Abstract
During the National Neurotrauma Symposium 2010, the DG Research of the European Commission and the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) organized a workshop on comparative effectiveness research (CER) in traumatic brain injury (TBI). This workshop reviewed existing approaches to improve outcomes of TBI patients. It had two main outcomes: First, it initiated a process of re-orientation of clinical research in TBI. Second, it provided ideas for a potential collaboration between the European Commission and the NIH/NINDS to stimulate research in TBI. Advances in provision of care for TBI patients have resulted from observational studies, guideline development, and meta-analyses of individual patient data. In contrast, randomized controlled trials have not led to any identifiable major advances. Rigorous protocols and tightly selected populations constrain generalizability. The workshop addressed additional research approaches, summarized the greatest unmet needs, and highlighted priorities for future research. The collection of high-quality clinical databases, associated with systems biology and CER, offers substantial opportunities. Systems biology aims to identify multiple factors contributing to a disease and addresses complex interactions. Effectiveness research aims to measure benefits and risks of systems of care and interventions in ordinary settings and broader populations. These approaches have great potential for TBI research. Although not new, they still need to be introduced to and accepted by TBI researchers as instruments for clinical research. As with therapeutic targets in individual patient management, so it is with research tools: one size does not fit all.
Key words: comparative effectiveness research, clinical research, clinical trials, methodology, systems biology, traumatic brain injury
Introduction
Epidemiologic considerations
The worldwide importance of traumatic brain injury (TBI) requires an effective, widely applicable response. The annual incidence of TBI is estimated at up to 500/100,000 in the U.S. and Europe, and results in over 200 per 100,000 individuals being admitted to hospitals each year in Europe (Langlois et al., 2006; Maas et al., 2008; Styrke et al., 2007; Tagliaferri et al., 2006). Epidemiological surveillance suggests that the nature of TBI is changing over time. Globally the incidence is increasing, due mainly to greater use of motor vehicles in low- and middle-income countries (Maas et al., 2008). Vulnerable road users (pedestrians, cyclists, and motorcyclists) are at high risk. The World Health Organization (WHO) predicts that deaths from road traffic incidents (primarily due to TBI) will double between 2000 and 2020, and that this increase will come exclusively in low- and middle-income countries (WHO/OMS, 2009).
In developed, westernized societies the occurrence of TBI is mainly increasing in people aged over 60 years (https://webgate.ec.europa.eu/idb/documents/2009-IDB-Report_screen.pdf; http://www.cdc.gov/traumaticbraininjury/tbi_ed.html). In the past, a TBI in this age group was thought to lead to a uniformly bad outcome; however, current experience is that a favorable outcome is possible and not uncommon. Nevertheless, the classic age range for inclusion in many clinical trials excludes such people and makes trial data less relevant to their treatment (Dhruva and Redberg, 2008). Another development is that the increasing burden of TBI due to military conflicts, and the exposure of civilians in combat zones has modified the epidemiology and clinical pattern of TBI (Risdall and Menon, 2011). Worldwide, TBI has devastating effects on patients and their relatives and results in high socioeconomic costs to society. This necessitates a widespread international effort to combat TBI and improve outcomes.
Discordance between experimental and clinical success: The complexity of clinical research in TBI
Research in basic science has disclosed that multiple mechanisms are involved in the pathophysiology of TBI (Marklund et al., 2006). This has led to the development of many neuroprotective agents with promising potential, but none have yielded benefits in clinical testing. The gap between bench and bedside raises the question of why benefits from seemingly effective interventions have not been seen in large randomized clinical trials. One explanation for this discrepancy may be that the clinical situation is far more complex and unpredictable than that seen with experimental models of TBI. Experimentally, the type and degree of injury can be standardized, while in the clinical situation wide variability exists, both in type (e.g., diffuse and focal injuries) and in severity of injury. In the clinical situation, pretreatment is impossible, and intervention within short therapeutic windows can be challenging. Adverse effects of systemic insults, such as hypoxia and hypotension, on outcome might easily overwhelm any benefit from a new therapy. The identification of relevant covariates and the development of robust prognostic models by the CRASH (MRC CRASH Trial Collaborators, 2008) and IMPACT (Steyerberg et al., 2008) collaborations are very useful for dealing with the heterogeneity of the TBI population. However, adjustments for baseline prognostic risk ignore the contribution of aspects of care, such as the quality of intensive care and rehabilitation, on outcome. For example, data from the NABISH-I study (Clifton et al., 2001) showed that inter-center variations in clinical care could produce substantial noise in results of clinical trials. Recent findings from the IMPACT studies have shown that the risk of poor outcomes could differ between centers, and is up to three times higher than would be expected by chance after adjustment for baseline prognostic risk (Lingsma et al., 2011). How much of the observed variability reflects variability in clinical management is unknown.
The impact that variability of care may have on clinical outcomes requires rigorous examination, because identification of underlying causes could result in real improvements in outcomes. Identification of best clinical practices and efforts to minimize recognized variances in care (Clifton et al., 2001), through, for example, “Six Sigma” processes (Schweikhart and Dembe, 2009), may yield benefits. These may exceed those provided by research addressing a single aspect in the continuum of care. It is therefore important to undertake more implementation-based research to ensure that current and future effective therapies are translated into improving clinical outcomes in everyday clinical practice. To quote Goethe, “Knowing is not enough; we must apply. Willing is not enough; we must do.”
These considerations provided the context for the DG Research of the European Commission and NIH/NINDS to organize a workshop during the National Neurotrauma Symposium 2010 on comparative effectiveness research (CER).
The aims of this workshop on CER were to: (1) perform a critical re-appraisal of approaches to clinical research in TBI; (2) identify the greatest unmet needs from a clinical perspective; (3) discuss the potential of CER in the field of TBI; and (4) explore the possibility and the added value of a joint EU-U.S. effort in this field. This article summarizes the results of this workshop.
Approaches to clinical research in TBI: What has (and has not) made a difference?
Clinical trials
Traditionally, the efficacy of new treatments has been investigated in randomized controlled trials (RCTs). In TBI, these trials have been conducted on a range of neuroprotective agents and surgical and medical approaches (Maas et al., 2010a). Whereas various single-center studies have reported benefits of a range of interventions (for example hyperbaric oxygen, mannitol, hypothermia, and decompressive craniectomy), none of these findings were generalizable in multicenter RCTs. Furthermore, substantial selection bias may have existed in reporting benefits in single-center studies (Maas et al., 2010a). For example, of the 20 multicenter studies on neuroprotective agents, only one reported a significant treatment benefit: the HIT III study researchers (Harders et al., 1996) found a beneficial effect of the calcium channel modulator nimodipine, but targeted only patients with traumatic subarachnoid hemorrhage. The generalizability of this relatively small study (n=123) is therefore questionable, and taken in combination with negative results from three other studies on nimodipine, these findings have not changed clinical practice.
At the other extreme, a mega-trial of steroids in TBI was halted early because of increased mortality at 14 days in patients treated with corticosteroids (Roberts et al., 2004), but did not show a significant effect on quality of outcome at 6 months (Edwards et al., 2005).
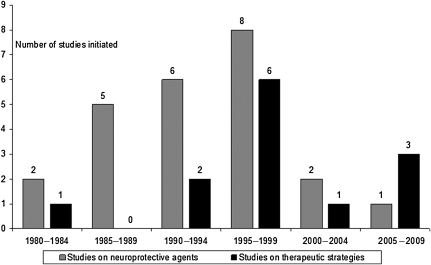
It is not surprising that the past decade has seen a sharp decline in the number of clinical trials initiated in TBI, particularly in those on neuroprotective agents (Fig. 1). This may be due to disappointment in the results of previous studies, along with the perception that TBI is a high-risk and costly indication. Increasing overhead costs required by academic research institutions in the EU and U.S. contribute significantly to the high cost of clinical trials, in some cases amounting to or even exceeding 100% overhead, which doubles the amount of funding required to conduct the trial. The result is that academic institutions are pricing themselves out of the market, and a clear shift in research has been seen towards the Far East and Latin America, where the potential for patient recruitment is higher and study costs are lower (Maas et al., 2010a).
FIG. 1.
Number of clinical trials initiated over the past 30 years, differentiated by studies of neuroprotective agents versus therapeutic strategies. Adapted from Maas et al., 2010a.
However, there is uncertainty whether findings obtained in these settings may be extrapolated to higher-income countries, and about the importance of components of care systems. As the search for effective therapies continues, there is a need for comparative studies in which differences, for example in access to acute and post-acute care, in trauma organization, and in treatment, may be explored and the consequences for generalizability estimated.
Guideline development
Some consistency in approach to care has been achieved by management guidelines (www.tbiguidelines.org; www.nice.org.uk; Maas et al., 1997). Most of these guidelines are evidence-based, and follow a rigorous and systematic analysis of the available literature. Despite isolated reports that suggest otherwise (Cremer et al., 2005), most reports show that adoption of such guidelines has resulted in improvements in TBI mortality and functional outcome (Bulger et al., 2002; Clayton et al., 2004; Elf et al., 2002; Fakhry et al., 2004; Patel et al., 2002; Stein et al., 2010; Suarez et al., 2004; Varelas et al., 2006). Nevertheless, the level of evidence underpinning the guidelines is, on average, only low. Of the 229 recommendations contained in seven guideline publications, only four are based on class I evidence (Table 1).
Table 1.
