown to regress tumors clinically, and was approved in 1976 for the treatment of cancers of the digestive organ, lung and breast. Clinical use in Japan was started in 1977. After reevaluation in 1989, PSK was approved for use in combination with chemotherapy to prolong survival of patients with gastric cancer (resected cases) or colorectal cancer (curatively resected cases), and to prolong remission of patients with small-cell lung carcinoma.
Along with advances in molecular biology and tumor immunology, many investigators have conducted research on the mechanisms of action of PSK and have built up substantial knowledge. Studies on clinical effects are still ongoing, mainly in accordance with the currently approved indications. The present article reviews the recent developments in research on the biological mechanisms of PSK and the major clinical results reported to date, to identify the challenges for the future.
Biological mechanism of PSK
Although the importance of BRMs in cancer treatment has been proven, better understanding of their mechanisms of action is essential for optimal application of these compounds. For PSK, three main mechanisms have been revealed (Fig. 1) [11]. First, PSK improves host immunocompetence by inhibiting the production of or neutralizing immunosuppressive substances that are increased in cancer. Second, PSK activates immune cells such as lymphocytes, either directly or by regulating the production of various cytokines. Third, PSK acts directly on cancer cells. These mechanisms are considered to support the clinical effectiveness of PSK in suppressing cancer relapse.
Fig. 1.
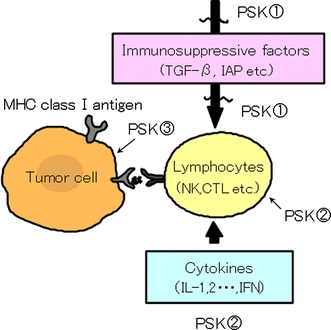
Tumor microenvironment and actions of PSK. ① Suppressed production or neutralization of immunosuppressive factors. ② Activation of immune cells and regulation of cytokine production. ③ Direct action on tumor cells [induction of apoptosis, enhanced expression of major histocompatibility complex (MHC) class I antigen]. Adapted from Fujii [11]
Suppressed production or neutralization of immunosuppressive factors (Table 1)
Table 1.
Suppressed production or neutralization of immunosuppressive factors
| Immunosuppressive factors | Subjects/animals/cells | Key observations | References |
|---|---|---|---|
| Cytokine | PBMCs of healthy volunteers | Abrogation of inhibition of LAK activities by TGF-β | Yamaguchi [19] |
| PBMCs of colorectal cancer patients | Suppression of IL-10 production | Shibata [20, 21] | |
| Cancer cell | CD8+ T cells in peripheral blood of healthy volunteers | Recovery of NKG2D expression downregulated by tumor cells | Tsujitani [22] |
| Tumor culture supernatant | CD14+ cells in peripheral blood of healthy volunteers | Prevention of defective maturation of DCs by tumor culture supernatant | Okuzawa [23] |
| Surgical stress | Mice | Correction of Th1/Th2 imbalance | Ooshiro [25] |
| Chemotherapy | Gastric cancer patients | Partial prevention of S1-induced peripheral blood T-cell apoptosis | Kono [27] |
PBMC peripheral blood mononuclear cell, LAK lymphokine-activated killer, TGF-β transforming growth factor-β, NKG2D natural killer (NK) group 2D, DC dendritic cell, S-1 tegafur–gimeracil–oteracil potassium
It is well known that various humoral factors induce immunosuppression in cancer-bearing individuals. PSK restores or attenuates this suppression, as demonstrated by a larger number of reports showing abrogation of the immunosuppressive effects of serum obtained from cancer-bearing animals or cancer patients, inhibition of transforming growth factor (TGF)-β production or antagonism against TGF-β, and lowering of serum level of immunosuppressive acidic protein (IAP) and prostaglandin E2 (PGE2) production [12–17]. In addition, PSK has been reported to possess antioxidant activity [18]. No other drug exhibits such diverse actions as a single agent, and this is the characteristic of PSK.
In an in vitro study using peripheral blood mononuclear cells (PBMCs) from healthy volunteers, Yamaguchi et al. [19] have reported that PSK enhances interleukin-2 (IL-2)-induced proliferation of lymphokine-activated killer (LAK) cells and their cytotoxic activity, and abolishes the TGF-β-induced inhibition of LAK activity. They speculated that PSK acted on TGF-β receptors to block the association between TGF-β and its receptors. Thus, PSK seems to inhibit the effects of TGF-β through various mechanisms including suppression of TGF-β production, direct binding with TGF-β, and acting on TGF-β receptors.
IL-10 shows various immunosuppressive activities, and elevated serum levels of IL-10 have been reported as a negative prognostic factor. Shibata et al. [20, 21] have studied the effect of PSK on IL-10. They found that IL-10 production was suppressed when PSK was added in vitro to phytohemagglutinin (PHA)-stimulated PBMCs from patients with advanced colorectal cancer, and that IL-10 production by PHA-stimulated PBMCs from advanced colorectal cancer patients was reduced after 2 months of immunochemotherapy with PSK, compared to that before treatment.
Tsujitani et al. [22] have examined the effect of PSK on the expression of natural killer group 2D (NKG2D), a receptor that activates CD8+ T cells and natural killer (NK) cells. When PBMCs from healthy volunteers were co-cultured with the human gastric cancer cell line MKN-45, NKG2D expression on CD8+ T cells was downregulated, while the addition of PSK restored NKG2D expression on CD8+ T cells. The authors suggested that direct contact between CD8+ T cells and gastric cancer cells was necessary for the downregulation of NKG2D expression. These findings raise interest on how PSK affects the interaction between tumor cells and T cells.
The suppressive effect of tumor-derived factors on DCs and the ability of PSK to overcome this effect have been studied. Okuzawa et al. [23] have studied the in vitro differentiation of peripheral blood CD14+ cells into DCs and reported that the addition of the culture supernatant of human gastric cancer cells MKN-45P downregulated the surface markers of DC maturation, decreased IL-12 production, and increased apoptosis of DC. Addition of PSK restored all these changes to the levels observed in the absence of the culture supernatant. These findings suggest that, by reversing the defective DC differentiation and function, PSK is able to augment the subsequent immune response.
It is well known that surgical stress lowers the immune response. PSK has been reported to attenuate the immunosuppression due to surgical invasion [24]. Using a mouse laparotomy model, Ooshiro et al. [25] have found that concanavalin-A-stimulated interferon (IFN)-γ (Th1 cytokine) and IL-4 (Th2 cytokine) production by spleen cells was decreased, and the decrease in IFN-γ was especially marked, which resulted in a low IFN-γ/IL-4 ratio. In mice treated with PSK before laparotomy, the decreases in IFN-γ and IL-4 production were ameliorated, the recovery of IFN-γ production was especially marked, and the IFN-γ/IL-4 ratio was restored. The resultant Th1/Th2 balance shifted to Th1 dominance. The ability of PSK to correct the Th1/Th2 imbalance, to be described later, could also work during the perioperative period.
Many basic studies on the effects of the combined use of PSK with chemotherapy have been reported. While chemotherapy induces leukopenia and depresses immunity, PSK inhibits these adverse effects [26]. The ability of PSK to prevent chemotherapy-depressed immunity is one of the mechanisms that accounts for the benefit of PSK in combined therapy. Kono et al. [27] have examined apoptosis of peripheral blood T cells in gastric cancer patients treated with oral fluoropyrimidine agent tegafur–gimeracil–oteracil potassium (S-1; TS-1®), alone or in combination with PSK. While an increase in T-cell apoptosis concomitant with elevation of caspase-3 and Bax expression was observed in patients treated with S-1 alone, these increases were partially prevented in patients treated with S-1 and PSK (Fig. 2). The mechanism has been speculated as ensuing from the antioxidant activity of PSK, which counteracts the increase in reactive oxygen species resulting from chemotherapy.
Fig. 2.
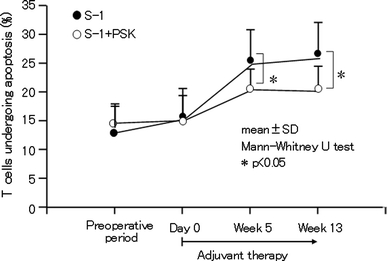
Effect of oral administration of PSK on apoptosis of circulating T cells induced by the anticancer drug S-1 in gastric cancer patients. Adapted and modified from Kono et al. [27]
Regulation of immune cells and cytokine production (Table 2)
Table 2.
Regulation of immune cells and cytokine production
| Immune cells | Subjects/animals/cells | Key observations | References |
|---|---|---|---|
| NK cells | Human NK cell line | Activation of PKCδ, PKCε, ERK2, ERK3, AP-1 and CRE transcriptional factor | García-Lora [39, 40] |
| T cells | CD4+ T cells in murine mesentric lymph node | Activation of LAT, ERK1/2, NFAT and AP-1 | Asai [41] |
| DCs | CD14+ mononuclear cells in PBMC of healthy volunteers | Promotion of maturation of DCs | Kanazawa [42], Ogihara [43] |
| Th1/Th2 | PBMC of human healthy volunteers | Increase of TNF-α production and decrease of IL-10 production by stimulation of human gastric cancer cell lysate | Sugiyama [45] |
| Colorectal cancer patients | Decrease of proportion of IL-10+ CD4+ T cells (Th2) in peripheral blood | Yoshino [46] | |
| B cells | Human B cell line | Enhancement of IgM production | Maruyama [49] |
| Monocytes | Human cord blood mononuclear cells | Activation of T cells and induction of CTLs through increase of IL-15 and LTB4 production by monocytes in EBV-infected cultures | Liu [50–52], Klein [53] |
| NKT cells | Gastric cancer patients | Decrease of CD57+ T cells in peripheral blood | Akagi [54] |
| Others | Murine peritoneal macrophages, etc. | Inhibition of binding of LPS to LBP, protection of LPS-induced lethality | Asai [55] |
| Mouse model of DSS and DMH-induced inflammatory bowel disease and colorectal carcinogenesis | Antagonistic effects on inflammation and carcinogenesis | Tsutsumi [57] |
NK natural killer, PKC protein kinase C, ERK extracellular signaling regulated kinase, AP-1 activator protein-1, CRE cAMP-response element, LAT linker for activation of T cells, NFAT nuclear factor of activated T cells, DC dendritic cell, PBMC peripheral blood mononuclear cell, TNF-α tumor necrosis factor-α, CTL cytotoxic T lymphocyte, LTB 4 leukotriene B4, EBV Epstein–Barr virus, LPS lipopolysaccharide, LBP LPS-binding protein, DSS dextran sodium sulfate, DMH 1,2-dimethylhydrazine
Antitumor immune responses involve complex interactions among various immune cells and cytokines (Fig. 3). PSK exerts various effects on the immune cells, and its effects on lymphocytes, helper T cells, CTLs, NK cells, LAK cells, and macrophages have been reported in numerous publications [28–36]. In addition, the effects of PSK on the production of various cytokines and nitric oxide (NO) have been reported [37, 38].
Fig. 3.
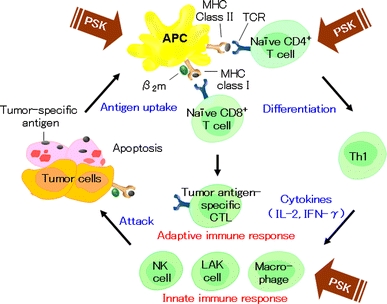
Schematic representation of the antitumor immune responses and actions of PSK. Tumor cells or their fragments are taken up by APCs. Antigen peptides bind to MHC class I or II on the APCs. The antigen peptides on MHC class I molecules activate naïve CD8+ T cell, whereas the antigen peptides on MHC class II molecules activate naïve CD4+ T cells (T-helper cell precursors). T-helper cell precursors differentiate into Th1 and Th2 cells. Th1 cells produce cytokines, including IL-2 and IFN-γ. These cytokines induce proliferation and activation of tumor antigen-specific CTLs, activation of NK cells and macrophages, and induction of LAK cells
García-Lora et al. [39, 40] have used the human NKL cell line to study the intracellular signal transduction pathway involved in the activation of NK cells by PSK. PSK activates protein kinase C (PKC)δ, PKCε, and extracellular signal-regulated kinase (ERK)2 and ERK3, and increases DNA binding of activator protein-1 (AP-1) and cAMP response element (CRE) transcriptional factor. Asai et al. [41] have shown that the addition of PSK in the stimulation of mesenteric lymph node CD4+ T cells with anti-CD3 antibody increased the production of IL-2, IFN-γ, and IL-4. They reported that the signaling pathway involved the activation of linker for activation of T cells (LAT), ERK1/2, nuclear factor of activated T cells, and AP-1. Although the actions of PSK on immune cells differ slightly depending on the cell type, these actions have started to unfold at the level of the signal transduction pathway. Further accumulation of data using different immune cells under various conditions might provide a comprehensive picture of the action of PSK.
