Abstract
The pathogenesis of minimal change disease (MCD), considered to be the simplest form of nephrotic syndrome, has been one of the major unsolved mysteries in kidney disease. In this review, recent landmark studies that have led to the unraveling of MCD are discussed. A study published earlier this year now explains the molecular basis of major clinical and morphological changes in MCD. The overproduction of angiopoietin-like 4 (ANGPTL4) in podocytes in MCD causes binding of ANGPTL4 to the glomerular basement membrane (GBM), development of nephrotic-range selective proteinuria, diffuse effacement of foot processes, and loss of GBM charge, but is not associated with changes shown by light microscopy in the glomerular and tubulointerstitial compartments. At least some of this ability of ANGPTL4 to induce proteinuria is linked to a deficiency of sialic acid residues, since oral supplementation with sialic acid precursor N-acetyl-D-mannosamine improves sialylation of podocyte secreted ANGPTL4 and significantly reduces proteinuria. Animal models of MCD, recent advances in potential biomarkers, and studies on upstream factors that may initiate glomerular changes are also discussed. In summary, recent progress in understanding MCD is likely to influence the diagnosis and treatment of MCD in the near future.
Index words: Podocyte, proteinuria, glomerulus, nephrotic syndrome, ManNAc, sialic acid
BACKGROUND
Minimal change disease is the primary cause of childhood nephrotic syndrome, and accounts for 10–15% of nephrotic syndrome cases seen in adults (1). MCD is characterized clinically by the explosive onset of edema (sometimes overnight), large amounts of selective proteinuria, and clinical response to glucocorticoid therapy in the majority of patients. Histological features include normal appearance of glomeruli on light microscopy, extensive effacement of foot processes of the podocytes (or glomerular visceral epithelial cells) noted on electron microscopy, and lack of tubulointerstitial fibrosis despite heavy proteinuria.
CASE VIGNETTE
A 19 year old white man presented with a two week history of abrupt onset of swelling of the feet that progressed towards the knee and was associated with facial puffiness. He did not report any shortness of breath, chest pain, skin rash or joint problems. He had gained 30 pounds in weight over this period. His medical history was significant for nephrotic syndrome at the age of 2 which was presumed to be MCD and treated with glucocorticoids. Remission and relapse were noted over the next 2 years, following which he received cyclophosphamide at age 4, and remained on variable doses of glucocorticoids for the next 5 years. Kidney biopsy at age 9 during a relapse revealed normal appearance of glomeruli on light microscopy, negative immunofluorescence, and diffuse effacement of foot processes by electron microscopy, consistent with the diagnosis of MCD. He received glucocorticoids and cyclophosphamide, and remained asymptomatic over the next 10 years until the current episode. Physical exam revealed normal blood pressure and anasarca, but was otherwise unremarkable. Laboratory assessment revealed creatinine 0.9 mg/dL (79.6 μmol/L); estimated GFR > 60 mL/min/1.73 m2 (>1 mL/s/1.73 m2); normal C3, C4, and CBC; total cholesterol 441 mg/dL (11.4 mmol/L); triglycerides 267 mg/dL (3.01 mmol/L); serum albumin 1.6 g/dL (16 g/L); and urinalysis with pH 7.0, specific gravity 1.020, protein (4+), and moderate blood with 4–10 RBC’s/HPF. 24 hour urine collection had 10.3 grams of protein.
He was started on prednisone 60 mg once daily, furosemide 40 mg twice daily, and a proton pump blocker. Within 2 weeks of therapy, anasarca had improved significantly, and urinalysis revealed trace protein. By the end of 4 weeks, urinary protein excretion was within the normal range. The patient received prednisone 60 mg daily for 8 weeks, after which it was gradually tapered. The patient has remained in complete remission for 6 months.
PATHOGENESIS
Changes in the glomerular capillary loop are critical to the pathogenesis of MCD. Extensive effacement of podocyte foot processes is the key morphological change noted in this disease (Figure 1). The GBM, while morphologically unremarkable, has reduced charge, which was thought to explain the development of selective proteinuria in MCD. The proposed role of charge in proteinuria has raised substantial controversy over the years, and is far from resolved (2).
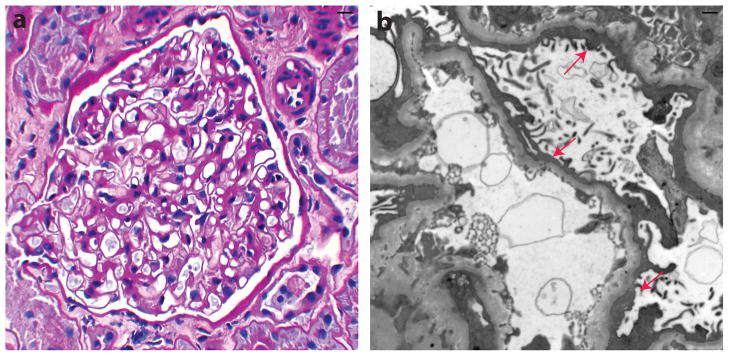
Figure 1.
