Abstract
Objective
Bcl-2 is widely expressed in a developing tooth organ and regulates tooth morphogenesis. However, whether Bcl-2 is related to tooth damage repair is unknown yet. Using an odontoblast-targeted Bcl-2 overexpression transgenic mouse (Col2.3Bcl-2) and artificial cavity preparation as a model system, the relationship between Bcl-2 and reparative dentinogenesis is investigated in this study.
Methods
The odontoblastic-like cell cultures derived from mouse molar pulps were established. The expression of transgenic human Bcl-2 (hBcl-2) and endogenous mouse Bcl-2 (mBcl-2) and mouse Bax (mBax, a Bcl-2 antagonist) was detected in vivo and in vitro by Western blot and immunocytochemistry, respectively. Basal level and artificial cavity-induced odontoblast apoptosis was detected by the Deoxynucleotidyl Transferase (TdT) dUTP Nick End labeling (TUNEL) technique. Reparative dentin formation induced by artificial cavity drilled to a half dentin thickness on mesial cervical region of mandibular first molars 2, 4, and 6 weeks post-op was evaluated histologically and via micro-CT.
Results
The transgenic hBcl-2 was stably expressed in odontoblasts of the transgenic animals without interference with the expression of mBcl-2 and mBax. Basal level as well as artificial cavity- induced odontoblast apoptosis was prevented by the transgene. Compared to the wild type, the transgenic animals produced reparative dentin with significantly higher mineral density 6 weeks after the operation.
Conclusions
Bcl-2 overexpression prevents odontoblast apoptosis and promotes dentin damage repair, indicating that genetic manipulation of Bcl-2 may be a novel strategy to maintain the vitality and function of dentine-pulp complex under detrimental mechanical stimuli.
Keywords: Odontoblasts, Bcl-2, Artificial cavity, Reparative, Dentin
Introduction
The development of teeth is under strict genetic control and is regulated by inductive interactions between oral epithelium and cranial neural crest-derived mesenchymal cells (1, 2). The reciprocal and sequential epithelial-mesenchymal interactions instruct a tooth germ to proceed through bud, cap, and bell stages, before the mesenchymal odontoblasts and epithelial ameloblasts differentiate terminally and deposit the organic matrices of dentin and enamel, respectively (3, 4).
Normally three types of dentin may be formed throughout the life of a tooth. Primary dentin is produced by odontoblasts at an active secretory phase during the formation of the crown and root(s) of the tooth. After completion of root formation, physiological secondary dentine is secreted by odontoblasts at a continuing, albeit much reduced rate. Under environmental stimuli, odontoblasts may generate tertiary dentin (reactionary or reparative variants) at the site of injury. In the case of mild injuries, such as non-cavitated or slowly progressing caries, mild abrasion/erosion, odontoblasts focally up-regulate their secretory activity and deposit reactionary dentin. However, more intensive stimuli, such as advanced dental caries, cavity preparation, and therapeutic or restorative dental materials, may induce odontoblast apoptosis and reparative dentin formation by odontoblastic-like cells derived from progenitor cells within the pulp (5–8). Reactive dentin shares many similarities to the primary and secondary dentin and can effectively oppose exogenous detrimental stimuli to protect the pulp (9). Reparative dentin is more diversified, which contains both atubular and tubular dentine (10). In clinical scenery, tooth damage repair is demonstrated as a mixture of reactionary and reparative dentin, which is indistinguishable at the in vivo level, nor from a biochemical and molecular point of view (8).
Dentinogenesis is under the regulation of a variety of growth factors and transcription factors. Strong parallels exist between many tooth developmental events and dental damage repair. BMPs, TGFβs, FGFs, IGF, Msx-1 and -2, c-jun, and jun-B are all found to regulate differentiation and functions of odontoblasts (11–14). Sequestration of these factors within dentin matrix will allow their release in carious and injured teeth, due to matrix demineralization and dissolution. These factors will further act as both mitogens and chemotactic factors for pulp cells to signal reparative process at the injured sites (15–19).
In addition to growth factors and other relevant mediators, apoptosis seems to play a pivotal role in developmental and damage-repair related dentin remodeling. Apoptosis in odontoblasts has been shown in rodent incisors and human molars of different ages (20). Massive programmed cell death may be the strategy employed by odontoblasts to cope with reduced pulp chamber size after secondary dentin deposition (20, 21). For teeth under violent stress, odontoblasts adjacent to the injured sites will undergo apoptosis and replaced by pulp progenitors-derived odontoblastic-like cells to elaborate reparative dentin (22–26). Among numerous apoptosis regulators, Bcl-2, an anti-apoptotic protein, is expressed at various stages of a developing tooth (27–29). In odontoblasts underlying cavity preparation, Bcl-2 expression was found to be increased significantly (24). These observations suggest that regulation of odontoblast apoptosis by Bcl-2 may contribute to dentin homeostasis and damage-related dentin remodeling. However, how Bcl-2 is related to these physiological and pathological processes is not entirely clear yet.
