Abstract
Long-term potentiation (LTP) is a synaptic mechanism underlying learning and memory that has been studied extensively in laboratory animals. The study of LTP recently has been extended into humans using repetitive sensory stimulation to induce cortical LTP. In this review paper we will discuss past results from our group demonstrating that repetitive sensory stimulation (visual or auditory) induces LTP within the sensory cortex (visual/auditory, respectively) and can be measured non-invasively using EEG or fMRI. We will discuss a number of studies that indicate that this form of LTP shares several characteristics with the synaptic LTP described in animals: it is frequency dependent, long-lasting (>1 hour), input specific, depotentiates with low-frequency stimulation, and is blocked by NMDA receptor blockers in rats. In this paper we also present new data regarding the behavioral significance of human sensory LTP.
These advances will permit enquiry into the functional significance of LTP that has been hindered by the absence of a human model. The ability to elicit LTP using a natural sensory stimulus non-invasively will provide a model system allowing the detailed examination of synaptic plasticity in normal subjects and may have future clinical applications in the diagnosis and assessment of neuropsychiatric and neurocognitive disorders.
Keywords: Long-term potentiation (LTP), Human, Electroencephalography (EEG), Sensory stimulation, Visual evoked potential (VEP), Cortical plasticity
INTRODUCTION
Until recently, the study of LTP in humans was limited to examination of its properties in excised cortical tissue. Techniques have now been developed, using rTMS (repetitive transcranial magnetic stimulation) and EEG (electroencephalography), which enable non-invasive investigation of LTP in healthy intact human cortical tissue (see refs 1 and 2 for reviews of this work). In this paper we provide an overview of the evidence that LTP can be induced by repetitive sensory stimulation that is similar to the repetitive electrical stimulation used in-vitro, and can be measured non-invasively using EEG and fMRI. An advantage of this approach is that a natural sensory stimulus can be used to non-invasively probe cortical plasticity. This may be useful in assessing whether abnormal degrees of synaptic plasticity may represent endophenotypes of disease in humans. As the same sensory stimuli can also be used to induce LTP in animals, it may be possible to test the pharmacology of candidate compounds regulating synaptic plasticity preclinically, before the translation to human clinical models.
Long-Term Potentiation
Cognitive and memory functions are generally thought to involve the adjustment of synaptic strength in networks of connected cortical neurons. Long-term potentiation (LTP) is a process whereby synaptic strength is rapidly increased and is currently the best candidate for the substrates of cognition and memory. While LTP has been studied extensively at the cellular and molecular level in laboratory animals (3), LTP had not, until recently, been shown in the intact human. LTP has been demonstrated in humans in isolated cortical tissue obtained from patients undergoing surgery, specifically from the hippocampus (4) and the temporal lobe (5), where it displays properties identical to that seen in animals. To date, investigation of synaptic plasticity in intact humans has been hindered by the lack of translational methodology. Before explaining the current state of a human LTP model we first will lay out the mechanisms, induction parameters and specifications of synaptic LTP as studied in animal models.
In animal studies, LTP is induced by high-frequency electrical stimulation (HFS or tetanus) of afferents. LTP is observed as an immediate and enduring increase in the postsynaptic response at glutamate synapses in cortical neurons both from single neurons and from populations. The HFS leads to increased excitability of the dendritic spine on the postsynaptic neuron. This, in turn, activates both the NMDA glutamate receptor subtype (NMDAR, which is normally quiescent) and voltage dependant calcium channels to briefly increase postsynaptic Ca2+ levels. This transient increase in Ca2+ concentration induces LTP by activating a molecular signaling cascade which results in the insertion of more glutamate AMPA receptors (a receptor subtype that is always active) into the postsynaptic membrane and increasing the ionic conductance of existing AMPA receptors – both resulting in larger postsynaptic excitatory responses (6,7,8). The cellular and molecular mechanisms underlying LTP induction and expression are reasonably well understood (9,10).
The maintenance phase of LTP is the continued expression of increased synaptic efficacy that can last for hours in vitro and for days in vivo (11). This phase is supported by post-synaptic insertion of additional AMPA and NMDA receptors, neurotrophins and synthesis of new proteins (12,13,14,15). The cellular mechanism of LTP is complex and subject to many modulators of the synaptic function, including mGluRs, NO, noradrenaline, serotonin, dopamine, acetylcholine, nicotine, sex hormones such as estrogen, cytokines, and mitochondrial enzymes (16,17,18). Other factors such as stress or sleep deprivation can diminish/block LTP (19,20). Generally, in adult animals, LTP obeys several rules: it is long-lasting, frequency dependent (stimulation with higher frequencies induce potentiation, while stimulation at low frequencies induce depotentiation to pre-LTP levels), input specific, dependent on increases in the intracellular calcium levels, and it is saturable (can reach a ceiling) (1).
In behaving animals: (a) LTP can be observed following learning a task (in the absence of HFS), (b) electrically inducing LTP results in improvements of subsequent learning and (c), pharmacologically blocking the NMDAR blocks LTP and (d) interferes with learning (1). All of these observations suggest that LTP is a normal substrate of learning and, when induced experimentally, improves subsequent performance. Recently, two methods for the non-invasive evaluation of cortical plasticity in humans have been developed: use of sensory evoked potentials to evaluate cortical areas (2,21) and use of TMS to probe the motor cortex (1). Studies from the Teyler and Bear labs have demonstrated that when rodents are exposed to a similar repetitive visual stimulation, the evoked response in the visual cortex is enhanced (22,23,24), and that visual cortex LTP is related to improved performance in a visual discrimination task (25).