As mentioned earlier, DCs play an important role in the immune response. In the peripheral blood, DCs exist in an immature state. Upon maturation by the actions of inflammatory cytokines, they activate T cells and induce acquired immunity. Kanazawa et al. [42] have reported that the addition of PSK to cultures of peripheral blood CD14+ mononuclear cells from healthy adults and advanced cancer patients with IL-4 and granulocyte-macrophage colony stimulating factor for DC differentiation potentiates the expression of maturation surface markers, such as CD83, production of IL-12, and induction of CTLs. Using a similar in vitro system, Ogihara et al. [43] have reported similar results with the addition of PSK.
Through the actions of the APCs, T cells are activated and naïve CD4+ T cells differentiate into Th1 or Th2 cells. In a cancer-bearing state, the Th1/Th2 balance shifts to Th2 dominance; therefore, cellular immunity, which is the main component of antitumor immunity, does not function efficiently [44]. Sugiyama et al. [45] have examined cytokine production of PBMCs from healthy adults stimulated with tumor antigen obtained by freezing and thawing a gastric cancer cell line after 1 week. Addition of PSK did not change IFN-γ production, but increased tumor necrosis factor-α (TNF-α) (Th1 cytokine) production and decreased IL-10 (Th2 cytokine) production. Yoshino et al. [46] treated colorectal cancer patients with PSK for 1 week before surgery and examined the peripheral blood Th1/Th2 balance by flow cytometry before and after treatment. After PSK treatment, although the proportion of IFN-γ-producing CD4+ T cells (Th1) did not change, the proportion of IL-10-producing CD4+ T cells (Th2) decreased. These studies suggested that PSK appeared to act mainly to suppress Th2, and PSK shifted the Th1/Th2 balance to Th1 dominance to augment antitumor immunity.
Various monoclonal antibodies have been developed for cancer treatment. PSK has been reported to augment antibody-dependent cell cytotoxicity [47]. The effect of PSK on antibody production is considered to act via helper T cells [48]. Maruyama et al. [49] have reported that PSK directly enhances the proliferation and IgM production of the human B cell line BALL-1, which suggests that PSK also acts directly on the humoral immunity pathway to potentiate acquired immunity.
Most of the studies on the effects of PSK on immune cells or cytokine production have been conducted by targeting individual immune cells or cytokines. In vivo, however, the immune response is a cascade reaction that involves many immune cells and cytokines. Liu et al. [50–52] have investigated the effect of PSK on the immune response against Epstein–Barr virus (EBV) infection, using human cord blood mononuclear cells (CBMCs) that contain various types of immune cells. The addition of PSK to EBV-infected CBMCs increases expression of signaling lymphocytic-activation molecule-associated protein (SAP), which is an indicator of T- and NK cell activation. PSK also induces EBV-specific CD4+ CTLs, and inhibits proliferation of the potentially malignant EBV-infected B cells. Liu et al. have further demonstrated that the presence of monocytes and monocyte-derived IL-15 and leukotriene B4 (LTB4) is necessary for the upregulation of SAP expression by PSK. Klein et al. [53]. have reported that the interaction between T cells activated by EBV-infected B cells and monocytes activated by PSK occurs via CD40 ligand and CD40, and that these cells together with NK cells activated by EBV-infected B cells progress to a functionally activated state, which produces IL-15, IFN-γ, and LTB4 to induce CD4+ CTLs. IL-15 has been attracting the most attention as an antitumor agent with potential for clinical application (NCI Immunotherapy Agent Workshop, 2007), and studies have demonstrated that PSK induces IL-15.
Akagi et al. [54] have examined the effect of postoperative immunochemotherapy with PSK on immune cells in peripheral blood of gastric cancer patients at 3 months after surgery, and have reported that administration of PSK decreases the proportion of CD57+ T cells that mediate suppressor T-cell functions.
On a slightly different note, Asai et al. [55] have examined the NF-κB activity (indicated by luciferase activity) when the pro-B cell line Ba/F3 transfected with TLR-4 and MD-2 was stimulated with LPS. They have reported that addition of PSK inhibited nuclear factor (NF)-κB activity, which was caused by inhibition of binding between LPS and LPS-binding protein (LBP) by PSK. Administration of PSK also protected mice from LPS-induced lethality, which indicates that PSK is useful for controlling sepsis, which is also interesting from a clinical viewpoint.
Association between chronic inflammation and cancer development has been well described, and various factors released from inflammatory cells have been reported to exacerbate cancer [56]. Using a dextran sodium sulfate (DSS) and 1,2-dimethylhydrazine-induced mouse model of inflammatory bowel disease and colorectal carcinogenesis, Tsutsumi et al. [57] have shown that PSK administration reduced DSS-induced colitis-related mortality and inhibited colorectal carcinogenesis, which indicates that PSK possesses dual antagonistic effects against inflammation and carcinogenesis. This study offers a new perspective on the mechanism of the action of PSK.
Direct action on tumor cells (Table 3)
Table 3.
Direct actions on tumor cells
| Targets of PSK action | Animals/cells | Key observations | References |
|---|---|---|---|
| Apoptosis | Burkitt lymphoma | Induction of apoptosis | Hattori [61] |
| Human gastric cancer cell line | Induction of G1 arrest and apoptosis | Jiménez-Medina [62] | |
| Metastasis | Human pancreas or gastric cancer cell line | Direct or indirect inhibition of MMP production through TGF-β production and suppression of latent TGF-β activation | Zhang [64] |
| Human colon cancer cell line | Suppression of nuclear localization of Smad2; decreased expression of TGF-β-targeted gene; inhibition of TGF-β-induced EMT | Hayashida [66] | |
| Neoangiogenesis | HUVEC, rat | Inhibition of bFGF-induced cell proliferation through direct binding to bFGF | Wada [68] |
| Gene expression | Human colorectal cancer cell line | Upregulation of expression of MRP3, lymphotactin, transgelin and pirin | Yoshikawa [70] |
| Combination with chemotherapy | Human pancreas cancer cell line | Inhibition of docetaxel-induced NF-κB activation and cIAP-1 expression, and enhancement of apoptosis induced by docetaxel | Zhang [71] |
| Human gastric cancer cell line | Inhibition of docetaxel-induced NF-κB activation and survivin expression | Kinoshita [72] |
MMP matrix metalloproteinase, TGF-β transforming growth factor-β, EMT epithelial–mesenchymal transition, HUVEC human umbilical vein endothelial cells, bFGF basic fibroblast growth factor, MRP3 multidrug resistance protein 3, NFκB nuclear factor-κB, cIAP-1 cellular inhibitor of apoptosis protein
It has been demonstrated that PSK directly inhibits the proliferation of cancer cells and induces apoptosis [24, 58, 59]. Also, PSK has been reported to upregulate the expression of human leukocyte antigen (HLA) on human gastric cancer cells [60]. Hattori et al. [61] have examined the effects of PSK on 33 hematological malignant cell lines and have reported that PSK strongly inhibits the proliferation of Namalwa (Burkitt lymphoma) cells and increases apoptosis. It has been shown that inhibition of cell proliferation by PSK is antagonized by the addition of galactose. When Namalwa cells are treated with galactosidase, the effect of PSK is enhanced, which suggests that the galactose-containing structure of the Namalwa cell surface plays an important role in the action of PSK. Jiménez-Medina et al. [62] also have examined the effects of PSK on seven human and mouse cancer cell lines. The inhibitory effect of PSK on cell proliferation differs depending on the cell line. For the AGS (human gastric cancer cell line) cells in which a strong inhibitory effect is observed, arrest of the cell cycle at G1, and increased apoptosis and caspase-3 expression have been observed. From the above results, it is apparent that PSK induces apoptosis of cancer cells, but the effect seems to differ depending on the type of cancer. Further studies should examine the properties of cells in which apoptosis is induced by PSK.
PSK is well known to exhibit anti-metastatic activity in various experimental metastasis models, and the mechanism of action has been reported for each step of metastasis [63]. Two new and interesting reports on the mechanism of the anti-metastatic action of PSK, from the viewpoint of TGF-β suppression, have been published. Zhang et al. [64] have reported that PSK decreases the invasiveness of the human pancreatic cancer cell line, NOR-P1, and the gastric cancer cell line, MK-1P3, by multiple mechanisms that include direct suppression of matrix metalloproteinase (MMP) production, inhibition of the activation of latent TGF-β, and indirect suppression of MMP production through inhibition of TGF-β production. In recent years, the association of epithelial–mesenchymal transition (EMT) with invasion and metastasis of cancer cells has been speculated, and TGF-β has been shown to play an important role in the EMT process [65]. Hayashida et al. [66] have treated SW837 cells (human colorectal cancer cell line with normal TGF-β receptor and signal transduction) with TGF-β followed by PSK, and have found that nuclear translocation of the TGF-β signal transducer Sma- and Mad-related protein 2 (Smad2) was inhibited, and that the expression of genes including the TGF-β target gene SERPINE-1 was also inhibited. In addition, they performed an EMT assay using TGF-β as the inducer and reported that PSK inhibited EMT.
PSK is known to inhibit angiogenesis [67] and new results on the mechanism have been reported. Wada et al. [68] have shown that PSK administration inhibits basic fibroblast growth factor (bFGF)-induced angiogenesis in rats, and from studies on human umbilical vein endothelial cells, they have speculated that the mechanism is due to direct binding of PSK with bFGF, which results in suppression of bFGF-induced proliferation of endothelial cells. The effect of PSK on vascular endothelial growth factor-induced angiogenesis [69] should also be examined.
Comprehensive gene expression analysis of PSK-treated cancer cells has been attempted. Yoshikawa et al. [70] have used cDNA microarrays to analyze the in vitro effect of PSK on the gene expression profile of the human colorectal adenocarcinoma cell line HCT116, the proliferation of which is inhibited by PSK. After PSK treatment, approximately 450 genes showed altered expression. The expression of genes that were significantly altered was examined further in two cell lines, HCT116 and SW480, using RT-PCR. Multidrug resistance protein 3, lymphotactin, transgelin, and pirin were upregulated in both cell lines. Studies on the relationship between PSK-induced altered gene expression and the biological response are anticipated.
Even when used in conjunction with chemotherapy, PSK directly acts on cancer cells to enhance the effect of combined therapy. Zhang et al. [71] have studied the effect of PSK and low-dose docetaxel on proliferation of the human pancreatic cancer cell line NOR-P1, and have reported the mechanisms as follows. Docetaxel induces apoptosis, but at the same time it also activates NF-κB and induces cellular inhibitor of apoptosis protein (cIAP)-1 expression, to suppress the induction of apoptosis. PSK inhibits the docetaxel-induced NF-κB activation to enhance induction of apoptosis. Kinoshita et al. [72] also have reported that PSK inhibits docetaxel-induced NF-κB activation and survivin expression in the human gastric cancer cell line TMK-1, and enhances antitumor effect of docetaxel through inhibition of survivin expression in tumor tissue in a xenograft model.
Clinical effect of PSK
Clinical trials of PSK were started from around 1972, and the early trials demonstrated the effectiveness of PSK as monotherapy or in combination with chemotherapy, which led to the approval of PSK for clinical use in Japan. In 1980, the criteria for the evaluation of immunotherapy for malignant tumors were published. According to these criteria, immunotherapy should be used in combination with other therapies such as chemotherapy, and the evaluation of its effect should include statistically estimating the overall survival (OS) or disease-free survival (DFS), based on randomized control trials (RCTs) using the base therapy as a control [73]. These criteria apply also to PSK. RCTs were planned and conducted, and submitted for reevaluation, which resulted in reapproval for current indications. In addition, the efficacy of PSK has been verified in multiple meta-analyses.
Gastric cancer (Tables 4, 6)
Table 4.
