Abstract
Epithelial cancers are the most common malignancies and the greatest cause of cancer mortality worldwide. The incidence of keratinocyte-derived (non-melanoma) skin cancers (NMSCa) is increasing rapidly. Despite access to abundant tumor tissue and ease of observation, acceptance of NMSCa as model carcinomas has been hindered by the lack of a reliable xenograft model. Herein we describe conditions that allow routine xenoengraftment of primary human squamous cell carcinoma (SCCa) cells. Tumor development required creation of an appropriate stromal bed prior to xenografting tumor tissue onto the backs of athymic nude mice. We also demonstrate that the stromal bed must be “humanized” if primary human SCCa is to be propagated from cell suspensions. SCCa xenografts recapitulated the histological grade and phenotype of the original tumors with considerable fidelity, even after serial passage, irrespective of the histological grade of the primary human SCCa. To our knowledge this previously unreported model can be used for drug testing, as well as for studies that are relevant to the biology of primary human SCCa and other epithelial cancers.
Keywords: Skin cancer, squamous cell carcinoma, mouse models
Introduction
Cancers of epithelial tissues (skin, stomach, lung, breast, prostate and colon) approximately account for 85% of all cancers and 75% of all cancer-related deaths in the USA and worldwide (Altekruse et al., 2009; Ferlay et al., 2010). Among carcinomas, although not included in these cancer statistics because of their relative low mortality, the incidence of non melanoma skin cancer (NMSCa) (basal cell carcinoma and squamous cell carcinoma (SCCa)) is rising faster than all other cancers (Christenson et al., 2005; Diepgen et al., 2002; Green et al., 1996; Buettner et al., 1998; Holme et al., 2000; Benton et al., 2008). Currently, NMSCa are the most frequent of all cancers and affect 1 million Americans per year, 250,000 of whom have SCCa (http://www.cancer.org/docroot/CRI/content/CRI_2_2_1X_How_many_people_get_nonmelanoma_skin_cancer_51.asp?sitearea=). Despite the high incidence and ease of observation, the study of primary human SCCa as a model of epithelial cancer has been hindered by the lack of reproducible in vivo tumor tissue growth assays.
Although the genetic basis of human SCCa has not been completely characterized and likely involves a number of different alterations (Boukamp., 2005), approximately 50% of all SCCa harbor a UV light-induced p53 mutation in one or both alleles (Ziegler et al., 1994; Brash et al., 1991). Also, since overexpression of the RAS signaling pathway is observed in 75% of all SCCa, RAS likely plays a critical role in SCCa initiation and maintenance (Dajee et al., 2003). Previously it has been suggested that a minimal set of alterations, including RAS activation and NFκB pathway inactivation or upregulation of cyclin dependent kinase 4, are sufficient to transform normal human keratinocytes resulting in SCCa-like tumors in a subcutaneous xenograft model (Dajee et al., 2003; Ortiz-Urda et al., 2005).
Primary human skin (either intact or as reconstituted human skin equivalents) can be successfully grafted onto the backs of immunocompromised mice (Guerret et al., 2003; Pouliot et al., 2002). These grafts retain many features of normal human skin: structure (Bohnert et al., 1989; Kim et al., 1992), epidermal differentiation (Demarchez et al., 1986) and function (Kim et al., 1992; Demarchez et al., 1986). However, establishing primary human SCCa xenografts has proved to be difficult. Most successful xenografts of human SCCa involve cell lines. Rheinwald and Beckett reported two primary human cutaneous SCCa as well as 4 orally-derived SCCa from cells that had been passaged in tissue culture prior to successful grafting onto athymic nude mice (Rheinwald et al., 1981). Intriguingly, reproducible tumor growth required implantation of ≥ 3×106 cultured SCCa cells. Similarly, the Fusenig laboratory reported engraftment of two poorly differentiated primary human SCCa from tissue cultured passaged cells onto athymic nude mice (Boukamp et al., 1982; Tilgen et al., 1983). Independent of these early successes and despite the subsequent availability of additionally immunocompromised mouse strains that have allowed successful xenografts of other tumor types, xeno-engraftment of primary human SCCa remains challanging (Takizawa et al., 1997).