Histological characteristics of human minimal change disease. (a) Periodic acid–Schiff stain shows normal appearance of glomeruli by light microscopy. (b) Electron microscopy reveals diffuse effacement of podocyte foot processes (arrows). Courtesy of Dr William Cook.
Existing animal models used to study MCD
The most commonly used model of MCD is the puromycin aminonucleoside nephrosis (PAN) model in rats, induced by a single intravenous injection of puromycin aminonucleoside (3). Whereas these rats develop explosive onset of proteinuria and have the classic morphological features, they differ from human MCD in some ways. Their proteinuria, even though in the nephrotic range, is non-selective and only partially glucocorticoid sensitive. This suggests that this model is comprised of a combination of glucocorticoid sensitive and glucocorticoid resistant pathways. Whereas these issues have been ignored in the past, further progress in this field requires use of more rigorous criteria to reassess traditional models of disease. One useful modification of the PAN model is to reduce the injected dose from 15 mg per 100 grams of body weight to 10 mg per 100 grams, in which case proteinuria tends to be more glucocorticoid sensitive.
The protamine sulfate model (4, 5) was developed in rats to mimic MCD-like changes in glomerular capillary loop charge by infusion of protamine sulfate and heparin sequentially into the renal artery. Foot process effacement and proteinuria were dependent upon the dose and duration of protamine infusion, and these changes were reversed with heparin. Initial studies suggested that these changes may be related to the direct neutralization of GBM charge by protamine, but subsequent studies suggest a major role of cellular components (podocyte and endothelium) in this model (6). Protamine sulfate nephropathy is no longer viewed as a model of MCD (3), but used to study molecular changes associated with reversible transient foot process effacement in rats and mice.
There are no acceptable models of MCD in mice. Adriamycin nephropathy, previously used to study MCD, is a model of FSGS in both rats and mice (3). In this less than perfect scenario, investigators have used other general models of proteinuria to assist in the study of genes relevant to MCD. The authors favor the use of the γ2 fraction of sheep anti-rat whole glomerular nephrotoxic serum, which has been extensively characterized and has reactivity to multiple podocyte and GBM proteins (7, 8). If factors reactive to known nephritogenic GBM proteins are removed from the γ2 fraction by adsorption to an affinity matrix, this does not abrogate its ability to induce proteinuria, a phenomenon that is most likely related to reactivity to podocyte proteins (7). Intravenous injection in rats and mice induces large amounts of proteinuria that is dose dependent and independent of complement, leukocytes, or fibrin during the heterologous phase (first 5–7 days). Light microscopy in this phase is unremarkable, and electron microscopy reveals extensive effacement of foot processes. ANGPTL4 upregulation was first noted in this model (9), and later investigated in other models of human glomerular disease. This serum is most useful for inducing proteinuria in knockout mice of candidate genes being assessed for their role in the pathogenesis of proteinuria. Lipopolysaccharide has also been used by some investigators for this purpose (10), but the proteinuria is mild and transient and the dose range narrow, beyond which mice develop ATN or the systemic effects of lipopolysaccharide.
Circulating factors in MCD
Four decades ago, Shalhoub (11) proposed that soluble factors secreted by T cells may form the critical upstream signal for initiating glomerular changes in MCD. The key observations behind this hypothesis were a lack of a humoral antibody response, remission brought about by measles (an infection that alters cell-mediated immunity), the benefits of steroids and cyclophosphamide therapy (which also lessen cell-mediated immunity), and occurrence in Hodgkins disease. Although this hypothesis was appropriate when proposed, it no longer receives substantial support. Glucocorticoids (9, 12) and cyclophosphamide (13) are now known to have direct effects in the kidney, independent of their effects on the immune system. Most patients with Hodgkins disease do not develop MCD, and the exponential growth in our knowledge of cell-mediated immunity and multiple genes co-expressed in immune cells and podocytes makes the measles-induced remission point weak. Recent studies published on interleukin 13 (IL-13) (14) have generated some interest in this field, especially since this cytokine expresses a receptor on the podocyte surface. Rats electroporated with IL-13 expression constructs develop very high circulating IL-13 levels, a mean 10-fold increase in albuminuria after 10 weeks, normal appearance of glomeruli on light microscopy, and 80% effacement of podocyte foot process revealed by electron microscopy. The IL-13 levels correlated more strongly with elevated plasma cholesterol levels than with albuminuria, and plasma triglyceride levels were not assessed. Even though a subset of rats developed a mean 30-fold increase in albuminuria, the amount of proteinuria is still 10–15 fold lower than the PAN model of MCD. It is unclear whether this IL-13 related albuminuria is glucocorticoid sensitive, or whether such high IL-13 levels have ever been noted in human MCD. Based on this data, it is hard to establish a direct relationship between human MCD and IL-13. Nevertheless, it is possible that IL-13 may be part of a circulating complex that causes MCD.
RECENT ADVANCES
Despite the extensive use of rat models of MCD to study nephrotic syndrome, the molecular pathogenesis of human MCD remained unclear until very recently. A study published from our laboratory (9) clarifies the molecular basis of many of the previously unexplained features of human and experimental MCD. In brief, this study shows that qualitative and quantitative changes in the expression of angiopoietin-like protein 4 (ANGPTL4) in podocytes induces most of the characteristic features of MCD.