Using a Col2.3Bcl-2 transgenic mouse, in which hBcl-2 is driven by the 2.3 kb fragment of rat type I collagen promoter, we have previously shown that primary dentinogenesis is impaired by odontoblast-targeted Bcl-2 overexpression, as demonstrated by much thinner and less dense dentin in the transgenics compared with age-matched wild type animals (30). In the present study, how dentin repair is affected by Bcl-2 was investigated with the same transgenic animals, using artificial cavity preparation as a model system for intense mechanical stimulus. These studies are anticipated to expand our fundamental understanding on tooth developmental biology and regeneration, and ultimately lead to novel therapeutic interventions for dental developmental anomalies and damage repair.
Materials and methods
Animals
The Col2.3Bcl-2 transgenic mice and their wild type littermates were imported from University of Connecticut Health Center. The methods of creating the transgenic mice and a PCR-based genotyping were described previously (31). Briefly, transgenic mice were identified using PCR of ear punch isolated genomic DNA with 5′-tgaagtcaacatgcctgcc and 3′-ctctaaaggtgcggcttcct primers that produce a 670-bp product specific to the 3′ untranslated region of hBcl-2. All animal-related experiments were approved by the Center for Laboratory Animal Medicine and Care at the University of Texas Health Science Center at Houston.
Pulp cell cultures
Coronal molar pulps of 5-day-old mice were isolated and digested with 0.05% trypsin and 0.1% collagenase P at 37 °C for 50 min. Cells were plated onto NALGE-NUNC Lab-Tek 4-well chamber slides (Nalge Nunc International, Rochester, NY) at a density of 104 cell/cm2 in α-MEM containing 10% fetal bovine serum (FBS), 100 U/ml of penicillin and 100 μg/ml of streptomycin. After confluence (normally on day 7), the cells were induced to differentiate with 10−8 M dexamethasone, 8 mM β-glycerophosphate (βGP), and 50 μg/ml ascorbic acid in α-MEM for 1 day, then the medium was switched to α-MEM+10% FBS+4 mM βGP+50 μg/ml ascorbic acid, and was changed every other day thereafter. The day of plating pulp cells was counted as day 1, and on subsequent days 7, 14, and 21, immunocytochemistry or TUNEL assay was performed on the cultures as mentioned below.
Immunocytochemistry
Pulp cells were fixed with 70% ethanol at −20ºC for 2hrs. The samples were air dried, and endogenous peroxidase activity was blocked by incubating 10 min with 3% H2O2. Nonspecific proteins were blocked with DAKO protein block (Dako, Carpinteria, CA) for 30 min at room temperature (RT). The primary antibodies used were monoclonal mouse anti-human Bcl-2 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), monoclonal mouse anti-mouse Bcl-2 (Santa Cruz), monoclonal mouse anti-mouse Bax antibody (Santa Cruz), or mouse immunoglobulin (negative control). The primary antibodies were diluted to 4 μg/ml in 1% BSA in TTBS (1% tween-20 in TBS). The cells were incubated at RT for 1 h with primary antibodies. Secondary antibody and substrate staining were performed with DAKO LSAB+ HRT kit and liquid DAB+ substrate-chromogen system according to the manufacturer’s instructions (Dako). The slides were counterstained by hematoxylin and mounted.
Western blotting
Tooth bud from E18 mouse embryo and teeth from mice at ages of 1-week, 1-month, and 6-month were collected under stereomicroscopy. Periodontal tissues, including gingiva, alveolar bone, and periodontal ligament were carefully removed when teeth were extracted. Teeth were homogenized in 0.5mM DTT, 0.1mM EDTA, 10mM Tris-HCl buffer (pH7.2), 250mM sucrose, 10μg/ml leupeptin, 10μg/ml aprotinin, 1mM PMSF and 0.1% Triton X-100 on ice. The protein extract was spun at 10,000 g for 10 min. The supernatant protein concentration was determined by BCA assay. Proteins (20 μg) was separated by a 10–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) system, and transferred to nitrocellulose membrane. The blots were incubated with 1:100 monoclonal mouse anti-human Bcl-2 (Santa Cruz), mouse anti-mouse Bcl-2 (Santa Cruz), mouse anti-mouse Bax (Santa Cruz), or polyclonal HRP-conjugated goat anti-actin antibodies (Santa Cruz) overnight at 4°C. The blots were washed, followed by incubation with 1:10,000 goat anti-mouse HRP conjugated secondary antibody for 1 h at RT. Protein bands were visualized using an Immun-Star™ HRP substrate kit (BioRad, Hercules, CA).