RCTs of postoperative adjuvant chemotherapy with PSK for resected gastric cancer
| References or trial | Stage | No. of patients | Treatment | Percent survival/years | P value | Suggestive data (percent survival/years) |
|---|---|---|---|---|---|---|
| Kondo [76] | III | 72 |
A: PSK B: Placebo |
Improvement of DFS | Significant | |
| 72 | ||||||
| Maehara [77] | Advanced stage | 137 |
A: MMC + FT + PSK B: Surgery alone |
56.9/15 45.7/15 |
0.0351 | |
| 118 | ||||||
|
Niimoto [79] SACG Chugoku/Kyushu |
a | 191 |
A: MMC+FT+PSK B: MMC+PSK C: MMC+FT |
71.7/5 64.1/5 58.5/5 |
3 groups <0.05 A versus C <0.01 |
|
| 189 | ||||||
| 199 | ||||||
|
Kondo [80] SACG Chubu |
a | 145 |
A: FT+PSK B: FT |
64.1/8 61.0/8 |
0.410 |
A: 86 casesb, 56.8/8 B: 90 casesb, 43.6/8 P = 0.071 |
| 159 | ||||||
|
Kondo [82] TGOG |
Advanced stage | 47 |
A: CQ+PSK B: CQ |
Not described | 0.827 |
A: 30 casesc, 43.4/7 B: 32 casesc, 32.3/7 P = 0.153 |
| 49 | ||||||
|
Nakazato [83] SIP |
T2, T3 | 124 |
A: MMC+5-FU+PSK B: MMC+5-FU |
73.0/5 60.0/5 |
0.044 | |
| 129 | ||||||
|
Hattori [13] JFMC01 |
II–III | 1,426 |
A: MMC+FT+PSK B: MMC+FT C: MMC+FT+OK-432+PSK D: MMC+FT+OK-432 |
71.6/3 69.6/3 69.1/3 68.7/3 |
NS | |
| 1,357 | ||||||
| 1,338 | ||||||
| 1,363 | ||||||
| Ogawa [84] | I-IV | 56 |
A: MMC+HCFU+PSK B: MMC+HCFU |
80.2/5 81.1/5 |
0.811 | |
| 55 | ||||||
| JFMC05 | T3, T4 | 215 |
A: FT+PSK B: FT+OK-432 C: FT |
52.8/5 49.3/5 47.0/5 |
NS | |
| 219 | ||||||
| 217 | ||||||
| JFMC11 | T1, T2 | 114 |
A: CPA+PSK B: surgery alone |
84.8/5 83.3/5 |
NS | |
| 112 |
DFS disease-free survival, MMC mitomycin C, FT tegafur, CQ carboquone, T tumor, 5-FU 5-fluorouracil, NS not significant, OK-432 picibanil, HCFU carmofur, CPA cyclophosphamide
aExcluding mucosal cancer having no lymph node metastasis
bTumors with serosal invasion
cTumors with serosal invasion with limited lymph node metastasis
Table 5.
RCTs of postoperative adjuvant chemotherapy with PSK for resected colorectal cancer
| References | Stage | No. of patients | Treatment | Percent survival/years | P value | Suggestive data (percent survival/years) |
|---|---|---|---|---|---|---|
| Torisu [88] | Stage III, IV | 55 |
A: PSK B: Placebo |
Improvement of DFS and OS | <0.05 | |
| 56 | ||||||
| Takashima [89] | Dukes’ A–C | 53 |
A: MMC + FT suppo + PSK B: MMC + FT suppo |
91.4/6 80.8/6 |
0.139 |
A: 30 casesa, 90.2/6 B: 39 casesa, 70.9/6 P < 0.05 |
| 71 | ||||||
| Mitomi [90] | Stage III | 221 |
A: MMC+5-FU+PSK B: MMC+5-FU |
78.5/5 69.7/5 |
0.0325 | |
| 227 | ||||||
| Ito [91] | Dukes’ C | 220 |
A: 5-FU+PSK B: 5-FU |
79.6/7 75.6/7 |
0.081 |
SCD A: 83.4/7 B: 78.5/7 P = 0.019 |
| 221 | ||||||
| Ohwada [92] | Stage II, III | 137 |
A: MMC+UFT+PSK B: MMC+UFT |
81.8/5 72.1/5 |
0.056 |
DFS A: 73.0/5 B: 58.8/5 P=0.016 |
| 68 |
DFS disease-free survival, OS overall survival, MMC mitomycin C, FT tegafur, 5-FU 5-fluorouracil, SCD survival for cancer-related death, UFT tegafur-uracil
aLymphatic vessel invasion (+)
Anticancer chemotherapy can be classified into two types: systemic chemotherapy for unresectable metastatic or recurrent cancer, and adjuvant chemotherapy for suppressing postoperative relapse. Almost all the clinical results of PSK belong to the latter type. As a study of the former type, Nakao et al. [74] have conducted a clinical trial to determine the efficacy of PSK in 54 patients with unresectable or postoperative recurrent gastric cancer. Patients were treated with intravenous mitomycin C (MMC) and 5-fluorouracil (5-FU) twice weekly for 2 weeks, followed by intravenous MMC/5-FU once weekly plus oral PSK 3 g/day on consecutive days (chemotherapy + PSK group), or MMC/5-FU alone (chemotherapy group). They reported that, although the response rates were not significantly different between the two groups, the survival duration was significantly (P = 0.03) prolonged in the chemotherapy + PSK group. Although unresectable, metastatic, and recurrent gastric cancers are not included in the approved indications for PSK at present, this study does suggest a direction for the potential use of PSK.
The results of postoperative adjuvant chemotherapy for gastric cancer have been reviewed by Maehara et al. [75]. In Japan, many controlled trials of postoperative adjuvant chemotherapy compared with surgery alone were conducted from the 1960s, but few studies have reported significant results in favor of postoperative adjuvant chemotherapy. Kondo et al. [76] have conducted a double-blind trial on 144 stage III surgical patients randomized to receive oral PSK 1–3 g/day from 10–15 days after surgery until relapse or distant metastasis (PSK group), or placebo (control group). They reported that PSK treatment significantly prolonged DFS. Their results indicate that PSK, even when used alone, is effective in some patients, although PSK monotherapy is not an approved usage at present. In the study of Maehara et al. [77], 225 patients who underwent histologically curative resection of advanced gastric cancer were treated postoperatively with intravenous MMC once every 3 months for 1 year, together with oral tegafur and PSK from 7 to 10 days after surgery, for long-term (postoperative long-term cancer chemotherapy group; PLCC), or surgery alone. The long-term (15-year) survival rate was 56.9% in the PLCC group and 45.7% in the surgery group, which showed a significant (P = 0.0351) survival benefit in the PLCC group (Fig. 4).
Fig. 4.
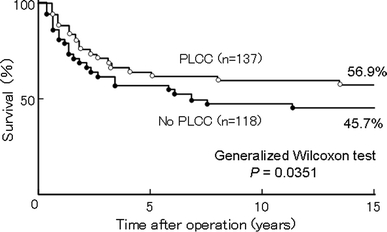
Effect of PLCC plus PSK on 15-year OS in patients with advanced gastric cancer after curative resection. Adapted and modified from Maehara et al. [77]
Since a subset analysis of multiple clinical trials has shown the effectiveness of postoperative adjuvant chemotherapy compared with surgery alone, studies from the 1980s have compared efficacy between different adjuvant chemotherapies. The effectiveness of PSK as an adjuvant therapy for gastric cancer has been evaluated mainly during this period, by comparing chemotherapy + PSK with chemotherapy alone. To examine the effectiveness of immunostimulators, the Co-operative Study Group of Surgical Adjuvant Chemotherapy for Gastric Cancer (SACG) divided Japan into six blocks in 1978, and each block conducted multicenter RCTs independently in patients with resected gastric cancer, excluding the patients with mucosal cancer having no lymph node metastasis, and compared immunochemotherapy with chemotherapy [78]. In the Chugoku/Kyushu block, 579 patients who underwent curative surgery were given intravenous MMC on the day of and 1 day after surgery, followed 2 weeks later by oral PSK 3 g/day plus tegafur (chemotherapy + PSK group), or PSK alone (PSK group) or tegafur alone (chemotherapy group) for 1 year. Niimoto et al. [79] have reported that the 5-year OS rate in the three groups was 71.7, 64.1, and 58.5%, respectively, and a significant difference (P < 0.05) was detected in a three-group comparison. In two-group comparisons, the survival rate was significantly (P < 0.01) higher in the chemotherapy + PSK group than in the chemotherapy group. The Chubu block also enrolled curative surgical cases. The subjects were randomized into two groups according to treatment from 2 weeks after surgery: oral tegafur for 3 months followed by PSK for 2 months (one course) for two courses or more (chemotherapy + PSK alternating therapy group), and tegafur for 3 months followed by no treatment for 2 months for two courses or more (intermittent chemotherapy group). Kondo et al. [80] have reported that a subset analysis of subjects having tumors with serosal invasion showed an improved 8-year survival rate in the chemotherapy + PSK alternating therapy group (56.8%) compared with the intermittent chemotherapy group (43.6%) (P = 0.071). Sakamoto et al. [81] also reported that subset analysis of subjects with preserved cellular immunity, as shown by a positive purified protein derivative of tuberculin (PPD) skin test before surgery in this trial, showed an improved survival rate in the chemotherapy + PSK alternating therapy group compared with the intermittent chemotherapy group (P = 0.254). In addition, Kondo et al. [82] have reported that a subset analysis of subjects having tumors with serosal invasion and limited lymph node metastasis showed an improved 7-year survival rate in the chemotherapy (carboquone) + PSK alternating therapy group (43.4%) compared with the intermittent chemotherapy group (32.3%) (P = 0.153) (Tokai Gastrointestinal Oncology Group; TGOG). Based on these findings, the Study Group of Immunochemotherapy with PSK for gastric cancer (SIP) conducted a multicenter RCT on 253 curative surgery cases with T2 or T3 lesions and positive preoperative PPD skin tests. After surgery, MMC was administered intravenously. Four weeks later, one group was given PSK 3 g/day for 4 weeks and then oral 5-FU for 4 weeks (one course) for a total of 10 courses (chemotherapy + PSK alternating therapy group), and the other group was given tegafur alone (intermittent chemotherapy group). As reported by Nakazato et al. [83], the 5-year OS rate in the PSK + chemotherapy alternating therapy and intermittent chemotherapy groups was 73.0 and 60.0%, respectively (P = 0.044), and the DFS rate was 70.7 and 59.4%, respectively (P = 0.047), which showed significantly better survival with PSK + chemotherapy alternating therapy (Fig. 5).
Fig. 5.
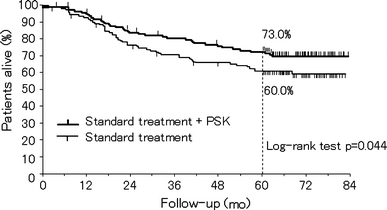
Effect of adjuvant immunochemotherapy with PSK on 5-year overall survival in patients with T2 or T3 gastric cancer after curative resection. Adapted and modified from Nakazato et al. [83]
Among the RCTs that have examined the efficacy of PSK in combination with chemotherapy, some could not confirm the efficacy of PSK. The Cooperative Project No. 1 of the Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC01) was a large-scale clinical trial that enrolled 7,637 patients in 267 facilities. To clarify the effectiveness of PSK and OK-432, two widely used immunostimulatory compounds, patients were administered MMC intravenously on the day of and 1 day after surgery. From 2 weeks after surgery, the patients received the following treatments for 8 months: oral tegafur only, oral tegafur + PSK 3 g/day, tegafur + intradermal or intramuscular OK-432 0.5–5 KE/day, or tegafur + PSK + OK-432. The 3-year survival rate tended to be higher in groups administered PSK and/or OK-432 [13]. The immune status of the patients was evaluated simultaneously, using the effect of patients’ serum on the PHA blastogenesis reaction of mouse lymphocytes as an indicator. Hattori et al. [13] have reported that the 4-year survival rate was significantly (P = 0.012) higher in patients with a high stimulation index, and many patients in the PSK-treated group had a high index.
Ogawa et al. [84] (Kumamoto Gastrointestinal Immunochemotherapy Study Group; KGSG) enrolled 111 patients who underwent curative surgery and treated them 2 weeks after surgery with oral carmofur and PSK 3 g/day (chemotherapy + PSK group), or carmofur alone (chemotherapy group) for 1 year, and observed no difference in 5-year survival rate between the two groups. In the JFMC05 trial, 651 patients with resected T3 or T4 gastric cancer received postoperative tegafur + PSK, tegafur + OK-432, or tegafur alone after surgery. The 5-year survival rate was 52.8% in the tegafur + PSK group, 49.3% in the tegafur + OK-432 group, and 47.0% in the tegafur alone group. Although the rate was apparently higher in the tegafur + PSK group, the difference was not significant (http://jfmc.or.jp/product/prod04/index.html). In the JFMC11 trial, 228 patients with resected T1 or T2 gastric cancer were enrolled. One group received one dose of cyclophosphamide (CPA) and PSK 3 g/day for 1 week before surgery, as well as PSK for 6 months after surgery, and the other group had surgery only. The survival rate did not differ between the two groups (http://jfmc.or.jp/product/prod04/index.html).
