Abstract
Hyperthermia may accentuate the detrimental consequences of brain injury and worsen the outcome of patients with acute head trauma, especially severe traumatic brain injury (TBI). We explored the effect of different magnitudes and durations of hyperthermia in the first 3 days after injury on the outcome of 7145 patients with acute head trauma, including 1626 with severe TBI. The differences in mortality and unfavorable outcome between the normothermia group, mild fever group, moderate fever group, and high fever group were statistically significant (p<0.001). The mortality and unfavorable outcome of severe TBI patients in the groups also differed significantly (p<0.001). The mortality and unfavorable outcome of patients with 1 day, 2 days, and 3 days of high fever were significantly increased (p<0.01). Our data strongly indicate that both degree and duration of early post-trauma hyperthermia are closely correlated with the outcome of acute TBI patients, especially severely injured ones, which indicates that hyperthermia may play a detrimental role in the delayed mechanisms of damage after acute TBI. Prevention of early hyperthermia after acute head trauma is therefore essential to the management of TBI patients.
Key words: acute head trauma patients, hyperthermia, outcome
Introduction
A number of factors may influence the outcome of head trauma patients, including age, gender, Glasgow Coma Scale (GCS) score, intracranial pressure (ICP), pupillary size and response, hypoxia, and computed tomographic (CT) findings (Braakman et al., 1980; Narayan et al., 1981; Young et al., 1981; Colohan at al., 1989; Muizelaar et al., 1991; Phuenpathom et al., 1993; Fearnside et al., 1993; Jiang et al.,2002; Low et al., 2009). Several studies have reported that hyperthermia may accentuate the detrimental consequences of brain injury and worsen the outcome of patients with stroke and severe traumatic brain injury (TBI) (Dietrich, 1992; Chatzipanteli et al., 2000; Jiang et al., 2002). However, hyperthermia may be not associated with increased risk of death in patients with severe TBI (Childs et al., 2006). In the present study, we used the Chinese Head Trauma Data Bank (CHTDB) to assess the effect of different degrees and different durations of hyperthermia over the first 3 days after head trauma on the outcome of 7145 acute head trauma patients, including 1626 cases of severe TBI.
Methods
Clinical characteristics of the patients
From December 1, 2008 through August 20, 2009, a total of 7145 patients with acute head trauma (GCS score 3–15) were admitted to 47 hospitals, enrolled in the CHTDB, and analyzed. Among this population, 5427 (75.96%) were males. The three most common causes of head trauma were motor vehicle crashes (3836/7145, 53.69%), falls (2381/7145, 29.13%), and violence (804/7145, 11.25%). The age ranged from 1–92 years, and most were adults (ages X–92) (5381/7145, 75.31%). GCS was assessed in all patients within 48 h after injury: 1626 cases had GCS scores of 3–8, 1222 had GCS scores of 9–12, and 4297 had GCS scores of 13–15. CT scanning was routinely performed in all 7145 patients. Intracranial hematomas were found in 2738 cases (38.32%), including epidural hematomas in 1076, subdural hematomas in 873, intracerebral hematomas in 425, and multiple hematomas in 364 cases. Cerebral contusion was found in 3691 cases (51.63%), and traumatic subarachnoid hemorrhage (tSAH) was found in 3566 cases (49.89%). A craniotomy for removal of intracranial hematomas or/and decompression was performed in 2589 cases (36.24%); the other 4556 (63.76%) received conservative treatment. The patients were treated according to the principles described in the guidelines for the management of severe head injury (Bullock et al., 2000).
The measurement of temperature and the definition of fever
All patient axilla temperatures were measured every hour over the first 3 days after trauma in neurosurgical intensive care units. Clinical temperature classification was as follows: normothermia 36.3–37.2°C, mild fever 37.3–38.0°C, moderate fever 38.1–39.0°C, and high fever >39.0°C. Patients were classified in these categories based on the highest temperature occurring over two consecutive hours each day.
The causes and management of fever
We concluded that the most common cause of fever was due to damage to the thermoregulatory centers (“central fever”), because hyperthermia occurred within the first 3 days after head trauma, a time frame less likely for hyperthermia to be attributable to infectious causes. In order to determine whether patients suffered from pneumonia, chest x-rays and white blood cell counts (WBC) were routinely assessed in all head trauma patients with fever. Furthermore, comatose head trauma patients with high fever were also routinely examined with chest CT scans. The results confirmed that pneumonia was absent in fever patients within 3 days after head trauma.