TUNEL staining
TUNEL staining was performed for both pulp cell cultures and mouse molar histological sections. For pulp cell cultures, the cells were fixed in 4% paraformaldehyde in PBS at days 7, 14, and 21 of the cultures. For mouse hemi-mandible samples, they were fixed in 10% neutral buffered formalin, decalcified in 3.4% sodium formate/15% formic acid, and embedded in paraffin. Series of 5 μm-thick tissue sections were cut along the sagittal plane of mandibular molars. The tissue sections were deparaffinized and rehydrated in decreasing concentrations of ethanol. The TUNEL procedure was performed with TACS TBL kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Nuclease treatment or exclusion of TdT enzyme was used as positive or negative control, respectively. Light microscopy exam revealed apoptotic cells as having condensed, blue-stained nuclei. Quantification of apoptotic cells was determined in a blinded nonbiased manner and expressed as percentage of total cell counted.
Artificial cavity preparation
Five to seven week-old mice was anesthetized by intraperitoneal injection of ketamine and zylazine at doseages of 135 mg/kg and 15 mg/kg, respectively. Enamel and dentin thicknesses were calculated based on the μCT measurement. Artificial cavities were prepared on the mesial cervical one third of mandibular first molars at approximately a half thickness of dentin. The procedure was performed under stereomicroscopy to ensure an optimal visualization of the tooth structures. The mice were sacrificed at different post-surgery intervals for histological and μCT analysis.
Micro-CT analysis
Two, four, and six weeks after the artificial cavity preparation, the mice were sacrificed, hemi-mandibles were isolated, fixed in 70% ethanol and scanned with an Explore Locus SP pre-clinical Specimen Scanner (GE Medical Systems, London Ontario). The images were reconstructed using a modified Feldkamp method (32). The mineral density of primary dentin was measured in crown underneath the enamel and on mesial roots close to apex, and then an average was taken. For the reparative dentin, its boundary was delineated manually on contiguous slices before a 3-D region of interest was configured. The mineral density of dentin was analyzed with the MicroView software (GE Medical Systems) and normalized with a hydroxyl apatite phantom.
Statistical analysis
All figures are representative of three independent experiments. Statistical analysis was performed by paired Student’s t test to determine significance between groups (p≤ 0.05).
Results
HBcl-2 is persistently expressed in odontoblasts of Col2.3Bcl-2 transgenic mice
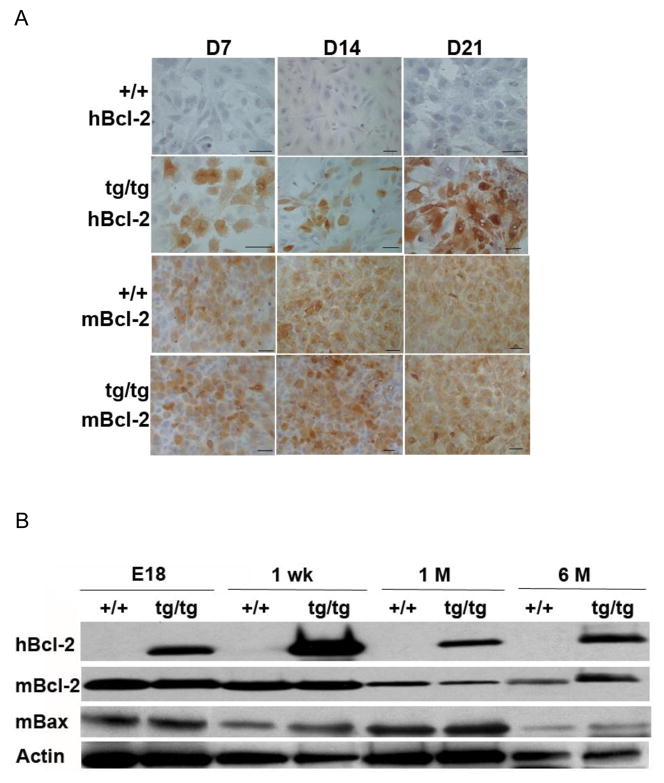
Immunocytochemical study revealed that many odontoblastic-like cells in the pulp cultures derived from the Col2.3Bcl-2 transgenic mice demonstrated strong cytoplasmic staining for hBcl-2, whereas the cultures derived from wild type animals were totally negative from day 7 to day 21. The expression of endogenous mouse Bcl-2 was comparable between the wild type and transgenic animals in the assayed time points (Fig 1A).
Fig 1.
HBcl-2 is expressed in odontoblasts of Col2.3Bcl-2 transgenic mice without interference with endogenous mBcl-2 and mBax expression in vivo and in vitro. A. Immunocytochemistry staining of pulp cell cultures derived from wild type and transgenic animals on days 7, 14, and 21. Brown indicates positive staining for the tested genes. Scar bar = 100 μm. B. Western blot detection of hBcl-2, mBcl-2, and mBax expressions in tooth proteins extracted from animals of various ages. Actin is the loading control. Abbreviations: hBcl-2, human Bcl-2; mBcl-2, mouse Bcl-2; mBax, mouse Bax; +/+, wild type; tg/tg, homozygous transgenic; D, day; E, embryonic day; wk, week; M, month.
