Abstract
Erectile dysfunction mechanisms in diabetic patients are multifactorial and often lead to resistance to current therapy. Animal toxins have been used as pharmacological tools to study penile erection. Human accidents involving the venom of Phoneutria nigriventer spider are characterized by priapism. We hypothesize that PnTx2-6 potentiates cavernosal relaxation in diabetic mice by increasing cGMP. This effect is nNOS dependent. Cavernosal strips were contracted with phenylephrine (10−5 M) and relaxed by electrical field stimulation (EFS, 20V, 1–32 Hz) in the presence or absence of PnTx2-6 (10−8 M).Cavernosal strips from nNOS and eNOS knocaut (KO) mice, besides nNOS inhibitor (10−5M), were used to evaluate the role of this enzyme in the potentiation effect evoked by PnTx2-6. Tissue cGMP levels were determined after stimulation with PnTx2-6 in presence or absence of L-NAME (10−4M) and ω-conotoxin GVIA (10−6M), an N-type calcium channel inhibitor. Results showed PnTx2-6 enhanced cavernosal relaxation in diabetic mice (65%) and eNOS KO mice, but not in nNOS KO mice. The toxin effect in the cavernosal relaxation was abolished by nNOS inhibitor. cGMP levels are increased by PnTx2-6, however L-NAME abolished this enhancement as well as ω-conotoxin GVIA. We conclude PnTx2-6 facilitates penile relaxation in diabetic mice through a mechanism dependent on nNOS, probably via increasing NO/cGMP production.
Keywords: Phoneutria nigriventer, erectile dysfunction, PnTx2-6 toxin, NO, cGMP
INTRODUCTION
Venoms from arthropods are rich source of bioactive molecules which can be useful as pharmacological tools (1). It has been described that patients bitten by Phoneutria nigriventer spider present various symptoms including priapism (2–6). The venom from this arthropod, referred to as the `armed spider' (6), is composed of a variety of distinct polypeptides which have been shown to elicit various biological activities. (7–10). The majority of isolated toxins from this venom have been described as neurotoxins which primarily induce effects on various ion channels (5, 11–13). The PhTx2 fraction as well as some of its isolated peptides has been shown to alter the kinetics of neuronal sodium (Na2+) channel inactivation (14–16).
PnTx2-6 a polypeptide isolated from this fraction has been shown a high affinity for Na2+ channels, as demonstrated in its affect on the voltage-dependent gating of the Na2+ channel. Previously published papers from our group have demonstrated that incubation of PnTx2-6 in cavernosal strips increases relaxation, and others have shown that direct injection of this toxin into the penis leads to an erectile response (7–8). Additionally, this fraction preferentially localizes in penile tissue (17), which makes PnTx2-6 a direct target for research into the mechanisms of erectile function and reversal of erectile dysfunction (ED).
It is widely accepted that regulation of erection is a complicated system under neuro-regulatory control and consists of cholinergic, adrenergic and nonadrenergic non-cholinergic (NANC) effector systems (18–19). Most investigators agree that nitric oxide (NO) is the primary mediator of the erectile response. (18, 20–26). Given that deregulated events in the NO/cGMP pathway can cause impaired cavernosal relaxation leading to ED which is defined as an inability to attain or to maintain a penile erection (NIH Consensus Conference, 1993). This condition has become increasingly prevalent throughout the US and the world, and is said to affect from 19–64% of men (27–28).
ED risk factors include cardiovascular disease (CVD), lifestyle choices, such as obesity, smoking and a sedentary lifestyle in addition to chronic illness (28–32). According to the Massachusetts Male Aging Study, one such chronic illness is diabetes mellitus (DM); these men were shown to have a 28% prevalence rate of ED as compared to 9.6% in the general population (33). DM is a metabolic disorder, where patients have hyperglycemia and insulin resistance and it frequently co-exists with a sedentary lifestyle and increased body mass index (BMI). In diabetic patients and animal models, vascular and endothelial dysfunction is a common theme, with current literature suggesting that there is an impairment of vasodilatory signaling, decreased NO in addition to NANC dysfunction (34–35).
PnTx2-6 has been demonstrated to mediate penile erection through an increase in NO; making it a possible therapeutic approach for diabetes induced ED. In addition, toxins have been used as pharmacological tool to study ED (1) and recently it has been demonstrated that PnTx2-6 recombinant toxin (rPnTx2-6) was active in rat erectile function (36) as well as PnTx2-6. In this manuscript, we propose that PnTx2-6 can increases NO via nNOS, as well as cGMP, which can reverse the cavernosal hyperreactivity in STZ-diabetic mice.
METHODS
Animals
Male C57Bl6 mice (10–12 weeks-old, 22–28g) and genetically altered mice which lack genes for nNOS and eNOS were used in these studies (Jackson Laboratories, Bar Harbor ME). All procedures were carried out in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Medical College of Georgia Committee and by the Federal University of Minas Gerais on the use of animals in research and education. The animals were housed 4 per cage on a 12-h light/dark cycle and fed a standard rat chow diet and water.