Evidence-Based Guidelines for Management of Traumatic Brain Injury (TBI): Strength of Recommendations
| |
|
|
Recommendations (n) |
||
|---|---|---|---|---|---|
| Guideline | Reference | Topics (n) | Class I | Class II | Class III |
| Prehospital management | Brain Trauma Foundation, 2000 | 7 | 0 | 5 | 12 |
| Penetrating brain injury | Aarabi et al., 2001 | 7 | 0 | 0 | 12 |
| Pediatric guidelines | Adelson et al., 2003 | 17 | 0 | 6 | 40 |
| U.K. guidelines for triage, assessment, investigation, and management of TBI | U.K. National Institute for Health and Clinical Excellence, 2003 | 27 | 3 | 16 | 107 |
| Field management of combat-related head trauma | Brain Trauma Foundation, 2005 | 5 | 0 | 3 | 15 |
| Surgical management of TBI | Bullock et al., 2006 | 5 | 0 | 0 | 26 |
| Revised guidelines for management of severe TBI | Brain Trauma Foundation, 2007 | 15 | 1 | 14 | 17 |
| Total | 83 | 4 | 44 | 229 | |
| Classification of evidence on therapeutic effectivenessa | |||||
| Class I lack sufficient patient numbers, or suffer from other methodological inadequacies that render them class II or III. | |||||
| Class II that were based on reliable data. Comparison of two or more groups must be clearly distinguished. Types of studies include observational, cohort, prevalence, and case-control. Class II evidence may also be derived from flawed RCTs. | |||||
| Class III Types of studies include case series, databases or registries, case reports, and expert opinions. Class III evidence may also be derived from flawed RCTs, cohort, or case-control studies. |
From Guidelines for the Management of Severe Traumatic Brain Injury, 3rd ed., Brain Trauma Foundation, www.tbiguidelines.org.
In the NICE guidelines (http://www.nice.org/), the grading scheme for level of recommendations was adapted from the Oxford Centre for Evidence Based Medicine levels of evidence as level A–D; for consistency, we considered grade A class I, grade B as class II, and grades C and D as class III.
The limited number of recommendations that are supported by high-quality evidence is not unique to TBI, but has also been noted in other fields of medicine. A recent review of practice guidelines developed by the American College of Cardiology and the American Heart Association found that relatively few recommendations were based on high-quality evidence from overviews of RCTs, and many were based solely on expert opinion, individual case studies, or standard of care (Tricoci et al., 2009).
The paucity of high-quality evidence reflects the many uncertainties about the benefits and risks of multiple treatment approaches, and the limited funding available for TBI clinical research. We will never be able to mount adequately powered trials to address all of these uncertainties. Furthermore, traditional clinical trials do not address the effect of different clinical practices available in the real world, and have limited external validity since the effect of treatment is evaluated in selected populations, with management protocols that are sometimes difficult to replicate in the wider clinical care spectrum. The discrepancy between clinical trial data and real world practice, and the need for other approaches to gathering evidence as a basis for clinical management, was highlighted 15 years ago (Black, 1996).
Observational studies
Although relatively small in comparison to the number of clinical trials conducted in TBI, observational studies have had a substantial impact upon clinical management.
The Glasgow group initiated prospective data collection on severe head injury in 1968, and expanded this to an international level in 1972, including centers from the U.S. and the Netherlands (Jennett et al., 1976, 1977, 1980). These studies were facilitated by the introduction of the Glasgow Coma Scale (GCS) and the Glasgow Outcome Scale (GOS), both of which are still universally accepted for classification of initial injury severity and outcome. Treatment approaches were compared, and extensive prognostic studies revealed that age, GCS score, and pupillary reactivity were the main predictors of outcome in severe TBI, and prognostic rules were developed.
The Traumatic Coma Data Bank (TCDB; 1984–1987) prospectively collected data from four U.S. centers (Foulkes et al., 1991). This study highlighted the importance of systemic hypoxia and hypotension as determinants of outcome (Chesnut et al., 1993).
More recent reports suggest that physiological insults continue to be an issue, both in the emergency room and in the critical care unit context (Hlatky et al., 2004; Manley et al., 2001). Numerous studies in animal models have confirmed the significance of these clinical observations, while adding to our understanding of the pathological mechanisms involved (DeWitt et al., 1995; Ishige et al., 1988; Statler et al., 2001). A major advance employed in the TCDB was the Marshall CT classification as a descriptive measure for the type of brain damage and the risk of increased intracranial pressure (ICP). Further studies have confirmed its importance as a prognostic parameter (Maas et al., 2007b).
The core data survey conducted by the European Brain Injury Consortium (EBIC) concerned a 3-month data collection period in moderate and severe TBI across 67 centers (Murray et al., 1999). This study resulted in four publications and demonstrated the evolution of CT lesions over time (Servadei et al., 2000), as well as the importance of traumatic subarachnoid hemorrhage (Servadei et al., 2002). Wide variations in intensive care management were reported across participating centers, without evidence of an association with improved outcomes (Stocchetti et al., 2001).
In an analysis of patients with head injury from the registry of the Trauma Audit and Research Network (TARN) in the U.K., Patel and associates (2005) found that improvements over time in outcomes of patients with head injuries were less than those observed in other trauma patients. Patients with severe head injuries treated in a hospital without a neurosurgeon had a 2.15-fold increase in the odds of death after adjustment for case mix, compared to those treated in a unit with neurosurgical facilities.
Meta-analysis of individual patient data
The IMPACT studies (Maas et al., 2007a) brought together individual patient data from eight RCTs and three observational studies (n=9205; Marmarou et al., 2007). Relevant parameters from the individual studies were merged into a large dataset, forming a “culture medium” for exploring concepts for improving the design of clinical trials in TBI (Maas et al., 2007a). The IMPACT studies have yielded important contributions to advance the field of clinical research in TBI. Extensive prognostic analysis has defined and quantified more precisely the predictive value of many known predictors, has yielded new predictors, and has resulted in the presentation of validated prognostic models for use in moderate and severe TBI (Steyerberg et al., 2008). These models have much wider applications than only in the context of trial design (Lingsma et al., 2010). Simulation studies demonstrated that broad inclusion criteria, pre-specified covariate adjustment, and an ordinal analysis all promote an efficient trial, yielding gains in statistical efficiency of over 40%. This means that smaller but clinically relevant treatment effects can now be detected without increasing trial size or duration, and these findings have the potential to revolutionize the design and analysis of clinical trials in TBI (Maas et al., 2010b).
In summary, major advances in understanding and improving TBI care have come not from clinical trials, but from observational studies, guideline development, and meta-analysis of individual patient data. Rigorously conducted observational studies in large and diverse populations have the potential to identify better clinical practices and to reshape care for patients with TBI in the future.
A critical re-appraisal of the experience within and across clinical trials in TBI illustrates the inadequacies of approaches to study design and analysis, clinical assessments, classification, and management.
Bridging the gap between bench and beside
The possible causes for translational failures in clinical neuroprotection in TBI have been addressed in many previous publications (Green, 2002; Ikonomidou and Turski, 2002; Maas et al., 1999; Narayan et al., 2002; Tolias and Bullock, 2004). One of the aspects highlighted during the panel discussions of the workshop was the uncertain relevance of results obtained from experimental studies in rodents to human subjects, reflecting differences in brain development, brain anatomy, and physiology. While studies in lissencephalic species may continue to be useful in elucidating and antagonizing disease mechanisms, a strong plea was heard to implement more intermediate studies in larger gyrencephalic mammals, such as pigs and sheep, before clinical translation is attempted. An alternative or adjunctive approach to such large animal studies may be more preparatory clinical research, including the use of strategies to optimize candidate therapies before initiation of formal Phase II trials in patients (Janowitz and Menon, 2010). In TBI, perhaps uniquely among acute CNS diseases, conventional protocols for clinical care provide unparalleled access to key biological compartments through techniques such as microdialysis, ventriculostomy, and jugular bulb catheterization. This access, and the common use of serial imaging (universally with computed tomography [CT], but increasingly with magnetic resonance imaging [MRI], and sometimes with positron emission tomography [PET]), provides opportunities to examine disease mechanisms, drug pharmacokinetics, and proof of principle of therapy in small, well-designed studies. We suggest that those monitoring and investigation tools that are commonly used in patients with TBI may provide a route to facilitate a more rational approach to translational research. This suggestion is underpinned by existing research data on disease biology, drug delivery, and treatment response obtained with these methods (Janowitz and Menon, 2010).
Genetic make-up and response to injury
A major gap in knowledge concerns different responses to similar injuries. Such patient-specific differences could in part be genetically determined, and much research will be needed in the areas of genomics and metabolomics to elucidate the wide variability in the response to brain injury. The relevance of genetics may be seen by the observation that recovery is poorer in patients with stroke or TBI who have the APOE ɛ4 allele than in those who do not have this allele (Alexander et al., 2007). Other genes for which evidence exists for associations with outcomes are the TP53, COMT, DRD2, CACNA1A, and BCL2 genotypes (Jordan, 2007; Zangrilli Hoh et al., 2010).
Premorbid factors and comorbidity
The importance of premorbid factors and comorbidity has often been neglected in TBI, on the assumption that primarily young and otherwise healthy subjects are afflicted. Awareness of the increase of TBI in older people and in those with prior problems has changed this. A recent analysis revealed that one in four U.S. citizens now lives with at least two chronic comorbidities (Parekh and Barton, 2010). These comorbidities imply impaired physiological reserve and a range of pre-existing medications (including anticoagulant therapy and platelet aggregation inhibitors). These factors will influence both disease course (e.g., via more hemorrhagic expansion), and the pharmacokinetic interactions and pharmacodynamic safety of new therapies in TBI.