What is ANGPTL4?
ANGPTL4, a secreted glycoprotein, belongs to the angiopoietin-like protein family, which shares structural and some functional similarities with angiopoietins, but also has distinct features that determine much of their biological role. After the discovery of ANGPTL4 over a decade ago, expression was reported in adipose tissue, liver, skeletal muscle, heart and placenta. The first biological role described was the inhibition of endothelium bound lipoprotein lipase activity (15). Since lipoprotein lipase promotes the tissue uptake of triglycerides, ANGPTL4-mediated lipoprotein lipase inhibition increases plasma triglyceride levels. Subsequent studies showed a role in tumor metastasis (16). Our laboratory observed expression in podocytes in vivo nearly 8 years ago, and over this period have investigated its role in the pathogenesis of nephrotic syndrome. We showed (9) that ANGPTL4 upregulation in the podocyte explains major clinical and histological features of MCD. Increased ANGPTL4 protein expression is noted in the glomeruli, urine, and serum in patients with MCD.
Consequences of podocyte ANGPTL4 upregulation in MCD
ANGPTL4 is normally expressed in podocytes at relatively low levels (9), but is highly upregulated in MCD. While some of this podocyte-secreted ANGPTL4 binds to the GBM and affects the endothelial surface, other enters the urinary space and is taken up by tubules or appears in urine. Despite heavy proteinuria, circulating ANGPTL4 levels are increased in MCD. Whether podocyte secreted ANGPTL4 enter the circulation in MCD has yet to be determined.
The development of 2 different tissue-specific ANGPTL4-overexpressing transgenic rats has helped in sorting out some of the biological properties of ANGPTL4 in nephrotic syndrome. Rats that specifically overexpress ANGPTL4 from adipose tissue (aP2-ANGPTL4 transgenic rats) have high circulating levels of ANGPTL4, but do not develop proteinuria and have normal glomerular morphology on light and electron microscopy. This suggests that high circulating levels of ANGPTL4, often observed in patients with MCD and in the PAN rat model, do not cause proteinuria. NPHS2 (podocin)-ANGPTL4 transgenic rats, which overproduce ANGPTL4 specifically from the podocyte, develop many features of human MCD (9). The extent of ANGPTL4 upregulation in heterozygous NPHS2-ANGPTL4 transgenic rats (120-fold) is comparable with upregulation noted in the single intravenous dose PAN model, which commonly peaks at 70 fold increase.
Proteinuria in MCD
NPHS2-ANGPTL4 transgenic rats develop selective proteinuria, which is recognized by the presence of albumin as the dominant protein in urine, with males developing more albuminuria than females (Figure 2). Albuminuria increases with age, and in some rats is more than 500 times higher than age and sex matched controls. While assessing for albuminuria using timed urine collections, albuminuria was stratified by fold increase in study rats (compared with control littermates) as microalbuminuria (up to 10-fold increase), sub-nephrotic range (10 – 99 fold increase), and nephrotic range (over 100-fold increase). The same criteria also apply to spot urine albumin-creatinine ratios used sometimes in mice. Several lines of evidence point towards the interaction of podocyte-secreted ANGPTL4 with the GBM as the principal cause of proteinuria. Albuminuria develops as early as age one month in NPHS2-ANGPTL4 transgenic rats, when foot processes are well preserved. Immunogold electron microscopy reveals transit of ANGPTL4 across the GBM, and a progressive increase in clustering of gold particles in the GBM as the animals grow older. An interesting trio of observations is noted at age 3 months when effacement of podocyte foot processes is first noted. Clustering of ANGPTL4 gold particles in the GBM is seen most often in areas where foot process effacement develops, whereas gold particles remain more diffuse in areas where foot processes are still intact (Figure 3). This is accompanied by acceleration in albuminuria. Without studying additional time points around age 3 months, it is hard to tease out the appropriate sequence of events between clustering, foot process effacement and acceleration in proteinuria. One explanation is that clustering represents progression of ANGPTL4 effects its interaction with GBM proteins and among themselves, resulting in increased proteinuria through further progressive GBM permeability and effacement of foot processes via outside-in signaling at the foot process–GBM interface. This hypothesis will be placed under rigorous scrutiny in future studies. Extensive foot process effacement is observed by age 5 months in most transgenic rats. This gradual onset of foot process effacement contrasts with extensive effacement noted on biopsy in classic human MCD, and offers unique insight into pathogenesis of MCD in “slow motion” (Figure 4), as is also sometimes seen in human biopsies.
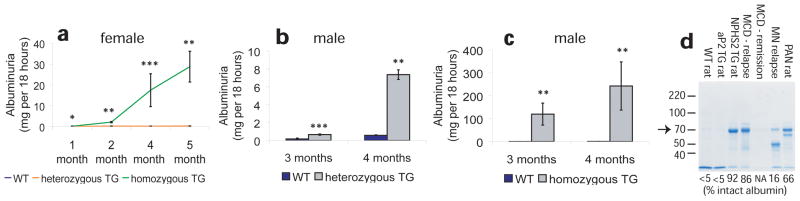
Figure 2.