All patients with hyperthermia were routinely treated if fever was > 38°C with routine medical management, including ice packs or cooling blankets, and oral or intramuscular injection of drugs (paracetamol, aspirin, and metamizole). Procedures to treat fever such as intravascular devices, infusion of cold IV fluids, lavage of cold fluids into nasogastric tubes, or bladder instillation of cold fluids were not used in these patients.
Assessment of neurological outcome
Patient neurological outcome was determined at discharge from the hospital (31±5.1 days). Patient neurological outcome was scored according to the Glasgow Outcome Scale (GOS; Teasdale and Jennet, 1974) as follows: 1, death; 2, vegetative state, unable to interact with the environment; 3, severe disability, unable to live independently but able to follow commands; 4, moderate disability, capable of living independently but unable to return to work or school; and 5, mild or no disability, able to return to work or school. Good recovery and moderate disability were designated as favorable outcomes; severe disability, a vegetative state, and death were designated as unfavorable outcomes (Marshall, 1991; Jiang, 2002).
Statistical analysis
The data were analyzed with SPSS 11.0. Patient outcomes in the different temperature groups were compared with the use of chi-square tests, Fisher's exact tests, or Student's t-test, as appropriate. A p value<0.05 was considered significant.
Results
Effects of hyperthermia on the outcome of 7145 patients with acute head trauma
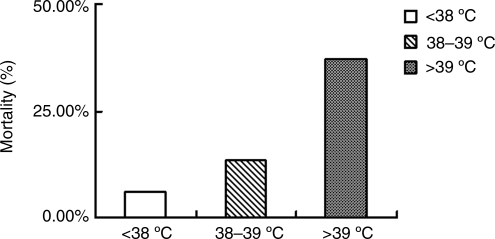
The mortality of patients with acute head trauma in the normothermia (36.3–37.2°C), and mild fever combined group (37.3–38°C), the moderate fever group (38–39°C), and the high fever group (>39°C), were 6.0% (374/1229), 13.6% (106/781), and 37.0% (50/135), respectively. Differences between the three groups were statistically significant (p<0.001; Fig. 1).
FIG. 1.
Effects of hyperthermia on the mortality of 7145 patients with acute head trauma.
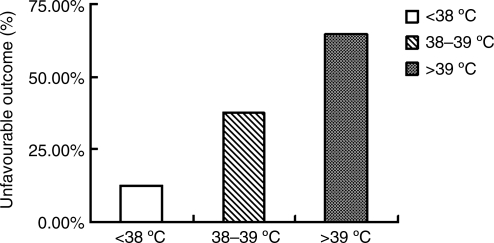
Percentages of patients with unfavorable outcomes in the normothermia and mild fever combined group, the moderate fever group, and the high fever group, were 12.6% (786/6229), 37.6% (294/781), and 64.4% (87/135), respectively. The differences between the three groups were statistically significant (p<0.001; Fig. 2).
FIG. 2.
Effects of hyperthermia on the unfavorable outcomes of 7145 patients with acute head trauma.
Effects of hyperthermia on the outcome of 1626 patients with severe head trauma
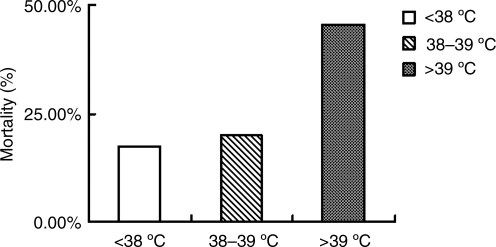
Mortality rates of patients with severe head trauma in the normothermia and mild fever combined group, the moderate fever group, and the high fever group, were 17.5% (193/1100), 20.0% (85/425), and 45.5% (46/101), respectively. The mortality of patients in the normothermia and mild fever combined group was significantly lower than that of the moderate fever group (p<0.05) and the high fever group (p<0.001; Fig. 3).
FIG. 3.
Effects of hyperthermia on the mortality of 1626 patients with severe head trauma.
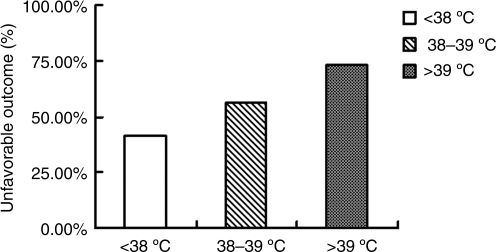
Unfavorable outcomes in patients with severe head trauma in the normothermia and mild fever combined group, the moderate fever group, and the high fever group, were 41.6% (458/1100), 56.2% (239/425), and 73.3% (74/101), respectively. The differences between the three groups were statistically significant (p<0.001; Fig. 4).