Classification
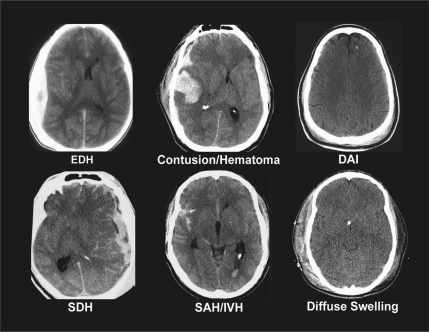
General approaches to classification of TBI are by cause (mechanistic) and severity. In clinical practice the conventional categorization of the severity of brain injury is primarily based on the GCS score. While the GCS represented a significant advance in the characterization of TBI, more recent studies suggest that its accuracy and prognostic power in more severely injured patients may be reduced due to effects of early sedation, neuromuscular blockade, and ventilation (Czosnyka et al., 2005; Stocchetti et al., 2004). Further, it should be realized that the commonly employed differentiation into mild (GCS score 13–15), moderate (GCS score 9–12), and severe (GCS score 8 or less) injury is artificial, and that clinical severity lies on a continuum. Pathoanatomical and pathophysiological processes may occur across the spectrum of clinical severity, but may vary in frequency and severity. Moreover, patients with similar clinical severity as assessed by the GCS may have widely differing types of injury (Fig. 2). Additional major issues are the physiological vulnerability of patients with TBI, particularly of those with moderate or severe injury and extracranial injuries. Thus the classification of TBI is multidimensional and complex. A recent authoritative workshop has made a strong case for a new and more comprehensive categorization of TBI in clinical trials (Saatman et al., 2008). The workshop also recommended the development of a common data set of TBI data elements, and the establishment of a large prospective patient database across the spectrum of injury severity. Common data elements have been proposed (Maas et al., 2011), but a large prospective data set is still required.
FIG. 2.
Types of brain injury may differ greatly in patients with similar initial clinical severity as assessed by the Glasgow Coma Scale. Adapted from Saatman et al., 2008 (EDH, extradural hematoma; DAI, diffuse axonal injury; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage).
Mechanistic targeting
Different pathophysiological processes may occur simultaneously or sequentially, and to varying degrees. The concept of mechanistic targeting—the ideal for clinical trials—will require reliable identification of occurrence and time course of pathophysiological mechanisms in individual patients. There is the emerging hope that multimodality monitoring may allow us to differentiate between pathophysiologies that appear superficially similar, but require different treatment. For example, the combination of conventional monitoring with brain tissue oximetry and microdialysis may allow us to differentiate between classical ischemia (which may respond to cerebral perfusion pressure [CPP] elevation), and diffusion hypoxia (Menon et al., 2004), or mitochondrial dysfunction (Vespa et al., 2005), which may respond to normobaric hyperoxia (Nortje et al., 2008). The emerging fields of advanced MRI imaging and of proteomic biomarkers offer opportunities for detection and tracking of pathophysiological processes.
Individualizing clinical management
Protocol-driven approaches are currently the standard in the treatment of TBI. These approaches are often poorly focused, utilizing a stepwise approach, with an escalating intensity of therapy, regardless of the underlying pathophysiology. They adhere to the concept of a “one pill for everybody” approach. The introduction of novel monitoring technology, and advances in neuroimaging techniques, now offer opportunities for advancing care from a one-size-fits-all approach, to a more focused approach, targeted to the needs of an individual patient. The concept of individualized management is closely related to the possibilities for mechanistic targeting. There are clear benefits to be gained from the employment of personalized approaches. For example, the curve of the relationship between CPP and outcome shows an inverted “U” shape, suggesting that there is an optimum level of CPP, and that higher or lower levels are disadvantageous (Balestreri et al., 2006). Indeed, the use of management schemes that incorporate vigorous CPP augmentation can result in significant cardiorespiratory complications (Robertson et al., 1999). Further, there is accumulating evidence that the optimum level of CPP varies between patients, and the optimum level in a given patient may be identifiable using autoregulatory indices (Howells et al., 2005; Steiner et al., 2002), or brain oximetry (Spiotta et al., 2010).
Early end-points and mechanistic targets
Major advances in the fields of cardiovascular medicine, oncology, and AIDS research have resulted from the use of early (mechanistic) end-points, for example troponin in myocardial infarction and CD4 counts in HIV. In TBI, however, early end-points that reliably predict quality of recovery are not yet available. In the past, ICP has been used, either explicitly or implicitly, as a surrogate end-point, especially in early-stage trials in clinical TBI. While this approach has some merit, it is important to recognize two important confounders.
First, modern neuro-ICU practices have substantially blunted our ability to use ICP as a surrogate marker in this way. It is possible to control ICP by intensifying ICP/CPP therapies until the system terminally decompensates and intracranial hypertension becomes refractory to therapy. In this context, the intensity of ICP/CPP-targeted therapy may be more relevant than the actual values of ICP. Second, the use of ICP as a surrogate outcome marker neglects the side effects of therapies (Coles et al., 2002, 2007). The importance of complications following, for example decompressive craniectomy, was recently demonstrated in the DECRA trial (Cooper et al. 2011).
A more rational option would be to use definitive markers of tissue fate in the brain. Biomarkers may have great potential in TBI (Table 2). Advances in this emerging field offer hope for the identification of biochemical and other markers that are clinically relevant for quantifying and tracking disease processes. Further, if we could use patients as their own controls in determining if an intervention altered the trajectory of neuronal loss, we could at least determine whether a neuroprotective intervention was initially effective, in that it reduced incremental tissue injury. Serial MRI with diffusion tensor imaging (DTI; Niogi et al., 2008), and MR spectroscopy (Ashwal et al., 2006; Govind et al., 2010), may provide clinically viable methods of assessing such incremental neuronal losses, but this approach needs to be tested in large prospective studies that map the temporal trajectories of lesion evolution with conventional treatment.
Table 2.
Potential Uses for Biomarkers in Traumatic Brain Injury (TBI)
| • Establishing a diagnosis of TBI (relevant to mild cases) |
| • Assessing the severity and nature of TBI |
| • Monitoring the evolution of injury and recovery in individual patients or groups of patients |
| • Defining treatments needed |
| • Monitoring of treatment effects |
| • Mechanistic target for clinical trials |
| • Prediction of outcome |
Post-acute assessments and care
Consistent standardized methods are largely lacking to track service utilization and changes in functional status following acute care discharge. Where they do exist, general use is often inhibited by the copyright protection imposed by developers. In comparison to acute care studies, post-acute studies are often small in size and research is fragmented. In particular, there is little continuity in research between acute and post-acute care studies. Nevertheless, disparities in access to post-acute care may influence the recovery process and confound interpretation of outcomes. A major challenge in the post-acute care phase is posed by the highly variable time periods at which data are recorded, confounding comparability of studies and interpretation of their results. Thus a great need exists for more prospective longitudinal studies bridging the gap between acute and post-acute research in TBI.
Outcome
The GOS is currently accepted by investigators and regulatory authorities alike as the standard end-point for judging efficacy in a cohort of patients with TBI. Outcome after TBI, however, is by definition multidimensional, including neurophysical disabilities, disturbances in mental functioning (e.g., cognitive and executive functioning), and consequent problems in social reintegration. Moreover, even the Extended GOS is relatively insensitive in its upper ranges, and may therefore be less suited for patients with milder injuries. Recently, the Neurological Outcome Scale for TBI (NOS-TBI) has been proposed as an effective and simple tool to quantify neurological deficits after TBI (Wilde et al., 2010). This approach already takes some multidimensional aspects of outcome assessment into consideration.
Relatively few TBI studies have utilized Health-Related Quality of Life (HRQoL) measures. There is a need for comprehensive disease-specific instruments for HRQoL assessment in persons after TBI, such as the novel disease-specific scale Quality of Life in Brain Injury (QOLIBRI; Truelle et al., 2010; von Steinbuechel et al., 2005, 2010a, 2010b). There is a need to develop a multidimensional approach to outcome assessment and classification, including the patient's quality of life.
Psychological health and TBI
The workshop on “An Integrated Approach to Research in Psychological Health and TBI,” organized by NIH/NINDS, the Department of Defense, the Department of Veteran Affairs, and the National Institute on Disability and Rehabilitation Research in October 2008, revealed the previously unrecognized relationship between these two seemingly unrelated disciplines (Thurmond et al., 2010). First, substance abuse is common in victims of TBI and may often be a contributing factor in injury mechanism. Second, depression and anxiety disorders are relatively frequent in survivors, and this may adversely affect social reintegration and confound outcome assessments. Mental health and chronic pain are considered key areas, both pre- and post-injury. Third, symptoms consistent with post-traumatic stress disorder (PTSD) are reported in up to 40% of military personnel returning from deployment following mild blast TBI (Hoge et al., 2008), although the study is controversial because it may have underestimated the percentage of patients with mild TBI who experienced PTSD (Correspondence NEJM, 2009). Questions persist concerning the best methods for the determination of the neurological and/or psychological aspects and etiologies of these interrelated disorders (Bryant, 2008). In one study using survey techniques for military personnel injured in Iraq and Afghanistan, researchers found that 12% of respondents reported a history consistent with mild TBI, and 11% screened positive for PTSD (Schneiderman et al., 2008). Advanced neuroimaging techniques such as MRI spectroscopy offer opportunities for the diagnosis of brain abnormalities in this population (Hoge et al., 2009). Civilian TBI studies have rarely included measures of PTSD, and consequently the incidence of this combined diagnosis is unknown in civilian populations. There is clearly a great need for further research in this area, including studies that allow us to differentiate more accurately between PTSD and the sequelae of TBI in all populations.
Pediatric considerations
Despite past and current prevention efforts, TBI continues to kill and disable more children and adolescents than any other cause (Forsyth et al., 2008; Langlois et al., 2005). There is a paucity of widely-applied, effective therapies, and results from adult studies may not be applicable to children. While conducting research in pediatric TBI, researchers need to carefully balance the need for better therapies and delivery of care with the consideration that children with TBI are a vulnerable population in whom to conduct research. There is only fragmented understanding of disease biology, and the frequency and geographical distribution of pediatric TBI create unique challenges for researchers (Natale et al., 2006).
Translation of knowledge and effective therapies from the bench to the bedside are of particular significance in pediatric TBI. While a body of research has provided important pre-clinical data, TBI models in immature animals have unique limitations, including differences in brain development between humans and the species used in the laboratory, injury mechanics, and evaluation of recovery (Prins and Hovda, 2003). Pre-clinical studies have and will continue to make contributions to the field, but more work needs to be done to improve our understanding of the biology of TBI in children. It is through this understanding that the suitability of a given mechanism as a target for therapy can best be defined. Ongoing efforts to understand the pathobiology of pediatric TBI will build on previous single-center studies with limited sample sizes, and the consequent need to use pooled samples (Kochanek et al., 2000). Such an approach has value, but results in fragmented descriptions of the time course of the pathological cascades under study, and limits external validity.