Western blot performed on tooth proteins extracted from E18, postnatal 1-week, 1-month, and 6-month old animals demonstrated persistent hBcl-2 expression in the teeth of transgenic mice, not but in the wild type animals (Fig 1B), which is consistent with continuous activation of Col2.3 promoter due to constant primary and secondary dentin formation. The expression of hBcl-2 appeared moderately decreased upon 1-month, presumably correlating with reduced dentin deposition rate at this age. The expressions of endogenous mouse Bcl-2 and Bax, a Bcl-2 antagonist, in teeth were similar between wild type and transgenic animals.
Bcl-2 overexpression prevented basal level and artificial cavity induced odontoblast apoptosis
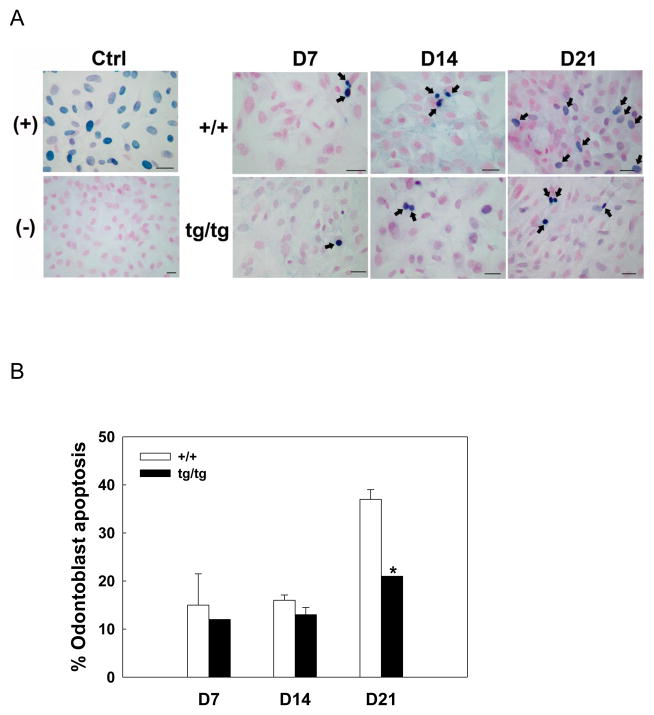
Transgenic odontoblasts demonstrated less apoptosis than the wild type in day 7–21 pulp cultures as assayed by TUNEL, especially on day 21, when prevalent odontoblast apoptosis was prevented in the transgenic cultures (Fig 2A and 2B).
Fig 2.
Bcl-2 prevents odontoblast apoptosis in vitro. A. TUNEL staining of pulp cell cultures derived from wild type and transgenic animals. Nuclear Fast Red was used for counterstaining. Black arrows point to apoptotic cells with condensed, blue-stained nuclei. Nuclease treatment or exclusion of TdT was used as (+) and (−) control, respectively. Transgenic cultures demonstrated much less apoptotic odontoblasts than wild type on day 21. Scale bar = 100 μm. B. Quantification of the data in A. *, P< 0.05 tg/tg compared with +/+. Abbreviations: (+), positive control; (−), negative control; D, day; +/+, wild type; tg/tg, homozygous transgenic.
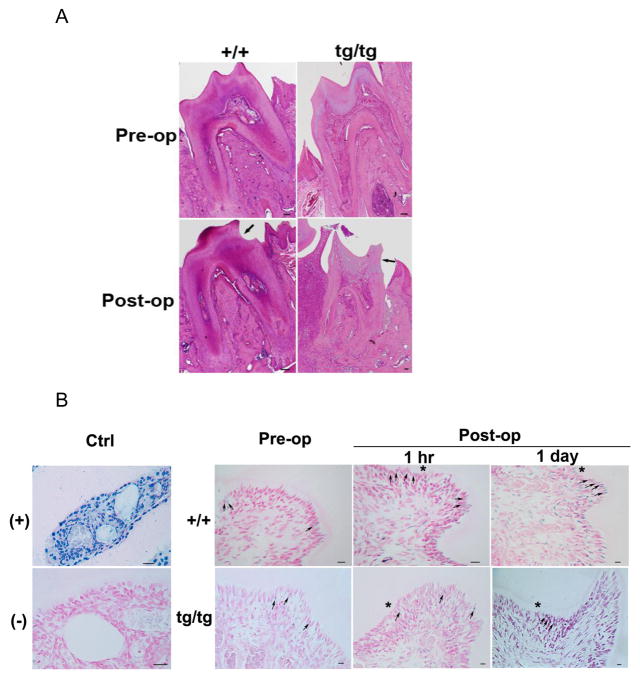
To evaluate how Bcl-2 affects odontoblast apoptosis elicited by mechanical stimuli, artificial cavity was drilled on the mesial cervical region of mouse mandibular first molar at about a half dentin thickness (Fig 3A). The number of apoptotic odontoblasts before and after operation was manually counted on TUNEL-stained tooth sections. The percentage of odontoblasts undergoing apoptosis before surgery was arbitrarily set as base line, and the one after surgery was normalized to it and defined as apoptotic index. The formula is as following: apoptotic index = (% odontoblasts undergoing apoptosis post-op)/(% odontoblasts undergoing apoptosis pre-op). The apoptosis of odontoblasts was examined 1-hour, 1-day, 1-week, and 2-week after the surgery. Wild type animals demonstrated significantly increased odontoblast apoptosis 1-hour postop relative to pre-op, whereas in the transgenics, the change was not statistically significant (Fig 3B and Table 1). Some scattered TUNEL-positive cells were also detected in the subodontoblastic region for both genotypes (Fig 3B).