FIG. 4.
Effects of hyperthermia on the unfavorable outcomes of 1626 patients with severe head trauma.
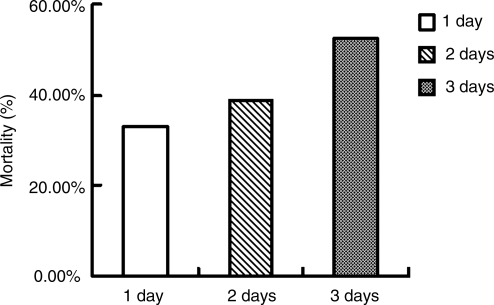
Effects of duration of high fever for 3 days after head trauma on the outcomes of 101 patients with severe head trauma
Mortality rates of patients with severe head trauma with 1 day, 2 days, and 3 days of high fever were 33.3% (18/54), 38.7% (12/31), and 53.5% (8/15), respectively. The mortality rates of patients with 3 days of high fever were significantly higher than those of the other two groups (p<0.05; Fig. 5).
FIG. 5.
Effects of duration of high fever for 3 days after head trauma on the mortality of 101 patients with severe head trauma.
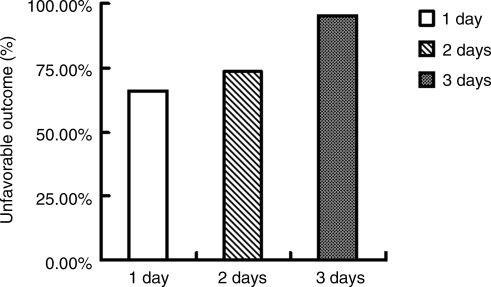
Percentages of unfavorable outcomes in patients with severe head trauma with 1 day, 2 days, and 3 days of high fever were 66.7% (36/54), 74.2% (23/31), and 93.3% (14/15), respectively. The differences between the three groups were statistically significant (p<0.01; Fig. 6).
FIG. 6.
Effects of duration of high fever for 3 days after head trauma on the unfavorable outcomes of 101 patients with severe head trauma.
Discussion
Both the degree and duration of early post-trauma hyperthermia were strongly related with the outcomes of patients with acute TBI. This indicates that hyperthermia may play a detrimental role in delayed mechanisms of damage after TBI. Our findings are consistent with those of previous reports (Dietrich, 1992; Chatzipanteli et al., 2000; Jiang et al., 2002).
Fever in the neurocritical care setting is common and is often associated with a negative impact on outcome of many disease types (Clifton, 1991; Dietrich, 1992; Dietrich et al., 1996). Meta-analyses have demonstrated that fever at onset and in the acute setting after ischemic brain injury, intracerebral hemorrhage, and cardiac arrest has a negative impact on morbidity and mortality (Badjatia, 2009). Data support the theory that the impact of fever is sustained for longer duration after subarachnoid hemorrhage and TBI. Recent advances in aggressive patient management have made eliminating fever and maintaining normothermia feasible (Badjatia, 2009).
The epidemiologic evidence from this study suggests that the vast majority of patients admitted to an ICU environment with TBI will develop fever. The development of fever is clearly associated with a worse prognosis. There is now a better understanding of the possible mechanism of harm behind fever (Cairns and Andrews, 2002). It has recently been reported that hyperthermia may accentuate the detrimental consequences of brain injury and worsen the outcome of patients with stroke and severe TBI (Dietrich, 1992; Chatzipanteli et al., 2000; Jiang et al., 2002). Previous studies have also demonstrated that hyperthermia may result in worse neurobehavioral outcomes following experimental fluid percussion brain injury in rats (Clifton et al., 1991; Dietrich et al., 1996). Dietrich and colleagues found that hyperthermia causes significant blood–brain barrier disruption and brain edema after experimental brain injury (Dietrich et al., 1990). Either of these consequences is likely to have detrimental effects on patient outcomes.
The morphological consequences of delayed post-traumatic brain hyperthermia (39°C) after fluid percussion brain injury were previously assessed in experimental studies in rats (Dietrich et al., 1996). At 4 days after TBI, delayed post-traumatic hyperthermia significantly increased both mortality and contusion volume compared to normothermia. Three hours of hyperthermia increased hemorrhage, blood–brain barrier permeability, axonal swelling, and thinning of myelination. These experimental animal results strongly suggest that post-traumatic brain hyperthermia might increase morbidity and mortality in patients with head injury by aggravating axonal and microvascular damage (Dietrich et al., 1996).