Drugs and Solutions
Physiological salt solution of the following composition was used: 130mM NaCl, 14.9 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4.7H2O, 1.6 mM CaCl2. 2H2O, and 0.026 mM EDTA. Bretylium tosylate [(o-bromobenzyl) ethyldimethylammonium p-toluenesulfonate], ω-conotoxin GVIA and 7-nitroindozole were purchased from Sigma Chemical Co. (St. Louis, MO, USA). N-Nitro-L-arginine methyl ester (L-NAME) was purchased from Cayman Chemical (Michigan, USA). All reagents used were of analytical grade. Stock solutions were prepared in deionized water and stored in aliquots at −20°C; dilutions were freshly prepared before use. The toxin PnTx2-6 was kindly provided by the Ezequiel Dias Foundation (Brazil) and purified as reference cited (37)..
Functional studies in cavernosal strips
The mice were anesthetized (ketamine/xylazine, (100:10 mg/kg, i.p.) and exsanguinated by aorta abdominal. After euthanasia, penes were excised and dissected in ice-cold buffer. The tunica albuginea was removed and one crural strip preparation (1×1×10 mm) was obtained from each corpus cavernosum (two crural strips from each penis). Cavernosal strips were mounted in 4-ml myograph chambers (Danish Myo Technology, Aarhus, Denmark) containing buffer at 37°C continuously aerated with a mixture of 95% O2 and 5% CO2. The tissue was stretched to a passive force of 3.0mN and allowed to equilibrate for 60 min; the solutions were replaced every 10 to 15 min. Changes in isomeric force were recorded using a PowerLab/8SP data acquisition system (Chart software, version 5.0; ADInstruments, Colorado Springs, CO). To verify the contractile ability of the preparations, a high potassium chloride (KCl) solution (120mM) was added to the organ bath at the end of the equilibration period. All preparations were incubated for 35 min with bretylium tosylate (30 μM) to block sympathetic nerve discharge. Cavernosal strips were contracted with phenylephrine (PE; 10 μM) and relaxation was evoked by electrical field stimulation (EFS). Electrical stimuli were applied to strips placed between platinum pin electrodes, which were attached to a stimulus splitter unit (Stimu-Splitter, USA) connected to a Grass S88 stimulator (Astro-Med, USA). EFS was conducted at 20 V, 1-ms pulse width and trains of stimuli lasting 10s at varying frequencies (1 to 32 Hz) before and after incubation with PnTx2-6 (10−8 M, 4min) and 7-nitroindozole (nNOS inhibitor, 10−5 M)
The calcium channel blocker ω-conotoxin GVIA (10−6M) was used to evaluate the participation of N-type Ca+2 channels in cGMP production in the presence of PnTx2-6. This specific Ca+2 channel inhibitor and/or the NOS inhibitor, L-NAME (10−4 M), were added 35 minutes before cGMP measure levels.
Streptozocin induced diabetes
Diabetes is chiefly characterized by hyperglycemia which was induced by using streptozocin (STZ). C57Bl6 mice were injected with STZ (125 mg/kg body weight in 10−1M citrate buffer, pH 4.5) for two days. Mice were allowed to become hyperglycemic for 8 weeks before use. The penis was removed and cavernosal strips isolated. Hyperglycemia was confirmed using the AccuCheck glucose meter (Roche Diagnostic Corporation, Indianapolis, IN, USA) and glucose test strips in fasted mice. Mice showing glucose levels up to 350 mg/dl were considered diabetic.
Determination of cGMP levels
Mice cavernosal strips were equilibrated for 20min in warmed and oxygenated Krebs' solution. Tissues were then stimulated for 35min with L-NAME (10−4M), ω-conotoxin GVIA (10−6M) in the presence and absence of PnTx2-6 (10−8M) added in the last 4 minutes. Next, strips were collected immediately by freezing the segments in liquid nitrogen. Some tissues were frozen following the addition of vehicle to obtain baseline readings. Frozen cavernosal tissue were pulverized, homogenized in trichloroacetic acid (TCA; 5% w/v) and then centrifuged at 1500 g for 10min at 4°C. The TCA was extracted from samples with three washes of water-saturated ether. The weights of the dried pellets were used to standardize the different samples. cGMP was extracted and quantified using a cGMP enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI). Assays were performed in duplicate using different dilutions of samples.
Statistical Analysis
Results are expressed as mean values ± SEM. Relaxation is presented as percentage change from the PE-induced contraction. Relaxation response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 4.0; GraphPAD Software Inc., San Diego, CA, USA). Differences were estimated by two-way analyses of variance (ANOVA) and Student's t-test. Values of P<0.05 were considered statistically significant.