Natural sensory stimulation had also been shown to induce LTP in visual areas of the developing tadpole (26). Therefore, in order to translate animal studies of LTP to humans, we reasoned that high-frequency sensory activity arriving at sensory cortex might initiate LTP by exciting neurons similar to the electrical induction of LTP by HFS common in animal studies and that it may be reflected in the sensory evoked potential (EP). The method involves substituting high-frequency electrical stimulation of afferents with rapid sensory stimulation, assuming that a sensory volley arriving at neocortex is analogous to the afferent volley elicited by electrical stimulation. We have presented subjects with both visual and auditory high-frequency stimulation and have measured the resultant potentiation as an alteration in the EP relative to a pre-stimulation baseline. The observed plasticity is localized to the appropriate regions of sensory neocortex and possesses corresponding properties to those observed in animal preparations leading us to conclude that we are observing the network manifestation of LTP with potentiation occurring in a large population of cortical synapses (1,2).
LTP induced by Visual Stimulation
In our initial demonstration of sensory induced LTP we presented healthy humans, fitted with an array of 128 EEG recording electrodes (Electrical Geodesics Inc., Eugene, OR), with a checkerboard visual stimulus (21). We first presented a block of checkerboard stimuli (subtending 4 degrees of visual angle) presented at low-frequency (1 Hz) to either the right or left visual hemifield in order to obtain a stable baseline visual evoked potential (VEP; Fig 1). Stimuli were presented to each hemifield to isolate whether the potentiation was unilateral (i.e. direct) or bilateral (i.e. direct + indirect). Next we presented the same checkerboard at high-frequency (9 Hz) for two minutes to induce LTP. To measure the resultant potentiation we again presented blocks of the checkerboard presented at low-frequency for up to 1 hour. We observed an increase in the VEP following the high-frequency stimulation (Fig 1), indicating that LTP was present. Since the VEP is a complex response consisting of several overlapping responses, we subjected it to an Independent Components Analysis, which breaks the overall response into its separate components. This analysis revealed that one of the components, a negative waveform peaking around 170 ms post- stimulus – termed N1b, was significantly potentiated (Fig 1 middle, inset). While the potentiated response might reflect LTP, it was also possible that the augmented response was simply due to differing levels of attention. Arguments against this possibility included: 1) the selective potentiation of only one of the components of the VEP, 2) the localization of the potentiation to restricted regions of visual cortex, and 3) the increase was sustained over an hour. Using the same paradigm, but examining the induced oscillations rather than the evoked potential, we have also shown that there is greater alpha desynchronization post visual tetanus (see below). Increased alpha desynchronization suggests that greater cortical resources are being activated in response to the baseline stimuli, consistent with the notion of network level LTP. As source localization of evoked potentials measured at the scalp can be somewhat unreliable, we replicated this experiment and measured the potential potentiation using functional magnetic resonance imaging (fMRI), which offers superior spatial resolution of sources (27). The results of this experiment showed that the hemodynamic (BOLD) response increased post-tetanus in extrastriate (area V2) visual cortex (Fig 1 Bottom panel). This increase in the hemodynamic response in the visual cortex after rapid sensory stimulation strongly indicated an increase in neuronal output as it has been suggested that an increase in BOLD signifies synchronized synaptic activity (28). These results also support the argument that we were able to induce and record LTP within human cortex non-invasively using visual stimulation.
Figure 1.
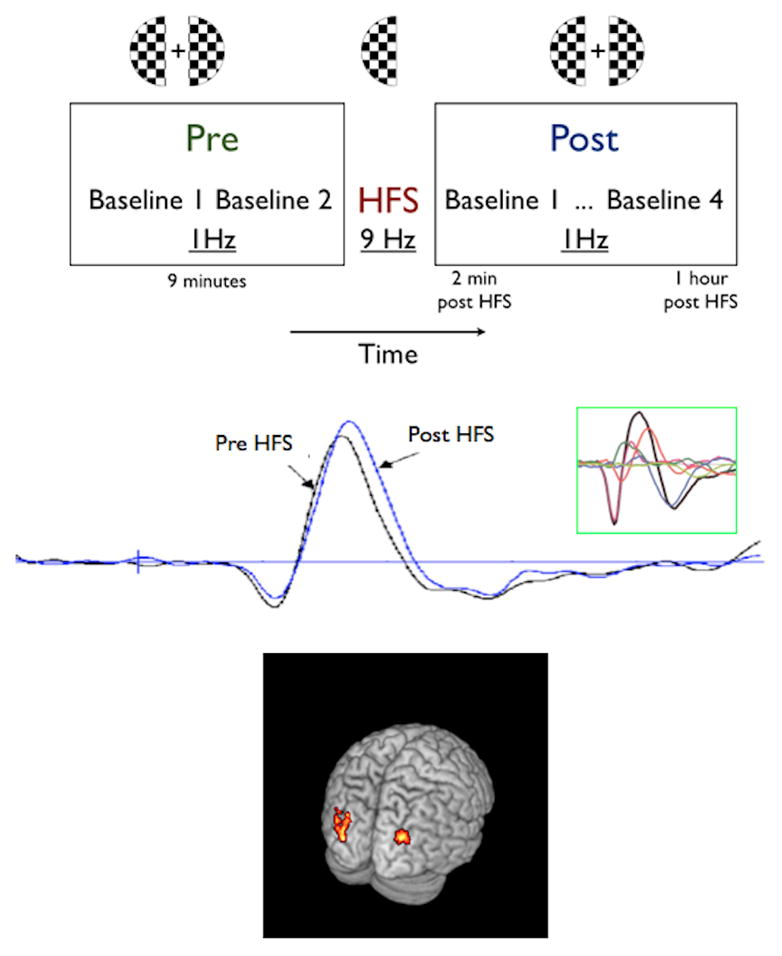