Variability in aspects of clinical care can affect the applicability of controlled studies to the general population. For example, children with severe TBI can be admitted to adult hospitals, adult trauma centers (with or without pediatric qualifications), pediatric hospitals, or pediatric trauma centers. Furthermore, it is estimated that 17,000,000 children in the United States do not have timely access to high-level pediatric trauma care (Carr and Nance, 2010). Transport decisions are influenced by geographic factors and emergency medical service practices. Variability of clinical care within and between pediatric centers adds to these challenges. Many of the challenges described in the adult TBI population are magnified when it comes to pediatric TBI. Specific considerations concern injury classification, the transition from acute to sub-acute care, and the selection of optimal outcome measures that include measurements of quality of life and evaluations of psychological health (Winthrop, 2010). As researchers address these challenges, the dynamic nature of human development needs to be taken into account when evaluating injury type, physiological values (such as CPP and cerebral blood flow [CBF]), and cell injury mechanisms and recovery (e.g., language, executive function, and measures of independence; Kapapa et al., 2010; Kochanek, 2006; Walker et al., 2009).
During the workshop, a review of the international networks available to conduct research in pediatric TBI revealed numerous opportunities and highlighted challenges. Identification and understanding of best clinical practices through CER may help optimize and homogenize care delivery, facilitating mechanistic targeting and the implementation of future effective therapies. Whether via RCTs, rigorously conducted observational studies, or other forms of CER, understanding pediatric TBI requires strong collaboration among multiple centers and across countries and continents.
Alternative Approaches and Priorities for Clinical Research in Traumatic Brain Injury
The issues summarized above highlight the complexity of the challenges facing clinical TBI research. Priorities for clinical research are summarized in Table 3. A strong collaborative international effort will be required to address these.
Table 3.
Summary of Unmet Needs and Priorities for Clinical Research
| • Bridge the gap between bench and bedside |
| - Promote interaction between basic scientists and clinical researchers in order to better scale experimental models to reflect human pathology both physically and pathologically |
| - Experimental work-up should include testing in larger animals |
| - Better optimization of candidate drug molecules in clinical disease through experimental medicine approaches |
| - Develop mechanistic end-points in human traumatic brain injury (TBI), such as biomarkers and advanced neuro-imaging |
| • Integrate acute and post-acute care research |
| • Develop a novel approach to the classification of TBI, also considering extracranial injuries |
| • Develop a multidimensional approach to outcome assessment, including the patient perspective (quality of life) |
| • Explore the influence of gender and genetic makeup on disease course and outcome |
| • Develop tools to better capture clinical variability |
| • Use information on clinical variability to develop and test strategies for individualized management |
| • Prediction research |
| - Outcome: update/validate prognostic models |
| - Prediction of treatment response |
| - Prediction of the expected trend using monitored parameters |
| - Prediction of the risk of hemorrhagic expansion |
| • Involve information technology personnel and other experts from unrelated fields in explorations of novel approaches to classification (pattern recognition), and prediction research (machine learning techniques) |
| • “Open source” research: data sharing and data standardization |
| • A particular focus on pediatric and elderly subpopulations |
| • Collaborate with psychological health and pain experts |
| • Ensure that improvements in therapy are applicable to settings where they are needed most (developing economies) |
| • Explore whether findings obtained in a particular setting (e.g., developing economies) may be extrapolated to other settings |
In the absence of possibilities for mechanistic targeting, the traditional approach to clinical trials in TBI has been to decrease heterogeneity in patient populations by employing restrictive enrollment criteria. The disadvantage of this approach is that it is statistically inefficient and decreases external validity. The question is if attempts to limit heterogeneity are appropriate, or alternatively, that the existing heterogeneity in patient populations, management approaches, and outcomes, may be used to advantage by exploring these differences and analyzing the underlying causes for a given outcome or individual patient response to a selected therapy or intervention. Classical clinical trials, with large numbers and substantial costs, may not be suitable vehicles for providing answers to all of the questions that we have. We need alternative approaches to address these questions. One direction to in which to proceed is the prospective collection of large, multi-scalar (demographics, physiology, proteomics, genomics, and outcome), longitudinal, high-quality clinical databases, associated with systems biology and CER methods.
A Systems Biology Approach
Traditional clinical trials and studies have relied upon a hypothesis-driven, model-based approach. While this reductionistic approach has been very successful in developing treatments for infectious diseases and cancer, where single organisms or cell types are responsible for the pathology, there has been only limited success using this approach for more heterogeneous complex diseases, such as inflammatory disease, diabetes, and cardiovascular disease. In these complex disorders there is likely no single factor that is responsible for the disease. This is particularly true for disorders of the central nervous system, such as traumatic brain injury, for which there is significant heterogeneity in the etiology, pathology, mechanisms, and outcome.
In contrast to the reductionistic, hypothesis-driven approach that seeks to target a single variable responsible for the disease, a systems biology approach aims to identify multiple factors that contribute to the disease. Systems biology also addresses the complex interactions of these multiple variables in a multivariate, multidimensional manner, over time. Systems biology is a rapidly growing interdisciplinary field that combines biology, mathematics, statistics, and computer science, to better understand complex biological processes.
In this regard systems biology may be better considered an “informational science” approach, in which hypotheses are data driven and seek to describe the behavior of the entire system. In lay terms, it is the description of the forest instead of the trees. There are now numerous examples of the application of the systems approach to complex biological problems, particularly in microorganisms (Bischofs et al., 2009; Spiro et al., 1997), and more recently complex human disease (Chen et al., 2009; Sears et al., 2009). These studies demonstrate how a systematic, integrative analysis that includes genes, proteins, and behavior over time, can solve complex problems that are insufficiently addressed by the conventional, reductionistic approach.
Comparative effectiveness research
RCTs—generally considered to be the gold standard—address efficacy rather than effectiveness. Efficacy reflects the degree to which an intervention produces the expected result under carefully controlled conditions chosen to maximize the likelihood of observing an effect if it exists. The study population and setting of efficacy studies may differ in important ways from those settings in which the interventions are likely to be used. By contrast, CER intends to measure differences in outcome and to relate these to the package of care and its constituent components in ordinary settings and broader populations. It can therefore be more relevant to policy evaluation and the health care decisions of providers and patients (Table 4; IOM, 2009).
Table 4.
The Different Intents of Randomized Controlled Trials (RCTs) and Comparative Effectiveness Studies (CERs)
| Efficacy | Can it work? | RCT |
| Effectiveness | Does it work? | CER |
Adapted from Drummond et al., 2008.
Many different official definitions of CER exist. Common characteristics are presented in Table 5.
Table 5.
Common Characteristics of Comparative Effectiveness Research
| • The objective of directly informing a specific clinical decision from the patient perspective, or a health policy decision from the population perspective |
| • Comparison of at least two alternative interventions, each with the potential to be a best practice |
| • Description of results at the population and subgroup levels |
| • The use of outcomes—both benefits and harms—that are important to patients |
| • Employment of methods and data sources appropriate for the decision of interest |
| • Interventions conducted in settings that are similar to those in which the intervention will be used in practice |
The call for CER does not mean that all research must have these characteristics. Early studies of an intervention should also compare it to a placebo, standard care, or no intervention. During early development of a new intervention, it is critical to determine safety and efficacy under a defined set of circumstances (IOM, 2009).
Examples of comparative effectiveness research in TBI
The concept of CER in TBI is not new. In 1983 Gelpke and associates analyzed differences in outcome between two centers from the Netherlands (Rotterdam and Groningen) participating in the International Data Bank of severe head injury. The 1-year survival rate in Rotterdam was 45% versus 63% in Groningen. The research question was whether the difference in survival rate was due to differences in the initial severity of the injury or to a difference in management efficacy. Of the 18% difference in survival rate, 10.5% was due to differences in severity of injury on admission. The remaining 7.5% difference in survival rate was not explained, but may have been caused by unmeasured variations in the initial determination of severity of injury or by differences in management. Groningen had a more conservative management approach than Rotterdam. For example, artificial ventilation was used in 43% of the Rotterdam cases and in 14% of the Groningen cases. The authors concluded that the results of their study did not support the concept that an aggressive management regimen would improve outcome. However, the efficacy with which aggressive management was implemented and the impact on target pathophysiological variables (such as cerebral perfusion pressure and carbon dioxide control) was not analyzed.
This study can be considered a CER study, although performed long before this type of research was recognized as a separate and important entity or even had a name. It used observational data from a setting that represents clinical practice. It measured a patient-relevant outcome, and the study aimed to inform medical decision making.
Similarly, the comparative analysis of treatment results between Charlottesville (U.S.) and New Delhi (India) reported by Colohan and colleagues in 1989 qualifies as CER. An almost 8% higher mortality was found in patients with a localizing motor response in New Delhi (12.5% versus 4.8%). The relative absence of pre-hospital emergency care and the delay in admission after head injury were considered as two possible causes for these differences (Colohan et al., 1989).
A more recent example of CER comes from the IMPACT studies. Here, differences in outcome between centers were quantified across 10 RCTs and three observational studies, containing data on 9578 patients with moderate and severe TBI (Lingsma et al., 2011). The between-center differences in unfavorable outcome (dead, vegetative state, or severe disability as measured with the Glasgow Outcome Scale) at 6 months were estimated with a random effects logistic regression model. An odds ratio (OR) was estimated for each center by comparing the number of patients with unfavorable outcome to the average, set at an OR of 1. The authors found that the 95% range of ORs among centers was 0.55–1.83, meaning that there are centers in which the odds of unfavorable outcome are almost half the average, and centers where the odds of unfavorable outcome approach twice the average. There is thus a more than threefold difference in the probability over and above chance effects to have an unfavorable outcome between the centers, which could not be explained by adjustment for the most important predictors of outcome in TBI: age, GCS motor score, and pupil reactivity.