RESULTS
PnTx2-6 toxin improves EFS-mediated relaxation in cavernosal tissue from WT mice and STZ-diabetic mice
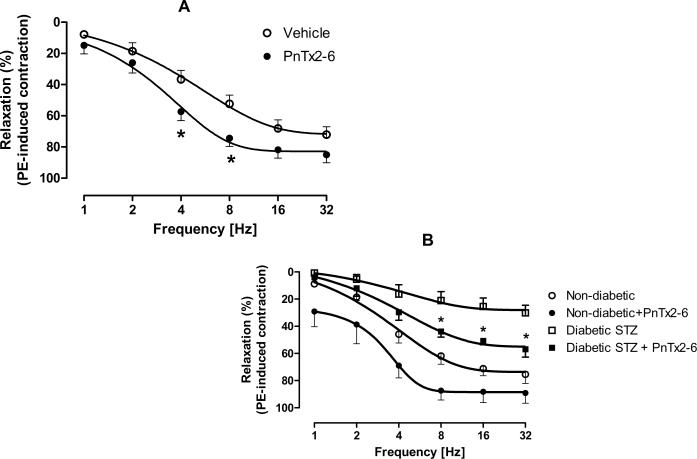
To determine if PnTx2-6 can increase relaxation in mice cavernosal strips, tissues were incubated with PnTx2-6 or vehicle for 4min, contracted to PE and a relaxation response curve to EFS performed. As shown in Figure 1A, cavernosal strips from WT incubated with PnTx2-6 exhibited a dose-dependent increase in cavernosal relaxation, reaching significance at 4Hz and 8Hz (n=9, p<0.05), suggesting that PnTx2-6 induces an increase around 23% in NANC mediated relaxation. Cavernosal strips from STZ-diabetic mice, animals found to be significantly hyperglycemic as compared to sham mice (Table 01, 135±34 dl/ml vs. 435±28 dl/ml), exhibited a significantly decreased NANC mediated relaxation response (Figure 1B). When the strips were incubated with PnTx2-6, both sham and STZ-diabetic mice exhibited a significantly increased relaxation response (Figure 1B). Interestingly, the maximal relaxation response achieved by strips from STZ-diabetic mice incubated with this toxin did not reach levels similar to cavernosal strips from normoglycemic mice (±55% vs ±75%), however it was almost twice as large if compared with non treated STZ-diabetic mice (±32%). These data demonstrate that PnTx2-6 can reverse the dysfunctional erectile phenotype seen in STZ-diabetic mice.
Figure 1.
PnTx2-6 improves non adrenergic-non cholinergic (NANC)-induced relaxation in cavernosum strips from Wt and STZ-diabetic mice. Cavernosal tissue from WT mice (A) and STZ-diabetic mice (B) incubated for 4 min with PnTx2-6 (10−8M) showed significant enhanced in cavernosal relaxation (n=9, p<0.05). As observed in the figure B, impaired cavernosal relaxation in STZ-diabetic mice strips were reversed by PnTx2-6 toxin.
Table 01.
Body weight and blood glucose levels of non-diabetic and STZ-diabetic mice. Plasma glucose measure showed rates to STZ-treated mice that indicated levels compatible with hyperglycemia (n=5, * P < 0.05). Mice with glucose levels up to 350 mg/dl were considered diabetic.
| Non-diabetic | STZ-diabetic | |
|---|---|---|
| Body weight (g) | 29.4±1.9 | 21.7±2.3 * |
| Glucose (mg/dl) | 135±34 | 435±28 * |
| Cavernosal strip | 1.76±0.3 | 1.74±0.2 |
| Weight (mg) |
PnTx2-6 improves cavernosal relaxation in eNOS KO mice, but not in nNOS KO mice
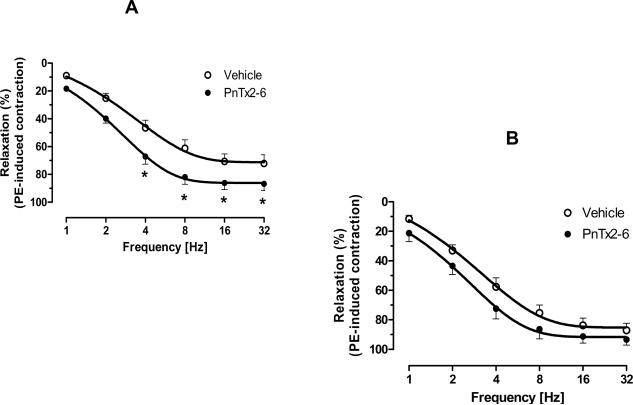
It has been previously demonstrated by our group that the PnTx2-6 toxin induces erection in rodent studies via NO; however, the source of NO has not been shown. Thus, EFS-mediated relaxation was performed in PE contracted cavernosal strips from eNOS (Figure 2a) and nNOS (Figure 2b) KO mice, incubated with vehicle or PnTx2-6. As shown in Figure 2, the toxin improves the relaxation in cavernosal strips from eNOS KO mice (n=9, p<0.05), but not from nNOS KO mice (n=8). This data suggest that PnTx2-6 acts on nitrergic nerves inducing increase in NO release from nNOS.
Figure 2.