Visual LTP: Top Panel: Experimental paradigm- 2 baseline blocks (1Hz) were delivered before the block of high frequency stimulation (HFS) at 9Hz. LTP was then tracked for 1 hour with 4 blocks of the low frequency baseline stimulation (10). Middle Panel: LTP of human visual evoked potential. Pre- and post-high-frequency stimulation average evoked potentials recorded over the occipital cortex contralateral to the visual stimulus. Inset: Independent Components Analysis showing the component waveforms – only the N1b component (red) displayed LTP (10). Bottom panel- fMRI results. Increased bilateral hemodynamic response in extrastriate visual cortex after a block of HFS. The figure shows the activation pattern of the comparison “post-HFS” (20 min post HFS) vs. “pre-HFS” to a left visual field stimulus. The results show increased BOLD activation bilaterally in the extrastriate visual cortex after the presentation of a block of HFS to one hemifield (left visual field in this case). Statistical Parametric Mapping (SPM(t)) maps are overlaid on a structural MRI brain (11).
LTP induced by Auditory Stimulation
To determine if sensory-induced LTP was limited to the visual system, we adapted the paradigm to the auditory system (29). Tone pips (tone frequency= 1 kHz) were used in all stages of the experiment – i.e. for baseline, tetanus, and post-tetanic recording. During the baseline recording these tone pips were presented with an interstimulus interval determined randomly with the constraint that the value fell within the range 1800–2600 ms (0.45 Hz). Following two sequential baseline recordings (to ensure stability), the auditory tetanus was delivered. The tetanus consisted of 2 minutes of the tone pips presented 13 times per second (13Hz). The auditory evoked potential (AEP) was then tracked with 4 blocks of the baseline stimulation parameters for the next hour. The results demonstrated a significant enhancement of the N1 component of the AEP (Fig 2) that was localized to auditory cortex by fMRI (30) and lasted beyond an hour. These results show that sensory LTP is not limited to visual cortex and may well be a property of all sensory cortices. Consistent with this idea is the fact that repetitive transcranial stimulation techniques suggest that LTP may be a property of motor cortex (1).
Figure 2.
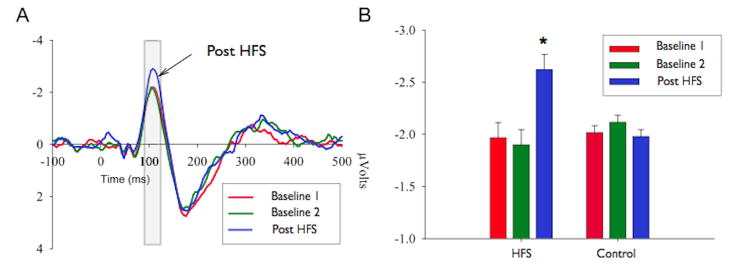
Auditory HFS induces LTP of the N1 component. A) Group average Auditory Evoked Potentials (AEP) to tone pip stimulation. The grey section indicates the time window of interest. The red and green lines represent the 2 baseline blocks (pre HFS) and the blue line indicates the post-HFS AEP. Note that the post-HFS response is substantially larger than the two baseline blocks in the time window of interest (N1). B) Group average N1 amplitudes are shown for the two pre-HFS baseline periods, and the post-HFS period. The data reveals that only the group of subjects that received an auditory HFS had a significantly larger N1 amplitude (p<.05). Error bars represent the standard error of the mean (SEM) (12).
Depotentiation
Animal studies have shown that a potentiated response, while lasting hours-days, can be actively eliminated (or depotentiated) with the application of low-frequency electrical stimulation. Depotentiation (and/or long term depression) may be involved in the homeostatic regulation of plasticity to prevent saturation, to facilitate extinction and forgetting (1), and to improve signal information processing by enhancing neuronal signal-to-noise ratios (31). In our initial visual LTP experiment (21) we found that the N1b response was significantly potentiated immediately following high-frequency visual stimulation, but declined slowly thereafter. This suggested that either our plasticity was transient (e.g. dissipating over the passage of time) or due to depotentiation because we were constantly stimulating the visual cortex with low frequency visual stimulation (in order to measure the plasticity effects). To differentiate between these possibilities, we repeated the experiment but withheld a number of blocks of baseline stimulation. By omitting select blocks of post-stimulation baseline the response did not decline, supporting the idea that the baseline stimulation resulted in an active depotentiation of the LTP. These results suggest that human sensory LTP can be depotentiated by low-frequency stimulation, as is the case in animal studies of LTP.