Relationship of ANGPTL4 overexpression with proteinuria. (a) Albuminuria in female NPHS2-ANGPTL4 transgenic rats. (b) Albuminuria in male heterozygous NPHS2-ANGPTL4 transgenic rats. (c) Albuminuria in male homozygous NPHS2-ANGPTL4 transgenic rats. (d) Gel electrophoresis of urinary protein from wild-type (WT) rats, transgenic (TG) rats, PAN (puromycin aminonucleoside nephrosis) rats, and individuals with minimal change disease (MCD) and membranous nephropathy (MN). Arrow points towards prominent 70 kDa intact albumin band. Mean percentage densitometry of intact albumin is shown for each lane. * P<0.05, ** P<0.01, *** P<0.001. Abbreviations: NPHS2, podocin; ANGPTL4, angiopoietin-like 4. Reproduced from Clement et al9.
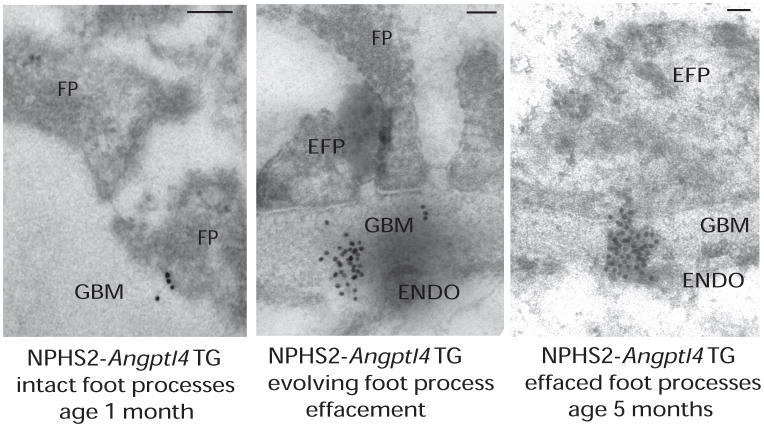
Figure 3.
Relationship of clustering of ANGPTL4 with effacement of podocyte foot processes. Immunogold electron microscopy of NPHS2-ANGPTL4 transgenic rats revealed a progression from (left) intact foot processes (FP) with gold particles just entering the glomerular basement membrane (GBM) in young rats, (middle) to partial effacement with GBM gold particle clusters opposite to effaced foot processes (EFP) reaching up to the endothelial (ENDO) surface (usually noted starting age 3 months), and finally (right) diffuse effacement with dense gold particle clusters in the GBM usually noted by age 5 months. Scale bars denote 0.2 μm. Abbreviation: TG, transgenic. Reproduced from Clement et al9.
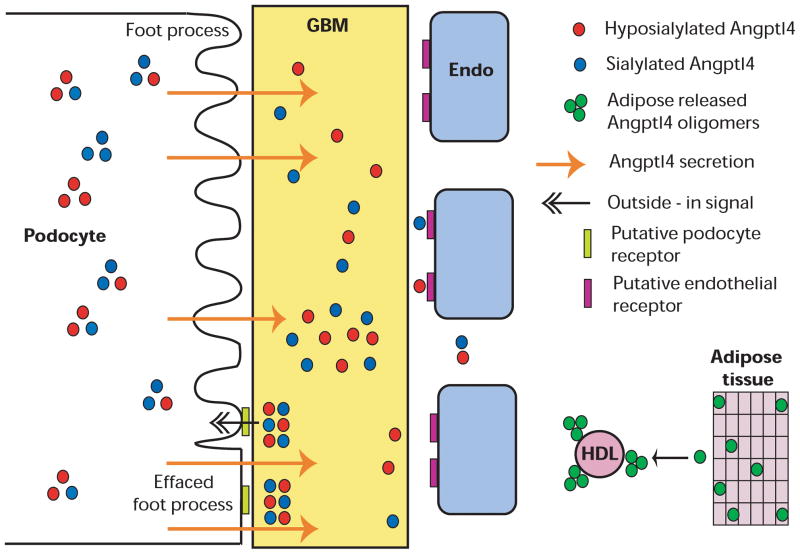
Figure 4.
Schematic representation of the role of podocyte secreted Angtpl4 in the pathogenesis of minimal change disease. Sequence of events arranged from top to bottom. Podocytes secrete neutral and high pI ANGPTL4 that binds to the glomerular basement membrane (GBM) to alter protein-protein interactions, resulting in proteinuria. Over time, ANGPTL4 reaches up to the endothelial (Endo) surface. Progressive accumulation and clustering of ANGPTL4 in the GBM likely activates signals at the podocyte-GBM interface and induces foot process effacement. Circulating ANGPTL4 secreted from other organs in disease states, e. g. adipose tissue, forms medium and high order oligomers that are bound to high-density lipoprotein (HDL) particles, migrate at neutral or low-neutral pI, and do not enter the GBM or cause proteinuria. Reproduced from Clement et al9.