PnTx2-6 failed to relax cavernosum strips from nNOS KO mice. Cavernosum tissue from eNOS and nNOS KO mice were incubated with PnTx2-6 (10−8M) for 4 min to verified the source of NO production in presence of this toxin. EFS-curve to cavernosal relaxation from eNOS KO mice in the presence of PnTx2-6 showed significant difference (Fig. 2A) and this effect was not observed in cavernosal tissue from nNOS KO mice (Fig. 2B), suggesting NO production mediated by nNOS in the presence of PnTx2-6 (n=8, p<0.05).
PnTx2-6 relaxation is dependent upon nNOS
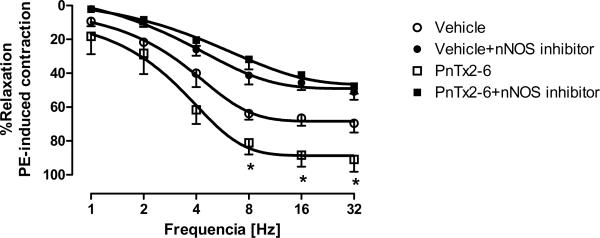
Even though the use of KO mice can increase our understanding, the whole animal KO of a particular gene can lead to alterations in other genes, producing compensation from alternate pathways. To confirm whether the increase in NO from PnTx2-6 can be from nNOS, a specific nNOS inhibitor (10−5M), 7-nitroindizole, was used. EFS-mediated relaxation curves were performed in PE contracted cavernosal tissue incubated with either vehicle, PnTx2-6, 7-nitroindizole (Figure 3). Incubation with PnTx2-6 increased EFS-mediated relaxation, while incubation with the nNOS inhibitor decreased relaxation. Interestingly, incubation with both PnTx2-6 and the nNOS inhibitor reversed the increased relaxation response seen in strips incubated with PnTx2-6 alone. These data suggest that nNOS production of NO may be mediating some of the effects of PnTx2-6.
Figure 3.
PnTx2-6 cavernosal relaxation enhancement was abolished by nNOS inhibitor. Mice cavernosal tissue previously incubated with nNOS inhibitor 7 nitroindizole (10−5M, 35 min) did not showed EFS-relaxation augment in the presence of PnTx2-6, suggesting an nNOS dependent effect (n=5, p<0.05).
PnTx2-6 incubation did not change cholinergic relaxation responses
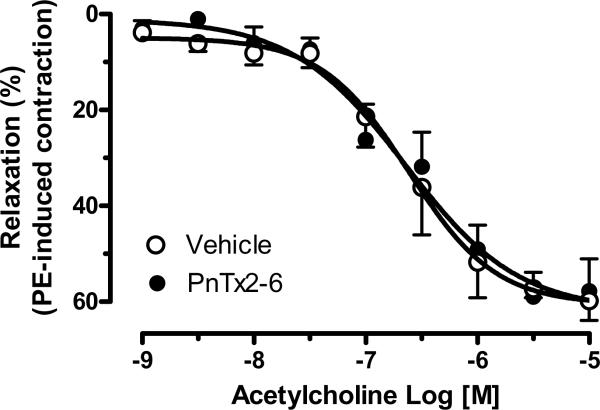
Relaxation in penile tissue is mainly mediated via NO release from the NANC system; however, endothelium mediated relaxation is an important component of erectile function. To determine whether PnTx2-6 alters ACh-mediated relaxation, concentration response curves (10−9 to 10−5 M) were performed in cavernosal tissue incubated with vehicle, or PnTx2-6 (n=9). We observed no increase in relaxation with this toxin, suggesting that PnTx2-6 cannot potentiate NO release from eNOS (Figure 4).
Figure 4.
PnTx2-6 did not interfere with cholinergic relaxation response. Concentration response curve to Ach (10−9 to 10−5 M) was performed in the presence and absence of PnTx2-6. Any difference was observed suggesting no cholinergic involvement in the relaxation evoked by this toxin (n=8).
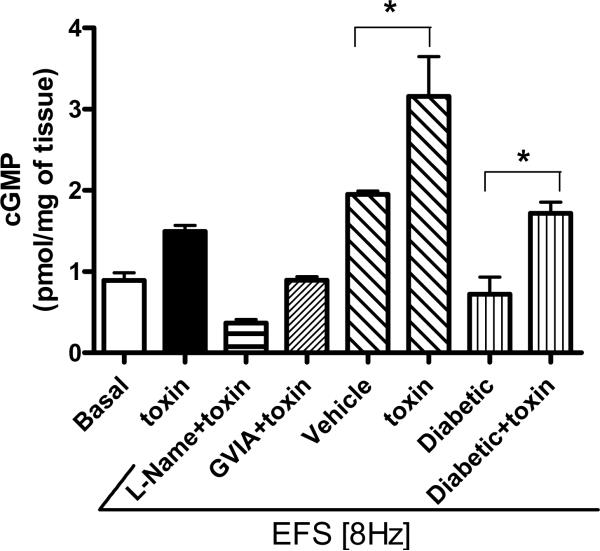
An increased cGMP level evoked by PhTx2-6 in cavernosal tissue was suppressed by L-NAME and ω-conotoxin GVIA
The levels of accumulated cGMP are an indicator of NO production and sGC activation. In cavernosal tissue incubation with PnTx2-6 induced an increase in cGMP levels, as compared to basal levels, further confirming an increase in NO by PnTx2-6. Cavernosal tissue incubated with L-NAME, the non-specific NOS inhibitor, and PnTx2-6 showed decreased levels of cGMP, which were lower than baseline. In addition, incubation with ω-GVIA conotoxin and PnTx2-6 together led to decreased cGMP levels, as compared to incubation with the toxin alone. Electrical stimulation (ES) leads to the release of NO, and cGMP levels were evaluated in ES cavernosal tissue (n=4, Figure 5). Also, in the presence of PnTx2-6, cGMP levels were further increased in diabetic penile tissue compared with non-diabetic tissue. Taken together, these data suggest that this toxin increases NO produced by NOS, which can be inhibited by the N-type calcium channel blocker ω-conotoxin. In addition, these data confirm our functional studies showing that PnTx2-6 mediates NO release through NANC mechanism, since incubation with toxin and ES lead to three times the amount of cGMP in cavernosal tissue.