Limitations resulting from the nature of the IMPACT database (inconsistent recording of relevant variables across multiple studies) unfortunately precluded a detailed comparative analysis aimed at exploring possible underlying causes in depth. The observed center differences clearly demonstrate the potential for CER in TBI and the importance of rigorously conducted, comprehensive, consistent, prospective data collection across multiple centers. One key step in such efforts will be the development of common data elements for TBI (CDEs), and the implementation of web-based, efficient data collection tools (Maas, 2009; Maas et al., 2010c, 2011).
A further example of the application of CER to TBI—and now in the setting of post-acute care—is based upon standardized data collection performed by the Kaiser Permanente health system in the U.S. Kaiser Permanente (KP) serves the health care needs of 8.2 million members in nine states and the District of Columbia, including California, Colorado, Georgia, Hawaii, Maryland, Ohio, Oregon, Virginia, and Washington. The KP system offers a unique opportunity to compare variation of care and outcomes. Much of the data concerning an encounter or episode of care is standardized, and permits comparison of populations based on demographic characteristics, care settings and trajectories, service delivery, and also outcomes by mining and analyzing data. In the KP Northern California Region (KP-NCAL), where membership currently stands at 3.2 million members, the annual incidence of acute brain injury is approximately 2500, and a large-scale genetics database is being created that will be available for TBI studies. In 2002, The Division of Research in Oakland (KP-NCAL) created a large mild TBI registry that is also available for research studies. KP has internally funded a study of TBI to determine the risk of development of Alzheimer's disease that was to be completed at the end of 2010.
With the availability of large databases and variability in utilization of services, an opportunity exists to study the variation in outcomes of care delivery. For KP-NCAL neurotrauma patients, care may be initially provided outside the system, in a non-KP trauma center or an emergency room. For example, KP-NCAL patients may receive acute trauma care at San Francisco General Hospital, a Level 1 trauma center, or Kaiser South Sacramento, a Level 2 trauma center, or in a KP emergency department. Patients with mild TBI are often seen in emergency departments, within or outside the KP integrated health care system, and released to home with a referral to their primary care physicians. Others are referred to specialists, for example at the regional rehabilitation center or elsewhere, by local trauma centers. Physiatric co-management with a primary care provider, or evaluation by a neuropsychologist, may also be an important model to study. Studies of inpatient hospital versus skilled nursing facility versus home or outpatient care may be further developed using this approach.
The future potential of comparative effectiveness research in TBI
There are many unanswered questions in TBI, that relate to the process of care, trauma organization, and specific approaches. Many uncertainties concerning best clinical practice exist in TBI.
How should acute trauma care and post-acute care be organized? Does time to referral to a specialist for post-acute care matter? Can any preference in the post-acute care setting be found for inpatient hospital versus skilled nursing facility versus home or outpatient care? Management issues in the acute care of more severely injured patients include which treatment modalities for treating ICP should be used and in what sequence. Uncertainty exists about the optimal timing for extracranial surgery, and for the indications and timing of surgery for contusions and for treatment of raised ICP.
There are four unique features of TBI that make CER a feasible approach to address these uncertainties.
First, there are large between-center differences and between-country differences in both outcome and management. On one hand these differences might be considered worrisome. On the other hand they provide a major opportunity to compare alternative interventions/management strategies/care organization that all are possible best practices, in everyday clinical practice.
Second, robust covariates and validated prognostic models have been developed specifically for TBI by the CRASH and IMPACT collaborations (MRC CRASH Trial Collaborators, 2008; Steyerberg et al., 2008). These models provide the possibility to adjust for patient characteristics that affect outcome.
Third, advanced statistical models, including random effects models, are available to analyze differences between centers.
Fourth, recommendations have been developed for standardization of data collection and coding of variables.
However, what is currently still lacking in TBI is the existence of a contemporary observational dataset with high-quality, uniformly-collected highly granular, prospective data. This is crucial to performing high-quality CER studies. It has been argued that data for CER research might be drawn from ongoing clinical trials. This approach would, however, violate a main principle of CER studies, namely that they should be conducted in settings similar to those in which the intervention will be used in practice. Moreover, the experience from the IMPACT studies illustrates that in-depth CER analysis of such data is not possible if specific research interests, such as treatment-specific effects, have not been predetermined and data collection targeted accordingly. We argue that contemporary prospective data collection is essential and should be carried out through a coordinated effort involving a large number of clinical centers in several countries. This initiative would require a significant investment in terms of time and money. However, such an investment would be certainly repaid by the results that would stem from the CER analysis of the collected data. CER research has the potential to answer the many open questions in TBI, and further offers opportunities for cost utility studies in the context of health technology assessment in TBI.
Conclusions
In many ways this workshop points to a paradigm shift in clinical research in TBI. First, the joint organization by European and U.S. funding agencies reflects the need for international collaboration. Second, we have come to realize that approaches other than the reductionistic methods of strictly controlled trials should be considered in clinical TBI research.
Improved clinical care of TBI patients will likely depend on a range of research approaches, including systems biology and CER. These approaches are not yet widely used in clinical TBI research, first because of unfamiliarity, second because of the lack of a rich and comprehensive human dataset that includes demographic, clinical, genomic, proteomic, imaging, and detailed outcome data across multiple time points. It is essential that CER studies comply with published guidelines for such studies (Sox et al., 2010). The lower costs of this type of approach make it particularly attractive for studies that otherwise would be cost-prohibitive. Well conducted studies will translate into clinically meaningful results, which will directly inform decision making in clinical practice, and thus improve care for TBI patients. An important barrier to systems biology approaches is cultural, in which this new data-driven systems approach challenges the current reductionistic approach to clinical research, and requires a new way of thinking about human biology and disease. It also requires multidisciplinary teams of investigators from disciplines that have not previously worked together to apply and refine these promising mathematical and computational tools. Overcoming existing prejudice will require vision and sufficient and sustained funding.
However relevant these approaches may be, it is important that we do not view them as the new panacea in TBI research. As with therapeutic targets in individual patient management, so it is with research tools in populations of patients: one size does not fit all.
The joint organization of the workshop by European and U.S. funding agencies holds promise. It reflects the agencies' interest in joining efforts to move the TBI field forward, and this was confirmed during the American Association for the Advancement of Science meeting (Washington, D.C., February 2011), when the EU commission and the NIH jointly organized a session on “transatlantic synergies to promote effective traumatic brain injury research.” An international collaborative initiative would be timely and of significant added value for the TBI field. Constituting an observational dataset with data of quality high enough to perform system biology approaches and CER studies requires a sizable economic effort. Given the observational nature of these approaches, there is a limited probability that it would be funded by industry. Governmental agencies are thus the most plausible source of funding. Furthermore, the necessity of enrolling a large number of clinical centers across different countries would rule out the possibility of the funding coming from a single country. The need for an international collaborative research effort is evident. We argue that this would offer a much-needed opportunity to provide answers to the many open questions in TBI research, and improve care for TBI patients.
Acknowledgments
The workshop on CER was supported by the European Commission DG Research and the National Institutes of Health/National Institute of Neurological Diseases and Stroke (NIH/NINDS). We are grateful to Dr. Ramona Hicks (NIH/NINDS) and Dr. Patrizia Tosetti (DG Research, European Commission) for their insightful planning and coordination of the workshop. The authors of this manuscript were all speakers at the workshop. We wish to express our gratitude to Dr. Kenneth Curley, Dr. Alan Faden, and Prof. Nicole von Steinbuechel for contributing to the panel discussions. We are grateful to all attendees who participated in the workshop, many of whom traveled from afar specifically to attend the workshop, and for their contributions to the general discussions. Their input was essential in making this workshop a success. A particular word of thanks is due to Dr. Toril Skandsen (Trondheim, Norway), for providing her notes of the discussions, to Dr. Kenneth Curley for input in drafting the manuscript, to Eno Lavrysen for administrative support in preparing the manuscript, and to Sir Graham Teasdale for advice in editing the final version.
Author Disclosure Statement
All of the authors conduct research in TBI and receive funding for this research from various sources. None of the authors received any honorarium or other compensation for their participation at the workshop or for their contributions in writing this manuscript.
References
- Aarabi B. Alden T.D. Chestnut R.M. Downs J.H. Ecklund J.M. Eisenberg H.M. Farace E. Florin R.E. Jane J.A. Krieger M.D. Maas A.I.R. Narayan R.K. Potapav A.A. Salazar A.M. Shaffrey M.E. Walters B.C. Management and prognosis of penetrating brain injury—guidelines. J. Trauma. 2001;51:S1–S86. [Google Scholar]
- Adelson P.D. Bratton S.L. Carney N.A. Chestnut R.M. du Coudray H.E. Goldstein B. Kochanek P.M. Miller H.C. Partingtan M.D. Seldon N.R. Warden C.R. Wright D.W. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr. Crit. Care Med. 2003;4:1–75. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- Alexander S. Kerr M.E. Kim Y. Kamboh M.I. Beers S.R. Conley Y.P. Apolipoprotein E4 allele presence and functional outcome after severe traumatic brain injury. J. Neurotrauma. 2007;24:790–797. doi: 10.1089/neu.2006.0133. [DOI] [PubMed] [Google Scholar]
- Ashwal S. Holshouser B.A. Tong K.A. Use of advanced neuroimaging techniques in the evaluation of pediatric traumatic brain injury. Dev. Neurosci. 2006;28:309–326. doi: 10.1159/000094157. [DOI] [PubMed] [Google Scholar]
- Balestreri M. Czosnyka M. Hutchinson P. Steiner L.A. Hiler M. Smielewski P. Pickard J.D. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit. Care. 2006;4:8–13. doi: 10.1385/NCC:4:1:008. [DOI] [PubMed] [Google Scholar]
- Bischofs I.B. Hug J.A. Liu A.W. Wolf D.M. Arkin A.P. Complexity in bacterial cell-cell communication: quorum signal integration and subpopulation signalling in the Bacillus subtilis phosphorelay. Proc. Natl. Acad. Sci. USA. 2009;106:6459–6464. doi: 10.1073/pnas.0810878106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Trauma Foundation, American Association of Neurological Surgeons (AANS), Congress of Neurological Surgeons (CNS), AANS/CNS Joint Section on Neurotrauma and Critical Care. Guidelines for the Management of Severe Traumatic Brain Injury, 3rd. J. Neurotrauma. 2007;24:1–106. [Google Scholar]
- Brain Trauma Foundation. http://www.tbiguidelines.org. [May 3;2011 ]. http://www.tbiguidelines.org Online TBI guidelines.