Glucocorticoid sensitivity in MCD
Up to 95 percent of patients with MCD are sensitive to glucocorticoid therapy (1), which suggests that the key mediators of this disease are either encoded by glucocorticoid sensitive genes or controlled by glucocorticoid sensitive pathways. Glomerular ANGPTL4 upregulation noted in the PAN model is highly glucocorticoid sensitive (9). Administration of glucocorticoids in PAN reduces glomerular ANGPTL4 expression by over 70% within 6 days, while reducing proteinuria by approximately 20%. The quantitative disparity between the reduction of proteinuria and ANGPTL4 upregulation by glucocorticoids is a limitation of the PAN model, and, as discussed earlier, related to this model being a combination of glucocorticoid sensitive and resistant pathways. There is limited value of treating the NPHS2-ANGPTL4 transgenic rat with glucocorticoids to study glucocorticoid-sensitivity in the context of MCD, since transgenic expression is not driven by the native ANGPTL4 promoter.
Loss of GBM charge
Loss of GBM charge in MCD was reported over 4 decades ago (17), and has now been explained by binding of ANGPTL4 to the GBM. We investigated GBM charge in NPHS2-ANGPTL4 transgenic rats, aP2-ANGPTL4 transgenic rats, and a mouse model of generalized ANGPTL4 transgenic expression that also has mild ANGPTL4 overexpression in podocytes (9). Using alcian blue staining, reduction of GBM charge was noted in NPHS2-ANGPTL4 rats and ANGPTL4 transgenic mice, whereas no changes were seen in aP2-ANGPTL4 rats. These results suggest that podocyte-secreted ANGPTL4, but not circulating ANGPTL4, can reduce GBM charge, and provide the first ever molecular basis for this phenomenon. The relationship between charge and proteinuria is unclear. Whereas the ANGPTL4 studies were not designed to look at this relationship, the comparable reduction in GBM charge between NPHS2-ANGPTL4 rats and ANGPTL4 transgenic mice despite presence of mild proteinuria only in the transgenic mice argues against a strong relationship between charge and proteinuria. It is more likely that neutralization of GBM charge is related to the transit of ANGPTL4, with its high isoelectric point (pI), across the GBM against the direction of fluid flow. We believe that ANGPTL4 binds the GBM by a combination of charge-independent and charge-dependent mechanisms.
In light of these observations, it is also important to consider other potential explanations for selective proteinuria in MCD, and lack of selectivity in other forms of glomerular disease. Selectivity may indeed be a result of uniform or symmetric increase in permeability of the GBM induced by podocyte secreted proteins in MCD. As a corollary, non-selective proteinuria would result from non-uniform involvement of one or more layers of the glomerular capillary loop in non-MCD patients, since histological lesions in these conditions are also asymmetric. Exploring this hypothesis may require purified individual intact GBM proteins, most of which are not currently available.
Molecular basis of ANGPTL4 effects: the role of sialylation
Analysis of PAN rat glomerular protein extracts using 2-dimensional gel electrophoresis shows that ANGPTL4 is overproduced in two distinct forms: a positively charged form that migrates at a high pI (8–8.5), and a neutral form that migrates at or just below pI 7. One important difference between these two forms of ANGPTL4 is the lack of adequate sialylation in the high pI form. NPHS2-ANGPTL4 transgenic rats similarly overexpress a mixture of neutral and high pI ANGPTL4 in glomeruli, making these rats biologically suitable to further investigate the role of ANGPTL4 in MCD. Whereas both forms of ANGPTL4 are likely to have significant direct effects on the pathogenesis of MCD, published studies have so far focused on the high pI, positively charged form of ANGPTL4. ANGPTL4 was previously shown to interact with heparan sulfate proteoglycans(18), and the presence of this class of proteins with negatively charged glycosaminoglycan chains in the GBM makes high pI ANGPTL4, among other things, a strong candidate to explain loss of GBM charge. Transit across the GBM from the podocyte towards the endothelial surface against the direction of fluid flow is likely to be facilitated by charge related binding to GBM proteins.