Input Specificity
Changes in the amplitude of a postsynaptic response may reflect the overall excitability of the neuron or network under study. It is possible that the high-frequency stimulation results in a non-specific heightened excitability which is then reflected as an enhanced response, as opposed to the synapse-specific enhancement seen in LTP. In animal studies this is controlled for by identifying two independent inputs to the same cell or network, obtaining baseline responses for both inputs, and then electrically stimulating just one of them. Only the input that receives the high-frequency stimulation shows LTP. This defining characteristic of LTP is termed Input Specificity.
Such manipulations cannot be carried out using the non-invasive approach we have adopted. Therefore we developed an analogous method by presenting two related, but distinct, visual stimuli. One experiment used horizontal sine grating stimuli with spatial frequencies of one or five cycles per degree (32), the other used stimuli differing in orientation (horizontal and vertical sine grating stimuli), each with a spatial frequency of one cycle per degree (33). For each experiment, using our standard paradigm, baselines were obtained to both stimuli (i.e. one and five cycles per degree horizontal sine gratings), but only one of the visual stimuli was presented at high-frequency (i.e., five cycles per degree horizontal sine grating). The critical measure is if the potentiation is specific to the stimulus that was presented at a high-frequency for 2 minutes. In both the spatial frequency and orientation specificity experiments only the stimulus that was presented rapidly for 2 minutes yielded LTP that lasted throughout the time tested (20 min post tetanus) (Fig 3), indicating that human sensory LTP conforms to input specificity - a defining characteristic of LTP. These results also further demonstrate that the potentiated responses do not reflect generalized changes in attention or arousal, as this would be expected to affect both stimuli.
Figure 3.
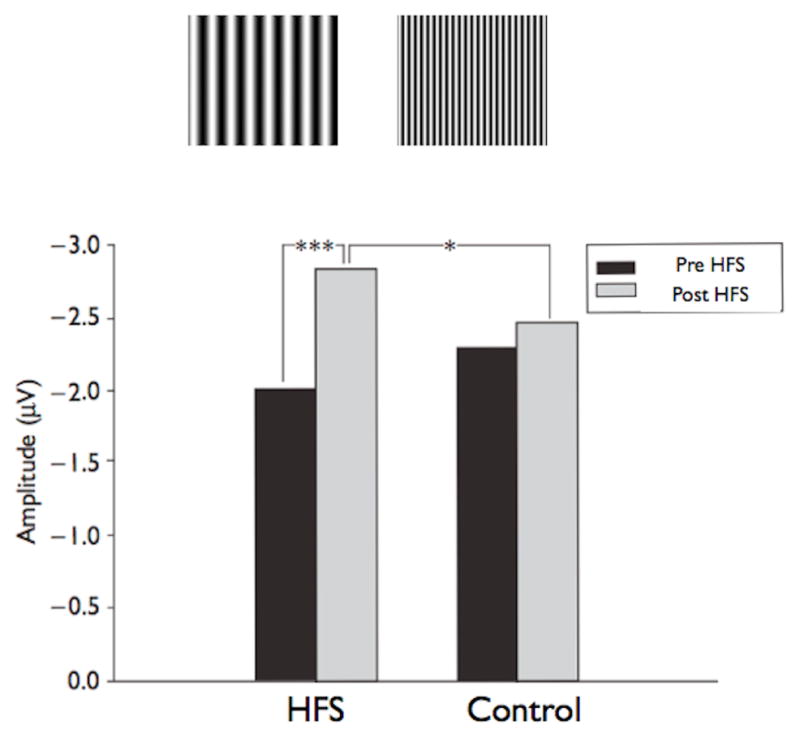
Top Panel: Low and High Frequency sine gratings. Bottom Panel: Amplitudes of the visual N1b potential for pre-HFS and post-HFS presentations of sine gratings that were (HFS) or were not given HFS (Control). A significant difference was observed between the N1b amplitudes for pre-HFS and post-HFS presentations of the grating that was tetanized with HFS (P<0.001) but not the grating that was presented only at the baseline rate (P=0.258). Additionally, the difference in N1b amplitude between the tetanized and non tetanized gratings was not significant for pre-HFS presentations (P=0.273), but was significant following the HFS (P=0.041) (14).
LTP Induction and Duration
Induction
Animal studies have explored the induction parameters of LTP and report a wide range of effective high-frequency electrical stimulation (from 12Hz to 400Hz) generally given in short (100msec – 1 sec) bursts. Using sensory systems to deliver afferent volleys to induce LTP in cortex limits the range considerably. In the visual system, flicker fusion (where rapidly flashing light is perceived as a steady light), generally limits the useful frequency rate to below 40Hz. In our experiments we empirically determined that a 9 Hz high-frequency stimulation, using our patterns, yielded significant LTP.