Fig 3.
Bcl-2 prevents artificial cavity induced odontoblast apoptosis as assayed by TUNEL. A. H/E staining demonstrating the artificial cavity drilled on molar 1 wk post-op. An artificial cavity of approximately half dentin thickness was drilled on the mesial cervical surface of mouse mandibular first molar. The black arrow denotes the cavity. B. TUNEL staining of odontoblast apoptosis before and after the operation. Nuclear Fast Red was used for counterstaining. Apoptotic cells are shown as having condensed, blue-stained nuclei as denoted by the black arrows. *,represents the side of pulp chamber close to the artificial cavity. Nuclease treatment or exclusion of TdT was used as (+) and (−) control, respectively. Scale bar = 100 μm. Abbreviations: (+), positive control; (−), negative control; +/+, wild type; tg/tg, homozygous transgenic; hr, hour.
Table 1.
Odontoblast-targeted Bcl-2 overexpression prevents artificial cavity induced odontoblast apoptosis as demonstrated by TUNEL (Quantification of the data in Fig 3B).
| Apoptotic index (post-op) | +/+ | tg/tg |
|---|---|---|
| 1 hr | 1.24±0.11 a | 1.15±0.09 |
| 1 day | 1.03±0.17 | 0.87±0.12 |
| 1 wk | 1.14±0.20 | 1.12±0.16 |
| 2 wk | 0.97±0.13 | 0.87±0.06 |
Apoptotic index = (% odontoblasts undergoing apoptosis post-op)/(% odontoblasts undergoing apoptosis pre-op). Data are presented as mean ± SEM.
, P< 0.05 compared with the pre-op basal line.
Abbreviations: +/+, wild type; tg/tg, homozygous transgenic; hr, hour; wk, week. n=6 per time point for each genotype.
Odontoblast-targeted Bcl-2 overexpression promoted dentin damage repair
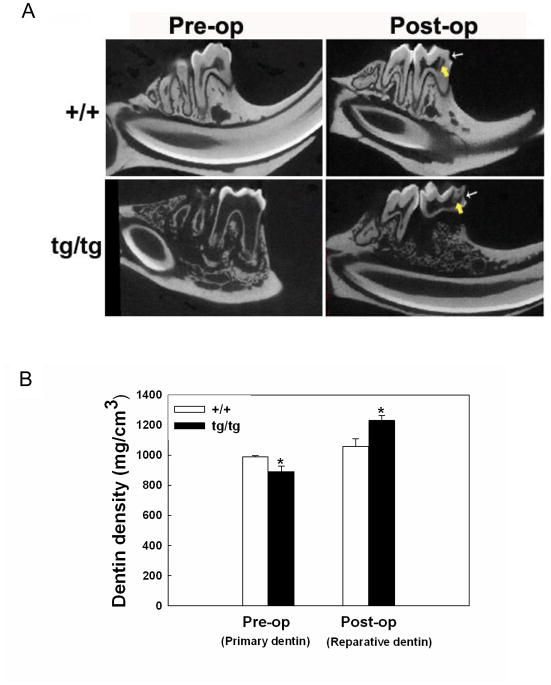
After cavity preparation, there was reparative dentin formation along the pulp surface opposite to the artificial cavity in both wild type and transgenic animals (Fig 4A). Wild type animals had primary dentin with significantly higher mineral density than that of the transgenics. However, the mineral density of reparative dentin produced by transgenics was significantly higher than that of the wild type 6 weeks post-op, as analyzed by micro-CT (Fig 4B). Although histological and micro-CT examinations showed slightly larger volume of reparative dentin in the transgenics compared to the wild type, the difference was not statistically significant (data not shown).
Fig 4.
Odontoblast-targeted Bcl-2 overexpression promotes dentin damage repair. A. Micro-CT images of sagittal planes of mouse mandibular first molar before and 6-week after artificial cavity preparation. Thin arrow points to artificial cavity drilled on the mesial surface of the molar at approximately a half dentin thickness. Thick arrow points to reparative dentin formed adjacent to the cavity. B. Quantification of the data in A. *, P< 0.05 tg/tg compared with +/+ at the same time point. Abbreviations: +/+, wild type; tg/tg, homozygous transgenic.