- Bryant R.A. Disentangling mild traumatic brain injury and stress reactions. N Engl. J. Med. 2008;358:525–527. doi: 10.1056/NEJMe078235. [DOI] [PubMed] [Google Scholar]
- Bulger E.M. Nathens A.B. Rivara F.P. Moore M. MacKenzie E.J. Jurkovich G.J. Brain Trauma Foundation. Management of severe head injury: Institutional variations in care and effect on outcome. Crit. Care Med. 2002;30:1870–1876. doi: 10.1097/00003246-200208000-00033. [DOI] [PubMed] [Google Scholar]
- Bullock R.M. Chesnut R. Ghajar J. Gordon D. Hartl R. Newell D.W. Servadei F. Walters B.C. Wilberger J.E. Guidelines for the surgical management of traumatic brain injury. Neurosurgery. 2006;58:1–58. doi: 10.1227/01.NEU.0000210365.36914.E3. [DOI] [PubMed] [Google Scholar]
- Carr B.G. Nance M.L. Access to pediatric trauma care: alignment of providers and health systems. Curr. Opin. Pediatr. 2010;22:326–331. doi: 10.1097/MOP.0b013e3283392a48. [DOI] [PubMed] [Google Scholar]
- Chen J. Arnow B.J. Jegga A.G. Disease candidate gene identification and prioritization using protein interaction networks. BMC Informatics. 2009;10:73. doi: 10.1186/1471-2105-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R.M. Marshall L.F. Klauber M.R. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Clayton T.J. Nelson R.J. Manara A.R. Reduction in mortality from severe head injury following introduction of a protocol for intensive care management. Br. J. Anaesth. 2004;93:761–767. doi: 10.1093/bja/aeh249. [DOI] [PubMed] [Google Scholar]
- Clifton G.L. Choi S.C. Miller E.R. Levin H.S. Smith K.R., Jr. Muizelaar J.P. Wagner F.C., Jr. Marion D.W. Luerssen T.G. Intercenter variance in clinical trials of head trauma—experience of the National Acute Brain Injury Study: Hypothermia. J. Neurosurg. 2001;95:751–755. doi: 10.3171/jns.2001.95.5.0751. [DOI] [PubMed] [Google Scholar]
- Coles J.P. Fryer T.D. Coleman M.R. Smielewski P. Gupta A.K. Minhas P.S. Aigbirhio F. Chatfield D.A. Williams G.B. Boniface S. Carpenter T.A. Clark J.C. Pickard J.D. Menon D.K. Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism. Crit. Care Med. 2007;35:568–578. doi: 10.1097/01.CCM.0000254066.37187.88. [DOI] [PubMed] [Google Scholar]
- Coles J.P. Minhas P.S. Fryer T.D. Smielewski P. Aigbirihio F. Donovan T. Downey S.P. Williams G. Chatfield D. Matthews J.C. Gupta A.K. Carpenter T.A. Clark J.C. Pickard J.D. Menon D.K. Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates. Crit. Care Med. 2002;30:1950–1959. doi: 10.1097/00003246-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Colohan A.R. Alves W.M. Gross C.R. Torner J.C. Mehta V.S. Tandon P.N. Jane J.A. Head injury mortality in two centers with different emergency medical services and intensive care. J. Neurosurg. 1989;71:202–207. doi: 10.3171/jns.1989.71.2.0202. [DOI] [PubMed] [Google Scholar]
- Cooper D.J. Rosenfeld J.V. Murray L. Arabi Y.M. Davies A.R. D'Urso P. Kossmann T. Ponsford J. Seppelt I. Reilly P. Wolfe R. the DECRA Trial Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- Correspondence NEJM. Care of war veterans with mild traumatic brain injury. N. Engl. J. Med. 2009;361:536–538. doi: 10.1056/NEJMvcm0803922. [DOI] [PubMed] [Google Scholar]
- Cremer O.L. van Dijk G.W. van Wensen E. Brekelmans G.J. Moons K.G. Leenen L.P. Kalkman C.J. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit. Care Med. 2005;33:2207–2213. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- Czosnyka M. Balestreri M. Steiner L. Smielewski P. Hutchinson P.J. Matta B. Pickard J.D. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J. Neurosurg. 2005;102:450–454. doi: 10.3171/jns.2005.102.3.0450. [DOI] [PubMed] [Google Scholar]
- DeWitt D.S. Jenkins L.W. Prough D.S. Enhanced vulnerability to secondary ischemic insults after experimental traumatic brain injury. New Horiz. 1995;3:376–383. [PubMed] [Google Scholar]
- Dhruva S.S. Redberg R.F. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch. Intern. Med. 2008;168:136–140. doi: 10.1001/archinternmed.2007.56. Erratum, Arch. Intern. Med. 168, 774. [DOI] [PubMed] [Google Scholar]
- Drummond M.F. Schwartz J.S. Jönsson B. Luce B.R. Neumann P.J. Siebert U. Sullivan S.D. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int. J. Technol. Assess. Health Care. 2008;24:244–258. doi: 10.1017/S0266462308080343. discussion 362–368. [DOI] [PubMed] [Google Scholar]
- Edwards P. Arango M. Balica L. Cottingham R. El-Sayed H. Farrell B. Fernandes J. Gogichaisvili T. Golden N. Hartzenberg B. Husain M. Ulloa M.I. Jerbi Z. Khamis H. Komolafe E. Laloë V. Lomas G. Ludwig S. Mazairac G. Muñoz Sanchéz Mde L. Nasi L. Olldashi F. Plunkett P. Roberts I. Sandercock P. Shakur H. Soler C. Stocker R. Svoboda P. Trenkler S. Venkataramana N.K. Wasserberg J. Yates D. Yutthakasemsunt S. MRC CRASH trial collaborators. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury—outcomes at 6 months. Lancet. 2005;365:1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- Elf K. Nilsson P. Enblad P. Outcome after traumatic brain injury improved by an organized secondary insult program and standardized neurointensive care. Crit. Care Med. 2002;30:2129–2134. doi: 10.1097/00003246-200209000-00029. [DOI] [PubMed] [Google Scholar]
- Fakhry S.M. Trask A.L. Waller M.A. Watts D.D. IRTC Neurotrauma Task Force. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J. Trauma. 2004;56:492–499. doi: 10.1097/01.ta.0000115650.07193.66. [DOI] [PubMed] [Google Scholar]
- Forsyth R.J. Parslow R.C. Tasker R.C. Hawley C.A. Morris K.P. Prediction of raised intracranial pressure complicating severe traumatic brain injury in children: implications for trial design. Pediatr. Crit. Care Med. 2008;9:8–14. doi: 10.1097/01.PCC.0000298759.78616.3A. [DOI] [PubMed] [Google Scholar]
- Foulkes M.A. Eisenberg H.M. Jane J.A. Marmarou A. Marshall L.F. the TCDB research group. The traumatic coma data bank: design, methods, and baseline characteristics. J. Neurosurg. 1991;75:8–13. [Google Scholar]
- Gelpke G.J. Braakman R. Habbema J.D. Hilden J. Comparison of outcome in two series of patients with severe head injuries. J. Neurosurg. 1983;59:745–750. doi: 10.3171/jns.1983.59.5.0745. [DOI] [PubMed] [Google Scholar]
- Govind V. Gold S. Kaliannan K. Saigal G. Falcone S. Arheart K.L. Harris L. Jagid J. Maudsley A.A. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J. Neurotrauma. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A.R. Why do neuroprotective drugs that are so promising in animals fail in the clinic? An industry perspective. Clin. Exp. Pharmacol. Physiol. 2002;29:1030–1034. doi: 10.1046/j.1440-1681.2002.03767.x. [DOI] [PubMed] [Google Scholar]
- Harders A. Kakarieka A. Braakman R. Traumatic subarachnoid hemorrhage and its treatment with nimodipine. German tSAH Study Group. J. Neurosurg. 1996;85:82–89. doi: 10.3171/jns.1996.85.1.0082. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Contant C.F. Diaz-Marchan P. Valadka A.B. Robertson C.S. Significance of a reduced cerebral blood flow during the first 12 hours after traumatic brain injury. Neurocrit. Care. 2004;1:69–83. doi: 10.1385/NCC:1:1:69. [DOI] [PubMed] [Google Scholar]
- Hoge C.W. Goldberg H.M. Castro C.A. Care of war veterans with mild traumatic brain injury. N. Engl. J. Med. 2009;360:1588–1591. doi: 10.1056/NEJMp0810606. [DOI] [PubMed] [Google Scholar]
- Hoge C.W. McGurk D. Thomas J.L. Cox A.L. Engel C.C. Castro C.A.V. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Howells T. Elf K. Jones P.A. Ronne-Engstrom E. Piper I. Nilsson P. Pressure reactivity as a guide in the treatment of cerebral perfusion pressure in patients with brain trauma. J. Neurosurg. 2005;102:311–317. doi: 10.3171/jns.2005.102.2.0311. [DOI] [PubMed] [Google Scholar]
- Traumatic brain injury in the United States: emergency department visits, hospitalisations and visits. http://www.cdc.gov/traumaticbraininjury/tbi_ed.html. 2002–2006. [May 3;2011 ]. http://www.cdc.gov/traumaticbraininjury/tbi_ed.html
- Injuries in the European Union: Statistic summary. https://webgate.ec.europa.eu/idb/documents/2009-IDB-Report_screen.pdf. 2005–2007. [May 3;2011 ]. https://webgate.ec.europa.eu/idb/documents/2009-IDB-Report_screen.pdf
- Ikonomidou C. Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) The National Academies Press; Washington, D.C: 2009. Initial National Priorities for Comparative Effectiveness Research. [Google Scholar]
- Ishige N. Pitts L.H. Berry I. Nishimura M.C. James T.L. The effects of hypovolemic hypotension on high-energy phosphate metabolism of traumatized brain in rats. J. Neurosurg. 1988;68:129–136. doi: 10.3171/jns.1988.68.1.0129. [DOI] [PubMed] [Google Scholar]
- Janowitz T. Menon D.K. Exploring new routes for neuroprotective drug development in traumatic brain injury. Sci. Transl. Med. 2010;2:27. doi: 10.1126/scitranslmed.3000330. rv1. [DOI] [PubMed] [Google Scholar]
