Abstract
Previously, it was shown that a novel 4-(N)-stearoyl gemcitabine nanoparticle formulation was more effective than gemcitabine hydrochloride in controlling the growth of model mouse or human tumors pre-established in mice. In the present study, the feasibility of targeting the stearoyl gemcitabine nanoparticles (GemC18-NPs) into tumor cells that over-express epidermal growth factor receptor (EGFR) to more effectively control tumor growth was evaluated. EGFR is over-expressed in a variety of tumor cells, and EGF is a known natural ligand of EGFR. Recombinant murine EGF was conjugated onto the GemC18-NPs. The ability of the EGF to target the GemC18-NPs to human breast adenocarcinoma cells that expressed different levels of EGFR was evaluated in vitro and in vivo. In culture, the extent to which the EGF-conjugated GemC18-NPs were taken up by tumor cells was correlated to the EGFR density on the tumor cells, whereas the uptake of untargeted GemC18-NPs exhibited no difference among those same cell lines. The relative cytotoxicity of the EGF-conjugated GemC18-NPs to tumor cells in culture was correlated to EGFR expression as well. In vivo, EGFR-over-expressing MDA-MB-468 tumors in mice treated with the EGF-conjugated GemC18-NPs grew significantly slower than in mice treated with untargeted GemC18-NPs, likely due to that the EGF-GemC18-NPs were more anti-proliferative, anti-angiogenic, and pro-apoptotic. Fluorescence intensity data from ex vivo imaging showed that the EGF on the nanoparticles helped increase the accumulation of the GemC18-NPs into MDA-MB-468 tumors pre-established in mice by more than 2-fold as compared to the un-targeted GemC18-NPs. In conclusion, active targeting of the GemC18-NPs into EGFR-over-expressed tumors can further enhance their anti-tumor activity.
Keywords: particle uptake, cytotoxicity, biodistribution, ex vivo imaging
1. Introduction
Gemzar®, gemcitabine hydrochloride for injection, is approved for the treatment of pancreatic, breast, lung, and ovarian cancers [1]. Although extremely toxic to tumor cells in culture, there continues to be a need to improve the clinical outcomes of Gemzar® therapy, and there are reports showing that formulating gemcitabine into nanoparticles may be an effective approach to address this need [2-10]. Previously, we reported a novel gemcitabine nanoparticle formulation and demonstrated that the nanoparticles were more effective than gemcitabine hydrochloride (HCl) in controlling the growth of model mouse or human tumors in mice, clearly indicating that the in vivo efficacy of gemcitabine can be improved using nanoparticles [8]. The nanoparticle delivery system for gemcitabine was prepared from lecithin/glycerol monostearate (GMS)-in-water emulsions. Gemcitabine is highly water soluble; and thus, in order to incorporate it into the solid lipid nanoparticles, gemcitabine was lipophilized by conjugating a stearoyl group to its N-terminus to form 4-(N)-stearoyl gemcitabine (GemC18) [5]. The enhanced anti-tumor activity from the stearoyl gemcitabine nanoparticles (GemC18-NPs) was not simply due to the GemC18 because the same dose of GemC18 in Tween 20 micelles did not show any significant anti-tumor activity [8].
In the present study, the feasibility of further improving the anti-tumor activity of the stearoyl gemcitabine nanoparticles by actively targeting them into tumors was evaluated. It was hypothesized that conjugation of epidermal growth factor (EGF) as a ligand to EGF receptor (EGFR) on the surface of the stearoyl gemcitabine nanoparticles will increase the delivery of the nanoparticles into tumor (cells), and thus improve the resultant anti-tumor activity. Among the earliest growth factors characterized and sequenced, EGFR (a 170 kD endogenous cell surface glycoprotein) has been inherently tied with normal cellular function including cell proliferation, survival, adhesion, migration, and differentiation. More than ten ligands, including EGF, have been shown to exhibit strong receptor-ligand affinity towards EGFR [11]. Although EGFR is expressed in a variety of normal cell types at ~1 × 104 per cell, there are numerous studies reporting the over-expression of EGFR in various tumor cells (i.e., 10-1,000-fold greater than in normal cells) [12]. For example, it has been discovered that EGFR is over-expressed in 80-100% of human head and neck cancer cells, 14-91% of human breast cancer cells, and 30-50% of human pancreatic cancer cells [13]. Multiple EGFR targeting agents have been developed for cancer therapy (e.g., anti-EGFR MAb 225, ZD1839 (Iressa)) [14, 15], and the clinical efficacy of those EGFR targeting agents have been promising [16]. In the present study, the over-expressed EGFR was exploited as a target to more specifically deliver the GemC18-NPs into tumor cells by conjugating EGF onto the surface of the nanoparticles. Previously, EGF and antibodies against EGFR had been conjugated onto liposomes to successfully target them to EGFR-over-expressing tumor cells [17-19]. For example, it was shown that treatment of nude mice with EGFR-over-expressing A549 human non-small cell lung cancer cells with gemcitabine-incorporated liposomes surface-conjugated with monoclonal antibodies against EGFR more effectively inhibited the tumor growth than un-targeted liposomes [19]. Similarly, Arya et al. (2011) reported that conjugation of anti-HER2, an antibody against human EGFR-2, onto gemcitabine-chitosan nanoparticles enhanced the anti-proliferative activity of the nanoparticles against HER2-expressing Mia PaCa-2 cells and PANC-1 tumor cells in culture [20].