The effectiveness of PSK as postoperative adjuvant therapy has been shown in meta-analysis. Oba et al. [85] have identified 47 papers that have compared the survival duration of chemotherapy + PSK with chemotherapy alone in patients who underwent curative surgery for gastric cancer. They excluded duplicated data and selected eight trials of high quality to conduct a meta-analysis. The hazard ratio of 5-year survival was 0.88 [95% confidential interval (CI): 0.79–0.98, P = 0.018] (Fig. 6), which verified that chemotherapy + PSK improved survival in patients with curatively resected gastric cancer.
Fig. 6.
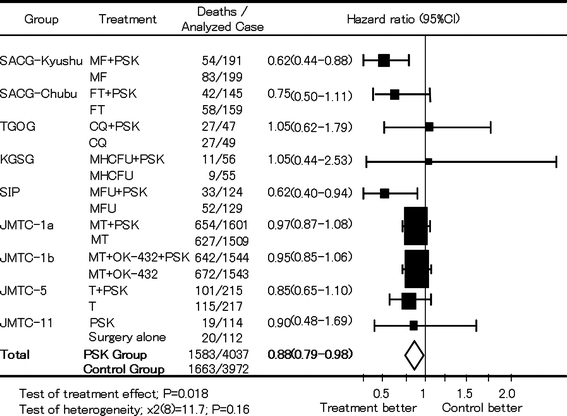
Meta-analysis of the effect of adjuvant immunochemotherapy with PSK on OS in patients with gastric cancer after curative resection. JMTC, Japanese Foundation for Multidisciplinary Treatment of Cancer; MF mitomycin C plus tegafur, MHCFU mitomycin C plus HCFU, MFU mitomycin C plus 5-fluorouracil, MT mitomycin C plus tegafur, T tegafur. Adapted from Oba et al. [85]
As shown above, the efficacy of PSK as postoperative adjuvant therapy has been verified by a large number of studies. Furthermore, Sugimachi et al. [86] have studied 196 patients with curatively resected, poorly differentiated stage II–IV gastric cancer and treated them with MMC and PSK 3 g/day as the basal postoperative therapy, in combination with two doses of UFT. The recurrence rate was significantly reduced and the 5-year DFS and cause-specific survival rates were significantly (P < 0.05) increased in the high-dose UFT (12 mg/kg) group compared with the moderate-dose (8 mg/kg) group. They concluded that combination therapy with high-dose UFT plus PSK improved the postoperative outcome, with no increase in toxicity for poorly differentiated gastric cancer.
After 1990, RCTs were again conducted to examine the effectiveness of postoperative adjuvant chemotherapy compared to surgery alone. At present, TS-1 has become the standard agent for postoperative adjuvant chemotherapy for gastric cancer in Japan [87].
For PSK, the Hokuriku-Kinki Immunochemotherapy Study Group Gastric Cancer (HKIT-GC) group is currently conducting a clinical trial on patients with curatively resected stage II and IIIA gastric cancer. Two groups are being compared: one group is receiving TS-1 for 2 weeks on and 1 week off for 6 months, followed by TS-1 for 2 weeks on and 2 weeks off for 6 months (TS-1 group); and another group is receiving PSK in combination with the above regimen for 1 year (TS-1 + PSK group)(http://clinicaltrials.gov/ct2/home). Another ongoing trial conducted by Tokyo Metropolitan Oncology Group (TMOG) is recruiting curatively resected stage II and III gastric cancer patients and comparing the outcome of treatment with TS-1 alone for 4 weeks on and 2 weeks off (TS-1 group) and PSK in combination with the above regimen (TS-1 + PSK group) for 1 year (http://clinicaltrials.gov/ct2/home).
Colorectal cancer (Tables 5, 6)
Table 6.
Ongoing RCTs with PSK
| Title of the study | Organizations/sponsors | Tumor | Stage | Treatment regimen |
|---|---|---|---|---|
| Randomized controlled study of postoperative adjuvant therapy for gastric cancer using TS-1 or TS-1 + PSK | Hokuriku-Kinki Immunochemotherapy Study Group Gastric Cancer (HKIT-GC) | Gastric cancer | II, IIIA |
TS-1(1 year) TS-1+PSK (1 year) |
| Study of TS-1 or TS-1 + PSK for gastric cancer patients | Tokyo Metropolitan Oncology Group (TMOG) | Gastric cancer | II, III |
TS-1 (1 year) TS-1+PSK (1 year) |
| Randomized phase III adjuvant study for stage III colorectal cancer | Hokkaido Gastrointestinal Cancer Study Group, Colorectal Adjuvant Chemotherapy Division (HGCSG-CAD) | Colorectal cancer | III |
UFT + LV (5 courses) UFT + LV (5 courses)/UFT (1 year) UFT + LV + PSK (5 courses)/UFT + PSK (1 year) |
| Uracil and tegafur/leucovorin (UFT/LV) versus UFT/LV+PSK for stage IIIa/IIIb colorectal cancer | Iwate Clinical Oncology Group, Colorectal Cancer (ICOG-CC) | Colorectal cancer | IIIa, IIIb |
UFT + LV UFT + LV + PSK |
| Phase III trial comparing UFT + PSK to UFT + LV in stage IIB, III colorectal cancer | Multicenter Clinical Study Group of Osaka, Colorectal Cancer Treatment Group (MCSGO-CCTG) | Colorectal cancer | IIB, III |
UFT+PSK (1 year) UFT+LV (6 months) |
| Phase III trial comparing surgery alone to UFT + PSK in stage II rectal cancer | Japanese Foundation for Multidisciplinary Treatment of Cancer | Rectal cancer | II |
Surgery alone UFT + PSK (1 year) |
TS-1 tegafur–gimeracil–oteracil potassium, UFT tegafur-uracil, LV leucovorin
The prognosis of surgical treatment for colon and rectal cancers is poor for stage III or higher colon cancer and stage II or higher rectal cancer. For these advanced cancers, postoperative adjuvant therapy is considered necessary. In Japan, many trials have examined the effectiveness of postoperative adjuvant chemotherapy for colorectal cancer using oral fluorinated pyrimidine agents, compared with surgery alone, but few studies have reported the effectiveness of these agents.
The efficacy of PSK on colorectal cancer has been evaluated as postoperative adjuvant therapy. Torisu et al. [88] have conducted a double-blind trial on patients with curatively resected stage III and IV colorectal cancer and compared PSK 1–3 g/day given after surgery until relapse or distant metastasis (PSK group) and placebo (control group). They reported significantly better OS (P < 0.05) and DFS (P < 0.05) rates in the PSK group.
Thereafter, comparative studies of chemotherapy versus chemotherapy + PSK have been conducted. The Colorectal Cancer Chemotherapy Group in Hokuriku has conducted a multicenter RCT of 124 patients with curatively resected advanced colorectal cancer and administered intravenous MMC on the day of and 1 day after surgery. The patients were randomized to receive tegafur suppository and oral PSK 3 g/day (chemotherapy + PSK group) or tegafur suppository alone (chemotherapy group) from 1–2 weeks after surgery for 1 year. Takashima et al. [89] have reported that in lymphatic vessel invasion (+) cases, the 6-year OS rates for the two groups were 90.2 and 70.9%, which was significantly (P < 0.05) higher in the chemotherapy + PSK group. The Cooperative Study Group of Surgical Adjuvant Immunochemotherapy for Cancer of Colon and Rectum (Kanagawa) has conducted a multicenter RCT on 448 patients who underwent macroscopic curative surgery for stage III and IV colorectal cancer. After treatment with intravenous MMC on the day of and 1 day after surgery, the subjects were randomized 2–3 weeks later to receive oral 5-FU for at least 6 months and PSK for at least 3 years (chemotherapy + PSK group), or 5-FU alone (chemotherapy group). Mitomi et al. [90] have reported that the 5-year DFS (P = 0.0302) and survival (P = 0.0325) rates were significantly higher in the chemotherapy + PSK group compared to the chemotherapy group (5-year DFS rates: 72.3 vs. 63.2%; 5-year survival rates: 78.5 vs. 69.7%).
The Study Group of Immunochemotherapy with PSK for colon cancer has conducted a multicenter RCT on 441 colon cancer patients with macroscopic Dukes’ C cancer after curative resection. After surgery, continuous intravenous infusion of 5-FU was administered and the patients were randomized at 4 weeks after surgery to receive oral PSK 3 g/day for 4 weeks, followed by oral 5-FU for 4 weeks (one course) for a total of 10 courses (chemotherapy + PSK alternating therapy), or 5-FU alone (intermittent chemotherapy). Ito et al. [91] have reported that the 7-year cancer death-free survival rate was significantly (P = 0.019) higher in the chemotherapy + PSK alternating therapy arm (83.4%) than in the intermittent chemotherapy arm (78.5%), with a risk reduction of 40.8%.
The Gunma Oncology Study Group has conducted a multicenter RCT on 205 patients with curatively resected stage II or III colorectal cancer. Two weeks after intravenous MMC (given on the day of surgery and the following day), one group received oral UFT and PSK 3 g/day for 2 years (chemotherapy + PSK group), and the other group received oral UFT alone (chemotherapy group). Ohwada et al. [92] have reported that the 5-year DFS rate was significantly (P = 0.016) higher in the chemotherapy + PSK group (73.0%) compared with the chemotherapy group (58.8%). Combined therapy with PSK reduced the risk of recurrence by 43.6%. The mean DFS period was significantly (P = 0.031) prolonged in the chemotherapy + PSK group (50.3 months) compared with the chemotherapy group (40.0 months). The 5-year OS rate was higher in the chemotherapy + PSK group (81.8%) than in the chemotherapy group (72.1%), although there was no significant difference (P = 0.056). Furthermore, they reported an adverse event rate of 15.1% for both groups, with no grade 3 or 4 events. They concluded that, compared with combined intravenous 5-FU and leucovorin (LV) treatment, for which many grade 3 or higher adverse events have been reported, UFT + PSK therapy was a useful adjuvant therapy that did not require frequent hospital visits and had few adverse reactions.
Sakamoto et al. [93] have performed a meta-analysis of three RCTs that were conducted and published between 1980 and 2004 on curatively resected colorectal cancer patients and compared chemotherapy + PSK with chemotherapy alone. The overall survival risk ratio was 0.71 (95% CI 0.55–0.90, P = 0.006) and the DFS risk ratio was 0.72 (95% CI 0.58–0.90, P = 0.003), which showed a significant survival benefit of combined therapy with PSK (Fig. 7). Their study confirms the significance of using PSK in postoperative adjuvant therapy for colorectal cancer and opens the possibility of developing improved therapy for colorectal cancer.
Fig. 7.
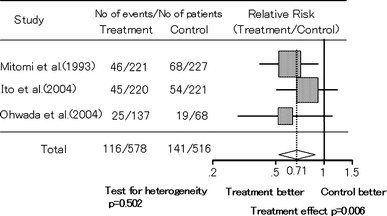
Meta-analysis of the effect of adjuvant immunochemotherapy with PSK on OS in patients with colorectal cancer after curative resection. Adapted from Sakamoto et al. [93]
Several ongoing studies on PSK have been examining various combination therapy regimens used as postoperative adjuvant therapy. The Hokkaido Gastrointestinal Cancer Study Group, Colorectal Adjuvant Chemotherapy Division (HGCSG-CAD) has been comparing 6 months UFT + LV therapy (UFT + LV group), 6-month UFT + LV with UFT extended for 1 year (UFT + LV/UFT group), and PSK added on to the latter regimen (UFT + LV + PSK/UFT + PSK group) in patients with curatively resected stage III colorectal cancer (http://clinicaltrials.gov/ct2/home). The Iwate Clinical Oncology Group, Colorectal Cancer (ICOG-CC) trial has been comparing UFT + LV with UFT + LV + PSK in patients with curatively resected stage IIIa and IIIb colorectal cancer (http://clinicaltrials.gov/ct2/home). The Multicenter Clinical Study Group of Osaka, Colorectal Cancer Treatment Group (MCSGO-CCTG) has been comparing UFT + PSK (1 year) with UFT + LV (6 months) in patients with curatively resected stage IIB and III colorectal cancer (http://clinicaltrials.gov/ct2/home). In the JFMC38-0901 trial, the effect of 12 months UFT + PSK therapy compared with surgery alone is being evaluated in patients with curatively resected stage II rectal cancer (http://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=search&action=list&language=J).