- Jennett B. Teasdale G. Braakman R. Minderhoud J. Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031–1034. doi: 10.1016/s0140-6736(76)92215-7. [DOI] [PubMed] [Google Scholar]
- Jennett B. Teasdale G. Galbraith S. Pickard J. Grant H. Braakman R. Avezaat C. Maas A. Minderhoud J. Vecht C.J. Heiden J. Small R. Caton W. Kurze T. Severe head injuries in three countries. J. Neurol. Neurosurg. Psychiatry. 1977;40:291–298. doi: 10.1136/jnnp.40.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennett B. Teasdale G. Fry J. Braakman R. Minderhoud J. Heiden J. Kurze T. Treatment for severe head injury. J. Neurol. Neurosurg. Psychiatry. 1980;43:289–295. doi: 10.1136/jnnp.43.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B.D. Genetic influences on outcome following traumatic brain injury. Neurochem. Res. 2007;4–5:905–915. doi: 10.1007/s11064-006-9251-3. [DOI] [PubMed] [Google Scholar]
- Kapapa T. Pfister U. Konig K. Sasse M. Woischneck D. Heissler H.E. Rickels E. Head trauma in children, part 3: clinical and psychosocial outcome after head trauma in children. J. Child Neurol. 2010;25:409–422. doi: 10.1177/0883073809340697. [DOI] [PubMed] [Google Scholar]
- Kochanek P.M. Clark R. Ruppel R.A. Adelson P.D. Bell M.J. Whalen M.J. Robertson C.L. Satchell M.A. Seidberg N.A. Marion D.W. Jenkins L.W. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatr. Crit. Care Med. 2000;1:4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Kochanek P.M. Pediatric traumatic brain injury: quo vadis? Dev. Neurosci. 2006;28:244–255. doi: 10.1159/000094151. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K.E. The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K.E. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta: 2006. [Google Scholar]
- Lingsma H.F. Roozenbeek B. Li B. Marmarou A. Murray G.D. Maas A.I. Steyerberg E.W. Large between-center differences in outcome after moderate and severe traumatic brain injury in the IMPACT study. Neurosurgery. 2011;68:601–607. doi: 10.1227/NEU.0b013e318209333b. discussion 607–608. [DOI] [PubMed] [Google Scholar]
- Lingsma H.F. Roozenbeek B. Steyerberg E.W. Murray G.D. Maas A.I. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9:543–554. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Dearden M. Teasdale G.M. Braakman R. Cohadon F. Iannotti F. Karimi A. Lapierre F. Murray G. Ohman J. Persson L. Servadei F. Stocchetti N. Unterberg A. EBIC—guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir. (Wien.) 1997;139:286–294. doi: 10.1007/BF01808823. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Harrison-Felix C.L. Menon D. Adelson P.D. Balkin T. Bullock R. Engel D.C. Gordon W. Langlois-Orman J. Lew H.L. Robertson C. Temkin N. Valadka A. Verfaellie M. Wainwright M. Wright D.W. Schwab K. Standardizing data collection in traumatic brain injury. J. Neurotrauma. 2011;28:177–187. doi: 10.1089/neu.2010.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A.I. Harrison-Felix C.L. Menon D.K. Adelson D. Balkin T. Bullock R. Engel D.C. Gordon W. Langlois J. Lew H.L. Robertson C. Temkin N. Valadka A. Verfaellie M. Wainwright M. Wright D.W. Schwab K. Common data elements for traumatic brain injury: Recommendations from the Interagency Working Group on Demographics and Clinical Assessment. Arch. Phys. Rehab. Med. 2010c;91:1641–1649. doi: 10.1016/j.apmr.2010.07.232. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Marmarou A. Murray G.D. Teasdale G.M. Steyerberg E.W. Prognosis and clinical trial design in traumatic brain injury: the IMPACT Study. J. Neurotrauma. 2007a;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Roozenbeek B. Manley G.T. Clinical trials in traumatic brain injury: Past experience and current developments. Neurotherapeutics. 2010a;7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A.I. Standardisation of data collection in traumatic brain injury: key to the future? Crit. Care. 2009;13:1016. doi: 10.1186/cc8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A.I. Steyerberg E.W. Butcher I. Dammers R. Lu J. Marmarou A. Mushkudiani N.A. McHugh G.S. Murray G.D. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007b;24:303–314. doi: 10.1089/neu.2006.0033. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Steyerberg E.W. Marmarou A. McHugh G.S. Lingsma H.F. Butcher I. Lu J. Weir J. Roozenbeek B. Murray G.D. IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics. 2010b;7:127–134. doi: 10.1016/j.nurt.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A.I. Steyerberg E.W. Murray G.D. Bullock R. Baethmann A. Marshall L.F. Teasdale G.M. Why have recent trials of neuroprotective agents in head injury failed to show convincing efficacy? A pragmatic analysis and theoretical considerations. Neurosurgery. 1999;44:1286–1298. [PubMed] [Google Scholar]
- Maas A.I. Stocchetti N. Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Manley G. Knudson M.M. Morabito D. Damron S. Erickson V. Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch. Surg. 2001;136:1118–1123. doi: 10.1001/archsurg.136.10.1118. [DOI] [PubMed] [Google Scholar]
- Marklund N. Bakshi A. Castelbuono D.J. Conte V. McIntosh T.K. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr. Pharm. Des. 2006;12:1645–1680. doi: 10.2174/138161206776843340. [DOI] [PubMed] [Google Scholar]
- Marmarou A. Lu J. Butcher I. McHugh G.S. Mushkudiani N.A. Murray G.D. Steyerberg E.W. Maas A.I. IMPACT database of traumatic brain injury: design and description. J. Neurotrauma. 2007;24:239–250. doi: 10.1089/neu.2006.0036. [DOI] [PubMed] [Google Scholar]
- Menon D.K. Coles J.P. Gupta A.K. Fryer T.D. Smielewski P. Chatfield D.A. Aigbirhio F. Skepper J.N. Minhas P.S. Hutchinson P.J. Carpenter T.A. Clark J.C. Pickard J.D. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004;32:1384–1390. doi: 10.1097/01.ccm.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- MRC CRASH Trial Collaborators. Perel P. Arango M. Clayton T. Edwards P. Komolafe E. Poccock S. Roberts I. Shakur H. Steyerberg E. Yutthakasemsunt S. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G. Teasdale G.M. Braakman R. Cohadon F. Dearden M. Iannotti F. Karimi A. Lapierre F. Maas A. Ohman J. Persson L. Servadei F. Stocchetti N. Trojanowski T. Unterberg A. The European Brain Injury Consortium survey of head injuries. Acta Neurochir. (Wien.) 1999;141:223–236. doi: 10.1007/s007010050292. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale J.E. Joseph J.G. Pretzlaff R.K. Silber T.J. Guerguerian A.M. Clinical trials in pediatric traumatic brain injury: unique challenges and potential responses. Dev. Neurosci. 2006;28:276–290. doi: 10.1159/000094154. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Sarkar R. Lee H. Meeker M. Zimmerman R.D. Manley G.T. McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nortje J. Coles J.P. Timofeev I. Fryer T.D. Aigbirhio F.I. Smielewski P. Outtrim J.G. Chatfield D.A. Pickard J.D. Hutchinson P.J. Gupta A.K. Menon D.K. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: preliminary findings. Crit. Care Med. 2008;36:273–281. doi: 10.1097/01.CCM.0000292014.60835.15. [DOI] [PubMed] [Google Scholar]
- Parekh A.K. Barton M.B. The challenge of multiple comorbidity for the US health care system. JAMA. 2010;303:1303–1304. doi: 10.1001/jama.2010.381. [DOI] [PubMed] [Google Scholar]
- Patel H.C. Bouamra O. Woodford M. King A.T. Yates D.W. Lecky F.E. Trauma Audit and Research Network. Trends in head injury outcome from 1989 to 2003 and the effect of neurosurgical care: an observational study. Lancet. 2005;366:1538–1544. doi: 10.1016/S0140-6736(05)67626-X. [DOI] [PubMed] [Google Scholar]
- Patel H.C. Menon D.K. Tebbs S. Hawker R. Hutchinson P.J. Kirkpatrick P.J. Specialist neurocritical care and outcome from head injury. Intensive Care Med. 2002;28:547–553. doi: 10.1007/s00134-002-1235-4. [DOI] [PubMed] [Google Scholar]