Orthotopic xenograft models are essential for investigation of tumor biology, notably in the identification of tumor initiating cell subpopulations, as well as for drug testing. The lack of standardized in vivo models that allow establishment and propagation of SCCa, and characterization of tumor initiating cells in SCCa has been a significant problem. To address this issue, we developed a reliable in vivo orthotopic xenograft assay that allows routine growth of tumors from directly implanted primary human SCCa tissue and cell suspensions.
Results
Orthotopic primary human SCCa xenografts rarely take
Normal skin can be xenografted onto immunocompromised mouse strains, either as intact tissue or alternatively reconstituted human skin equivalents (Guerret et al., 2003; Pouliot et al., 2002; Bohnert et al., 1989; Kim et al., 1992; Demarchez et al., 1986). To develop an in vivo assay for primary human SCCa, we first attempted tumor growth by xenografting intact tumor tissue (0.5cm3) in which epithelial-stromal relationships are maintained onto various immunocompromised mice. Despite using nude, SCID-beige and NOD-SCID mice strains, onto which we subcutaneously implanted 22 different primary human SCCa’s, reproducible tumor growth was not achieved (Row 1, Table 1). Although surprising, our observation is consistent with the literature (Rheinwald et al., 1981; Takizawa et al., 1997).
Table 1.
Development of an in vivo SCCa model
| SCCa tumor xenograft |
Graft site preparation |
Xenograft tumor frequency in different mouse strains |
||
|---|---|---|---|---|
| Nude | SCID- beige |
NOD- SCID |
||
| Intact tumor tissue | None | 1 of 22 | 1 of 5 | 0 of 5 |
| Intact tumor tissue | Creation of a stromal bed |
22 of 25 | 0 of 4 | ND |
| Minced tumor tissue | Creation of a stromal bed |
3 of 3 | ND | ND |
| Single cell suspension | Creation of a stromal bed |
3 of 24 | ND | ND |
| Single cell suspension with 106 primary human fibroblasts |
Creation of a stromal bed |
0 of 8 | ND | ND |
| Single cell suspension with 106 primary human fibroblasts |
Creation of a “humanized” stromal bed |
28 of 29 | ND | 0 of 4 |
ND = Experiment not performed
Creation of a stromal bed facilitates primary human SCCa xenograft growth in vivo
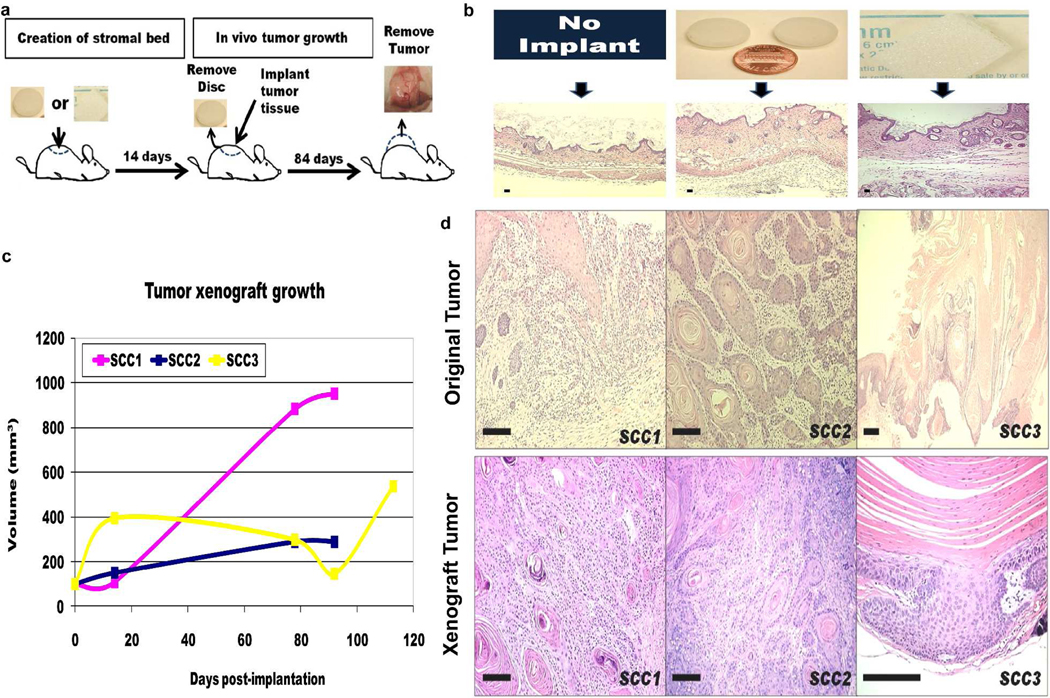