Discussion
Dental pulp from postnatal mice contains progenitor cells capable of differentiating into odontoblastic-like cells secreting tubular dentin in vitro when induced by ascorbic acid, β-glycerophosphate, and dexamethasone in the media (33, 34). Our previous study has shown sequential expression of type I collagen, dentin matrix protein-1, osteocalcin, and dentin sialophosphoprotein in the pulp culture (30), indicating pulp progenitors are developing along odontoblastic lineage, and primary cultures established from digested pulp is a good system to evaluate odontoblast development and function. Since the expression of transgene hBcl-2 was sustained during the 3-week culture period, the transgenic cultures may develop/maintain more anti-apoptotic odontoblasts than the wild type, especially in the later stages. This may explain why transgenic cultures had significantly less odontoblast apoptosis than the wild type on day 21.
In Col2.3Bcl-2 mice, transgene hBcl-2 is specifically expressed in tissues producing type I collagen, such as bone, tooth, and skin (31). The transgenic mice have been found to have thinner and less dense dentin compared to the wild type, indicating an impairment of primary dentinogenesis by Bcl-2 (30). However, the reparative dentin is much denser in the transgenics relative to wild type, suggesting a protective effect of Bcl-2 upon adverse stimuli. This observation in teeth is very similar to what is seen in the skeletal system. The Col2.3Bcl-2 mice have shorter and thinner bone compared with the age-matched wild type animals, but age-induced bone loss seen in the wild type animals due to excessive osteoblast apoptosis is prevented in the transgenics (31). These results confirm that physiological apoptosis is essential for the normal development and homeostasis of hard tissues, and interference with this process may negatively impact their formation and function. On the contrary, pathological apoptosis induced by external or internal stimuli is detrimental, and it will be beneficial to these tissues if excessive apoptosis is prevented. Creation of temporally inducible Bcl-2 overexpression transgenic mouse may help to further validate the above observations.
In this study, Western blot shows persistent hBcl-2 expression in the teeth of Col2.3Bcl-2 transgenic mice, and the levels of endogenous mouse Bcl-2 and Bax were similar between wild type and transgenics, which is consistent with in vivo immunohistochemistry result reported before (30). In vitro pulp cell cultures demonstrate similar expression pattern. Since transgenic odontoblasts have a higher Bcl-2/Bax ration than that of the wild type, they have a survival advantage relative to the wild type cells. This is manifested as less odontoblast apoptosis at basal level and after artificial cavity preparation in transgenic animals compared to the wild type.
Basal level of odontoblast apoptosis has been detected in vivo and in vitro for wild type and transgenic animals. Other investigators have identified no TUNEL-positive apoptotic odontoblasts in normal rat molars (24) and hamster incisors (23). This discrepancy may be related to the differences in the species and ages of animals, sample preparation, and the sensitivity of the technique employed. Odontoblast apoptosis is found to be significantly increased 1-hour after cavity preparation in wild type animals, which is consistent with what is reported in the literature (24). Since the depth of artificial cavity seems to affect the magnitude of induced odontoblast apoptosis (35), drilling cavities of a half dentin thickness was implemented as the standard of operation. The post-op specimens were checked histologically and via micro-CT examinations, to ensure that the mechanical stress is comparable and results are interpretable between the two genotypes.
With TUNEL technique, distinct individual apoptotic odontoblasts adjacent to the cavity and some scattered apoptotic sub-odontoblastic cells were detected 1 hr to 2 week post-op. Other study reported more contiguous positive staining for the odontoblast layer and underlying pulpal cells below the cavity (23). Hydroxyapatite has a high affinity for acidic proteins as well as for nucleic acids (36). Apoptotic odontoblasts can release fragmented DNA into surrounding mineralized dentin matrix, which might contribute to a contiguous false positive staining for the matrix, especially if decalcification of the specimens is not sufficient. In addition, phagocytosis of the apoptotic odontoblasts by scavenger cells may also result in the discontinuous staining pattern seen in our study.
Our data show that the primary dentin density in transgenic animals is significantly lower than that of the wild type. After cavity preparation, there is a 7% and 38% increase of reparative dentin density relative to the primary dentin for wild type and transgenics, respectively, which results in a much higher reparative dentin density in the transgenics compared to the wild type. It is speculated that odontoblasts in the transgenic animals have “double defenses”. On one hand they are much more damage-resistant and death-proof that, instead of becoming apoptotic, they are stimulated to produce reactionary dentin under cavity preparation. On the other hand growth factors released from demineralized dentin matrix will recruit pulp progenitors to differentiate and lay down reparative dentin at the injured site. Presence of both repair processes in the transgenic animals may explain why they have a higher reparative dentin density than that of the wild type. In another artificial cavity study, Bcl-2 expression is found to be increased significantly in odontoblasts underlying the cavity (24), suggesting that elevation of Bcl-2 is an important mechanism for odontoblasts to handle injury and maintain their structural and functional integrity.