In a multi-synaptic network composed of feedback loops like the visual and auditory systems, the high-frequency visual stimulation may set off a burst of higher frequency firing (50–100Hz for example) within the thalamocortical or cortico-cortical pathways. Thus, while not determining the intraburst frequency, the high-frequency visual stimulation may determine the interburst period (or burst frequency (2)). For example, the visual stimulation, presented at a rate of 9Hz, may actually be initiating a higher-frequency burst nine times a second. In this way the visual high-frequency stimulation may be closer to traditional electrical stimulation, where bursts of higher frequency stimulation are used to induce LTP. Electrical stimulation in the hippocampus of animals (a favorite structure for the study of LTP) typically consists of a train of theta-burst stimulation (e.g., 4 pulses at 100 Hz repeated with 200 ms inter-burst-intervals) designed to mimic hippocampal firing patterns during exploration. We have found that the most effective visual LTP stimulation frequency is in the alpha range. It is possible our stimuli are entraining alpha-burst (9 Hz) activity (2). Certainly, neurons in the visual cortex are most sensitive to alpha range oscillations (7–12 Hz) (34,35). Additionally, 7–8Hz is the natural resonance of the thalamocortical pathway (34,35), and the 9Hz visual stimulation sits on the high end of this resonating frequency. In the auditory modality it has been found that auditory cortex is most sensitive to 10–14Hz stimulation (36), and our auditory LTP stimulation was delivered at 13Hz. It seems likely that by delivering a sensory tetanus at the rate at which a particular sensory cortex is most sensitive would induce rhythmic thalamocortical/cortico-cortical high frequency bursts that, similar to electrical tetanic bursts, induce LTP (2).
Also of note, in most contemporary animal studies of LTP scientists use high-frequency bursts of electrical stimulation (such as theta-burst stimulation mentioned above) to rapidly induce maximum LTP. In the early days of LTP research in animals (37), scientists used separated bursts of much lower rates of stimulation which resulted in a step-like expression of potentiation - a paradigm arguably closer to natural patterns of neural activity than high-frequency stimulation which immediately induces maximal LTP. The experiments of Cooke and Bear (38) have demonstrated that the daily exposure to a visual stimulus is also capable of inducing potentiation.
Duration
Animal studies have shown electrically induced LTP to persist for hours to months, depending in the induction parameters (39). In our human studies, practical considerations limited the observation period following high-frequency stimulation to generally an hour. Thus from the human data alone we cannot determine the longevity of human sensory LTP. In a rat study using the same paradigm, we were able to induce cortical LTP using a visual pattern and demonstrate that it persisted 5 hours.
Alpha Desynchronization
A visual stimulus induces not only an evoked potential phase-locked to the stimulus, but also desynchronizes ongoing alpha-range EEG activity (40). A prominent theory of alpha desynchronization (41) holds that the neuronal assemblies involved are more readily propagating their signal through cortico-cortical oscillations, creating a stronger neuronal assembly. If our paradigm induces LTP over a network, then alpha desyncronization should be greater as the network increases its efficiency in propagating the signal. This prediction was confirmed when we examined alpha desychronization in the context of our visual LTP paradigm (40). This suggests that the induction of LTP has the effect of inducing long-term changes in the neuronal assemblies responsible for visual processing.
NMDAR Dependence
Animal studies of LTP have clearly shown that the most prevalent form of LTP across species and in several mammalian brain regions, including hippocampus and cortex is dependent on activation of the glutamatergic NMDAR. To address this defining characteristic of LTP we used our standard visual LTP paradigm in rats (42). Anesthetized rats stimulated with a block of high-frequency visual checkerboard stimulation demonstrated potentiation of the intracortical visual cortex field potential, showing that sensory stimulation is capable of inducing LTP in rodent sensory cortex as well. To test for NMDAR dependence, we administered the NMDAR antagonist CPP (3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid) which blocked the potentiation without affecting the baseline responses (Fig 4). This result, and that of the Bear lab (25), demonstrates that visual cortex LTP in rat was blocked by NMDAR antagonists and suggests that the cellular mechanisms underlying both human and non-human sensory LTP may be similar.
Figure 4.
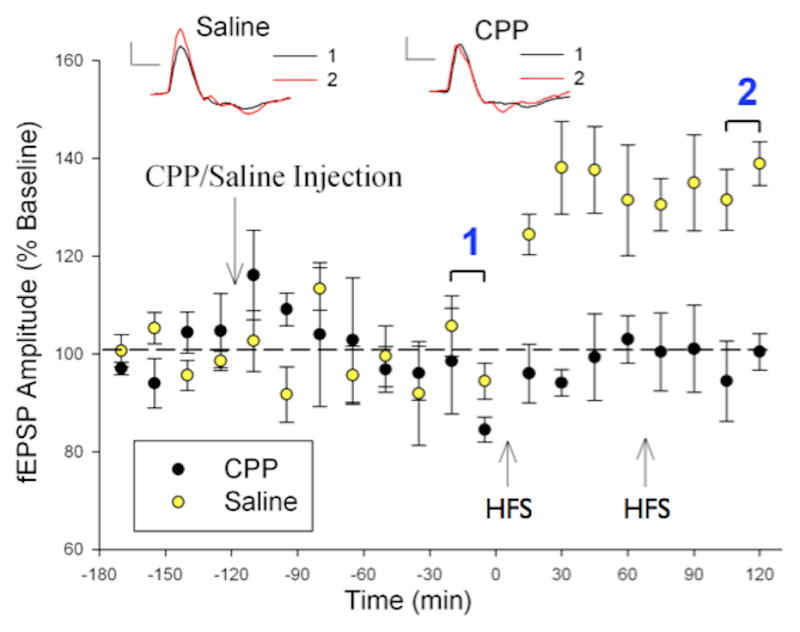
NMDA receptor-dependence of visual cortex LTP in rat. Baseline responses to a checkerboard stimulus were collected 1 hour before injection of either CPP (an NMDAR blocker) or saline. A block of HFS was delivered at the times marked by the arrows. In animals that received saline (n=5), a significant increase in evoked potential amplitude is recorded immediately after the delivery of HFS and remained increased throughout the testing (p<.05), indicating that LTP had been induced. CPP (n=4) completely blocked the induction of sensory LTP. Inset, averaged evoked potentials from baseline checkerboard stimulation pre- (black) and post-HFS (red) in representative animals that received either an injection of saline (left) or CPP (right). The evoked potentials are taken from the time periods denoted by 1 (pre) and 2 (post). Scale bars: 0.1 mV, 100 ms (20).