We hypothesized that since primary human SCCa are highly vascularised tumors, insufficient blood supply at the subcutaneous graft site might be responsible for graft failure. To facilitate xenograft engraftment and to increase vascularity, we induced a stromal reaction by first implanting either a subcutaneous glass disc (Roop et al,, 1986) or Gelfoam™ dressing (Zeamari et al., 2004) 2 weeks prior to the intact tumor tissue xenograft (Fig. 1a and b). We utilized athymic nude and SCID-beige mouse strains since these had allowed growth of a single clinically aggressive SCCa. Of 25 different primary human SCCa tissue xenografts, 22 demonstrated tumor growth as confirmed by serial tumor measurements and subsequent histology (Fig. 1c and Row 2, Table 1). All histological grades of primary human SCCa successfully engrafted and grew, including: 4 of 6 poorly, 7 of 7 moderately and 11 of 12 well-differentiated SCCa. All tumors maintained their original morphology in vivo, including large well-differentiated tumors (Fig. 1d). The growth rates of human SCCa xenografts were consistent with the relatively slow growth rate of in situ SCCa in patients (Fig. 1c). Intriguingly, xenograft growth was observed in athymic nude, but not in more immunocompromised SCID-beige, mice. None of the four different SCCa samples that grew as xenografts in athymic nude mice grew in SCID-beige mice, despite simultaneous engraftment using the identical experimental protocol. Although the number of SCID-beige mice is low, one would have expected at least an equal if not greater rate of graft takes in the more immunocompromised mice. Thus, primary human SCCa xenograft growth was not achievable in more severely immunocompromised mice strains, and was clearly dependent upon the prior creation of a stromal reaction.
Figure 1. Creation of a SCCa in vivo xenograft model that can accurately recapitulate and propagate human SCCa from intact tumor tissue.
a, Successful engraftment of primary human SCCa tissue required prior creation of a stromal bed, into which the tissue is implanted after 14 days. After 12 weeks, tumor growth was determined by histological sampling of all xenograft sites. b, H&E stained sections of athymic nude mouse skin demonstrate a fibrovascular proliferation following subcutaneous implantation of either a glass disk or Gelfoam™ dressing for 2 weeks. c, Tumor growth after implantation of similar size intact SCCa tissue. d, Xenograft tumors that developed from intact, primary human SCCa preserved the original tumor morphology. All scale bars are 100μm.