Despite a sound physiological argument for controlling fever in the brain-injured patient, there is presently no direct evidence that doing so will improve clinical outcome (Cairns and Andrews, 2002). Furthermore, there is abundant experimental evidence suggesting that hyperthermia leads to, or exacerbates, neuronal injury in conditions such as cerebral ischemia and TBI. However, conclusive evidence linking prevention of hyperthermia with improved outcomes of TBI patients is lacking (Aiyagari and Diringer, 2007). It has been difficult to design studies evaluating the impact of hyperthermia control on outcome, in part because traditional methods of hyperthermia control are ineffective. Recently, several new devices to control temperature have become available. These devices appear to be more effective than conventional means, and might allow for the design of studies that directly address this question. Randomized, controlled clinical trials are necessary to determine whether or not control of early hyperthermia after trauma improves the outcome of acute head trauma patients.
In summary, our study demonstrated that both the degree and duration of early post-trauma hyperthermia are strongly related with the outcome of patients with acute TBI. The detection of early post-traumatic hyperthermia may be helpful for neurosurgeons in predicting the outcome of severe traumatic brain-injured patients.
Chinese Head Trauma Study Collaborators
Li Jing,* Xu Shi-yi,** Jiang Ji-yao, Shanghai Renji Hospital (*first author, **co-first author); Yang Yi-lin, Qing Hua-ping, Jiangsu Changzhou First People's Hospital; Qian Suo-kai, Nanchang No. 94 Hospital; Yang Xiao-feng, Zhejiang University the First Affiliated Hospital; Feng Hua, Chongqing Xinan Hospital; Yu Ru-tong, Xuzhou Medical College Affiliated Hospital; Liu Zhi-xiong, Changsha Xiangya Hospital; Liu Jian-ming, Shanghai Changhai Hospital; Yang Hua-tang, Hebei Hangdan Hospital; Yang Chao-hua, Sichuan Huaxi Hospital; Long Lian-shen, Zhejiang Huzhou No. 98 Hospital; Zhang Jun, Shenzhen Longgang Hospital; Zhu Xiao-jiang, Shanghai First People's Hospital; Huang Qiang, Zhejiang Qiuzhou Hospital; Liu Bai-yun, Beijing Tiantan Hospital; Tong Wu-song, Shanghai Pudong Hospital; Sun Xiao-chun, Chongqing Medical University Affiliated Hospital; Yang Mu-lin, Nanchang Wujing Hospital; Zhang Nu, Wenzhou Medical College; Fang Nai-cheng, Zhejiang Zhuji Hospital; Qi Song-tao, Guangzhou Nanfang Hospital; Song Xi-wen, Shanghai Fengcheng Hospital; Tu Chuan-jian, Zhejiang Shaoxin County Hospital; Wang Ning, Harbin Medical University; Wu Tian-shun, Jiangxi Qianshan Hospital; Song Guang-lin, Shandong Jiaozhou Hospital; Tong Zheng-zhong, Jilin Tonghua Hospital; Fu Xian-an, Suzhou North Hospital; Fan Yong-jun, Jiangsu Lianyungang Second Hospital; Ni Xiang-yang, Jiangsu Yancheng Chinese Hospital; Cui Jian-zhong, Hebei Tangshan Worker's Hospital; Liang En-he, Tianjin Huanhu Hospital; Bao Nan, Shanghai Children's Hospital; Feng Dong-fu, Shanghai No. 3 Hospital; Xu Wei, Kunming Medical College; Li Wei-ping, Shenzhen Second People's Hospital; Fu Zheng, Jiangsu People's Hospital; Wang Zhong, Suzhou University Affiliated Hospital; Wang Yu-hai, Jiangsu Wuxie No. 101 Hospital; Yuan Jian-lie, Zhejiang Jinhua Hospital; Jin Guo-liang, Zhejiang Shaoxin Hospital; Chen Lin-bao, Shanghai No. 7 Hospital; Li Shi-ting, Shanghai Xinhua Hospital; Sun Yu-hai, Shanghai Zhoupu Hospital; Zhang Jian-lin, Tianjin General Hospital; Lei Ting, Wuhan Tongji Hospital; Du Hang-geng, Hangzhou Xinhua Hospital.
Acknowledgments
This work was supported by funding from a National Health Science Grant (no. 200802093), the National Key Basic Research Project (no. 2012CB518100), the Science and Technology Committee of Shanghai (no. 10JC1409800 and 08411951900), and the Program for Shanghai Outstanding Medical Academic Leaders.