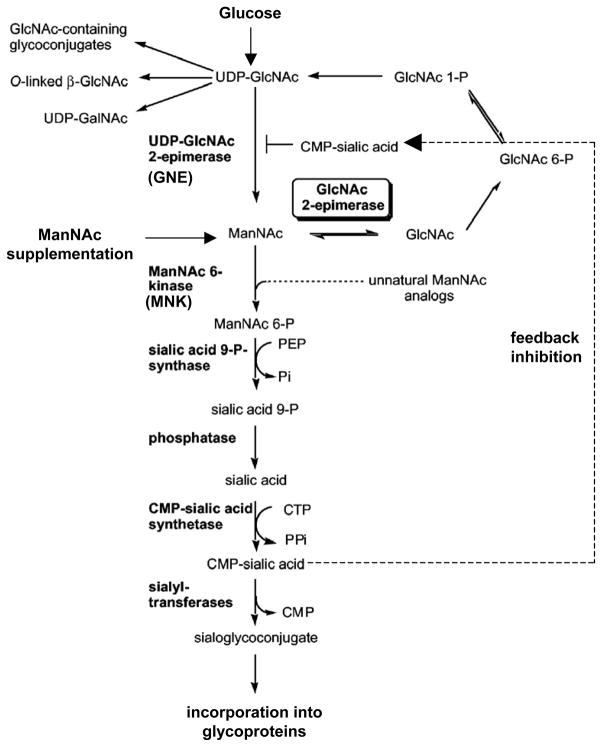
We developed two types of stable cell lines that secrete high pI ANGPTL4 into the supernatant, from where it can be concentrated or purified. To convert high pI, hyposialylated ANGPTL4 into neutral pI, sialylated ANGPTL4, these cell lines were incubated with N-acetyl-D-mannosamine (ManNAc), a precursor of sialic acid. ManNAc enters the cells, is converted into sialic acid, which is then incorporated into ANGPTL4 (9). The choice of ManNAc over sialic acid or other sialic acid precursors is governed by its ability to cross cell membranes easily and enter the sialic acid biosynthesis pathway following the first rate-limiting enzymatic step catalyzed by GNE (UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase) (Figure 5) (19). The biological significance of ANGPTL4 sialylation in vivo can be appreciated by studying its effects on albuminuria. The treatment of NPHS2-ANGPTL4 transgenic rats with oral ManNAc results in over 40% reduction in albuminuria and conversion of significant amounts of high pI to neutral pI ANGPTL4. By contrast, the overall charge of podocalyxin, a sialylated transmembrane glomerular glycoprotein, is unchanged by ManNAc therapy (9). This study provides the first example of sialylation based therapeutics for MCD, which, in contrast to glucocorticoids, acts by altering ANGPTL4 protein and not by reducing ANGPTL4 gene expression. There could be several reasons for the lack of sialylation of ANGPTL4 in MCD. The simplest possible explanation is based on the relatively low constitutive expression of ANGPTL4 in the normal podocyte, and therefore low requirements for sialic acid precursors by this pathway at baseline. Severe upregulation (70-fold increase in mRNA expression) in experimental MCD would encounter a relative sialic acid substrate deficiency during the post-translational modification of newly formed ANGPTL4, resulting in the secretion of hyposialylated high pI protein. Treatment with ManNAc is effective in sialylating ANGPTL4, since it enters the sialic acid biosynthesis pathway following the rate-limiting GNE-catalyzed step. Another possibility is that the activity of a class of enzymes called sialyltransferases, which add sialic acid residues to proteins, is reduced in the podocyte in MCD. However, this defect would perhaps not respond as dramatically to ManNAc therapy as has been noted. Finally, concomitant defects in sialic acid biosynthetic enzymes are possible, but in humans known mutations of at least one key enzyme, the dual-functional GNE, result in hereditary inclusion body myopathy, a disease with muscle weakness (20) but no effect on glomerular function. Knockout mice that are transgenic for the human aspartate to valine mutation at amino acid 176 of GNE mutant gene develop a classic phenotype of hereditary inclusion body myopathy (21, 22), but no glomerular disease. By contrast, a corresponding mutation in the mouse GNE gene results in abnormal development of glomeruli, that is partially rescued by ManNAc (23).
Figure 5.
Brief schematic representation of the sialic acid biosynthesis pathway. Glucose is converted into uridine diphosphate–N-acetylglucosamine (UDP-GlcNAc) through a number of enzymatic steps (not shown), which is converted to N-acetyl-D-mannosamine (ManNAc) via a rate-limiting reaction calalyzed by GNE. ManNAc undergoes conversion to cytidine monophosphate–sialic acid (CMP-sialic acid), which provides feedback inhibition to the rate-limiting GNE-calalyzed reaction. Exogenous ManNAc supplements enters the pathway after this rate limiting step. A substantial part of the sialic acid is incorporated into N- and O-linked glycan residues in structural and secreted glycoproteins. Modified from Luchansky et al19 with permission of The American Society for Biochemistry and Molecular Biology.
Reduced sialylation of podocalyxin, a transmembrane sialylated glycoprotein expressed in the glomerular capillary loop (24), has been well documented in experimental MCD (25). However, its relevance to human MCD is not well established. Earlier studies (25) proposed that podocalyxin-related negative cell surface charge contributed by sialic acid and sulfate residues (26) plays a major role in maintaining normal podocyte architecture, including the arch-like structure of foot processes. Studies that used sialidases(27) with the intention of removing charge from podocyte surface proteins to induce podocyte foot process effacement and proteinuria were not specific for podocalyxin, and are likely to have effects on additional proteins, and indeed, all glomerular cell types. Literature published over the past decade shows that podocalyxin is linked to the actin cytoskeleton through ezrin and NHERF2 (sodium-hydrogen exchange regulatory cofactor) (28). Mice deficient in podocalyxin are anuric, die at age 24 hours, and fail to develop podocyte foot processes (29), which suggests a much more severe phenotype than MCD. Therefore, the biological role of podocalyxin in glomerular disease is more likely to be related to its interaction with the actin cytoskeleton than its surface charge. Moreover, changes in the charge of podocalyxin do not explain any changes in GBM charge, which we now know are related to binding of podocyte secreted ANGPTL4. Podocalyxin may contribute to the development of foot process effacement, and data from NPHS2-ANGPTL4 transgenic rats suggest that this is unlikely to be the major determinant of proteinuria in MCD.