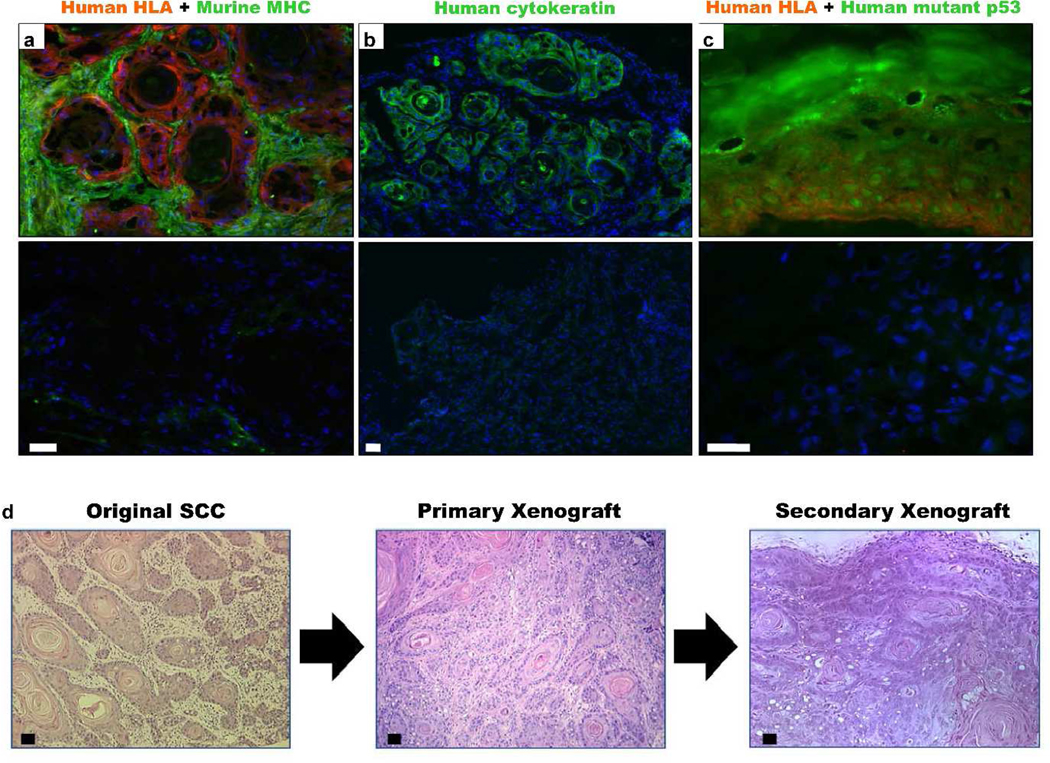
To confirm that xenograft tumors contained primary human SCCa epithelial cells, we stained tumor xenograft sections with antibodies to human HLA antigens, murine MHC class 1 antigens (Fig. 2a), human cytokeratins (Fig. 2b) and human p53 (Fig. 2c). The p53 antibody clone used binds to the DNA binding domain and specifically recognizes the mutated protein. After 12 weeks, most tumor stroma was comprised of murine cells. Primary human SCCa xenografts retained human HLA, keratin and mutant p53 protein expression demonstrating the survival and growth of primary human SCCa keratinocytes. The xenograft tumors were also amenable to serial passage (Fig. 2d). We concluded that athymic nude mice supported subcutaneous primary human SCCa xenografts, after creation of fibrovascular beds by implantation of either glass discs or Gelfoam™ dressings.
Figure 2. Xenografts express human markers and can be passage in vivo.
Primary human SCCa-derived xenograft tumors expressed: a, human HLA antigen, b, cytokeratin and c, nuclear accumulation of mutant p53. Respective isotype controls are shown in panels underneath. Xenograft tumor stroma was predominantly replaced by murine cells that expressed murine MHC class 1. Non- specific staining was observed within the dead squamous accumulations. d, Recreation of the original tumor morphology upon in vivo serial passage of the previously xenografted tumor. Histology after 12 weeks was similar to the original and primary xenograft tumors. All scale bars are 100 μm.