2. Materials and Methods
2.1. Materials and cell lines
Gemcitabine HCl was from U.S. Pharmacopeia (Rockville, MD). Soy lecithin was from Alfa Aesar (Ward Hill, MA). Geleol™ (Glycerol-monostearate, GMS) was from Gattefosse Corp. (Paramus, NJ). The 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol)-2000] (DSPE-PEG(2000)-maleimide) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein) (ammonium salt) (DOPE-fluorescein) were from Avanti Polar Lipids, Inc. (Alabaster, AL). Ovalbumin (OVA), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), poly-D-lysine hydrobromide, 2-iminothiolane hydrochloride (Traut’s Reagent), and Tween 20 was from Sigma-Aldrich (St. Louis, MO). CBQCA Protein Quantification Kit, cell culture medium, fetal bovine serum (FBS), and antibiotics were from Invitrogen (Carlsbad, CA). Sephadex™ G-25 column (PD-10) was from GE Biosciences (Piscataway, NJ). Recombinant murine EGF was from Peprotech Inc. (Rocky Hills, NJ). Human breast adenocarcinoma cell lines, MDA-MB-468 (HTB-132), MDA-MB-231 (HTB-26), and MCF-7 (HTB-22) were from the American Type Culture Collection (ATCC, Rockville, MD). It was reported that those cells express 1 × 106, 2 × 105, and 1 × 104 EGFR per cell, respectively [21, 22], and our RT-PCR data confirmed the differential expression as well (Rodriguez and Cui, unpublished data). All tumor cells were grown in Dulbecco’s modification of Eagle’s medium (DMEM) supplemented with 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin.
2.2. Preparation and characterization of EGFR-targeted stearoyl gemcitabine nanoparticles
Recombinant murine EGF was conjugated onto nanoparticles by forming a stable thioether group. GemC18 and GemC18-containing nanoparticles (GemC18-NPs) were prepared as previously described [23]. Briefly, 3.5 mg of soy lecithin, 0.5 mg of GMS, 5 mg of GemC18, and 0.875 mg of DSPE-PEG (2000)-maleimide were placed into a 7 mL glass vial. One mL of water was added into the mixture, which was then maintained at 70-75 °C while stirring until the formation of homogeneous slurry. Tween 20 was added in a step wise manner to achieve a final concentration of 1% (v/v). The resultant emulsions were allowed to stay at room temperature while stirring to form nanoparticles. To thiolate EGF, EGF was diluted into phosphate buffer saline (PBS, 0.1 M, pH 8.0) with ethylenediaminetetraacetic acid (EDTA, 5 mM), followed by the addition of Traut’s reagent (2 mg/mL) and a 1 h incubation at room temperature. Thiolated EGF was purified/desalted using a Sephadex™ G-25 column and mixed with pre-prepared GemC18-nanoparticles. The mixture was incubated overnight in a nitrogen-enriched atmosphere. Un-conjugated EGF was removed using a Sepharose® 4B column. The purified EGF-conjugated GemC18-NPs are named EGF-GemC18-NPs. As a control, OVA was conjugated onto the GemC18-NPs using the same procedure to prepare OVA-GemC18-NPs. To fluorescently label the nanoparticles, DOPE-fluorescein (0.95 mg) was included in the lipid mixture during the nanoparticle preparation. The concentration of the EGF and OVA in the nanoparticle preparations was determined using the CBQCA Protein Quantitation Kit following the manufacturer’s instruction(s). The concentration of the GemC18 in the final nanoparticle preparations was determined using Agilent high performance liquid chromatography (HPLC) with an Agilent ZORBAX Eclipse Plus C18 column (4.6 × 150 mm, 5 μm; Santa Clara, CA) after the nanoparticles were dissolved into tetrahydrofuran. The mobile phase was methanol (100%). The flow rate was 1 mL/min. The detection wavelength was 248 nm [5, 8].
The particle size and zeta potential of the nanoparticles were determined using a Malvern Zetasizer® Nano ZS (Westborough, MA). The nanoparticles in suspension were stored in ambient condition for 20 days, and their particle sizes were measured every five days.
2.3. In vitro cellular uptake assay
MDA-MB-468, MDA-MB-231, or MCF-7 cells were seeded in 12-well plate (2 × 105cells/well) (n = 6) and incubated at 37 °C, 5% CO2 for 24 h. Cells were then incubated with fluorescein-labeled EGF-GemC18-NPs or fluorescein-labeled OVA-GemC18-NPs (50 °L) for 6 h at 37 °C, 5% CO2, washed three times with PBS, and re-suspended in a cell lysis buffer (20 mM Tris, 100 mM NaCl, 1 mM EDTA, 0.5% Triton X-100). The 6 h incubation time was chosen based on data from previous studies [8, 24]. The fluorescence intensity of the samples in a black bottom plate (Corning, NY) was measured at 492/518 nm using a BioTek Synergy® Multi-Mode Microplate Reader (Winooski, CT). To confirm that the uptake was mediated by the EGF-EGFR interaction, the cells were incubated with free EGF (0.1 mg/mL) for 1 h prior to the addition of the nanoparticles. Data are reported as the absolute fluorescence intensity values or the % of uptake, which was calculated using the following formula: % uptake = (fluorescence intensity value in the cell lysates) / (total fluorescence intensity value of the nanoparticles added into the cell culture).
2.5. Fluorescence microscopy for the detection of the uptake of nanoparticles
MDA-MB-468 or MCF-7 cells (2 × 105) were seeded on poly-D-lysine-coated glass cover slips and incubated in 6-well plates at 37 °C, 5% CO2 for 24 h. After complete adherence, cells and cover slips were incubated with fluorescein-labeled EGF-GemC18-NPs or fluorescein-labeled OVA-GemC18-NPs for 6 h at 37 °C and 5% CO2, washed three times with PBS, and fixed with paraformaldehyde (3% in PBS) for 20 min at room temperature. The cover slips were washed with PBS an additional three times and mounted onto ethanol-cleaned glass slides using Vectashield H-1200 with 4′,6-diamidino-2-phenylindole (DAPI) (Vector laboratories, Burlingame, CA). Cells were examined using an Olympus BX60 microscope (Center Valley, PA).