Behavioral Consequences
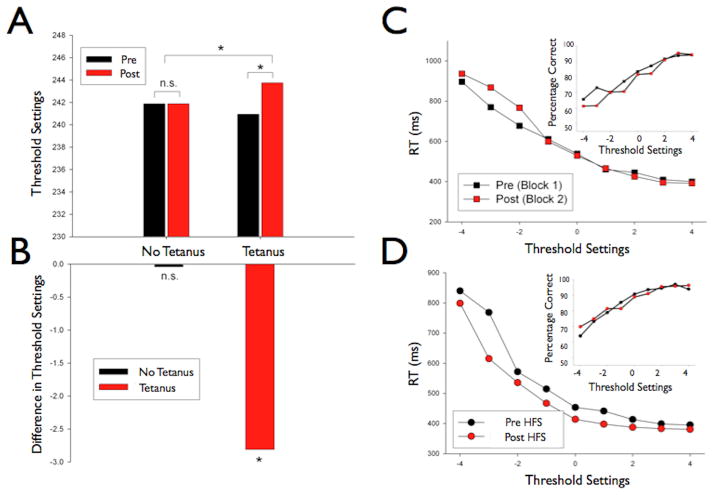
It is possible that the high frequency visual stimulation we used to induce LTP could be accompanied by a change in visual performance. To investigate this possibility, we performed an experiment in which participants’ visual detection thresholds were measured both before and after the presence of a high frequency stimulation (9 Hz visual tetanus) (see Supplementary Material for all methods and results) or no visual tetanus. This within-subject experiment revealed that when no HFS was delivered, two independent measurements of visual detection remained the same, yet when HFS was delivered (standard 2 minutes of stimulation at 9Hz) the detection threshold, as set by the descending method of limits, was shown to drop significantly (see Fig 5), however the accuracy on a two alternative forced choice task remained unchanged. Despite this lack of change in accuracy for the two alternative forced choice task, there was an improvement in response times. Thus, there is an encouraging indication that presenting visual checkerboards at a rapid rate not only results in detectable LTP in the system but may also result in a parallel improvement in visual detection thresholds. Once potentiated, these neuronal assemblies may be more likely to respond to previously subthreshold stimuli, possibly allowing subjects to see what they previously could not. It should be stated however, that these data must be treated with some caution. The methods used do not control for a change in response strategy for example. Although promising, there needs to be further behavioral work employing more rigorous psychophysical methods.
Figure 5.
Behavioral performance altered by LTP. Left: Lowering of detection thresholds after HFS. A) Threshold settings on Control day (no HFS/tetanus) and Experimental day (HFS/tetanus). B) Change in Threshold from Block A to Block B for both conditions. This figure reveals that when subjects were in the Control condition, their threshold settings from Block A and Block B remained the same (p>.05). Whereas when subjects received an HFS their threshold settings in Block B were much lower than from Block A, indicating a change in perceptual performance due to the HFS (p<0.001). Right: Detection performance increases after the administration of an HFS/tetanus. C) Block A Median Reaction Times (RT) in the Control condition, both before and after a 2 minute pause in testing. There was very little difference between the blocks when no HFS is administered. Inset- Accuracy data demonstrating that accuracies were very similar between the two blocks and there was a slight decrease in accuracy in the second block. D) Block B Median Reaction Times in the session when subject’s received an HFS. The reaction times are significantly decreased after the HFS, suggesting that subjects’ visual detection thresholds had lowered and thus they were significantly faster in responding to near threshold stimuli. Inset- Accuracy data revealing that accuracy did not change in the HFS condition between blocks signifying no evidence for a speed accuracy trade-off.
SUMMARY
This series of experiments demonstrates that rapidly presented sensory stimulation can induce LTP in specific regions of sensory cortex noninvasively in healthy humans. This LTP conforms to the characteristics and mechanisms of cellular studies of LTP in animal preparations, and thus may be considered as a network correlate of LTP. The paper by Cooke and Bear (38) demonstrates that cortical LTP can be induced by more naturalistic visual experience, arguing that it is a normal constituent of visual information processing in the brain. It can be anticipated that methods will be developed to extend the study of human LTP beyond sensory systems to tasks with an explicit cognitive component. This will allow for the study of the functional role of LTP in human subjects.
These results suggest that this methodology could have utility in rehabilitation of visual deficits such as agnosia, amblyopia and other signal detection deficient behaviors. Additionally, since deficits in synaptic plasticity are believed to underlie human neuropsychiatric disorders such as schizophrenia, this approach may provide a useful method of assessing the status of synaptic plasticity in patients. To the extent that human neurocognitive disorders involve defects in synaptic plasticity, this method may be used as a biomarker to reveal differences between normal humans and patients suffering from a variety of disorders of neocortical origin.