Primary human SCCa-derived cell suspensions require human fibroblasts to form a xenograft
We next attempted to adapt the in vivo model that we had created for intact tumor tissue to allow tumor growth in vivo from primary human SCCa-derived cell suspensions. First, we demonstrated that tumor xenograft growth was possible in 3 of 3 SCCa xenografts with fragments of tumor tissue (Fig. 3a–c, Row 3 Table 1). To derive single SCCa cell suspensions, we modified the enzymatic digestion protocol for normal skin dissociation (Fig. 3d,e), resulting in between 30 and 70% cell viability by FACS analysis (Fig 3f). However, when we attempted to initiate SCCa tumor growth by xenografting unsorted cell suspensions derived from human SCCa, we achieved growth in only 3 of 24 SCCa cell suspension xenografts after > 106 cells were resuspended in Matrigel™ and injected into the subcutaneous fibrovascular bed created in athymic nude mice (Row 4 Table 1). To improve the proximity of stromal and tumor cells, we next supplemented primary human SCCa cell suspensions with an additional 106 primary normal human or SCCa associated fibroblasts prior to implantation. With this approach, we achieved no tumor growth from 8 different primary human SCCa (Row 5 Table 1), however we did not attempt implantation with greater numbers of fibroblasts.
Figure 3. SCCa mechanical dissociation and generation of a single cell suspension.
a, Primary human SCCa was b, mechanically dissociated using surgical scissors, c, the minced tumor tissue was then implanted onto wound beds created by either a glass disc or gelfoam dressing that had been implanted for 14 days in the athymic nude mice. Shown is a typical tumor after 12 weeks. d, cell suspensions were created by additional enzymatic dissociation so that few cells remained in the remnant tissue. e, tumor cells could be labeled with a human pancytokeratin antibody and f, approximately 70% of the tumor dissociated cells were viable, as assessed by FACS. All scale bars are 100 μm.
As success in xenografting primary human breast cancer cells required cleared murine mammary fat pads to be first “humanized” by the pre-implantation of human fibroblasts (Kuperwasser et al., 2004), we hypothesized that primary human SCCa xenografts from single cell suspensions might also require “humanization”. Successful and reproducible xenografts of human SCCa cell suspensions were ultimately achieved when large numbers of normal human fibroblasts were used to “humanize” xenograft recipient sites. One million (106) primary normal human fibroblasts were first suspended in Matrigel™ and implanted with the glass disc or Gelfoam™ dressing. After 14 days, SCCa xenograft cell suspensions were co-injected with an additional 106 primary human normal fibroblasts suspended in Matrigel™ into the prepared xenograft sites (Fig. 4a). This approach yielded 28 successful xenograft tumors in 29 attempts (Table 1) using cell suspensions derived from 29 different primary human SCCa’s: 16 well differentiated SCCa, 7 moderately differentiated SCCa and 6 poorly differentiated SCCa. The histological patterns of these xenograft tumors recapitulated the original primary human SCCa histologies irrespective of the histological classification of the tumors (Fig. 4b). For success, a minimum number of SCCa cells were required such that xenograft success was assured if ≥ 3×106 unsorted primary human SCCa cells were xenografted. Tumor growth was not reproducible with xenografts containing < 3×106 unsorted primary human SCCa independent of the histological grade of the original tumor (Fig. 4c). Robust xenograft growth was observed in athymic nude mice and not the more immunocompromised NOD-SCID strain (Row 6 Table 1), in 0/4 attempts. Primary human SCCa xenograft tumors could also be serially passaged into new athymic mice as cell suspensions (Fig. 4d). Hence, we successfully created an orthotopic in vivo xenograft model using primary human SCCa cell suspensions that reproducibly recreated the original tumor phenotype, independent of the original histological grade.
Figure 4. Xenografts can accurately recapitulate and propagate human SCCa from cell suspensions.
a, Schematic b, SCCa xenografts from cell suspensions recreated the original tumor morphology c, with no difference in xenograft growth based upon the original tumor histological grade. Xenograft success was only dependent upon the number of tumor cells implanted and d, harvested tumor tissue could be dissociated and successfully implanted into naive athymic nude mice. All scale bars are 100 μm.