2.6. Flow cytometry
MDA-MB-468, MDA-MB-231, and MCF-7 cells (1 × 106/well) were incubated with fluorescein-labeled EGF-GemC18-NPs or fluorescein-labeled OVA-GemC18-NPs for 6 h at 37 °C, 5% CO2. Cells were washed three times with PBS, re-suspended in PBS, and analyzed using a BD FACSCalibur Flow Cytometer (San Jose, CA). Data was analyzed using the FlowJo Flow Cytometry Analysis software (Tree Star Inc., Ashland, OR).
2.7. In vitro cytotoxicity assay
Cells (5,000/well) were seeded in a 96-well plate and incubated overnight at 37 °C, 5% CO2.Cells were then incubated in the presence of various concentrations of EGF-GemC18-NPs, OVA-GemC18-NPs, or sterile PBS for 48 h. Cell viability was determined using an MTT assay following the manufacturer’s instructions. OD570 nm and OD630 nm values were measured using a BioTek Synergy™ HT Multi-Mode Microplate Reader. To understand whether the cells underwent apoptosis after treatment with the GemC18-NPs, MDA-MB-468 cells (5 × 104) were incubated with EGF-GemC18-NPs or OVA-GemC18-NPs for 48 h, washed, stained using a Guava Nexin kit that contains annexin V and 7-amino actinomycin D (7-AAD) according to the manufacturer’s protocol, and analyzed using a Guava Easycyte 8HT Flow Cytometry System (Millipore, Hayward, CA).
2.8. Evaluation of the in vivo anti-tumor activity of the EGF-GemC18-NPs
National Institutes of Health guidelines for animal use and care were followed. Animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Female athymic Nu/Nu mice (18-20 g) were from Charles River Laboratories (Wilmington, MA). MDA-MB-468 tumor cells (1 × 107 cells/mouse) were mixed with BD Matrigel™ (50:50, v/v) and subcutaneously injected in the right flank of the mice on day 0. On day 11, mice (n = 7, 5 for control) were randomized and injected intravenously (i.v.) via the tail vein with 200 °L of EGF-GemC18-NPs or OVA-GemC18-NPs in sterile mannitol (5%, w/v) or sterile mannitol alone as a negative control. Injection was repeated on days 17, 28, and 37. The dose of the GemC18 was about 860 μg per mouse per injection. Tumor growth and mouse survival was monitored. Tumor size was calculated based on the following equation: tumor diameter (mm) = (length × width)/2.
2.9. Ex vivo imaging using IVIS® spectrum
The biodistribution of EGF-GemC18-NPs and OVA-GemC18-NPs were evaluated in Nu/Nu mice with pre-established MDA-MB-468 tumors using an IVIS® Spectrum from Caliper (Hopkinton, MA). When the tumor size reached 8-10 mm, fluorescein-labeled EGF-GemC18-NPs or fluorescein-labeled OVA-GemC18-NPs were injected intravenously via the tail vein into mice (n = 3). As a control, mice were injected with sterile mannitol (5%, w/v). Mice were euthanized 24 h later to collect tumor, heart, lung, liver, spleen, and kidney. Samples were imaged immediately on non-fluorescent black Strathmore Artagain paper (Neenah, WI). Region of interest (ROI) values were recorded using Living Image® software (ver. 4.0). Fluorescence intensity (total counts) was determined in a fixed, circular ROI and reported after normalizing to the weight of the tumors or organs.
To determine the half-lives of the EGF-GemC18-NPs and OVA-GemC18-NPs, healthy, tumor-free C57BL/6 mice were injected intravenously with fluorescein-labeled EGF-GemC18-NPs or fluorescein-labeled OVA-GemC18-NPs and euthanized 5 min, 3 h, 6 h, 12 h, or 24 h later to collect blood samples (500 μL/mouse), which were placed into a multi-well plate and imaged using the IVIS® Spectrum to determine the fluorescence intensity. Pharmacokinetic data were analyzed based on the fluorescence intensity values using the PKsolver assuming a two compartment model [25].
2.10. Histology
Nu/Nu mice (n = 3) with pre-established MDA-MB-468 tumors were dosed (i.v.) with sterile mannitol, OVA-GemC18-NPs, or EGF-GemC18-NPs 20, 30, 40, and 53 days after the tumor cell injection. On day 54, mice were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU, 100 μg/g body weight) in sterile PBS and euthanized 30 min later. Tumor tissues were collected, fixed in formalin, embedded, sectioned, and stained at the University of Texas MD Anderson Cancer Center Science Park Research Division (Smithville, TX) with antibodies against BrdU, CD31, and caspase 3 as markers of cell proliferation, angiogenesis, and apoptosis, respectively. Slides were examined under a bright-field microscope. In addition, a slide was also stained with anti-EGFR-Alexa fluor 488 (Millipore, diluted 1/500) and DAPI, and then examined using a Leica TCS SP5 X Supercontinuum confocal microscope (Buffalo Grove, IL).
2.11. Statistical analysis
Statistical analyses were completed by performing analysis of variance (ANOVA) followed by Fisher’s protected least significant procedure. Mouse survival curves were analyzed using the Mantel-Cox log-rank method (GraphPad Prism®). A p-value of ≤ 0.05 was considered significant.