Other MCD relevant features in NPHS2-ANGPTL4 transgenic rats
The epitope tag of the transgene-expressed ANGPTL4 is not detectable in the plasma of NPHS2-ANGPTL4 transgenic rats. This suggests either that the overexpressed protein remains confined to the glomerulus, or that it leaks into the circulation in small amounts but gets filtered rapidly into the urine, since these rats are proteinuric and even have lower circulating levels of normal cleaved extrarenal secreted ANGPTL4. Proximal tubular uptake of ANGPTL4 is noted in these rats (9). The absence of circulating tagged ANGPTL4 in these rats may also suggest that additional putative factors secreted by podocytes in MCD, but not upregulated in NPHS2-ANGPTL4 transgenic rats, may facilitate the transit of ANGPTL4 across the glomerular capillary loop.
A key hallmark of MCD is the absence of tubulointerstitial fibrosis despite heavy proteinuria. No changes in the tubulointerstitial compartment suggestive of fibrosis were noted on light microscopy in NPHS2-ANGPTL4 transgenic rats up to age 6 months, despite the presence of severe proteinuria. This aspect of ANGPTL4 biology is under investigation.
Limitations of the NPHS2-ANGPTL4 transgenic rat model
NPHS2-ANGPTL4 transgenic rats develop the most features of human MCD, and are therefore the most accurate model for this condition. However, one of the features lacking in this model is the explosive onset of disease, noted commonly in humans. This suggests that changes in the expression of additional genes/proteins may be involved in the development of this feature.
Novel therapeutics and biomarkers in MCD
Sialic acid precursors like ManNAc have potential as therapeutic agents in MCD, since they complement the effects of glucocorticoids on reducing podocyte ANGPTL4 gene expression by improving sialylation of podocyte-secreted ANGPTL4 protein. ManNAc therapy may be especially useful in patients who have frequent relapses, those developing resistance to glucocorticoids, or patients in whom complete remission is not achieved or requires very prolonged glucocorticoid therapy. The index case discussed in this review had multiple relapses as a child, and would almost certainly have benefited from ManNAc therapy in the past episodes. In addition, ManNAc could also have shortened the duration of glucocorticoid therapy in the most recent relapse.
Development of novel biomarkers for MCD is also important. 2-dimensional gel electrophoresis and Western blot of human plasma reveals the presence of small amounts of high pI circulating ANGPTL4 in MCD patients in relapse, and its absence in plasma from patients with MCD in remission, membranous nephropathy and FSGS (9). If this is confirmed in larger studies, this could serve as a biomarker for MCD and even allow for early detection of relapse in the index case, prompting early therapy. Two additional studies on urinary B7.1/CD80 excretion suggested that this protein may be a reasonable biomarker for human MCD (30, 31). A relationship between ANGPTL4 and B7.1 has not been explored.
Areas of future investigation
There are many future areas of investigation for the role of ANGPTL4 in MCD. Of prime importance is a detailed study of the interaction of neutral and high pI ANGPTL4 with GBM proteins, its effects on GBM composition, structure and function, and the effects of all of these changes on the podocyte-GBM interface. It is likely the 2 different forms of ANGPTL4 will have qualitative and quantitative differences in interactions with matrix proteins, and these interactions are unlikely to be limited to heparan sulfate proteoglycans. It would be equally important to determine the factors that induce severe upregulation of podocyte ANGPTL4 expression in MCD. The prime candidates for this upregulation are the zinc finger and homeoboxes family of transcriptional factors, since they are known to be involved in the pathogenesis of MCD (8), and could directly transmit signals from the cell membrane to the nucleus during disease pathogenesis. More work needs to be done on the biological role in nephrotic syndrome of the circulating neutral pI form of ANGPTL4 that is likely contributed to by multiple organs listed earlier. Lastly, sialylation based therapeutics for MCD (9) represent a unique non-immunosuppressive modality of treating this condition, and requires investigation in human studies.
SUMMARY
The discovery of the role of ANGPTL4 in the pathogenesis of MCD has clarified many of the classic features of this disease, including glucocorticoid sensitivity, selective proteinuria and loss of GBM charge. In addition, conceptual advances were made clarifying the effect of podocyte secreted ANGPTL4 on the GBM in development of proteinuria in MCD. Most importantly, identification of defects in posttranslational modification of ANGPTL4 has initiated the development of novel mechanism based therapeutic strategies using precursors of sialic acid.
Acknowledgments
We thank Dr William Cook, Department of Pathology, University of Alabama at Birmingham, for providing human MCD biopsy images.
Support: Funded in part by National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK077073 to Dr Chugh.