Lung cancer (Table 7)
Table 7.
RCTs of chemotherapy and/or radiotherapy with PSK for lung cancer
| References | Tumor characteristics | No. of patients | Treatment | Response rate (%) or percentage survival/year | P value | Suggestive data |
|---|---|---|---|---|---|---|
| Duration of tumor response | ||||||
| Konno [94] | Small-cell lung cancer | 48 |
A: VCR+CPA+MMC (radiation)+PSK B: VCR+CPA+MMC (radiation) |
45 46 |
NS |
A: 25 weeks B: 13 weeks P = 0.042 |
| 49 | ||||||
| Ikeda [95] | Non-small cell lung cancer stage I–IV | 27 |
A: VCR+MMC+MTX (CPA, 5-FU)+PSK B: VCR+MMC+MTX (CPA, 5-FU)+OK-432 C: VCR+MMC+MTX (CPA, 5-FU) |
55.9/4 36.7/4 34.7/4 |
3 groups <0.05 A versus C <0.05 |
|
| 39 | ||||||
| 47 | ||||||
| Hayakawa [96] |
Non-small cell lung cancer Stage I–III |
77 |
A: Radiation+PSK B: Radiation |
30/5 9/5 |
<0.001 | |
| 111 |
VCR vincristine, CPA cyclophosphamide, MMC mitomycin C, NS not significant, MTX methotrexate, 5-FU 5-fluorouracil, OK-432 picibanil
Konno et al. [94] have compared the combination of intravenous vincristine (VCR), cyclophosphamide (CPA), and MMC once weekly for 8 weeks with or without 8–10 Gy radiotherapy (chemotherapy/radiotherapy group) with the combination of PSK 3 g/day added to the above regimen (chemotherapy/radiotherapy + PSK group) in 97 patients with small-cell lung carcinoma. Although the response rates did not differ between the two groups, the median response duration was significantly (P = 0.042) longer in the chemotherapy/radiotherapy + PSK group (25 weeks) than in the chemotherapy/radiotherapy group (13 weeks). Evidence for combined use of PSK and currently used chemotherapy regimen for extensive-stage small-cell lung cancer is anticipated; therefore, the Research Network for Chemotherapy of Lung Cancer (RNCLC) has been conducting a phase II trial to examine the effect of cisplatin + irinotecan + PSK compared with historical controls (http://clinicaltrials.gov/ct2/home).
To examine the usefulness of PSK as postoperative adjuvant therapy for lung cancer, Ikeda et al. [95] have studied 113 patients with non-small cell lung cancer randomized after surgery into three groups: chemotherapy with VCR, MMC, methotrexate, CPA or 5-FU combined with PSK 3 g/day (chemotherapy + PSK group); chemotherapy combined with OK-432 (chemotherapy + OK-432 group); and chemotherapy alone (chemotherapy group). The three-group comparison detected a significant difference (P < 0.05) in the 4-year survival, and the two-group comparisons found a significant difference (P < 0.05) between the chemotherapy + PSK and chemotherapy groups.
Hayakawa et al. [96] have investigated PSK therapy in 188 patients with non-small cell lung cancer, mainly stages I–III squamous cell cancer, who had achieved complete or partial response after radiotherapy. The patients were randomized to receive adjuvant treatment with PSK intermittent administration of 3 g/day for 2 weeks followed by 2 weeks off, or no adjuvant treatment. The 5-year survival rate was significantly (P < 0.001) higher in the group given PSK. As demonstrated in these studies, PSK is effective also for non-small cell lung cancer.
Other clinical uses (Tables 8, 9)
Table 8.
RCTs of chemotherapy and/or radiotherapy with PSK for various cancers
| References | Tumor characteristics | No. of patiens | Treatment | Results | P value | |
|---|---|---|---|---|---|---|
| 5-year survival rates | ||||||
| Go [97] | Nasopharyngeal carcinoma |
17 17 |
A: RT(±CTa) + PSK B: RT(±CT) |
28% 15% |
0.043 | |
| 3-year DFS rates | 4 groups | |||||
| Matsumoto [98] |
Superficial bladder tumor pTa, pT1 G1, G2 |
65 67 65 66 |
A: Resection+PSK B: Resection only C: Resection+CQ+PSK D: Resection+CQ |
56.3% 32.6% 43.1% 35.6% |
0.026 A versus B 0.008 A versus D 0.006 |
|
| 5-year survival rates | ||||||
| Ogoshi [99] |
Esophageal cancer stage I–IV |
38 31 56 49 |
A: Resection+RT+PSK B: Resection+RT C: Resection+RT+CTb+PSK D: Resection+RT+CT |
42.3% 40.0% 37.2% 29.1% |
C versus D 0.1930 |
|
| 10-year survival rates | 3 groups | |||||
| Iino [100] | Breast cancer |
74 76 77 |
A: Resection+FEMPc+PSK B: Resection+FEMP+Levamisole C: Resection+FEMP |
81.8% 76.9% 64.6% |
0.1686 A versus C 0.0706 |
|
RT radiotherapy, CT chemotherapy, DFS disease-free survival, pT pathologic tumor, G grade, CQ carboquone
aCisplatin or 5-FU or methotrexate or vincristine
bBleomycin or pepleomycin+tegafur
c5-FU+CPA+MMC+prednisolone
Table 9.
Other effects of PSK in cancer therapy
| References | Evaluation | Tumor characteristics | No. of patients | Treatment | Results |
|---|---|---|---|---|---|
| Kohara [102] | Toxicity of chemotherapy | Solid tumors |
20 49 |
A: 5-FU (dry syrup) + PSK B: 5-FU (dry syrup) |
Frequency of chemotherapy toxicity A: 7 cases (35.0%) B: 26 cases (53.1%) |
| Sadahiro [103] | Immune cells | Rectal cancer |
15 15 |
A: RT+S-1+PSK (preoperative) B: RT+S-1 (preoperative) |
Increases of the proportion of NK cells in the peripheral blood (P = 0.003) and cytotoxic T-cell counts in the peri-tumoral and normal mucosa (P=0.005, 0.003) |
| Yoshimura [104] | QOL | Stage III, IV adenocarcinoma of the lung, inoperable |
10 10 |
A: CDDP+VDS+PSK B: CDDP+VDS |
Good score of QOL A > B |
| Motai [105] | Cancerous pain | Nasopharyngeal carcinoma |
31 31 |
A: PSK B: Non-PSK |
Frequency of analgesic use A: 72.9 ± 16.2 B: 146.9 ± 43.6 P < 0.05 |
5-FU 5-fluorouracil, RT radiation therapy, S-1 tegafur–gimeracil–oteracil potassium, CDDP cisplatin, VDS vindesine, QOL quality of life
A number of reports on the effects of PSK on cancers not included in the approved indications have been published. In one report, nasopharyngeal carcinoma patients who had undergone radiotherapy or radiotherapy + chemotherapy were given PSK or no further treatment. The 5-year survival rate was significantly (P = 0.043) improved in the PSK-treated group compared to the non-PSK-treated group [97]. Patients with primary or relapsed superficial bladder cancer were randomized after surgery to receive PSK, chemotherapy with carboquone, or chemotherapy + PSK, and were compared with surgery alone. The 3-year DFS rate was significantly better in the PSK-treated group compared with surgery alone (P = 0.008) or the chemotherapy-treated group (P = 0.006) [98]. In esophageal cancer patients treated postoperatively with radiotherapy, or radiotherapy combined with PSK or radiotherapy + chemotherapy (bleomycin, or pepleomycin + tegafur), or radiotherapy + chemotherapy combined with PSK, the 5-year survival tended to be prolonged in the radiotherapy + chemotherapy + PSK group compared with the radiotherapy + chemotherapy group, although the difference was not significant (P = 0.1034) [99]. Breast cancer patients were treated with postoperative chemotherapy (5-FU + CPA + MMC + prednisolone) with or without PSK. The 10-year OS rate tended to be higher (P = 0.0706) in the PSK-treated group compared with the group given chemotherapy alone [100]. Although BRMs such as PSK have a direct effect on tumors, their major action is to enhance and modulate the immune response, which can account for a reasonable degree of effectiveness against various tumor types.
Few adverse reactions from the use of PSK have been reported, and most of them are gastrointestinal symptoms with no report of serious toxicity such as bone marrow suppression, or liver or renal function impairment [76, 88, 101]. PSK is relatively non-toxic and it has been reported to attenuate the adverse reactions or immunosuppression induced by chemotherapy or radiotherapy [102, 103]. Furthermore, Yoshimura et al. [104] have examined the quality of life (QOL) of 20 patients with unresectable stage III and IV lung adenocarcinoma treated with chemotherapy (cisplatin + vindesine) + PSK or chemotherapy alone, and found that good QOL was maintained in the patients treated with chemotherapy + PSK. Motai et al. [105] have investigated the frequency of prescription of analgesics for cancer pain in head and neck cancer, by comparing those treated and not treated with PSK. They reported significantly (P < 0.05) reduced frequency of analgesic use in patients treated with PSK, which suggested that PSK had some effect on cancer pain relief. Apart from its anticancer effects, PSK is a useful agent in cancer treatment as shown by the above studies.
Concluding remarks and future perspectives
Recent developments in the investigations of the mechanisms of action of PSK and its main clinical effects have been reviewed. Regarding the action of PSK against immunosuppression, PSK has been reported to restore or attenuate immunosuppression due to various factors. With regard to the actions on immune cells, the induction of DC maturation, the correction of Th1/Th2 imbalance, etc. have been reported. Furthermore, the involvement of PSK in intracellular signal transduction pathways also begins to unfold. With regard to the direct action on tumors, considerable knowledge about apoptosis induction by PSK has been accumulating, and the anti-metastatic effect and chemotherapy potentiating effect due to direct action on tumors have been reported. Future studies of the actions of PSK on immune cells and tumor cells at the molecular level under various conditions, and identification of the target molecules of PSK, are necessary to clearly define the whole mechanisms of action of PSK. Furthermore, it is essential to investigate the actions of PSK along with recent advances in molecular biology and tumor immunology. Although many research results on the mechanisms of action of PSK have been reported, it is undeniable that the main mechanism of action is unclear. It seems that PSK is an immunomodulator rather than a purely immunopotentiator [106]. PSK probably does not exert the same actions in all patients. The effects of PSK under different local (tumor site) or systemic immune conditions and tumor cell properties, and the mechanism of action of PSK in each circumstance should be studied.
The beneficial effect of PSK as postoperative adjuvant therapy for gastric and colorectal cancer has been shown in multiple RCTs. In addition, the effects of PSK in gastric and colorectal cancer have been verified in multiple meta-analyses. Prolongation of the remission period has also been found in small-cell lung cancer. The combined effect or comparison of combined formulation and biochemical modulation of fluoropyrimidine anticancer agents is being examined by RCTs, and the results will be available in the near future. Besides gastric, colorectal and small-cell lung cancer, PSK also exhibits reasonable effects on other cancers. These results are expected, because, unlike chemotherapeutic agents, PSK exerts anticancer effects through acting on host immunity. Also, PSK causes few adverse reactions and has been reported to reduce those associated with chemotherapy, improve QOL, and mitigate cancer pain. The next step of PSK research is to define which type of patient and disease conditions will allow PSK to exert its optimal effect, irrespective of cancer type. In this regard, there is an urgent need to elucidate the mechanisms of action of PSK at the molecular level so as to identify the biomarkers. Achievement of these goals will benefit patients from the personalized medicine point of view.