3. Results and discussion
3.1. Preparation and characterization of EGF-GemC18-NPs and OVA-GemC18-NPs
The EGFR-targeted GemC18-NPs were prepared by conjugating murine EGF onto pre-prepared GemC18-NPs. It was shown that murine EGF and human EGF bind to human EGFR with similar affinity at pH 7.4, and they have similar biological activity, but murine EGF is preferred due to the absence of lysine residues, which minimizes cross reaction [26, 27]. As a control, OVA was also conjugated onto the GemC18-NPs to generate OVA-GemC18-NPs. OVA was used as a control to EGF because the isoelectric point (pI) of OVA is similar to that of the murine EGF (pI = 4.6) [28, 29], and there is no report showing that OVA is a ligand to EGFR. Data that will be presented later also demonstrated that OVA did not affect the EGF-EGFR interaction.
The particle size, polydispersity index, and zeta potential of the EGF-GemC18-NPs and OVA-GemC18-NPs are shown in Table 1. Also shown in Table 1 are the final concentrations of GemC18 and EGF (or OVA) in the EGF-GemC18-NPs and OVA-GemC18-NPs. Statistical analyses did not reveal any significant difference between the EGF-GemC18-NPs and OVA-GemC18-NPs in those parameters, except the EGF or OVA protein concentration, which was likely due to the difference in the molecular weights of OVA and murine EGF. In a short 20-day stability study, when the nanoparticles were stored in ambient condition in an aqueous suspension, the particle size of both EGF-GemC18-NPs and OVA-GemC18-NPs did not change (data not shown).
Table 1. Characterization of nanoparticles.
Data shown are mean ± S.D. (n ≥ 3).
| Size (nm) | Polydispersity index |
Zeta potential (mV) |
[GemC18] (mg/mL) |
[EGF or OVA] (mM) |
|
|---|---|---|---|---|---|
| EGF-GemC18-NPs | 215 ± 8 | 0.24 ± 0.06 | −26.0 ± 0.5 | 4.4 ± 0.2 | 462 ± 39 |
| OVA-GemC18-NPs | 219 ± 3 | 0.32 ± 0.08 | −28.4 ± 0.5 | 4.2 ± 0.1 | 120 ± 42 |
3.2. In vitro uptake of the EGF-GemC18-NPs by tumor cells expressing different levels of EGFR
Three human breast adenocarcinoma cell lines, MDA-MB-468, MDA-MB-231, and MCF-7, were used in the in vitro uptake studies because they express different levels of EGFR per cell (1 × 106, 2 × 105 and 1 × 104, respectively) [21, 22]. As shown in Fig. 1A, after 6 h of incubation, the cellular uptakes of the OVA-GemC18-NPs by the three different cell lines were not different (p = 0.71, ANOVA). However, the extent of the uptake of the EGF-GemC18-NPs by the same cell lines was dependent on the number of EGFR per cell (Fig. 1A). In other words, the MDA-MB-468 tumor cells took up more EGF-GemC18-NPs than the MDA-MB-231 cells, and the MDA-MB-231 cells took up more EGF-GemC18-NPs than the MCF-7 cells (Fig. 1A). Data in Fig. 1B showed that pre-incubation of the MDA-MB-468 cells with free EGF for 1 h significantly inhibited the cellular uptake of the EGF-GemC18-NPs, but not the OVA-GemC18-NPs, by the MDA-MB-468 cells, demonstrating that the uptake of EGF-GemC18-NPs was mediated by the EGF-EGFR interaction. This is in agreement with Blessing et al, who reported that the competition of free EGF with EGF-containing plasmid DNA-polyethylenimine complexes resulted in decreased transfer of the plasmid into EGFR-over-expressing cells [30].
Figure 1. In vitro uptake of EGF-GemC18-NPs by tumor cells expressing different levels of EGFR.
(A). MCF-7, MDA-MB-231, and MDA-MB-468 cells were incubated with fluorescein-labeled EGF-GemC18-NPs (EGF-NPs) or fluorescein-labeled OVA-GemC18-NPs (OVA-NPs) for 6 h, and the extent of nanoparticle uptake was determined by measuring the fluorescence intensity (* p = 0.0001; ** p = 0.03).
(B). The uptake of the EGF-GemC18-NPs or OVA-GemC18-NPs by MDA-MB-468 cells with (EGF+) or without pre-incubation of the cells with free EGF (*** p = 0.009, EGF-NPs vs. EGF+ EGF-NPs). The initial fluorescence intensity values of the EGF-NPs and the OVA-NPs were not different. Data shown are mean ± S.D. (n = 4 in A, and 3 in B).
Flow cytometry and fluorescence microscopy were also employed to confirm that the uptake of the EGF-GemC18-NPs, but not the OVA-GemC18-NPs, was dependent on the density of the EGFR on tumor cells. As shown by the right-shifting of the fluorescein-positive peak in Fig. 2A, the uptake of the EGF-GemC18-NPs by the MDA-MB-468 cells was much more extensive than that of the OVA-GemC18-NPs, but it was only slightly different in the MDA-MB-231 and M‘CF-7 cells. Moreover, as indicated by the green fluorescence intensity in the micrographs in Fig. 2B, after 6 h of incubation, the uptake of the EGF-GemC18-NPs by the MDA-MB-468 cells was significantly greater than the uptake of the OVA-GemC18-NPs. However, the uptake of both nanoparticles by the MCF-7 cells was weak (Fig. 2B). The microscopic and flow cytometric data also showed that there is a slight increase in the uptake of the EGF-GemC18-NPs over the OVA-GemC18-NPs by MCF-7 cells (Fig. 2), which was expected because the MCF-7 cells express EGFR at a level of 1 × 104 per cell [21]. Taken together, data in Figs. 1 and 2 showed that the murine EGF conjugated onto the GemC18-NPs helped facilitate the uptake of nanoparticles by EGFR-expressing tumor cells through the EGF-EGFR interaction.