Discussion
Herein we describe an in vivo xenograft model for primary human SCCa using intact tumor tissue and cell suspensions in which in vivo tumor growth is reliable and recreates the original tumor hierarchical architecture, irrespective of the SCCa histological grade. In contrast to previously described models, in vitro passage was not required and primary tumor tissue or cell suspensions could be directly implanted. The high frequency and relative ease with which primary human SCCa tissue can be accessed, combined with the simplicity of this model, will facilitate the study of primary human epithelial cancer growth and drug testing. Since, in vivo growth can be assured with implantation of primary human SCCa cell, this assay can also be used for the study of potential tumor initiating cell populations.
Because no standardized assay has been established for growing primary human cutaneous SCCa xenografts, we describe our extensive efforts to develop an efficient in vivo xenograft mouse model in some detail. In our model, creation of a fibrovascular stromal bed by pre-implantation of glass discs or Gelfoam™ dressings was critical, suggesting the relative importance of a well developed tumor niche environment. These approaches were adapted from previous models for murine SCCa (Roop et al,, 1986) and peritoneal metastases (Zeamari et al., 2004) respectively, in which enhanced efficiency of tumor take was demonstrated. Likewise, in vivo growth from primary human SCCa cell suspensions required co-implantation of 106 primary human fibroblasts prior to, and along with, human cells derived from SCCa. Previously, the “humanization” of murine stroma was found to be necessary for successful human breast cancer xenografts (Kuperwasser et al., 2004). The requirement for pre-implanted human stromal cells is not well understood, since there is abundant vascularization and murine stroma in place even in the absence of human cells.
We were surprised that the success rate for establishing human SCCa xenografts was consistently higher in athymic nude mice than in more immunocompromised mice, including SCID-beige and NOD-SCID mice, even though all mice developed a fibrovascular stroma. Athymic nude mice retain some residual immunity with functional NK cells, but when attempting to xenograft tumor pieces after the creation of a stromal bed the administration of NK neutralizing antibody did not hinder nor enhance tumor growth (n=3). Athymic nude mice also retain functional B-lymphocytes, suggesting this could facilitate tumor growth. Interestingly, the paradoxical role of both the adaptive and innate immune responses, particularly B-lymphocytes, in facilitating mouse SCCa growth has been previously noted (Clevers, 2004; De Visser et al., 2006; Karin et al., 2006) and SCCa growth is observed in inflamed human skin (e.g.: chronic wounds, burn injury, discoid lupus erythematosus, lichen sclerosus, lichen planus, hidradenitis supprativa and lupus vulgaris (Alam et al., 2001)). Thus, the reactive tissue milieu in the prepared graft site may have been more inflammatory in the athymic immunodeficient nude mice recipients and facilitated SCCa growth (de Visser et al., 2005). However, patients receiving immunosuppressants for the management of renal transplants are at greater risk for developing clinically aggressive SCCa once tumors have been established. The development of a SCCa in vivo xenograft model using athymic nude mice is consistent with what is known about the role of inflammation in the pathogenesis of SCCa and the promoting effect of immunodeficiency. SCCa xenografts grew slowly and none of these tumors metastasized or were lethal within 12 weeks in vivo, consistent with the SCCa clinical phenotype in patients.
In conclusion, we have created a simple, reliable in vivo xenograft model for primary human SCCa. This assay will be widely applicable for drug testing, as well as for studies to elucidate the biology of primary human SCCa and other epithelial cancers.
Material and Methods
Immunofluorescence
Immunofluorescence was performed using standard techniques with the following primary antibodies: pancytokeratin (Dako, clone AE1/3), Mouse MHC class I (AbD Serotec, clone 2G5), Human HLA (AbD Serotec, clone YTH 862.2) and mutant p53 (Calbiochem, clone Pab240).
For cytospin analysis, normal human skin keratinocytes, RS4 lymphocyte cells (ATCC) or SCCa single cells were washed and resuspended in PBS at a concentration of 105 cells per ml. 100 μl aliquots of cells were added to each slide cytofunnel (Shandon, Thermo Scientific), spun at 800 rpm for 5 minutes in a cytocentrifuge (Shandon Cytospin 2 cytocentrifuge), fixed in acetone and labelled with a pancytokeratin antibody as above.