Several reports have already suggested that the patient’s immune function, as indicated by blood IAP level, peripheral granulocyte/lymphocyte ratio, and DC infiltration of tumor tissue, as well as HLA type, is a potential biomarker of response to PSK therapy [107–110]. The recent advances in biomarker research are summarized in Table 10. Yoshikawa et al. [111] have examined the action of PSK on human colorectal cancer cell line, using protein microarray with antibodies against 500 human proteins and found that expression of ECA39 protein was reduced. ECA39 expression in resected tumors is associated with poor DFS and OS; therefore, these findings suggest that ECA expression is a marker of response to PSK therapy. Yamashita et al. [112] have examined nuclear translocation of β-catenin in cancer tissues of colorectal cancer patients and reported that OS was improved with PSK immunochemotherapy in patients showing diffuse β-catenin nuclear accumulation in tumor tissues. Takahashi et al. [113] have reported that, in colon cancer patients with preoperative peripheral blood carcinoembryonic antigen level: ≥3.0 ng/ml or PPD skin reaction level: <19.0 mm, 7-year DFS and OS were significantly better with postoperative adjuvant immunochemotherapy using PSK than in patients treated with chemotherapy alone. Ohwada et al. [114] have reported that colorectal cancer patients with an increase in NK cell population at 3 months after surgery had more favorable DFS when treated with PSK immunochemotherapy than chemotherapy alone. Yoshino et al. [46] have found that relapse (3 years) did not occur in colorectal cancer patients with peripheral blood CD4+ IL-10+ T-cell ratios (post-/pre-PSK treatment): <0.8 when PSK was administered 1 week before surgery, and reported that these patients might be candidate PSK responders. We have examined the outcome in gastric and colorectal cancer patients using the PSK-induced peripheral blood lymphocyte blastogenesis reaction as an indicator for response to PSK therapy and gained an impression that patients with high reactivity had better survival outcome (Fig. 8) [115, 116]. We have also investigated the in vitro effect of PSK on PBMC gene expression in healthy individuals using DNA microarray analysis and observed changes in expression of six genes in four of five individuals. Using real-time RT-PCR, we found increased expression of IL-18BP, CCL2, IL-8, and vesicle amine transport 1 homolog and reduced expression of chondroitin sulfate proteoglycan in all five individuals [117]. The relationship between expression of these genes and relapse suppression needs to be examined further. We speculate that more than one biomarker might indicate response to PSK therapy. Identification of these biomarkers one by one will pave the way for the future use of PSK in cancer treatment.
Table 10.
Potential biomarkers of PSK
| Subjects | Potential biomarker | References |
|---|---|---|
| Human colon cancer cell line (in vitro) | Expression of ECA39 protein in tumor cells | Yoshikawa [111] |
| Colon cancer patients | Diffuse nuclear accumulation of β-catenin activation in primary tumor | Yamashita [112] |
| Colon cancer patients |
Preoperative peripheral blood CEA level: ≥3.0 ng/ml Preoperative PPD skin reaction level: <19.0 nm |
Takahashi [113] |
| Colorectal cancer patients | Increase of NK cell population in peripheral blood after PSK administration | Ohwada [114] |
| Colorectal cancer patients | Ratio of CD4+ IL-10+ T-cell percentage in peripheral blood before and after PSK treatment: <0.8 | Yoshino [46] |
| Gastric or colorectal cancer patients | In vitro activation level of peripheral blood lymphocytes by PSK | Yoshinaga [116] |
CEA carcinoembryonic antigen, PPD purified protein derivative of tuberculin, NK natural killer
Fig. 8.
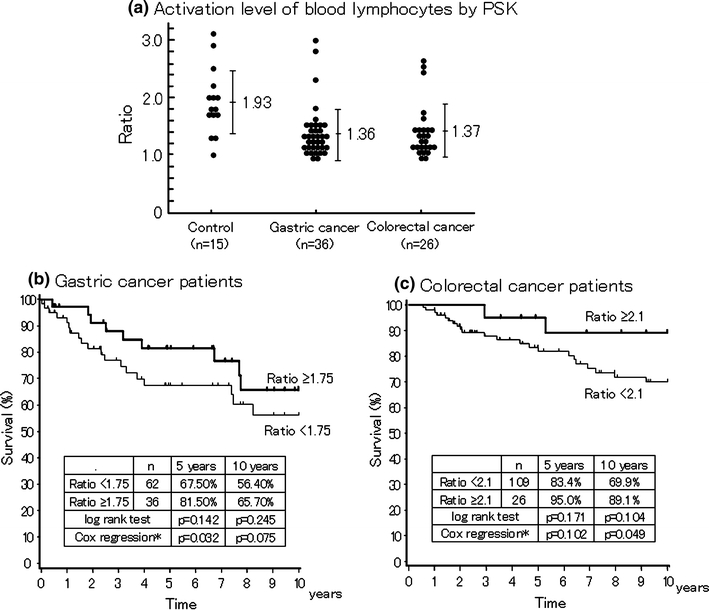
PSK-stimulated activation of blood lymphocytes in healthy volunteers and cancer patients (a). Effects of adjuvant immunochemotherapy with PSK on 10-year OS in gastric cancer (b) and colorectal cancer (c) patients with low or high PSK-induced lymphocyte activation level. The increase in DNA synthesis of lymphocytes was defined as ratio of the level of PSK-treated lymphocytes versus PSK-non-treated lymphocytes. Asterisk adjusted by gender, age, Dukes’ stage, tumor size, lymphatic vessel invasion, and venous invasion. Adapted from Sugimachi et al. [115] and Yoshinaga et al. [116]
Conflict of interest
Y.M. has received honoraria and research funding from Daiichi Sankyo Co. Ltd. and Kureha Corp. H.B. has received research funding from Daiichi Sankyo Co. Ltd. All other authors declare no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura K, Olino K, Edil BH, Schulick RD, Oka M. Immuno- and gene-therapeutic strategies targeted against cancer (mainly focusing on pancreatic cancer) Surg Today. 2010;40:404–410. doi: 10.1007/s00595-009-4120-8. [DOI] [PubMed] [Google Scholar]
- 6.Sargent DJ, Köhne CH, Sanoff HK, Bot BM, Seymour MT, de Gramont A, et al. Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:1948–1955. doi: 10.1200/JCO.2008.20.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson SS, Lenon RA. Alterations of nutritional status: impact of chemotherapy and radiation therapy. Cancer. 1979;43:2036–2052. doi: 10.1002/1097-0142(197905)43:5+<2036::AID-CNCR2820430712>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto J, Teramukai S, Koike A, Saji S, Ohashi Y, Nakazato H. Prognostic value of preoperative immunosuppressive acidic protein in patients with gastric carcinoma: findings from three independent clinical trials. Cancer. 1996;77:2206–2212. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2206::AID-CNCR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 10.Tsukagoshi S, Hashimoto Y, Fujii G, Kobayashi H, Nomoto K, Orita K. Krestin (PSK) Cancer Treat Rev. 1984;11:31–55. doi: 10.1016/0305-7372(84)90005-7. [DOI] [PubMed] [Google Scholar]
- 11.Fujii M. Krestin (PSK). Biotherapy. 1996;10:315–7 (in Japanese with English abstract).
- 12.Matsunaga K, Morita I, Iijima H, Endo H, Oguchi Y, Yoshimura M, et al. Competitive action of biological response modifier, PSK, on a humoral immunosuppressive factor produced in tumor-bearing hosts. J Clin Lab Immunol. 1990;31:127–136. [PubMed] [Google Scholar]
- 13.Hattori T, Nakajima T, Nakazato H, Tanabe T, Kikuchi K, Abe O, et al. Postoperative adjuvant immunochemotherapy with mitomycin C, tegafur, PSK and/or OK-432 for gastric cancer, with special reference to the change in stimulation index after gastrectomy. Jpn J Surg. 1990;20:127–136. doi: 10.1007/BF02470759. [DOI] [PubMed] [Google Scholar]
- 14.Harada M, Matsunaga K, Oguchi Y, Iijima H, Tamada K, Abe K, et al. Oral administration of PSK can improve the impaired anti-tumor CD4+ T-cell response in gut-associated lymphoid tissue (GALT) of specific-pathogen-free mice. Int J Cancer. 1997;70:362–372. doi: 10.1002/(SICI)1097-0215(19970127)70:3<362::AID-IJC19>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga K, Hosokawa A, Oohara M, Sugita N, Harada M, Nomoto K. Direct action of a protein-bound polysaccharide, PSK, on transforming growth factor-β. Immunopharmacology. 1998;40:219–230. doi: 10.1016/S0162-3109(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 16.Inokuchi K, Kumashiro R. Chemotherapy for gastric cancer. Surg Therapy. 1980;42:40–6 (in Japanese).
- 17.Yamanaka M, Takahata K, Oka H, Yoshino F, Sugita N, Yoshikumi C. Studies on the mechanisms of antitumor activities of PSK (Krestin): effects on prostaglandin metabolism of tumor cells. In: Ishigami J, editor. Recent advances in chemotherapy. Proceedings of the 14th ICC. Tokyo: University of Tokyo Press; 1985. pp. 896–897. [Google Scholar]
- 18.Kobayashi Y, Kariya K, Saigenji K, Nakamura K. Oxidative stress relief for cancer-bearing hosts by the protein-bound polysaccharide of Coriolus versicolor Quel with SOD mimicking activity. Cancer Biother. 1994;9:55–62. doi: 10.1089/cbr.1994.9.55. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, Minami K, Ohshita A, Kawabuchi Y, Noma K, Toge T. Enhancing effect of PS-K on IL-2-induced lymphocyte activation: possible involvement of antagonistic action against TGF-beta. Anticancer Res. 2004;24:639–648. [PubMed] [Google Scholar]
- 20.Shibata M, Abe H, Kanou H, Azuhata T, Nezu T, Ohara M, et al. In vitro and in vivo immune-modulating effects of polysaccharide-K (PSK) in patients with colorectal cancer. Proc AACR. 2002;43:448.
- 21.Shibata M, Nezu T, Kanou H, Nagata Y, Kimura T, Takekawa M, et al. Immunomodulatory effects of low dose cis-diaminedichloroplatinum (cisplatin) combined with UFT and PSK in patients with advanced colorectal cancer. Cancer Invest. 2002;20:166–173. doi: 10.1081/CNV-120001142. [DOI] [PubMed] [Google Scholar]
- 22.Tsujitani S, Ozaki T, Saito H, Fukuda K, Tatebe S, Ikeguchi M. The NKG2D expression on CD8+ T cells and efficacy of polysaccharide K (PSK) in gastric cancer. J Clin Oncol. 2008;26:148s. [Google Scholar]
- 23.Okuzawa M, Shinohara H, Kobayashi T, Iwamoto M, Toyoda M, Tanigawa N. PSK, a protein-bound polysaccharide, overcomes defective maturation of dendritic cells exposed to tumor-derived factors in vitro. Int J Oncol. 2002;20:1189–1195. [PubMed] [Google Scholar]
- 24.Sugiyama Y, Saji S, Kunieda K, Yamada M, Nagata M, Ri S, et al. Effect of PSK on either immunocytes or tumor cells. Biotherapy. 1996;10:18–25 (in Japanese with English abstract).
- 25.Ooshiro M, Sugishita Y, Tanaka H, Koide K, Nagashima M, Katoh R. Regulation of perioperative immunological changes following laparotomy: effects of biological response modifier (BRM) on surgical stress. Immunol Lett. 2004;93:33–38. doi: 10.1016/j.imlet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Tsukagoshi S. Fundamental approaches to cancer immunotherapy using a protein-bound polysaccharide, PS-K, with special reference to its clinical application. In: Mizuno D, Chihara G, Fukuoka F, Yamamoto T, Yamamura Y, editors. Host defense against cancer and its potentiation. Tokyo: University of Tokyo Press; 1975. pp. 365–377. [Google Scholar]
- 27.Kono K, Kawaguchi Y, Mizukami Y, Mimura K, Sugai H, Akaike H, et al. Protein-bound polysaccharide K partially prevents apoptosis of circulating T cells induced by anti-cancer drugs S-1 in patients with gastric cancer. Oncology. 2008;74:143–149. doi: 10.1159/000151361. [DOI] [PubMed] [Google Scholar]
- 28.Kariya Y, Okamoto N, Fujimoto T, Inoue N, Kihara T, Sugie K, et al. Lysis of fresh human tumor cells by autologous peripheral blood lymphocytes and tumor-infiltrating lymphocytes activated by PSK. Jpn J Cancer Res. 1991;82:1044–1050. doi: 10.1111/j.1349-7006.1991.tb01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nio Y, Shiraishi T, Tsubono M, Morimoto H, Tseng CC, Imai C, et al. In vitro immunomodulating effect of protein-bound polysaccharide, PSK on peripheral blood, regional nodes, and spleen lymphocytes in patients with gastric cancer. Cancer Immunol Immunother. 1991;32:335–341. doi: 10.1007/BF01741328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vánky F, Wang P, Klein E. The polysaccharide K (PSK) potentiates in vitro activation of the cytotoxic function in human blood lymphocytes by autologous tumor cells. Cancer Immunol Immunother. 1992;353:193–198. doi: 10.1007/BF01756187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebina T, Kohya H. Antitumor effector mechanism at a distant site in the double grafted tumor system of PSK, a protein-bound polysaccharide preparation. Jpn J Cancer Res. 1988;79:957–964. doi: 10.1111/j.1349-7006.1988.tb00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuru S, Nomoto K. Effects of PSK on specific tumor immunity to syngeneic tumor cells. J Clin Lab Immunol. 1983;10:215–219. [PubMed] [Google Scholar]
- 33.Algarra I, Collado A, Garrido F. Protein bound polysaccharide PSK abrogates more efficiently experimental metastasis derived from H-2 negative than from H-2 positive fibrosarcoma tumor clones. J Exp Clin Cancer Res. 1997;16:373–380. [PubMed] [Google Scholar]
- 34.Ueda Y, Naito K, Kobayashi M, Omori K, Shimode Y, Matsuda A, et al. In vitro induction of LAK cells with PSK. Biotherapy. 1991;5:861–3 (in Japanese with English abstract).