Figure 2. Flow cytometric and fluorescent microscopic analyses of the uptake of EGF-GemC18-NPs by tumor cells expressing different levels of EGFR.
(A). Typical flow cytometric graphs of cells after 6 h of incubation with fluorescein-labeled EGF-GemC18-NPs (green, far right), fluorescein-labeled OVA-GemC18-NPs (gray, middle), or sterile PBS (solid gray area). Experiment was repeated three times with similar results.
(B). Fluorescent microscopic images of MDA-MB-468 and MCF-7 cells after 6 h of incubation with EGF-GemC18-NPs, OVA-GemC18-NPs, or sterile PBS (control). Cell nucleus was stained with DAPI (blue). Nanoparticles were labeled with fluorescein and shown in green.
3.3. Correlation of the in vitro cytotoxicity of the EGF-GemC18-NPs with the density of the EGFR on tumor cells
The cytotoxicities of the EGF-GemC18-NPs and OVA-GemC18-NPs in MDA-MB-468, MDA-MB-231, and MCF-7 cells were evaluated and compared. As expected, in all three cell lines, the EGF-GemC18-NPs were more cytotoxic than the OVA-GemC18-NPs (Fig. 3A). Moreover, it appeared that the extent to which the EGF-GemC18-NPs were more cytotoxic than the OVA-GemC18-NPs was dependent on the density of the EGFR on the cells (Fig. 3A). In other words, the difference in the cytotoxicities between the EGF-GemC18-NPs and the OVA-GemC18-NPs was much greater in the MDA-MB-468 cells than in the MDA-MB-231 cells; and it was greater in the MDA-MB-231 cells than in the MCF-7 cells (Fig. 3A). Finally, data in Fig. 3B showed that the tumors cells treated with the GemC18-NPs underwent apoptosis as well. The cytotoxicity data are in agreement with the cell uptake data shown in Figs. 1-2. It is likely that the high density of EGFR on the surface of the MDA-MB-468 cells allowed the cells to take up more EGF-GemC18-NPs. The more EGF-GemC18-NPs that are internalized into the cells, the more cytotoxicity is expected.
Figure 3. In vitro cytotoxicity of EGF-GemC18-NPs.
(A). Percent of cells alive after 48 h of incubation with different concentration of GemC18 in EGF-GemC18-NPs or OVA-GemC18-NPs (n = 4).
(B). Flow cytometric graphs of MDA-MB-468 cells after 24 h of incubation with EGF-GemC18-NPs or OVA-GemC18-NPs and stained with Annexin V and 7-AAD. Numbers in the quadrants are % of cells in early apoptosis (LR), late apoptosis (UR), and cell debris (UL). Experiment was repeated 3 times with similar results.
3.4. The EGFR-targeting GemC18-NPs more effectively controlled the growth of pre-established EGFR-over-expressing tumors in mice
To investigate whether the EGF-GemC18-NPs are more cytotoxic than the OVA-GemC18-NPs to EGFR-over-expressing tumors in vivo, their ability to control tumor growth in vivo was evaluated and compared in mice with pre-established MDA-MB-468 tumors. As shown in Fig. 4A, tumors in mice that were treated with the EGF-GemC18-NPs grew significantly slower than in mice that were treated with the OVA-GemC18-NPs. From day 45 to day 50, the mean diameter of tumors in mice treated with the EGF-GemC18-NPs was significantly smaller than in mice treated with the OVA-GemC18-NPs (p < 0.05) (Fig. 4A). Statistical comparison of the size of tumors in mice treated with EGF-GemC18-NPs and that in mice treated with OVA-GemC18-NPs beyond day 50 was not carried out due to the death of mice in both groups around day 50. In fact, it seemed that the 4 doses of OVA-GemC18-NPs simply inhibited the growth of the MDA-MB-468 tumors for about 40 days. When the treatment was stopped - last dose on day 37, the tumors started to grow again (Fig 4A). In contrast, when the treatment with the EGF-GemC18-NPs was terminated, the mean diameter of the tumors continued to decrease (Fig. 4A). Mice that were treated with the EGF-GemC18-NPs also survived longer than those treated with the OVA-GemC18-NPs (Fig. 4B). The median survival time for mice that were treated with the EGF-GemC18-NPs was 160 days, only 69 days for mice treated with the OVA-GemC18-NPs. A comparison of the survival curves of the EGF-GemC18-NPs and OVA-GemC18-NPs revealed a p value of 0.053, which would have been smaller (i.e., 0.023) if one of the mice that were treated with the OVA-GemC18-NPs did not accidently pass away during the i.v. injection of the nanoparticles on day 37. Nonetheless, data in Fig. 4 showed that the EGF-conjugated GemC18-NPs were more effective than the control GemC18-NPs in controlling the growth of EGFR-over-expressing tumors in vivo. It is worth mentioning that gemcitabine HCl at the molar equivalent dose (28.3 mg/kg, 4 doses) caused serious toxicity to nude mice with MDA-MB-468 tumors because mice that were treated with gemcitabine HCl did not survive as long as mice that were injected with sterile mannitol (Supplemental Figure S1).
Figure 4. Anti-tumor activity of EGF-GemC18-NPs in nude mice with pre-established MDA-MB-468 tumors.
(A). Tumor growth curves.