As is shown in the paper by Cavus, et al (43) the ability to assess functional neurophysiological changes in the human cortex revealed that patients suffering from schizophrenia were impaired in their ability to express LTP. Additionally, in this issue it is shown by Mears and Spencer that auditory LTP is disrupted in schizophrenic patients (44). A study on patients suffering from major depression demonstrated that this group also failed to demonstrate normal LTP to a visual tetanus (45). The extent to which these results reflect the underlying neuropathology of the diseases remains for further study, but as synaptic plasticity is a fundamental neurobiological process it is conceivable that future research will reveal cortical constellations of defective synaptic plasticity unique to each disorder. The ability to detect sensory LTP by fMRI suggests that it may be possible to utilize this approach to scan brains for abnormal patterns of synaptic plasticity. It is currently unknown how early in the course of these disorders these neurophysiological changes can be observed, but it may be possible to use these changes as early biomarkers of the disorder in order to apply appropriate interventions at an early time. Similarly, the effectiveness of medications can be evaluated and perhaps predicted by their ability to affect neocortical synaptic plasticity. Early studies into this possibility suggest that effective anti-depression medications act to facilitate cortical LTP thus counteracting the decrement seen in the disorder (42). Our observation of the behavioral consequences of repetitive sensory stimulation also opens the possibility of developing treatment modalities to treat these disorders.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health (TJT, IJK, JPH) grant R01 MH064508, the NZ Neurological Foundation (JPH, IJK) grant no. 0311-SPG, and the NZ Royal Society Marsden Fund (IJK).
Footnotes
Financial Disclosures
Wesley C. Clapp reported that his name is included under a provisional patent titled “A Noninvasive Biomarker of Human Cortical Plasticity and Cognition”. Jeff P. Hamm reported research funding from the NIH and the NZ Neurological Foundation and that his name is included under a provisional patent titled “A Noninvasive Biomarker of Human Cortical Plasticity and Cognition”. Ian J. Kirk reported research funding from the NIH, the NZ Neurological Foundation, the NZ Royal Society Marsden Fund and that his name is included under a provisional patent titled “A Noninvasive Biomarker of Human Cortical Plasticity and Cognition”. Timothy J. Teyler reported research funding from the NIH, a consulting appointment with Pfizer Laboratories, and that his name is included under a provisional patent titled “A Noninvasive Biomarker of Human Cortical Plasticity and Cognition”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooke SF, Bliss TVP. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 2.Kirk IJ, McNair NA, Hamm JP, Clapp WC, Mathalon DH, Cavus I, Teyler TJ. Long-term potentiation of human sensory-evoked potentials. WIRES Cognitive Science. 2010;1(5):766–773. doi: 10.1002/wcs.62. Wiley Interscience On-line Reviews. [DOI] [PubMed] [Google Scholar]
- 3.Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232(2):331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck H, Goussakov IV, Lie A, Helmstaedter C, Elger CE. Synaptic plasticity in the human dentate gyrus. J Neurosci. 2000;20(18):7080–6. doi: 10.1523/JNEUROSCI.20-18-07080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen WR, Lee S, Kato K, Spencer DD, Shepherd GM, Williamson A. Long-term modifications of synaptic efficacy in the human inferior and middle temporal cortex. Proc Natl Acad Sci U S A. 1996;93(15):8011–5. doi: 10.1073/pnas.93.15.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luscher C, Frerking M. Restless AMPA receptors: implications for synaptic transmission and plasticity. Trends Neurosci. 2001;24(11):665–70. doi: 10.1016/s0166-2236(00)01959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):707–14. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 9.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;12:364(1515):357–67. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313(5790):1141–4. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 13.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–26. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 14.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–25. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–40. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem. 2007;87:372–84. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abel T, Nguyen PV. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog Brain Res. 2008;169:97–115. doi: 10.1016/S0079-6123(07)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleppisch T, Feil R. cGMP signalling in the mammalian brain: role in synaptic plasticity and behaviour. Handb Exp Pharmacol. 2009;191:549–79. doi: 10.1007/978-3-540-68964-5_24. [DOI] [PubMed] [Google Scholar]
- 19.Artola A, von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, Spruijt BM. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci. 2006;23:261–72. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 20.Foy MR, Baudry M, Diaz Brinton R, Thompson RF. Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis. 2008;15:589–603. doi: 10.3233/jad-2008-15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teyler TJ, Hamm JP, Clapp WC, Johnson BW, Corballis MC, Kirk IJ. Long-term potentiation of human visual evoked responses. Eur J Neurosci. 2005;21:2045–2050. doi: 10.1111/j.1460-9568.2005.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaknin G, DiScenna P, Teyler TJ. A method for calculating current source density (CSD) analysis without resorting to recording sites outside the sampling volume. J Neurosci Meth. 1988;24:131–135. doi: 10.1016/0165-0270(88)90056-8. [DOI] [PubMed] [Google Scholar]