Cell preparation
Normal foreskins and human squamous cell carcinoma were obtained after IRB approval of experiments, written, informed patient consent and adherence to the Helsinki Guidelines. Tumor tissue was mechanically dissociated, and then incubated for 2 hours at 37°C in a 100 ml sterile conical flask containing medium 199 with 200 u/ml Dispase (BD Biosciences) and 200 u/ml Ultrapure Collagenase Type III (Sigma) with stirring on a magnetic stirring plate in a tissue culture CO2 incubator. The semi-dissociated mixture was pipetted into sterile 50 ml tubes and, after centrifugation at 200g for 5 minutes, supernatants were discarded. The remaining tissue was then incubated at 37°C for 5 minute with 0.05% trypsin with EDTA. Tissue remnants were removed from cell suspensions by filtration through a 40 μm cell strainer.
Flow cytometry
Samples were assessed using a FACSCalibur flow cytometer (BD Biosciences) and mouse flurochrome-conjugated IgG subtype isotype controls (BD Pharmingen). A live cell gate was created using 7-Amino-Actinomycin D (BD Pharmingen) to label dead cells.
Transplantation of human SCCa cell suspensions
Athymic nude homozygous foxn1nu (Jackson Lab), SCID-beige and NOD-SCID (Taconics) mice were housed and utilized under conditions approved by the Animal Care and Use Committee at the National Cancer Institute. Mice were anaesthetized and either Gelfoam™ dressing (Johnson & Johnson) or sterilized glass discs were implanted into the dorsal subcutaneous space, together with 106 primary human fibroblasts suspended in 100 μl of Matrigel (BD Biosciences) for single cell suspension experiments, and wounds were closed with surgical staples (Mikron). After 14 days, mice were anaesthetized, glass discs were removed and SCCa cells that had been suspended in 100 μl of Matrigel was injected into the subcutaneous space or alternatively into the in-situ gelfoam™ dressing. After 12 weeks, mice were euthanized via CO2 inhalation, and tumors were removed for analysis.
Acknowledgements
We would like to thank all the members of the Dermatology Branch (National Cancer Institute, NIH, Bethesda, Maryland), including Dr. Mark Udey for many helpful discussions and comments and, in particular, Vogel laboratory members for their helpful comments. We thank Drs Andrew Montemarano (Rockledge Skin Cancer Clinic, Bethesda, Maryland), Kurt Maggio (Walter Reed Army Medical Center Dermatology Service, Washington DC) and Martin Braun (Braun & Braun MDs, Washington DC) for providing tumor tissue samples. It is with great sadness that we also inform you of the recent and untimely death of Dr Jonathan Vogel, the lead Prinicpal Investigator of this paper.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflict of Interest
The authors declare that no financial conflict of interest exists.