(B). Mouse survival curves (p = 0.053, EGF-GemC18-NPs vs. OVA-GemC18-NPs). In A and B, mice were dosed 11, 17, 28, and 37 days after tumor cell injection.
(C). Tumor growth curves in mice used for immunohistology analysis.
In A and C, * p < 0.05, EGF-GemC18-NPs vs. OVA-GemC18-NPs.
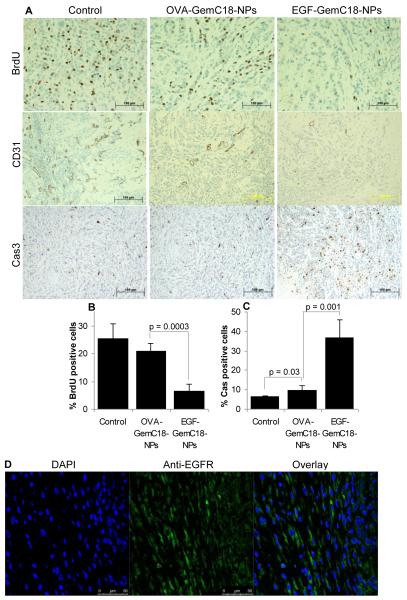
For immunohistology analysis, another mouse study was carried out by treating MDA-MB-468 tumor-bearing mice with sterile mannitol, OVA-GemC18-NPs, or EGF-GemC18-NPs 20, 30, 40, and 53 days after tumor cell injection. Similar to what was shown in Fig. 4A, tumors in mice that were treated with the EGF-GemC18-NPs grew significantly slower than in mice that were treated with the OVA-GemC18-NPs (Fig. 4C). Tumors were harvested on day 54 for analysis. Anti-BrdU staining showed that cell proliferation was less extensive in tumors in mice that were treated with the GemC18-NPs (Fig. 5A). More importantly, BrdU positive staining was significantly less extensive in tumors in mice that were treated with the EGF-GemC18-NPs than in tumors in mice that were treated with the OVA-GemC18-NPs (Figs. 5A, B). Anti-CD31 staining, a marker of endothelial cells, revealed extensive vascularization in tumors in mice that were treated with sterile mannitol and to a less extent in tumors in mice that were treated with OVA-GemC18-NPs (Fig. 5A). However, CD31 positive staining was very rare in tumors in mice that were treated with EGF-GemC18-NPs (Fig. 5A). Finally, caspase3 positive staining, an apoptosis marker, was extensive in tumors in mice that were treated with the EGF-GemC18-NPs, but significantly less extensive in tumors in mice that were treated with OVA-GemC18-NPs or sterile mannitol (Figs. 5A, C). Data in Fig. 5D showed that almost all the tumor cells were EGFR positive, because almost all cell nuclei (DAPI positive) were surrounded by EGFR positive staining. Therefore, it is likely that the caspase 3 positive cells in Fig. 5A were EGFR positive. Taken together, it appeared that, compared to the OVA-GemC18-NPs, the EGFR-targeted EGF-GemC18-NPs were more anti-proliferative, anti-angiogenic, and pro-apoptotic, which may explain why the EGF-GemC18-NPs were more effective than the OVA-GemC18-NPs in controlling the growth of the MDA-MB-468 tumors as shown in Fig. 4.
Figure 5. Immunohistographs of MDA-MB-468 tumors after treatment with EGF-GemC18-NPs or OVA-GemC18-NPs.
(A). Tumor tissues were staining with antibodies against BrdU, CD31, or caspase 3 (Cas 3). Scale bars = 100 μm.
(B). The % of BrdU positive cells.
(C). The % of caspase 3 positive cells.
(D). Tumor tissues after anti-EGFR antibody staining (green) and DAPI staining (blue).
3.5. The EGF on the EGF-GemC18-NPs increased the accumulation of the nanoparticles in tumors in mice
To evaluate the biodistribution of the EGF-GemC18-NPs and OVA-GemC18-NPs in mice, fluorescein-labeled nanoparticles were injected (i.v.) into nude mice with pre-established MDA-MB-468 tumors with an average diameter of 7-10 mm. Twenty four hours later, mice were euthanized to harvest tumor, lung, liver, spleen, kidney, and heart, and the fluorescence intensity in them were determined using an IVIS Spectrum. It was reported that the fluorescence intensity determined in ex vivo imaging is an accurate reflection of fluorescence-labeled nanoparticles accumulated inside organs due to the deep penetration in the near-infrared region and negligible auto-fluorescence in ex vivo organs [31]. Shown in Fig. 6A are the fluorescence images of different organs and tumor tissues. The values of the fluorescence intensity, after normalization to tumor or organ weights, are shown in Fig. 6B. The fluorescence intensity in tumors in mice that were treated with the EGF-GemC18-NPs was more than 2-fold greater than that in tumors in mice that were treated with the OVA-GemC18-NPs (p = 0.0003), clearly demonstrating that the EGF on the EGF-GemC18-NPs enhanced the accumulation of the EGF-GemC18-NPs into EGFR-over-expressing tumors. It is noticed that the fluorescence intensity in the livers in mice that were injected with the EGF-GemC18-NPs tended to be higher than that in mice that were injected with the OVA-GemC18-NPs (Fig. 6A), which may be explained by the fact that the EGFR expression is slightly higher in cells in the liver and kidney (i.e., 1 × 105 EGFR/cell). Due to the variations in the size (or weight) of the organs from different mice, the fluorescence intensity values reported in Figure 6B were normalized by organ weight. Interestingly, the fluorescence intensity values in livers after normalizing to weight were no longer significantly different in mice that were injected with EGF-GemC18-NPs or OVA-GemC18-NPs (Fig. 6B).