Figure 5.
Enhanced cGMP levels in mice tissue treated with PnTx2-6 were contained by L-NAME and ω-conotoxin GVIA. The first and second bars showed basal and cGMP levels in the presence of PnTx2-6 without EFS. From third to the last bar, experiments were performed in the presence of EFS (8Hz). Cavernosal tissue from diabetic and non-diabetic mice treated with toxin showed increased cGMP levels compared to non-treated. L-NAME (10−4 M) and ω-conotoxin GVIA (10−6 M) suppressed cGMP levels increased by PnTx2-6 incubation. These results point to participation of N-type Ca+2 channels in the improved relaxation mediated by NO/cGMP pathway due to PnTx2-6 (n=4 per group, p<0.05).
DISCUSSION
This study was performed to further characterize mechanisms underlying PnTx2-6 induced penile erection and to determine the source of the PnTx2-6-induced increases in NO. Additionally, we have previously shown that this toxin increases local NO levels in the penis, as well as potentiating the erectile response demonstrated by stimulation of the major pelvic ganglion (MPG) and measuring intracavernosal pressure (ICP) (7). All these data support clinical evidence that patients bitten by P. nigriventer exhibit priapism.
We show that incubation of cavernosal strips with PnTx2-6 potentiates the NANC-mediated relaxation response, and that incubation with an inhibitor of nNOS inhibitor abolishes this response. This shows a definitive role for PnTx2-6 in potentiating nitrergic NO release. NO release has been postulated to occur not only from nNOS, but from the endothelium of the sinusoidal cavities. The relative contribution of eNOS to erectile function has been investigated; however, much of the data are confounding. It is well known that mice lacking the eNOS gene exhibit hypertension, presumably due to the lack of vascular NO from NOS, and a study performed by Burnett and colleagues demonstrate that these mice also do not achieve penile erection in response to carbachol suggesting that this isoform is crucial for erection (38). In this manuscript we used cavernosal strips from eNOS KO mice to perform relaxation response curves to EFS. We observed that the NANC-mediated relaxation response in the eNOS KO mice was similar to wild-type (Wt) mice, and that incubation with PnTx2-6 can increase the relaxation response.
However, concentration response curves to ACh reveal that PnTx2-6 incubation does not change relaxation, suggesting that a primary mechanism for PnTx2-6 is not through cholinergic stimulation. Although we did not demonstrate a profound decrease in relaxation in the eNOS KO mice, various compensatory mechanisms in local vasodilators may be an explanation. We also performed similar experiments in nNOS KO mice and while we did not observe a potentiation of relaxation with PnTx2-6 incubation, we found that the relaxation responses in these mice were also similar to those in their Wt counterparts. While these data are surprising, our experiments using a specific nNOS inhibitor were shown to inhibit the increase in NANC-mediated relaxation by PnTx2-6 suggesting that nNOS is not only responsible for cavernosal relaxation, but that PnTx2-6 can potentiate this response. Current literature has demonstrated at least two splice variants of nNOS, of which a particular variant has been cloned from the rat penis (PnNOS) and seems to survive in mice with nNOS gene deletion (39). The discovery of these splice variants complicates interpretation of data obtained using nNOS KO mice. Furthermore, investigators must take into account the physiological effects that a lifelong deletion of a gene may have.
It has been previously demonstrated that both the fraction PhTx2 and the purified toxin (PnTx2-6) can delay Na2+ channel inactivation (14–15). With a decrease in Na2+, the nerve fiber will maintain a state of depolarization, leading to increased Ca2+ flux into the cell and increased release of neurotransmitters (7, 40). Studies using canine and human corpus cavernosum have shown a role for the N-type Ca2+ channel in the nitrergic nerve terminals, and suggest that these channels are responsible for the Ca2+ flux leading to NOS activation (26). Our data showed that the increase in locally accumulated cGMP with PnTx2-6 incubation is decreased when the slices of cavernosum corpus are incubated with ω-conotoxin, a selective N-type Ca2+ channel blocker. Our data add to the current body of literature directly demonstrating that PnTx2-6 potentiates the NO/cGMP signaling pathway indirectly via these N-type Ca2+ channels (Figure 7).