We thank Dr .Jin-fen Zhu for assistance with statistical analysis. We also thank Prof. Bruce Lyeth, University of California–Davis for English editing.
Author Disclosure Statement
No competing financial interests exist.
References
- Aiyagari V. Diringer M.N. Fever. J. Neurol. Sci. 2007;261:39–46. doi: 10.1016/j.jns.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Badjatia N. Hyperthermia and fever control in brain injury. Crit. Care Med. 2009;37:S250–S257. doi: 10.1097/CCM.0b013e3181aa5e8d. [DOI] [PubMed] [Google Scholar]
- Braakman R. Glepke G.J. Habberna J.D.F. Maas A., I Minderhound J.M. Systematic selection of prognostic features in patients with severe head injury. Neurosurgery. 1980;6:362–370. [PubMed] [Google Scholar]
- Bullock R. Chesnut R.M. Clifton G.L. Guidelines for the management of severe head injury. Brain Trauma Foundation. J. Neurotrauma. 2000;17:449–627. doi: 10.1097/00063110-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Cairns C.J. Andrews P.J. Management of hyperthermia in traumatic brain injury. Curr. Opin. Crit. Care. 2002;8:106–110. doi: 10.1097/00075198-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K. Alonso O.F. Kraydieh S. Dietrich W.D. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immuocytochemical studies. J. Cereb. Blood Flow Metab. 2000;20:531–542. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Childs C. Vail A. Leach P. Rainey T. Protheroe R. King A. Brain temperature and outcome after severe traumatic brain injury. Neurocrit. Care. 2006;5:10–14. doi: 10.1385/NCC:5:1:10. [DOI] [PubMed] [Google Scholar]
- Clifton G.L. Jiang J.Y. Lyeth B.G. Jenkins L.W. Hamm R.J. Hayes R.L. Marked protection by moderate hypothermia after experimental TBI. J. Cereb. Blood Flow Metab. 1991;11:114–121. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- Colohan A.R.T. Alves W.M. Gross C.R. Jane J.A. Head injury mortality in two centers with different emergency medical services and intensive care. J. Neurosurg. 1989;71:202–207. doi: 10.3171/jns.1989.71.2.0202. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Halley M. Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38:533–541. doi: 10.1097/00006123-199603000-00023. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Busto R. Halley M. Valdes I. The importance of brain temperature in alteration of the blood–brain barrier following cerebral ischemia. J. Neuropath. Exp. Neurol. 1990;49:495–500. doi: 10.1097/00005072-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. The importance of brain temperature in cerebral injury. J. Neurotrauma. 1992;9:S475–S485. [PubMed] [Google Scholar]
- Fearnside M.R. Cook R.J. McDougall P. Lewis R.J. The Westmead head injury project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br. J. Neurosurg. 1993;7:267–279. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Guo G.Y. Li W.P. Yu M.K. Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Xu W. Li W.P. Xu W.H. Zhang J. Bao Y.H. Ying Y.H. Luo Q.Z. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: A multicenter, prospective, randomized controlled study. J. Neurotrauma. 2005;22:623–628. doi: 10.1089/neu.2005.22.623. [DOI] [PubMed] [Google Scholar]
- Low D. Kuralmani V. Ng S.K. Ang B.T. Prediction of outcome utilizing both physiological and biochemical parameters in severe traumatic brain injury. J. Neurotrauma. 2009;26:1177–1182. doi: 10.1089/neu.2008.0841. [DOI] [PubMed] [Google Scholar]
- Muizelaar J.P. Mamarou A. Anderson R.L. Ward J.D. Kontos H.A. Choi S.C. Becker D.P. Young H.F. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J. Neurosurg. 1991;75:S59–S66. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Greenberg R.P. Miller J.D. Enas G.G. Choi S.C. Kishore P.R. Selhorst J.B. Lutz H.A. Becker D.P. Further experience in the management of severe head injury. J. Neurosurg. 1981;54:751–762. doi: 10.3171/jns.1981.54.6.0751. [DOI] [PubMed] [Google Scholar]
- Phuenpathom N. Choomuang M. Ratanalert S. Outcome and outcome prediction in acute subdural hematoma. Surg. Neurol. 1993;40:22–25. doi: 10.1016/0090-3019(93)90164-v. [DOI] [PubMed] [Google Scholar]
- Teasdale G. Jennet B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Young B. Rapp R.P. Norton J.A. Haack D. Tibbs D.A. Bean R. Early prediction of outcome in head-injured patients. J. Neurosurg. 1981;54:300–303. doi: 10.3171/jns.1981.54.3.0300. [DOI] [PubMed] [Google Scholar]