Figure 6. Biodistribution of EGF-GemC18-NPs.
(A). Ex vivo fluorescence IVIS images of MDA-MB-468 tumors and organs 24 h after injection (T = tumor, K = kidney, H = heart, and S = spleen).
(B). A comparison of the normalized fluorescence intensity of EGF-GemC18-NPs or OVA-GemC18-NPs in tumors and different organs 24 h after injection (*, p = 0.0003 in tumors).
(C). Fluorescence intensity in the blood of healthy C57BL/6 mice at different time points after i.v. injection of nanoparticles. Data shown in B and C are mean ± S.D. from three replicates.
Shown in Fig. 6C are the kinetics of the fluorescence intensity in the blood samples in healthy C57BL/6 mice injected with fluorescein-labeled EGF-GemC18-NPs or fluorescein-labeled OVA-GemC18-NPs, and there appears to be no difference between those two nanoparticle formulations. In fact, the half-lives of the EGF-GemC18-NPs and the OVA-GemC18-NPs at the elimination phase were not different (5.9 ± 2 h vs. 6.2 ± 1.5 h, respectively, p = 0.86). The half-lives of the EGF-GemC18-NPs and the OVA-GemC18-NPs have the potential to be significantly increased, because the original GemC18-NPs exhibited a half-life of around 24 h at the elimination phase [8]. Conjugation of the EGF or the OVA to the terminal of the PEG chains on the GemC18-NPs was likely responsible for the shorter half-life observed. It is known that the ability of the PEG to extend the blood circulation time of a drug carrier is related to parameters such as the amount and the molecular weight of the PEG [32]. Therefore, further future research efforts are underway to examine the feasibility of extending the half-life of the EGF-GemC18-NPs by increasing the amount of PEG(2000) in the formulation or using longer PEG molecules. The inclusion of certain percent of PEG(2000) that lacks the maleimide group, and thus does not allow the conjugation of the EGF protein, is expected to help as well.
As aforementioned, Gemzar® is commonly used as a single agent or in combination with additional anti-cancer drugs to treat pancreatic, breast, and lung cancers. Although relatively efficacious, there is still room to improve the anti-tumor activity of the gemcitabine HCl for injection. Data from several previous studies showed that formulating gemcitabine or its derivatives into nanoparticles (e.g., liposomes, polymeric nanoparticles, solid lipid nanoparticles) can potentially improve the resultant anti-tumor activity in vivo [2-10]. Previously, our group reported that change of the gemcitabine formulation from the gemcitabine HCl solution to a stearoyl gemcitabine in solid lipid nanoparticles significantly improved the resultant anti-tumor activity [8], and the data in the present study clearly demonstrated that further surface modification of the nanoparticles by conjugating EGF as a ligand to actively target the GemC18-NPs to tumor cells significantly increased the anti-tumor activity of the GemC18-NPs against the EGFR-over-expressing MDA-MB-468 tumors in mice (Fig. 4), very likely due to the EGF’s ability to increase the accumulation of the EGF-GemC18-NPs into the MDA-MB-468 tumors in vivo (Fig. 6). It remains unknown to what extent the EGF on the GemC18-NPs also increased the uptake or internalization of the GemC18-NPs by the tumor cells in tumor tissues. Data from a previous study showed that anti-HER2 antibody targeting of long-circulating lipidic nanoparticles increased the cellular uptake of the nanoparticles by the tumor cells in animal models, although they did not increase tumor localization (accumulation) [33]. Nonetheless, it is clear that the in vivo anti-tumor efficacy of the gemcitabine can be improved by formulating it into a tumor-targeting nanoparticle formulation.
The nanoparticles used in the present study were prepared from lecithin/GMS-in-water emulsions. Lecithin is a complex mixture of phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol and other substances such as triglycerides and fatty acids. It is generally regarded as safe (GRAS) and accepted in the FDA Inactive Ingredients Guide for parenterals [34]. GMS is used in a variety of pharmaceutical applications and is GRAS listed as well [34]. Tween 20 was used in the nanoparticle preparation as an emulsifier. Tween 20 is also GRAS listed and included in the FDA Inactive Ingredients Guide for parenterals [34]. Therefore, it is expected that the nanoparticles have a favorable toxicity profile, and data from our preliminary toxicity studies are promising. For example, it was previously shown that the GemC18-NPs did not induce detectable acute or subacute liver toxicity when injected intravenously into mice [8]. Similar nanoparticles did not cause any significant red blood cell lysis or platelet aggregation [24]. Moreover, in a 1.5-year study, the pathological and histological parameters of mice that received three doses of similar nanoparticles by subcutaneous injection were evaluated, and an examination by a board-certified veterinary pathologist did not reveal any significant difference between the treated and untreated mice (Sloat and Cui, unpublished data). Therefore, the possibility exists for the translation of these GemC18-NPs and/or the EGF-GemC18-NPs from bench to clinic as a novel and more efficacious gemcitabine formulation. Similar alternative GemC18 nanoparticle formulations may also be developed to improve the clinical outcome of gemcitabine therapy.
The EGFR was chosen as the target to actively deliver the GemC18-NPs into tumors in the present study. The majority of the normal cells express EGFR at 1 × 104 per cell, but EGFR is over-expressed in a variety of cancer cells [35]. In case targeting EGFR is not feasible, alternative targets including transferrin receptors, folate receptors, or vascular cell adhesion molecule-1 (VCAM-1) may be utilized [36-38]. Other tumor-specific ligands may be identified using phage display as well.