References
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. [Google Scholar]
- Benton EC, Kerr OA, Fisher A, Fraser SJ, McCormack SKA, Tidman MJ. The changing face of dermatological practice: 25 years’ experience. Br J Dermatol. 2008;159:413–418. doi: 10.1111/j.1365-2133.2008.08701.x. [DOI] [PubMed] [Google Scholar]
- Bohnert A, Hornung J, Mackenzie IC, Fusenig NE. Epithelial-mesenchymal interactions control basement membrane production and differentiation in cultured and transplanted mouse keratinocyte. Cell Tissue Research. 1989;244:413–429. doi: 10.1007/BF00219217. [DOI] [PubMed] [Google Scholar]
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Tilgen W, Dzarlieva RT, Breitkreutz D, Haag D, Riehl RK, Bohnert A, Fusenig NE. Phenotypic and genotypic characterization of a cell line from a squamous cell carcinoma of human skin. JNCI. 1982;68:415–427. [PubMed] [Google Scholar]
- Brash DE, Rudolph JA, Simon JA, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner PG, Raasch BA. Incidence rates of skin cancer in Townsville, Australia. Int J Cancer. 1998;78:587–593. doi: 10.1002/(sici)1097-0215(19981123)78:5<587::aid-ijc10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, et al. NF-κB blockade and oncogenic RAS trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- de Visser K, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- De Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Demarchez M, Sengel P, Prunieras M. Wound healing of human skin transplanted onto the nude mouse. I. An immunohistological study of the reepithelialization process. Developmental Biology. 1986;113:90–96. doi: 10.1016/0012-1606(86)90110-7. [DOI] [PubMed] [Google Scholar]
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl. 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM GLOBOCAN. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Lyon, France: International Agency for Research on Cancer; 2008. [accessed Dec 2010]. 2010. Available from: http://globocan.iarc.fr. [Google Scholar]
- Green A, Battistutta D, Hart V, Leslie D, Weedon D. Skin cancer in a subtropical Australian population: incidence and lack of association with occupation. Am J Epidemiol. 1996;144:1034–1040. doi: 10.1093/oxfordjournals.aje.a008875. [DOI] [PubMed] [Google Scholar]
- Guerret S, Govignon E, Hartmann DJ, Ronfard V. Long-term remodeling of a bilayered living human skin equivalent (Apligraf®) grafted onto nude mice: immunolocalization of human cells and characterization of extracellular matrix. Wounds Repair Regeneration. 2003;11:35–45. doi: 10.1046/j.1524-475x.2003.11107.x. [DOI] [PubMed] [Google Scholar]
- Holme SA, Malinovszky K, Roberts DL. Changing trends in non-melanoma skin cancer in South Wales 1988–1998. Br J Dermatol. 2000;143:1224–1229. doi: 10.1046/j.1365-2133.2000.03892.x. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kim YH, Woodley DT, Wynn KC, Giomi W, Bauer EA. Recessive dystrophic epidermolysis bullosa phenotype is preserved in xenografts using SCID mice: development of an experimental in vivo model. J Invest Dermatol. 1992;98:191–197. doi: 10.1111/1523-1747.ep12555849. [DOI] [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Urda S, Garcia J, Green CL, Chen L, Lin Q, Veitch DP, Sakai LY, Lee H, Marinkovich MP, Khavari PA. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773–1776. doi: 10.1126/science.1106209. [DOI] [PubMed] [Google Scholar]
- Pouliot R, Larouche D, Auger FA, Juhasz J, Xu W, Li H, Germain L. Reconstructed human skin produced in vitro and grafted on athymic mice. Transplantation. 2002;73:1751–1757. doi: 10.1097/00007890-200206150-00010. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Can Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- Roop DR, Lowy DR, Tambourin PE, et al. An activated Harvey ras oncogene produces benign tumors on mouse epidermal tissue. Nature. 1986;323:822–824. doi: 10.1038/323822a0. [DOI] [PubMed] [Google Scholar]
- Takizawa Y, Saida T, Tokuda Y, Dohi S, Wang YL, Urano K, Hioki K, Ueyama Y. New immunodeficient (nude-scid, beige-scid) mice as excellent recipients of human skin grafts containing intraepidermal neoplasms. Arch Dermatol Res. 1997;289:213–218. doi: 10.1007/s004030050182. [DOI] [PubMed] [Google Scholar]
- Tilgen W, Boukamp P, Breitkreutz D, Dzarlieva RT, Engstner M, Haag D, Fusenig NE. Preservation of morphological, functional, and karyotypic traits during long-term culture and in vivo passage of two human skin squamous cell carcinomas. Can Res. 1983;43:5995–6011. [PubMed] [Google Scholar]
- Zeamari S, Roos E, Stewart FA. Tumor seeding in peritoneal wound sites in relation to growth-factor expression in early granulation tissue. Eur. J. Cancer. 2004;41:1431–1440. doi: 10.1016/j.ejca.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]