Current clinical therapies for damaged or missing teeth include restorative procedures, prostheses, and implants. However, these methods are incapable of replacing all the functions of the original tooth and often fail overtime. It appears that genetic manipulation of Bcl-2 in odontoblasts is a novel strategy to protect a tooth under traumatic stress. Augmentation of Bcl-2 expression in time of needed will save the life of a tooth so that it can function at its full extends.
In summary, our study demonstrates that Bcl-2 promotes dentin damage repair via a prevention of excess odontoblast apoptosis induced by mechanical challenge. It seems that regulation of Bcl-2 expression in odontoblasts is a valuable alternative to preserve a damaged tooth.
Acknowledgments
This research was supported by NIH R03DE019663 (WZ). The authors thank Small Animal Imaging Facility at University of Texas M.D. Anderson Cancer Center for acquiring tooth Micro-CT images.
Abbreviations
- α-MEM
alpha-Minimum Essential Medium
- BCA
Bicinchoninic Acid Assay
- Bcl-2
B-cell Lymphoma-2
- hBcl-2
human Bcl-2
- mBcl-2
mouse Bcl-2
- mBax
mouse Bcl-2–associated X Protein
- βGP
beta-glycerophosphate
- BMP
Bone Morphogenetic Protein
- BSA
Bovine Serum Albumin
- DTT
Dithiothreitol
- EDTA
Ethylenediaminetetraacetic Acid
- E18
Embryonic Day 18
- FGF
Fibroblast Growth Factor
- HRP
Horseradish Peroxidase
- IGF
Insulin-like Growth Factor
- μCT
Micro X-ray Computed Tomography
- Msx
msh Homeobox
- PCR
Polymerase Chain Reaction
- RT
Room Temperature
- SDS-PAGE
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
- TdT
Terminal Deoxynucleotidyl Transferase
- TGFβ
Transforming Growth Factor beta
- TTBS
Tween 20 in Tris Buffered Saline
- TUNEL
Deoxynucleotidyl Transferase dUTP Nick End labeling
- +/+
Wild Type
- tg/tg
Homozygous Transgenic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruch JV. Tooth crown morphogenesis and cytodifferentiations: candid questions and critical comments. Connect Tissue Res. 1995;32(1–4):1–8. doi: 10.3109/03008209509013699. [DOI] [PubMed] [Google Scholar]
- 2.Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol. 1995;39(1):35–50. [PubMed] [Google Scholar]
- 3.Cate ART. Oral Histology: Development, Structure, and Function. 5. Saint Louis: Mosby; 1998. [Google Scholar]
- 4.Thesleff I, Nieminen P. Tooth morphogenesis and cell differentiation. Curr Opin Cell Biol. 1996;8(6):844–850. doi: 10.1016/s0955-0674(96)80086-x. [DOI] [PubMed] [Google Scholar]
- 5.About I, Mitsiadis TA. Molecular aspects of tooth pathogenesis and repair: in vivo and in vitro models. Adv Dent Res. 2001;15:59–62. doi: 10.1177/08959374010150011501. [DOI] [PubMed] [Google Scholar]
- 6.Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007;13(2):151–157. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med. 2001;12(5):425–437. doi: 10.1177/10454411010120050501. [DOI] [PubMed] [Google Scholar]
- 8.Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004;38(3):314–320. doi: 10.1159/000077771. [DOI] [PubMed] [Google Scholar]
- 9.Smith AJ. Pulpal responses to caries and dental repair. Caries Res. 2002;36(4):223–232. doi: 10.1159/000063930. [DOI] [PubMed] [Google Scholar]
- 10.Bjorndal L, Darvann T. A light microscopic study of odontoblastic and non-odontoblastic cells involved in tertiary dentinogenesis in well-defined cavitated carious lesions. Caries Res. 1999;33(1):50–60. doi: 10.1159/000016495. [DOI] [PubMed] [Google Scholar]
- 11.Begue-Kirn C, Smith AJ, Loriot M, Kupferle C, Ruch JV, Lesot H. Comparative analysis of TGF beta s, BMPs, IGF1, msxs, fibronectin, osteonectin and bone sialoprotein gene expression during normal and in vitro-induced odontoblast differentiation. Int J Dev Biol. 1994;38(3):405–420. [PubMed] [Google Scholar]
- 12.Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development (Cambridge, England) 1998;125(21):4325–4333. doi: 10.1242/dev.125.21.4325. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura C, Terashita M. Expressions of c-jun and jun-B proto-oncogenes in odontoblasts during development of bovine tooth germs. J Dent Res. 1997;76(4):822–830. doi: 10.1177/00220345970760040201. [DOI] [PubMed] [Google Scholar]
- 14.Ruch JV. Odontoblast commitment and differentiation. Biochem Cell Biol. 1998;76(6):923–938. [PubMed] [Google Scholar]
- 15.Cassidy N, Fahey M, Prime SS, Smith AJ. Comparative analysis of transforming growth factor-beta isoforms 1–3 in human and rabbit dentine matrices. Arch Oral Biol. 1997;42(3):219–223. doi: 10.1016/S0003-9969(96)00115-X. [DOI] [PubMed] [Google Scholar]