- 35.Baba N, Yamaguchi Y, Sato Y, Takayama T, Yanagawa E, Toge T. The enhancement of tumoricidal activities of macrophages by protein-bound polysaccharide in tumor bearing mice. Biotherapy. 1990;4:123–8 (in Japanese with English abstract).
- 36.Kato H, Kin R, Yamamura Y, Tanigawa M, Sano H, Sugino S, et al. Tumor inhibitory effect of polymorphonuclear leukocytes (PMN) induced by PSK in the peritoneal cavity of tumor-bearing mice. J Kyoto Pref Univ Med. 1987;96:927–938. [Google Scholar]
- 37.Hirose K, Zachariae C, Oppenheim J, Kouji M. Induction of gene expression and production of immunomodulating cytokines by PSK in human peripheral blood mononuclear cells. Lymphokine Res. 1990;94:475–483. [PubMed] [Google Scholar]
- 38.Asai K, Kato H, Kimura S, Mukai S, Kawahito Y, Sano H, et al. Induction of gene expression for nitric oxide synthase by immunomodulating drugs in the RAW264.7 murine macrophage cell line. Cancer Immunol Immunother. 1996;42:275–279. doi: 10.1007/s002620050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Lora A, Pedrinaci S, Garrido F. Protein-bound polysaccharide K and interleukin-2 regulate different nuclear transcription factors in the NKL human natural killer cell line. Cancer Immunol Immunother. 2001;50:191–198. doi: 10.1007/s002620100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Lora A, Martinez M, Pedrinaci S, Garrido F. Different regulation of PKC isoenzymes and MAPK by PSK and IL-2 in the proliferative and cytotoxic activities of the NKL human natural killer cell line. Cancer Immunol Immunother. 2003;52:59–64. doi: 10.1007/s00262-002-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asai H, Iijima H, Matsunaga K, Oguchi Y, Katsuno H, Maeda K. Protein-bound polysaccharide K augments IL-2 production from murine mesenteric lymph node CD4+ T cells by modulating T cell receptor signaling. Cancer Immunol Immunother. 2008;57:1647–1655. doi: 10.1007/s00262-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanazawa M, Mori Y, Yoshihara K, Iwadate M, Suzuki S, Endoh Y, et al. Effect of PSK on the maturation of dendritic cells derived from human peripheral blood monocytes. Immunol Lett. 2004;91:229–238. doi: 10.1016/j.imlet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Ogihara T, Iinuma H, Okinaga K. Usefulness of immunomodulators for maturation of dendritic cells. Int J Oncol. 2004;25:453–459. [PubMed] [Google Scholar]
- 44.Schoof DD, Terashima Y, Peoples GE, Goedegebuure PS, Andrews JV, Richie JP, et al. CD4+ T cell clones isolated from human renal cell carcinoma possess the functional characteristics of Th2 helper cells. Cell Immunol. 1993;150:114–123. doi: 10.1006/cimm.1993.1183. [DOI] [PubMed] [Google Scholar]
- 45.Sugiyama Y, Osada S, Yamaguchi K, Nagao N, Takahashi T, Sakashita F. Evidence-based biotherapy by use of PSK. Biotherapy. 2006;20:396–402 (in Japanese with English abstract).
- 46.Yoshino S, Hazama S, Shimizu R, Fukuda S, Kudoh A, Mizuta E, et al. Usefulness in predicting parameters for the selection of responders who received immunochemotherapy with PSK in patients with colorectal cancer. Jpn J Cancer Chemother. 2005;32:1568–70 (in Japanese with English abstract). [PubMed]
- 47.Kanoh T, Saito K, Matsunaga K, Oguchi Y, Taniguchi N, Endoh H, et al. Enhancement of the antitumor effect by the concurrent use of a monoclonal antibody the protein-bound polysaccharide PSK in mice bearing a human cancer cell line. In Vivo. 1994;8:241–245. [PubMed] [Google Scholar]
- 48.Nomoto K, Yoshikumi C, Matsunaga K, Fujii T, Takeya K. Restoration of antibody-forming capacities by PS-K in tumor bearing mice. Gann. 1975;66:365–374. [PubMed] [Google Scholar]
- 49.Maruyama S, Akasaka T, Yamada K, Tachibana H. Protein-bound polysaccharide-K (PSK) directly enhanced IgM production in the human B cell line BALL-1. Biomed Pharmacother. 2009;63:409–412. doi: 10.1016/j.biopha.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Liu A, Klein G, Bandobashi K, Klein E, Nagy N. SH2D1A expression reflects activation of T and NK cells in cord blood lymphocytes infected with EBV and treated with the immunomodulator PSK. Immunol Lett. 2002;80:81–88. doi: 10.1016/S0165-2478(01)00312-1. [DOI] [PubMed] [Google Scholar]
- 51.Liu A, Arbiser JL, Holmgren A, Klein G, Klein E. PSK and Trx80 inhibit B-cell growth in EBV-infected cord blood mononuclear cells through T cells activated by the monocyte products IL-15 and IL-12. Blood. 2005;105:1606–1613. doi: 10.1182/blood-2004-06-2406. [DOI] [PubMed] [Google Scholar]
- 52.Liu A, Claesson HE, Mahshid Y, Klein G, Klein E. Leukotrien B4 activates T cells that inhibit B-cell proliferation in EBV-infected cord blood-derived mononuclear cell cultures. Blood. 2008;111:2693–2703. doi: 10.1182/blood-2007-08-102319. [DOI] [PubMed] [Google Scholar]
- 53.Klein E, Liu A, Claesson HE. Activation of innate immunity by the leukotriene B4 inhibits EBV induced B-cell transformation in cord-blood derived mononuclear cultures. Immunol Lett. 2008;116:174–177. doi: 10.1016/j.imlet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Akagi J, Baba H. PSK may suppress CD57+ T cells to improve survival of advanced gastric cancer patients. Int J Clin Oncol. 2010;15:145–152. doi: 10.1007/s10147-010-0033-1. [DOI] [PubMed] [Google Scholar]
- 55.Asai Y, Takaori K, Yamamoto T, Ogawa T. Protein-bound polysaccharide isolated from basidiomycetes inhibits endotoxin-induced activation by blocking lipopolysaccharide-binding protein and CD14 functions. FEMS Immunol Med Microbiol. 2005;43:91–98. doi: 10.1016/j.femsim.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Miki C, Tanaka K, Inoue Y, Araki T, Ohi M, Mohri Y, et al. Perioperative host–tumor inflammatory interactions: a potential trigger for disease recurrence following a curative resection for colorectal cancer. Surg Today. 2008;38:579–584. doi: 10.1007/s00595-007-3674-6. [DOI] [PubMed] [Google Scholar]
- 57.Tsutsumi N, Kohnoe S, Sonoda H, Guntani A, Rikimaru T, Taguchi K, et al. Protein-bound polysaccharide-K reduces colitic tumors and improves survival of inflammatory bowel disease in vivo. Oncol Lett. 2011;2:791–796. doi: 10.3892/ol.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanagawa T, Oguro M, Takagi T, Takenaga K. Direct antitumor activity of biological response modifiers (B.R.M.) proven by an in vitro sensitivity test. Jpn J Cancer Chemother. 1984;11:2155–62 (in Japanese with English abstract). [PubMed]
- 59.Yefenof E, Gafanovitch I, Oron E, Bar M, Klein E. Prophylactic intervention in radiation-leukemia-virus-induced murine lymphoma by the biological response modifier polysaccharide K. Cancer Immunol Immunother. 1995;41:389–396. doi: 10.1007/BF01526559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Araya S, Nio Y, Hayashi H, Masai Y, Tsubono M, Ishigami S, et al. Various plant-derived polysaccharides augment the expression of HLA on Colo 205 human colonic cancer line. J Jpn Soc Cancer Ther. 1994;29:1965–73 (in Japanese with English abstract).
- 61.Hattori ST, Komatsu N, Shichijo S, Itoh K. Protein-bound polysaccharide K induced apoptosis of the human Burkitt lymphoma cell line, Namalwa. Biomed Pharmacother. 2004;58:226–230. doi: 10.1016/j.biopha.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Jiménez-Medina E, Berruguilla E, Romero I, Algarra I, Collado A, Garrido F, et al. The immunomodulator PSK induces in vitro cytotoxic activity in tumor cell lines via arrest of cell cycle, induction of apoptosis. BMC Cancer. 2008;8:78. doi: 10.1186/1471-2407-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi H, Matsunaga K, Oguchi Y. Antimetastatic effects of PSK (Krestin), a protein-bound polysaccharide obtained from Basidiomycetes: an overview. Cancer Epidemiol Biomarkers Prev. 1995;4:275–281. [PubMed] [Google Scholar]
- 64.Zhang H, Morisaki T, Matsunaga H, Sato N, Uchiyam A, Hashizume K, et al. Protein-bound polysaccharide PSK inhibits tumor invasiveness by down-regulation of TGF-β1 and MMPs. Clin Exp Metastasis. 2000;18:343–352. doi: 10.1023/a:1010897432244. [DOI] [PubMed] [Google Scholar]
- 65.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 66.Hayashida T, Konagi A, Hasegawa H, IshiiY, Endo T, Okabayashi K, et al. Inhibitory ability of a protein-bound polysaccharide, PSK, on transforming growth factor beta pathway and EMT. In: Proceedings of the JCA. 2009; p. 47.
- 67.Kumar S, Saitoh K, Kumar P. Angiogenesis: key principles–science–technology–medicine. In: Steiner R, Weisz PB, Langer R, editors. Antiangiogenesis strategies in cancer therapy with special reference to Krestin. Basel: Birkhauser Verlag; 1992. pp. 463–470. [DOI] [PubMed] [Google Scholar]
- 68.Wada T, Wakamatsu Y, Bannai K, Kato M, Oguchi Y, Matsunaga K, et al. Suppression mechanism of angiogenesis by PSK. Ann Cancer Res Ther. 2002;10:93–106. [Google Scholar]
- 69.Konno H, Yamamoto M, Ohta M. Recent concepts of antiangiogenic therapy. Surg Today. 2010;40:494–500. doi: 10.1007/s00595-009-4150-2. [DOI] [PubMed] [Google Scholar]
- 70.Yoshikawa R, Yanagi H, Hashimoto-Tamaoki T, Morinaga T, Nakano Y, Noda M, et al. Gene expression in response to anti-tumour intervention by polysaccharide-K (PSK) in colorectal carcinoma cells. Oncology Rep. 2004;12:1287–1293. [PubMed] [Google Scholar]
- 71.Zhang H, Morisaki T, Nakahara C, Matsunaga H, Sato N, Nagumo F, et al. PSK-mediated NF-κB inhibition augments docetaxel-induced apoptosis in human pancreatic cancer cells NOR-P1. Oncogene. 2003;22:2088–2096. doi: 10.1038/sj.onc.1206310. [DOI] [PubMed] [Google Scholar]
- 72.Kinoshita J, Fushida S, Harada S, Makino I, Nakamura K, Oyama K, et al. PSK enhances the efficacy of docetaxel in human gastric cancer cells through inhibition of nuclear factor-kappaB activation and survivin expression. Int J Oncol. 2010;36:593–600. doi: 10.3892/ijo_00000534. [DOI] [PubMed] [Google Scholar]
- 73.Sakurai Y, Azuma I, Ishihara K, Ogura T, Saito T, Simoyama M, et al. Method for evaluation of efficacy of immunopotentiating agent on malignant tumor. Pharm Regul Sci. 1980;11:764–8 (in Japanese).
- 74.Nakao I, Yokoyama T, Urushizaki I, Wakui A, Furue H, Koyama Y, et al. Clinical outcome of PSK for advanced gastric cancer—results of randomized control trial. Oncologia. 1985;14:163–9 (in Japanese).