As shown in Figure 2, cavernosal strips from eNOS KO mice incubated with PnTx2-6 exhibited an increase in NANC-mediated relaxation responses. The increase in relaxation was maintained from 4Hz, and culminated in a difference in the maximal response (n=9, p<0.05). In contrast, no statistical difference in relaxation was seen in tissues from nNOS KO mice incubated with this toxin (n=8). Though these data demonstrate that PnTx2-6 can increase cavernosal relaxation in eNOS mice, the data from nNOS mice is not surprising. There is significant overlap in NO production in the cavernosum, and investigators suggest that the lifelong deletion of eNOS could lead to an increase in local vasodilatory mechanisms (38).
Hypertension and diabetes are known risk factors for ED. A previous study, published by the author, revealed that use of PnTx2-6 can reverse hypertension induced ED. Deoxycorticosterone (DOCA) was used in that study to mimic mineralocorticoid-induced hypertension in rats. Those rats were shown to exhibit profound hypertension and exhibit ED, which was reversed with this toxin treatment. That study was the first to demonstrate a therapeutic effect of PnTx2-6 and to correlate this toxin to increased NO production and/or bioavailability. In this paper, we reveal that incubation of cavernosal strips not only increases NANC-mediated relaxation, but that the decreased relaxation responses observed in cavernosal strips from STZ-diabetic mice can be reversed with PnTx2-6 incubation.
PnTx2-6, this naturally existing polypeptide has the potential to emerge as a new pharmacological tool in conditions where vascular and endothelial dysfunction persists, and where increasing NO bioavailability may be able to reverse the dysfunction. ED is one such disorder. Our data suggested a primary role for PnTx2-6 in increasing NO/cGMP and the NANC mediated relaxation in cavernosal strips (Figure 07). Given that incubation with PnTx2-6 was able to reverse the dysfunctional phenotype of cavernosal strips from STZ-diabetic, PnTx2-6 should be investigated further for possible therapeutic interventions.
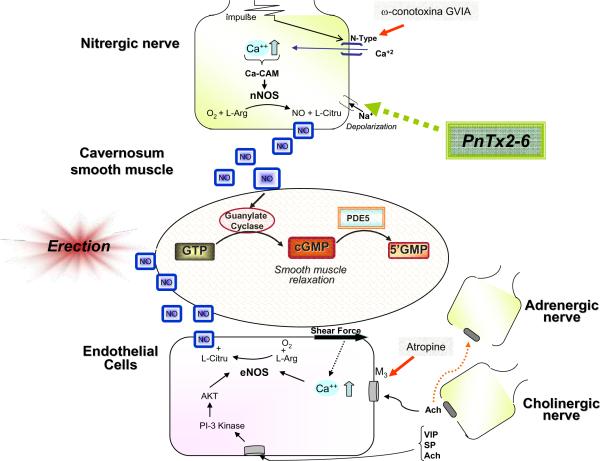
Figure 6.
Possible mechanism of action of PnTx2-6 toxin in the penis. The neuronal depolarization caused by PnTx2-6 (since this toxin delay the inactivation period of Na+ channels) lead to an increase in Ca+ influx, probably via activation of N-type Ca+ channels, which in turn activates nNOS inducing NO production and improving relaxation of vascular smooth muscle, causing penile erection. Red arrows mean inhibition.