- 16.Finkelman RD, Mohan S, Jennings JC, Taylor AK, Jepsen S, Baylink DJ. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J Bone Miner Res. 1990;5(7):717–723. doi: 10.1002/jbmr.5650050708. [DOI] [PubMed] [Google Scholar]
- 17.Murray PE, About I, Lumley PJ, Smith G, Franquin JC, Smith AJ. Postoperative pulpal and repair responses. J Am Dent Assoc (1939) 2000;131(3):321–329. doi: 10.14219/jada.archive.2000.0175. [DOI] [PubMed] [Google Scholar]
- 18.Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32(2):115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 19.Sloan AJ, Rutherford RB, Smith AJ. Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch Oral Biol. 2000;45(2):173–177. doi: 10.1016/s0003-9969(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 20.Franquin JC, Remusat M, Abou Hashieh I, Dejou J. Immunocytochemical detection of apoptosis in human odontoblasts. Eur J Oral Sci. 1998;106 (Suppl 1):384–387. doi: 10.1111/j.1600-0722.1998.tb02202.x. [DOI] [PubMed] [Google Scholar]
- 21.Vermelin L, Lecolle S, Septier D, Lasfargues JJ, Goldberg M. Apoptosis in human and rat dental pulp. Eur J Oral Sci. 1996;104(5–6):547–553. doi: 10.1111/j.1600-0722.1996.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 22.Mitsiadis TA, De Bari C, About I. Apoptosis in developmental and repair-related human tooth remodeling: a view from the inside. Exp Cell Res. 2008;314(4):869–877. doi: 10.1016/j.yexcr.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Bronckers AL, Lyaruu DM, Goei W, Litz M, Luo G, Karsenty G, et al. Nuclear DNA fragmentation during postnatal tooth development of mouse and hamster and during dentin repair in the rat. Eur J Oral Sci. 1996;104(2 Pt 1):102–111. doi: 10.1111/j.1600-0722.1996.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura C, Kimura K, Nakayama T, Toyoshima K, Terashita M. Primary and secondary induction of apoptosis in odontoblasts after cavity preparation of rat molars. J Dent Res. 2001;80(6):1530–1534. doi: 10.1177/00220345010800061001. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura C, Ogawa Y, Morotomi T, Terashita M. Differential induction of apoptosis by capping agents during pulp wound healing. J Endod. 2003;29(1):41–43. doi: 10.1097/00004770-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Wang PL, Shirasu S, Daito M, Ohura K. Streptococcus mutans lipoteichoic acid-induced apoptosis in cultured dental pulp cells from human deciduous teeth. Biochem Biophys Res Commun. 2001;281(4):957–961. doi: 10.1006/bbrc.2001.4451. [DOI] [PubMed] [Google Scholar]
- 27.Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92(1):19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 28.Piattelli A, Rubini C, Fioroni M, Ciavarelli L, De Fazio P. bcl-2, p53, and MIB-1 in human adult dental pulp. J Endod. 2000;26(4):225–227. doi: 10.1097/00004770-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Slootweg PJ, de Weger RA. Immunohistochemical demonstration of bcl-2 protein in human tooth germs. Arch Oral Biol. 1994;39(7):545–550. doi: 10.1016/0003-9969(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Ju J, Gronowicz G. Odontoblast-targeted Bcl-2 overexpression impairs dentin formation. J Cell Biochem. 2010;111(2):425–432. doi: 10.1002/jcb.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantschenko AG, Zhang W, Nahounou M, McCarthy MB, Stover ML, Lichtler AC, et al. Effect of osteoblast-targeted expression of bcl-2 in bone: differential response in male and female mice. J Bone Miner Res. 2005;20(8):1414–1429. doi: 10.1359/JBMR.050315. [DOI] [PubMed] [Google Scholar]
- 32.Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am. 1984;A1:612–619. [Google Scholar]
- 33.Balic A, Mina M. Analysis of developmental potentials of dental pulp in vitro using GFP transgenes. Orthod Craniofac Res. 2005;8(4):252–258. doi: 10.1111/j.1601-6343.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 34.Balic A, Rodgers B, Mina M. Mineralization and expression of Col1a1-3.6GFP transgene in primary dental pulp culture. Cells, tissues, organs. 2009;189(1–4):163–168. doi: 10.1159/000154813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitamura C, Ogawa Y, Morotomi T, Terashita M. Effects of cavity size on apoptosis-induction during pulp wound healing. Oper Dent. 2003;28(1):75–79. [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch EF, Maniatis T. Commonly used techniques in molecular cloning. 2. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]