- 75.Maehara Y, Baba H, Sugimachi K. Adjuvant chemotherapy for gastric cancer: a comprehensive review. Gastric Cancer. 2001;4:175–184. doi: 10.1007/s10120-001-8008-6. [DOI] [PubMed] [Google Scholar]
- 76.Kondo M, Torisu M, Evaluation of an anticancer activity of a protein-bound polysaccharide PS-K (Krestin). In: Torisu M, Yoshida T, editors. Basic mechanisms and clinical treatment of tumor metastasis. Orlando: Academic Press; 1985. p. 623–36.
- 77.Maehara Y, Moriguchi S, Sakaguchi Y, Emi Y, Kohnoe S, Tsujitani S, et al. Adjuvant chemotherapy enhances long-term survival of patients with advanced gastric cancer following curative resection. J Surg Oncol. 1990;45:169–172. doi: 10.1002/jso.2930450307. [DOI] [PubMed] [Google Scholar]
- 78.Nakajima T, Inokuchi K, Hattori T, Inoue K, Taguchi T, Kondou T, et.al. Multi-institutional cooperative study of adjuvant immunochemotherapy for gastric cancer—five-year survival rate. Jpn J Cancer Chemother. 1989;16:799–806 (in Japanese with English abstract). [PubMed]
- 79.Niimoto M, Hattori T, Tamada R, Sugimachi K, Inokuchi K, Ogawa N. Postoperative adjuvant immunochemotherapy with mitomycin C, futraful and PSK for gastric cancer. An analysis of data on 579 patients followed for five years. Jpn J Surg. 1988;18:681–686. doi: 10.1007/BF02471530. [DOI] [PubMed] [Google Scholar]
- 80.Kondo T, Ichihashi H, Nakazato H, Ogawa N. Results of adjuvant immunochemotherapy on 8-year survival using KRESTIN® and Futraful® for gastric cancer patients who underwent radical gastrectomy—a randomized controlled trial by cooperative study group. Biotherapy. 1989;3:655–64 (in Japanese with English abstract).
- 81.Sakamoto J, Nakazato H, Koike A, Saji S. Pitfalls in the clinical evaluation of surgical adjuvant immunochemotherapy for the multidisciplinary treatment of gastrointestinal cancers. Biotherapy. 1993;7:1759–64 (in Japanese with English abstract).
- 82.Kondo T, Sakamoto J, Nakazato H. Alternating immunochemotherapy of advanced gastric carcinoma: a randomized comparison of carbazilquinone and PSK to carbazilquinone in patients with curative gastric resection. Biotherapy. 1991;3:287–295. doi: 10.1007/BF02221321. [DOI] [PubMed] [Google Scholar]
- 83.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Lancet. 1994;343:1122–1126. doi: 10.1016/S0140-6736(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 84.Ogawa M, Kako H, Kitano K, Matsukane H, Nagao K, Misumi A, et al. Cooperative study of adjuvant immunochemotherapy using HUFU (Mifurol®) for gastric cancer patients who underwent curative resection. Rinsyo To Kenkyu. 1994;71:201–6 (in Japanese).
- 85.Oba K, Teramukai S, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resections of gastric cancer. Cancer Immunol Immunother. 2007;56:905–911. doi: 10.1007/s00262-006-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugimachi K, Maehara Y, Ogawa M, Kakegawa T, Tomita M. Dose intensity of uracil and tegafur in postoperative chemotherapy for patients with poorly differentiated gastric cancer. Cancer Chemother Pharmacol. 1997;40:233–238. doi: 10.1007/s002800050652. [DOI] [PubMed] [Google Scholar]
- 87.Fujii M, Kochi M, Takayama T. Recent advances in chemotherapy for advanced gastric cancer in Japan. Surg Today. 2010;40:295–300. doi: 10.1007/s00595-009-4148-9. [DOI] [PubMed] [Google Scholar]
- 88.Torisu M, Hayashi Y, Ishimitsu T, Fujimura T, Iwasaki K, Katano M, et al. Significant prolongation of disease-free period gained by oral polysaccharide K(PSK) administration after curative surgical operation of colorectal cancer. Cancer Immunol Immunother. 1990;31:261–268. doi: 10.1007/BF01740932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takashima S, Kinami Y, Miyazaki I. Clinical effect of postoperative adjuvant immunochemotherapy with FT-207 suppository and PSK for colo-rectal cancer patients. Jpn J Cancer Chemother. 1988;15:2229–36 (in Japanese with English abstract). [PubMed]
- 90.Mitomi T, Tsuchiya S, Iijima N, Aso K, Nishiyama K, Amano T, et al. Randomized controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer—5 years follow-up after surgery (a final report). J Jpn Soc Cancer Ther. 1993;28:71–83 (in Japanese with English abstract).
- 91.Ito K, Nakazato H, Koike A, Takagi H, Saji S, Baba S, et al. Long-term effect of 5-fluorouracil enhanced by intermittent administration of polysaccharide K after curative resection of colon cancer. A randomized controlled trial for 7-year follow-up. Int J Colorectal Dis. 2004;19:157–164. doi: 10.1007/s00384-003-0532-x. [DOI] [PubMed] [Google Scholar]
- 92.Ohwada S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, Takahashi T, et al. Adjuvant immunochemotherapy with oral tegafur/uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. Br J Cancer. 2004;90:1003–1010. doi: 10.1038/sj.bjc.6601619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sakamoto J, Morita S, Oba K, Matsui T, Kobayashi M, Nakazato H, et al. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunol Immunother. 2006;55:404–411. doi: 10.1007/s00262-005-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Konno K, Motomiya M, Oizumi K, Sato M, Yamamoto F, Tamiya K, et al. Effects of protein-bound polysaccharide preparation (PSK) in small cell carcinoma of the lung. JJLC. 1988;28:19–28 (in Japanese with English abstract).
- 95.Ikeda T, Sakai T, SuitoT, Kosaki G. Evaluation of postoperative immunochemotherapy for lung cancer patients. Jpn J Cancer Chemother. 1986;13:1044–9 (in Japanese with English abstract). [PubMed]
- 96.Hayakawa K, Mitsuhashi N, Saito Y, Nakayama Y, Furuta M, Nakamoto S, et al. Effect of Krestin as adjuvant treatment following radical radiotherapy in non-small cell lung cancer patients. Cancer Detect Prev. 1997;21:71–77. [PubMed] [Google Scholar]
- 97.Go P, Chung CH. Adjuvant PSK immunotherapy in patients with carcinoma of the nasopharynx. J Int Med Res. 1989;17:141–149. doi: 10.1177/030006058901700205. [DOI] [PubMed] [Google Scholar]
- 98.Matsumoto K, Nakagami Y, Kishimoto T. The prevention of recurrence of superficical bladder tumours with immunochemotherapy. In: deKernion JB, editor. International society of urology reports. Immunotherapy of urological tumours. Edinburgh: Churchill Livingstone; 1990. p. 149–55.
- 99.Ogoshi K, Satou H, Isono K, Mitomi T, Endoh M, Sugita M. Immunotherapy for esophageal cancer. A randomized trial in combination with radiotherapy and radiochemotherapy. Am J Clin Oncol. 1995;18:216–222. doi: 10.1097/00000421-199506000-00007. [DOI] [PubMed] [Google Scholar]
- 100.Iino Y, Yokoe T, Maemura M, Horiguchi J, Takei H, Ohwada S, et al. Immunochemotherapies versus chemotherapy as adjuvant treatment after curative resection of operable breast cancer. Anticancer Res. 1995;15:2907–2911. [PubMed] [Google Scholar]
- 101.Pharmaceutical Affairs Bureau, Ministry of Health and Welfare (Japan). Ethical drug side effect information no. 48. 1982; p. 220–3 (in Japanese).
- 102.Kohara K, Arima S, Tamesue N, Shimura H. Toxicities in single chemotherapy with 5-fluorouracil dry syrup and combination chemotherapy with 5-fluorouracil dry syrup and PSK for cancer patients. Jpn J Cancer Chemother. 1979;6:379–85 (in Japanese with English abstract).
- 103.Sadahiro S, Suzuki T, Maeda Y, Tanaka A, Kamijo A, Murayama C, Nakayama Y, Akiba T, et al. Effects of preoperative immunochemoradiotherapy and chemoradiotherapy on immune responses in patients with rectal adenocarcinoma. Anticancer Res. 2010;30:993–999. [PubMed] [Google Scholar]
- 104.Yoshimura A, Asakawa M, Nakai H, Sakai H, Nishiwaki Y, Furuse K, et al. Combined effects of PSK with chemotherapy in the treatment of adenocarcinoma of the lung. Assessment of quality of life (QOL). Biotherapy 1992;6:256–7 (in Japanese with English abstract).
- 105.Motai H. PSK effectiveness for cancer pain relief: clinical study covering the last 11 years. Biotherapy. 1992;6:950–6 (in Japanese with English abstract).
- 106.Nomoto K, Matsuo K. Role of immunopotentiators in immunotherapy. Jpn J Cancer Chemother. 1978;5:1135–41 (in Japanese with English abstract).
- 107.Sakamoto J, Koike A, Saji S, Teramukai S, Ohashi Y, Nakazato H. Preoperative serum immunosuppressive acidic protein (IAP) test for the prognosis of gastric cancer: a statistical study of the threshold level and evaluation of the effect of the biological response modifier PSK. Surg Today. 1992;22:530–536. doi: 10.1007/BF00308899. [DOI] [PubMed] [Google Scholar]
- 108.Toge T, Yamaguchi Y. Protein-bound polysaccharide increases survival in resected gastric cancer cases stratified with a preoperative granulocyte and lymphocyte count. Oncol Rep. 2000;75:1157–1161. doi: 10.3892/or.7.5.1157. [DOI] [PubMed] [Google Scholar]
- 109.Tsujitani S, Kakeji Y, Orita H, Watanabe A, Kohnoe S, Baba H, et al. Postoperative adjuvant immunochemotherapy and infiltration of dendritic cells for patients with advanced gastric cancer. Anticancer Res. 1992;12:645–648. [PubMed] [Google Scholar]
- 110.Ogoshi K, Tajima T, Mitomi T, Makuuchi, Tsuji K. HLA-A2 antigen status predicts metastasis and response to immunotherapy in gastric cancer. Cancer Immunol Immunother. 1997;45:53–9. [DOI] [PMC free article] [PubMed]
- 111.Yoshikawa R, Yanagi H, Shen CH, Fujiwara Y, Noda M, Yagyu T, et al. ECA39 is a novel distant metastasis-related biomarker in colorectal cancer. World J Gastroenterol. 2006;12:5884–5889. doi: 10.3748/wjg.v12.i36.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamashita K, Ougolkov AV, Nakazato H, Ito K, Ohashi Y, Kitakata H, et al. Adjuvant immunochemotherapy with protein-bound polysaccharide K for colon cancer in relation to oncogenic β-catenin activation. Dis Colon Rectum. 2007;50:1169–1181. doi: 10.1007/s10350-006-0842-5. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi Y, Mai M, Nakazato H. Preoperative CEA and PPD values as prognostic factors for immunochemotherapy using PSK and 5-FU. Anticancer Res. 2005;25:1377–1384. [PubMed] [Google Scholar]
- 114.Ohwada S, Ogawa T, Makita F, Tanahashi Y, Ohya T, Tomizawa N, et al. Beneficial effects of protein-bound polysaccharide K plus tegafur/uracil in patients with stage II or III colorectal cancer: analysis of immunological parameters. Oncol Rep. 2006;15:861–868. [PubMed] [Google Scholar]
- 115.Sugimachi K, Maehara Y, Kusumoto T, Okamura T, Korenaga D, Kohnoe S, et al. In vitro reactivity to a protein-bound polysaccharide PSK of peripheral blood lymphocytes from patients with gastrointestinal cancer. Anticancer Res. 1995;15:2175–2180. [PubMed] [Google Scholar]
- 116.Yoshinaga K, Saeki H, Endo K, Morita M, Emi Y, Kakeji Y, et al. Biomarker research of Krestin (PSK) therapy in gastric and colorectal cancer patients. J Jpn Soc Clin Oncol. 2008;43:900. [Google Scholar]
- 117.Sugiyama M, Yoshino I, Kakeji Y, Maehara Y. Gene expression profile in human peripheral blood mononuclear cells upon PSK stimulation. In: Proceedings of the JCA. 2008; p. 452.