- Prins M.L. Hovda D.A. Developing experimental models to address traumatic brain injury in children. J. Neurotrauma. 2003;20:123–137. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- Risdall J.E. Menon D.K. Traumatic brain injury. Phil. Trans. R. Soc. 2011;366:241–250. doi: 10.1098/rstb.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I. Yates D. Sandercock P. Farrell B. Wasserberg J. Lomas G. Cottingham R. Svoboda P. Brayley N. Mazairac G. Laloe V. Munoz-Sanchez A. Arango M. Hartzenberg B. Khamis H. Yutthakasemsunt S. Komolafe E. Olldashi F. Yadav Y. Murillo-Cabezas F. Shakur H. Edwards P. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- Robertson C.S. Valadka A.B. Hannay H.J. Contant C.F. Gopinath S.P. Cormio M. Uzura M. Grossman R.G. Prevention of secondary ischemic insults after severe head injury. Crit. Care Med. 1999;27:2086–2095. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Workshop Scientific Team and Advisory Panel Members. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman A.I. Braver E.R. Kang H.K. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: Persistent postconcussive symptoms and posttraumatic stress disorder. Am. J. Epidemiol. 2008;167:1446–1452. doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- Schweikhart S.A. Dembe A.E. The applicability of Lean and Six Sigma techniques to clinical and translational research. J. Investig. Med. 2009;57:748–755. doi: 10.231/JIM.0b013e3181b91b3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears D.D. Hsiao A. Yu J.G. Courtney C.H. Ofrecio J.M. Chapman J. Subramaniam S. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc. Natl. Acad. Sci. USA. 2009;106:18745–18750. doi: 10.1073/pnas.0903032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servadei F. Murray G. Penny K. Teasdale G.M. Dearden M. Iannotti F. Lapierre F. Maas A. Karimi A. Ohman J. Persson L. Stocchetti N. Trojanowski T. Unterberg A. The value of the “worst” computed tomographic scan in clinical studies of moderate and severe head injury. European Brain Injury Consortium. Neurosurgery. 2000;46:70–75. doi: 10.1097/00006123-200001000-00014. discussion 75–77. [DOI] [PubMed] [Google Scholar]
- Servadei F. Murray G. Teasdale G.M. Dearden M. Iannotti F. Lapierre F. Maas A. Karimi A. Ohman J. Persson L. Stocchetti N. Trojanowski T. Unterberg A. Traumatic subarachnoid hemorrhage: demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery. 2002;50:261–267. doi: 10.1097/00006123-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Sox H.C. Helfand M. Grimshaw J. Dickersin K. PLoS Medicine Editors. Tovey D. Knottnerus J.A. Tugwell P. Comparative effectiveness research: challenges for medical journals. Croat. Med. J. 2010;51:191–194. doi: 10.3325/cmj.2010.51.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiotta A.M. Stiefel M.F. Gracias V.H. Garuffe A.M. Kofke W.A. Maloney-Wilensky E. Troxel A.B. Levine J.M. Le Roux P.D. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J. Neurosurg. 2010;113:571–580. doi: 10.3171/2010.1.JNS09506. [DOI] [PubMed] [Google Scholar]
- Spiro P. Parkinson J. Othmer H. A model of excitation and adaptation in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA. 1997;94:7263–7268. doi: 10.1073/pnas.94.14.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler K.D. Jenkins L.W. Dixon C.E. Clark R.S. Marion D.W. Kochanek P.M. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J. Neurotrauma. 2001;18:1195–1206. doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- Steiner L.A. Czosnyka M. Piechnik S.K. Smielewski P. Chatfield D. Menon D.K. Pickard J.D. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med. 2002;30:733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Stein S.C. Georgoff P. Meghan S. Mirza K.L. El Falaky O.M. Relationship of aggressive monitoring and treatment to improved outcomes in severe traumatic brain injury. J. Neurosurg. 2010;112:1105–1112. doi: 10.3171/2009.8.JNS09738. [DOI] [PubMed] [Google Scholar]
- Steyerberg E.W. Mushkudiani N. Perel P. Butcher I. Lu J. McHugh G.S. Murray G.D. Marmarou A. Roberts I. Habbema J.D. Maas A.I. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchetti N. Pagan F. Calappi E. Canavesi K. Beretta L. Citerio G. Cormio M. Colombo A. Inaccurate early assessment of neurological severity in head injury. J. Neurotrauma. 2004;21:1131–1140. doi: 10.1089/neu.2004.21.1131. [DOI] [PubMed] [Google Scholar]
- Stocchetti N. Penny K. Dearden M. Braakman R. Cohadon F. Iannotti F. Lapierre F. Karimi A. Maas A. Murray G.D. Ohman J. Persson L. Servadei F. Teasdale G.M. Trojanowski T. Unterberg A. Intensive care management of head-injured patients in Europe: a survey from the European Brain Injury Consortium. Intensive Care Med. 2001;27:400–406. doi: 10.1007/s001340000825. [DOI] [PubMed] [Google Scholar]
- Styrke J. Stalnacke B.M. Sojka P. Bjornstig U. Traumatic brain injuries in a well-defined population: epidemiological aspects and severity. J. Neurotrauma. 2007;24:1425–1436. doi: 10.1089/neu.2007.0266. [DOI] [PubMed] [Google Scholar]
- Suarez J.I. Zaidat O.O. Suri M.F. Feen E.S. Lynch G. Hickman J. Georgiadis A. Selman W.R. Length of stay and mortality in neurocritically ill patients: impact of a specialized neurocritical care team. Crit. Care Med. 2004;32:2311–2317. doi: 10.1097/01.ccm.0000146132.29042.4c. [DOI] [PubMed] [Google Scholar]
- Tagliaferri F. Compagnone C. Korsic M. Servadei F. Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. (Wien.) 2006;148:255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- Thurmond V.A. Hicks R.A. Gleason T. Miller A.C. Szuflita N. Orman J. Schwab K. Advancing integrated research in psychological health and Traumatic Brain Injury: Common Data Elements (CDE) Arch. Phys. Rehab. Med. 2010;91:1633–1636. doi: 10.1016/j.apmr.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Tolias C.M. Bullock M.R. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1:71–79. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoci P. Allen J.M. Kramer J.M. Califf R.M. Smith S.C., Jr Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301:831–841. doi: 10.1001/jama.2009.205. [DOI] [PubMed] [Google Scholar]
- Truelle J. Koskinen S. Hawthorne G. Sarajuuri J. Formisano R. von Wild K. Neugebauer E. Wilson L. Gibbons H. Powell J. Bullinger M. Höfer S. Maas A.I. Zitnay G. von Steinbüchel N. the QOLIBRI Task Force. Quality of life after traumatic brain injury: the clinical use of the QOLIBRI, a novel disease-specific instrument. Brain Inj. 2010;24:1272–1291. doi: 10.3109/02699052.2010.506865. [DOI] [PubMed] [Google Scholar]
- U.K. National Institute for Health and Clinical Excellence. Head injury: Triage, assessment, investigation and early management of head injury in infants, children and adults: Clinical Guideline. 2003. http://www.nice.org.uk/cg056. [May 3;2011 ]. http://www.nice.org.uk/cg056
- Varelas P.N. Eastwood D. Yun H.J. Spanaki M.V. Hacein Bey L. Kessaris C. Gennarelli T.A. Impact of a neurointensivist on outcomes in patients with head trauma treated in a neurosciences intensive care unit. J. Neurosurg. 2006;104:713–719. doi: 10.3171/jns.2006.104.5.713. [DOI] [PubMed] [Google Scholar]
- Vespa P. Bergsneider M. Hattori N. Wu H.M. Huang S.C. Martin N.A. Glenn T.C. McArthur D.L. Hovda D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Steinbuechel N. Petersen C. Bullinger M. the QOLIBRI Group. Assessment of health-related quality of life in persons after traumatic brain injury—development of the QOLIBRI, a specific measure. Acta Neurochir. Suppl. 2005;93:43–49. doi: 10.1007/3-211-27577-0_6. [DOI] [PubMed] [Google Scholar]
- von Steinbuechel N. Wilson L. Gibbons H. Hawthorne G. Höfer S. Schmidt S. Bullinger M. Maas A. Neugebauer E. Powell J. von Wild K. Zitnay G. Bakx W. Christensen A.L. Koskinen S. Formisano R. Sarajuuri J. Sasse N. Truelle J.L. Quality of Life after Brain Injury (QOLIBRI) -— Scale Validity and Correlates of Quality of Life. J. Neurotrauma. 2010b;27:1157–1165. doi: 10.1089/neu.2009.1077. [DOI] [PubMed] [Google Scholar]
- von Steinbuechel N. Wilson L. Gibbons H. Hawthorne G. Höfer S. Schmidt S. Bullinger M. Maas A. Neugebauer E. Powell J. von Wild K. Zitnay G. Bakx W. Christensen A.L. Koskinen S. Sarajuuri J. Formisano R. Sasse N. Truelle J.L. Quality of Life after Brain Injury (QOLIBRI)—Scale Development and Metric Properties. J. Neurotrauma. 2010a;27:1167–1185. doi: 10.1089/neu.2009.1076. [DOI] [PubMed] [Google Scholar]
- Walker P.A. Harting M.T. Baumgartner J.E. Fletcher S. Strobel N. Cox C.S., Jr Modern approaches to pediatric brain injury therapy. J. Trauma. 2009;67:S120–S127. doi: 10.1097/TA.0b013e3181ad323a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/OMS. Geneva: World Health Organisation; 2009. Global status report on road safety: Time for action. [Google Scholar]
- Wilde E.A. McCauley S.R. Kelly T.M. Levin H.S. Pedroza C. Clifton G.L. Robertson C.S. Moretti P. Feasibility of the Neurological Outcome Scale for Traumatic Brain Injury (NOS-TBI) in adults. J. Neurotrauma. 2010;27:975–981. doi: 10.1089/neu.2009.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winthrop A.L. Health-related quality of life after pediatric trauma. Curr. Opin. Pediatr. 2010;22:346–351. doi: 10.1097/MOP.0b013e3283394351. [DOI] [PubMed] [Google Scholar]
- Zangrilli Hoh N.Z. Wagner A.K. Alexander S.A. Clark R.B. Beers S.R. Okonkwo D.O. Ren D. Conley Y.P. BCL2 genotypes: Functional and neurobehavioral outcomes after severe traumatic brain injury. J. Neurotrauma. 2010;27:1413–1427. doi: 10.1089/neu.2009.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]