- 23.Kenan-Vaknin G, Teyler TJ. Laminar pattern of synaptic activity in rat primary visual cortex: Comparison of in-vivo and in-vitro studies employing the current source density analysis. Brain Res. 1994;635:37–48. doi: 10.1016/0006-8993(94)91421-4. [DOI] [PubMed] [Google Scholar]
- 24.Frenkel MY, Sawtell NB, Diogo ACM, Yoon B, Neve RL, Bear MF. Instructive Effect of Visual Experience in Mouse Visual Cortex. Neuron. 2006;51:339–349. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Heynen AJ, Bear MF. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J Neurosci. 2001;21:9801–13. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LI, Tao HW, Poo M. Visual input induces long-term potentiation of developing retinotectal synapses. Nat Neurosci. 2000;3:708–715. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]
- 27.Clapp WC, Zaehle T, Lutz K, Marcar VL, Kirk IJ, Hamm JP, Teyler TJ, Corballis MC, Jancke L. Effects of long-term potentiation in the human visual cortex: a functional magnetic resonance imaging study. Neuroreport. 2005;16:1977–1980. doi: 10.1097/00001756-200512190-00001. [DOI] [PubMed] [Google Scholar]
- 28.Arthurs OJ, Williams EJ, Carpenter TA, Pickard JD, Boniface SJ. Linear coupling between functional magnetic resonance imaging and evoked potential amplitude in human somatosensory cortex. Neurosci. 2000;101:803–806. doi: 10.1016/s0306-4522(00)00511-x. [DOI] [PubMed] [Google Scholar]
- 29.Clapp WC, Kirk IJ, Hamm JP, Shepherd D, Teyler TJ. Induction of LTP in the human auditory cortex by sensory stimulation. Eur J Neurosci. 2005;22:1135–1140. doi: 10.1111/j.1460-9568.2005.04293.x. [DOI] [PubMed] [Google Scholar]
- 30.Zaehle T, Clapp WC, Hamm JP, Meyer M, Kirk IJ. Induction of LTP-like changes in human auditory cortex by rapid auditory stimulation: an FMRI study. Restor Neurol Neurosci. 2007;25:251–259. [PubMed] [Google Scholar]
- 31.Gladding CM, Fitzjohn SM, Molnár E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNair NA, Clapp WC, Hamm JP, Teyler TJ, Corballis MC, Kirk IJ. Spatial frequency-specific potentiation of human visual-evoked potentials. Neuroreport. 2006;17:739–741. doi: 10.1097/01.wnr.0000215775.53732.9f. [DOI] [PubMed] [Google Scholar]
- 33.Ross RM, McNair NA, Fairhall SL, Clapp WC, Hamm JP, Teyler TJ, Kirk IJ. Induction of orientation-specific LTP-like changes in human visual evoked potentials by rapid sensory stimulation. Brain Res Bull. 2008;76:97–101. doi: 10.1016/j.brainresbull.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Destexhe A. Modelling corticothalamic feedback and the gating of the thalamus by the cerebral cortex. J Physiol Paris. 2000;94:391–410. doi: 10.1016/s0928-4257(00)01093-7. [DOI] [PubMed] [Google Scholar]
- 35.Destexhe A, Rudolph M, Fellous JM, Sejnowski TJ. Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience. 2001;107:13–24. doi: 10.1016/s0306-4522(01)00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hose B, Langner G, Scheich H. Topographic representation of periodicities in the forebrain of the mynah bird: one map for pitch and rhythm? Brain Res. 1987;422(2):367–73. doi: 10.1016/0006-8993(87)90946-2. [DOI] [PubMed] [Google Scholar]
- 37.Andersen P, Teyler TJ, Wester K. Long-lasting change of synaptic transmission in a specialized cortical pathway. Acta Physiol Scand Suppl. 1973;396:A38. [Google Scholar]
- 38.Cooke SF, Bear MF. Stimulus-selective response plasticity in the visual cortex: A potential biomarker for the assessment of pathophysiology and treatment of psychiatric disorders. 2011 doi: 10.1016/j.biopsych.2011.09.006. This Vol. [DOI] [PubMed] [Google Scholar]
- 39.Abraham WC. How long will long-term potentiation last? Phil Trans Royal Soc London (Series B) 2003;358(1432):735–744. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clapp WC, Muthukumaraswamya SD, Hamm JP, Teyler TJ, Kirk IJ. Long-term enhanced desynchronization of the alpha rhythm following tetanic stimulation of human visual cortex. Neurosci Lett. 2006;398:220–223. doi: 10.1016/j.neulet.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 41.Lopes da Silva F. Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalogr Clin Neurophysiol. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- 42.Clapp WC, Eckert MJ, Teyler TJ, Abraham WC. Rapid visual stimulation induces N-methyl-Daspartate receptor-dependent sensory long-term potentiation in the rat cortex. NeuroReport. 2006;17:511–515. doi: 10.1097/01.wnr.0000209004.63352.10. [DOI] [PubMed] [Google Scholar]
- 43.Cavus I, Reinhart RMG, Ford JM, Roach BJ, Gueorguieva R, Teyler T, Clapp WC, Krystal JH, Mathalon DH. Impaired Visual Cortical Plasticity in Schizophrenia. 2011 doi: 10.1016/j.biopsych.2012.01.013. This Vol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mears RP, Spencer KM. Electrophysiological assessment of auditory stimulus-specific plasticity in schizophrenia. 2011 doi: 10.1016/j.biopsych.2011.12.016. This Vol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Normann C, Schmitz D, Fürmaier A, Döing C, Bach M. Long-Term Plasticity of Visually Evoked Potentials in Humans Is Altered in Major Depression. Biol Psychiat. 2007;62:373–80. doi: 10.1016/j.biopsych.2006.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.