Acknowledgments
This work was supported by grants from NIH (RO1-HL083685) and AHA in USA and grants from CAPES, CNPq, FAPEMIG and INCTTOX-FAPESP in Brazil. Dr. Nunes is supported by AHA Post-Doctoral fellowship.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Nunes KP. Animal toxins as potential pharmacologycal tools for treatment of erectile dysfunction. In: De Lima ME, editor. Animal toxins: State of the art Perspectives in health and biotechnology. Belo Horizonte: UFMG Editora; 2009. pp. 314–22. [Google Scholar]
- 2.Bucaretchi F, Deus Reinaldo CR, Hyslop S, Madureira PR, De Capitani EM, Vieira RJ. A clinico-epidemiological study of bites by spiders of the genus Phoneutria. Rev Inst Med Trop Sao Paulo. 2000 Jan-Feb;42(1):17–21. doi: 10.1590/s0036-46652000000100003. [DOI] [PubMed] [Google Scholar]
- 3.Bucaretchi F, Mello SM, Vieira RJ, Mamoni RL, Blotta MH, Antunes E, et al. Systemic envenomation caused by the wandering spider Phoneutria nigriventer, with quantification of circulating venom. Clin Toxicol (Phila) 2008 Nov;46(9):885–9. doi: 10.1080/15563650802258524. [DOI] [PubMed] [Google Scholar]
- 4.Mattiello-Sverzut AC, Fontana MD, Diniz CR, da Cruz-Hofling MA. Pathological changes induced by PhTx1 from Phoneutria nigriventer spider venom in mouse skeletal muscle in vitro. Toxicon. 1998 Oct;36(10):1349–61. doi: 10.1016/s0041-0101(98)00012-9. [DOI] [PubMed] [Google Scholar]
- 5.Mattiello-Sverzuta AC, da Cruz-Hofling MA. Toxin 2 (PhTx2), a neurotoxic fraction from Phoneutria nigriventer spider venom, causes acute morphological changes in mouse skeletal muscle. Toxicon. 2000 Jun;38(6):793–812. doi: 10.1016/s0041-0101(99)00188-9. [DOI] [PubMed] [Google Scholar]
- 6.Borges MH. Structural and functional diversity in the venom of spiders of the genus Phoneutria. Animal Toxins: State of the Art Perspectives in health and biotechnology. 2009;2:291–312. [Google Scholar]
- 7.Nunes KP, Costa-Goncalves A, Lanza LF, Cortes SF, Cordeiro MN, Richardson M, et al. Tx2-6 toxin of the Phoneutria nigriventer spider potentiates rat erectile function. Toxicon. 2008 Jun 1;51(7):1197–206. doi: 10.1016/j.toxicon.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade E, Villanova F, Borra P, Leite K, Troncone L, Cortez I, et al. Penile erection induced in vivo by a purified toxin from the Brazilian spider Phoneutria nigriventer. BJU Int. 2008 Sep;102(7):835–7. doi: 10.1111/j.1464-410X.2008.07762.x. [DOI] [PubMed] [Google Scholar]
- 9.Villanova FE, Andrade E, Leal E, Andrade PM, Borra RC, Troncone LR, et al. Erection induced by Tx2-6 toxin of Phoneutria nigriventer spider: expression profile of genes in the nitric oxide pathway of penile tissue of mice. Toxicon. 2009 Nov;54(6):793–801. doi: 10.1016/j.toxicon.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Nunes KP, Cardoso FL, Cardoso HC, Jr, Pimenta AMC, De Lima ME. Animal toxins as a potential pharmacological tools for the tratment of erectile dysfunction. In: de Lima ME, Pimenta AMC, Martin-Eauclaire MF, Zingali R, Rochat H, editors. Animal Toxins: State of the Art Perspectives in Health and Biotechnology. Belo Horizonte: Editora UFMG; 2009. pp. 131–22. [Google Scholar]
- 11.Diniz MR, Theakston RD, Crampton JM, Nascimento Cordeiro M, Pimenta AM, De Lima ME, et al. Functional expression and purification of recombinant Tx1, a sodium channel blocker neurotoxin from the venom of the Brazilian “armed” spider, Phoneutria nigriventer. Protein Expr Purif. 2006 Nov;50(1):18–24. doi: 10.1016/j.pep.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Moutot N, Mansuelle P, Alcaraz G, Dos Santos RG, Cordeiro MN, De Lima ME, et al. Phoneutria nigriventer toxin 1: a novel, state-dependent inhibitor of neuronal sodium channels that interacts with micro conotoxin binding sites. Mol Pharmacol. 2006 Jun;69(6):1931–7. doi: 10.1124/mol.105.021147. [DOI] [PubMed] [Google Scholar]
- 13.Kushmerick C, Kalapothakis E, Beirao PS, Penaforte CL, Prado VF, Cruz JS, et al. Phoneutria nigriventer toxin Tx3-1 blocks A-type K+ currents controlling Ca2+ oscillation frequency in GH3 cells. J Neurochem. 1999 Apr;72(4):1472–81. doi: 10.1046/j.1471-4159.1999.721472.x. [DOI] [PubMed] [Google Scholar]
- 14.Araujo DA, Cordeiro MN, Diniz CR, Beirao PS. Effects of a toxic fraction, PhTx2, from the spider Phoneutria nigriventer on the sodium current. Naunyn Schmiedebergs Arch Pharmacol. 1993 Feb;347(2):205–8. doi: 10.1007/BF00169268. [DOI] [PubMed] [Google Scholar]
- 15.Matavel A, Fleury C, Oliveira LC, Molina F, de Lima ME, Cruz JS, et al. Structure and activity analysis of two spider toxins that alter sodium channel inactivation kinetics. Biochemistry. 2009 Apr 14;48(14):3078–88. doi: 10.1021/bi802158p. [DOI] [PubMed] [Google Scholar]
- 16.Matavel A, Cruz JS, Penaforte CL, Araujo DA, Kalapothakis E, Prado VF, et al. Electrophysiological characterization and molecular identification of the Phoneutria nigriventer peptide toxin PnTx2-6. FEBS Lett. 2002 Jul 17;523(1–3):219–23. doi: 10.1016/s0014-5793(02)02988-5. [DOI] [PubMed] [Google Scholar]