Footnotes
Financial Disclosure: Dr Chugh is founder and president of GDTHERAPY LLC. The use of sialic acid precursors, including ManNAc, to treat proteinuria and nephrotic syndrome in MCD and diabetic nephropathy is covered by US patent application 13/152,169 and PCT/US2011/039058 filed by Dr Chugh. The remaining authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nachman PH, Jennette JC, Falk R. Primary glomerular disease. In: Brenner BM, editor. The Kidney. 8. Philadelphia, PA: Elsevier; 2008. pp. 987–1066. [Google Scholar]
- 2.Miner JH. Glomerular filtration: the charge debate charges ahead. Kidney Int. 2008;74(3):259–261. doi: 10.1038/ki.2008.260. [DOI] [PubMed] [Google Scholar]
- 3.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, et al. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296(2):F213–F229. doi: 10.1152/ajprenal.90421.2008. [DOI] [PubMed] [Google Scholar]
- 4.Seiler MW, Venkatachalam MA, Cotran RS. Glomerular epithelium: structural alterations induced by polycations. Science. 1975;189(4200):390–393. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- 5.Seiler MW, Rennke HG, Venkatachalam MA, Cotran RS. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab Invest. 1977;36(1):48–61. [PubMed] [Google Scholar]
- 6.Daniels BS. Increased albumin permeability in vitro following alterations of glomerular charge is mediated by the cells of the filtration barrier. J Lab Clin Med. 1994;124(2):224–230. [PubMed] [Google Scholar]
- 7.Chugh S, Yuan H, Topham PS, et al. Aminopeptidase A: A nephritogenic target antigen of nephrotoxic serum. Kidney Int. 2001;59(2):601–613. doi: 10.1046/j.1523-1755.2001.059002601.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu G, Clement LC, Kanwar YS, Avila-Casado C, Chugh SS. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J Biol Chem. 2006;281(51):39681–39692. doi: 10.1074/jbc.M606664200. [DOI] [PubMed] [Google Scholar]
- 9.Clement LC, Avila-Casado C, Macé C, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17(1):117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei C, Möller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 11.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2(7880):556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 12.Clement L, Liu G, Perez-Torres I, Kanwar YS, Avila-Casado C, Chugh SS. Early changes in gene expression that influence the course of primary glomerular disease. Kidney Int. 2007;72(3):337–347. doi: 10.1038/sj.ki.5002302. [DOI] [PubMed] [Google Scholar]
- 13.Abraham P, Isaac B. Ultrastructural changes in the rat kidney after single dose of cyclophosphamide---Possible roles for peroxisome proliferation and lysosomal dysfunction in cyclophosphamide induced renal damage [published online ahead of print March 18, 2011] Hum Exp Toxicol. doi: 10.1177/0960327111402240. [DOI] [PubMed] [Google Scholar]
- 14.Lai KW, Wei CL, Tan LK, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18(5):1476–1485. doi: 10.1681/ASN.2006070710. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res. 2002;43(11):1770–1772. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 16.Padua D, Zhang XH, Wang Q, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael AF, Blau E, Vernier RL. Glomerular polyanion. Alteration in aminonucleosidenephrosis. Lab Invest. 1970;23(6):649–657. [PubMed] [Google Scholar]
- 18.Cazes A, Galaup A, Chomel C, et al. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res. 2006;99(11):1207–1215. doi: 10.1161/01.RES.0000250758.63358.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luchansky SJ, Yarema KJ, Takahashi S, Bertozzi CR. GlcNAc 2-epimerase can serve a catabolic role in sialic acid metabolism. J Biol Chem. 2003;278(10):8035–8042. doi: 10.1074/jbc.M212127200. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg I, Avidan N, Potikha T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29(1):83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- 21.Malicdan MC, Noguchi S, Nonaka I, Hayashi YK, Nishino I. AGne knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Hum Mol Genet. 2007;16(22):2669–2682. doi: 10.1093/hmg/ddm220. [DOI] [PubMed] [Google Scholar]
- 22.Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med. 2009;15(6):690–695. doi: 10.1038/nm.1956. [DOI] [PubMed] [Google Scholar]
- 23.Galeano B, Klootwijk R, Manoli I, et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117(6):1585–1594. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984;98(4):1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerjaschki D, Vernillo AT, Farquhar MG. Reduced sialylation of podocalyxin--the major sialoprotein of the rat kidney glomerulus--in aminonucleosidenephrosis. Am J Pathol. 1985;118(3):343–349. [PMC free article] [PubMed] [Google Scholar]
- 26.Dekan G, Gabel C, Farquhar MG. Sulfate contributes to the negative charge of podocalyxin, the major sialoglycoprotein of the glomerular filtration slits. ProcNatlAcadSci U S A. 1991;88(12):5398–5402. doi: 10.1073/pnas.88.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelberg H, Healy L, Whiteley H, Miller LA, Vimr E. In vivo enzymatic removal of alpha 2-->6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab Invest. 1996;74(5):907–920. [PubMed] [Google Scholar]
- 28.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108(2):289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyonnas R, Kershaw DB, Duhme C, et al. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med. 2001;194(1):13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garin EH, Diaz LN, Mu W, et al. Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol. 2009;20(2):260–266. doi: 10.1681/ASN.2007080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garin EH, Mu W, Arthur JM, et al. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78(3):296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]