- 17.Nunes KP, Cordeiro MN, Richardson M, Borges MN, Diniz SOF, Cardoso VN, Tostes R, de Lima ME, Webb RC, Leite R. NO-induced vasorelaxation in response to PnTx2-6 toxin from Phoneutria nigriventer spider in rat cavernosal tissue. J Sex Medicine. 2010 doi: 10.1111/j.1743-6109.2010.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnett AL. Nitric oxide regulation of penile erection: biology and therapeutic implications. J Androl. 2002 Sep-Oct;23(5):S20–6. [PubMed] [Google Scholar]
- 19.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005 Nov;32(4):379–95. v. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992 Jul 17;257(5068):401–3. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 21.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–42. [PubMed] [Google Scholar]
- 22.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 23.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11–17;327(6122):524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 24.Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990 Jul 31;170(2):843–50. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 25.Burnett AL. The role of nitric oxide in erectile dysfunction: implications for medical therapy. J Clin Hypertens (Greenwich) 2006 Dec;8(12 Suppl 4):53–62. doi: 10.1111/j.1524-6175.2006.06026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toda N, Ayajiki K, Okamura T. Nitric oxide and penile erectile function. Pharmacol Ther. 2005 May;106(2):233–66. doi: 10.1016/j.pharmthera.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Leite R, Giachini FR, Carneiro FS, Nunes KP, Tostes RC, Webb RC. Targets for the treatment of erectile dysfunction: is NO/cGMP still the answer? Recent Pat Cardiovasc Drug Discov. 2007 Jun;2(2):119–32. doi: 10.2174/157489007780832579. [DOI] [PubMed] [Google Scholar]
- 28.Priviero FB, Leite R, Webb RC, Teixeira CE. Neurophysiological basis of penile erection. Acta Pharmacol Sin. 2007 Jun;28(6):751–5. doi: 10.1111/j.1745-7254.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 29.Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, Stefanadis C. The triad: erectile dysfunction--endothelial dysfunction--cardiovascular disease. Curr Pharm Des. 2008;14(35):3700–14. doi: 10.2174/138161208786898716. [DOI] [PubMed] [Google Scholar]
- 30.Carneiro FS, Webb RC, Tostes RC. Emerging Role for TNF-alpha in Erectile Dysfunction. J Sex Med. 2010 Mar 15; doi: 10.1111/j.1743-6109.2010.01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller A, Mulhall JP. Cardiovascular disease, metabolic syndrome and erectile dysfunction. Curr Opin Urol. 2006 Nov;16(6):435–43. doi: 10.1097/01.mou.0000250284.83108.a6. [DOI] [PubMed] [Google Scholar]
- 32.Wespes E, Schulman CC. Erectile dysfunction and cardiovascular diseases. Arch Esp Urol. 2010 Oct;63(8):649–54. [PubMed] [Google Scholar]
- 33.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Construction of a surrogate variable for impotence in the Massachusetts Male Aging Study. J Clin Epidemiol. 1994 May;47(5):457–67. doi: 10.1016/0895-4356(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 34.Moore CR, Wang R. Pathophysiology and treatment of diabetic erectile dysfunction. Asian J Androl. 2006 Nov;8(6):675–84. doi: 10.1111/j.1745-7262.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 35.Hidalgo-Tamola J, Chitaley K. Review type 2 diabetes mellitus and erectile dysfunction. J Sex Med. 2009 Apr;6(4):916–26. doi: 10.1111/j.1743-6109.2008.01116.x. [DOI] [PubMed] [Google Scholar]
- 36.Torres FS, Silva CN, Lanza LF, Santos AV, Pimenta AM, De Lima ME, et al. Functional expression of a recombinant toxin - rPnTx2-6 - active in erectile function in rat. Toxicon. 2010 Apr 24; doi: 10.1016/j.toxicon.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Cordeiro MN. The purification and amino acid sequences of four Tx2 neurotoxins from the venom of the Brazilian “armed” spider Phoneutria nigriventer (keys) FEBS Lett. 1995;32(28):153–6. doi: 10.1016/0014-5793(92)81318-g. [DOI] [PubMed] [Google Scholar]
- 38.Burnett AL, Chang AG, Crone JK, Huang PL, Sezen SE. Noncholinergic penile erection in mice lacking the gene for endothelial nitric oxide synthase. J Androl. 2002 Jan-Feb;23(1):92–7. doi: 10.1002/j.1939-4640.2002.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Cadavid NF, Burnett AL, Magee TR, Zeller CB, Vernet D, Smith N, et al. Expression of penile neuronal nitric oxide synthase variants in the rat and mouse penile nerves. Biol Reprod. 2000 Sep;63(3):704–14. doi: 10.1095/biolreprod63.3.704. [DOI] [PubMed] [Google Scholar]
- 40.Carneiro DS, Vieira LB, Cordeiro MN, Richardson M, Castro-Junior CJ, Gomez MV, et al. Effects of new Phoneutria spider toxins on glutamate release and [Ca2+]i in rat cortical synaptosomes. Cell Mol Biol (Noisy-le-grand) 2010;56(Suppl):OL1223–30. [PubMed] [Google Scholar]