4. Conclusions
It was previously shown that formulating a stearoyl gemcitabine derivative into nanoparticles significantly improved the resultant anti-tumor activity. Data in the present study showed that the anti-tumor activity of stearoyl gemcitabine nanoparticles can be further improved by actively targeting them into tumors.
Supplementary Material
Acknowledgment
This work was supported in part by a National Cancer Institute grant (CA135274) to ZC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Barton-Burke M. Gemcitabine: a pharmacologic and clinical overview. Cancer Nurs. 1999;22:176–183. doi: 10.1097/00002820-199904000-00011. [DOI] [PubMed] [Google Scholar]
- [2].Arias JL, Reddy LH, Couvreur P. Polymeric nanoparticulate system augmented the anticancer therapeutic efficacy of gemcitabine. J Drug Target. 2009;17:586–598. doi: 10.1080/10611860903105739. [DOI] [PubMed] [Google Scholar]
- [3].Arias JL, Reddy LH, Couvreur P. Superior preclinical efficacy of gemcitabine developed as chitosan nanoparticulate system. Biomacromolecules. 2011;12:97–104. doi: 10.1021/bm101044h. [DOI] [PubMed] [Google Scholar]
- [4].Celano M, Calvagno MG, Bulotta S, Paolino D, Arturi F, Rotiroti D, Filetti S, Fresta M, Russo D. Cytotoxic effects of gemcitabine-loaded liposomes in human anaplastic thyroid carcinoma cells. BMC Cancer. 2004;4:63. doi: 10.1186/1471-2407-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Immordino ML, Brusa P, Rocco F, Arpicco S, Ceruti M, Cattel L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J Control Release. 2004;100:331–346. doi: 10.1016/j.jconrel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [6].Jantscheff P, Ziroli V, Esser N, Graeser R, Kluth J, Sukolinskaya A, Taylor LA, Unger C, Massing U. Anti-metastatic effects of liposomal gemcitabine in a human orthotopic LNCaP prostate cancer xenograft model. Clin Exp Metastasis. 2009;26:981–992. doi: 10.1007/s10585-009-9288-1. [DOI] [PubMed] [Google Scholar]
- [7].Paolino D, Cosco D, Racanicchi L, Trapasso E, Celia C, Iannone M, Puxeddu E, Costante G, Filetti S, Russo D, Fresta M. Gemcitabine-loaded PEGylated unilamellar liposomes vs GEMZAR: biodistribution, pharmacokinetic features and in vivo antitumor activity. J Control Release. 2010;144:144–150. doi: 10.1016/j.jconrel.2010.02.021. [DOI] [PubMed] [Google Scholar]
- [8].Sloat BR, Sandoval MA, Li D, Chung WG, Lansakara-P DSP, Proteau PJ, Kiguchi K, DiGiovanni J, Cui Z. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. Int. J. Pharm. 2011;409:278–288. doi: 10.1016/j.ijpharm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stella B, Arpicco S, Rocco F, Marsaud V, Renoir JM, Cattel L, Couvreur P. Encapsulation of gemcitabine lipophilic derivatives into polycyanoacrylate nanospheres and nanocapsules. Int J Pharm. 2007;344:71–77. doi: 10.1016/j.ijpharm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- [10].Wang CX, Huang LS, Hou LB, Jiang L, Yan ZT, Wang YL, Chen ZL. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009;1261:91–99. doi: 10.1016/j.brainres.2009.01.011. [DOI] [PubMed] [Google Scholar]
- [11].Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [12].LeMaistre CF, Meneghetti C, Howes L, Osborne CK. Targeting the EGF receptor in breast cancer treatment. Breast Cancer Res Treat. 1994;32:97–103. doi: 10.1007/BF00666210. [DOI] [PubMed] [Google Scholar]
- [13].Klijn JG, Berns PM, Schmitz PI, Foekens JA. The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev. 1992;13:3–17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- [14].Baselga J. Monoclonal antibodies directed at growth factor receptors. Ann Oncol. 2000;11(Suppl 3):187–190. doi: 10.1093/annonc/11.suppl_3.187. [DOI] [PubMed] [Google Scholar]
- [15].Ranson M, Hammond LA, Ferry D, Kris M, Tullo A, Murray PI, Miller V, Averbuch S, Ochs J, Morris C, Feyereislova A, Swaisland H, Rowinsky EK. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002;20:2240–2250. doi: 10.1200/JCO.2002.10.112. [DOI] [PubMed] [Google Scholar]
- [16].Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003;39:1348–1354. doi: 10.1016/s0959-8049(03)00235-1. [DOI] [PubMed] [Google Scholar]
- [17].Kullberg EB, Nestor M, Gedda L. Tumor-cell targeted epiderimal growth factor liposomes loaded with boronated acridine: uptake and processing. Pharm Res. 2003;20:229–236. doi: 10.1023/a:1022223204460. [DOI] [PubMed] [Google Scholar]
- [18].Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD, Park JW. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63:3154–3161. [PubMed] [Google Scholar]
- [19].Kim IY, Kang YS, Lee DS, Park HJ, Choi EK, Oh YK, Son HJ, Kim JS. Antitumor activity of EGFR targeted pH-sensitive immunoliposomes encapsulating gemcitabine in A549 xenograft nude mice. J Control Release. 2009;140:55–60. doi: 10.1016/j.jconrel.2009.07.005. [DOI] [PubMed] [Google Scholar]
- [20].Arya G, Vandana M, Acharya S, Sahoo SK. Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine. 2011 doi: 10.1016/j.nano.2011.03.009. doi: 10.1016/j.nano.2011.03.009. [DOI] [PubMed] [Google Scholar]
- [21].Reilly RM, Kiarash R, Cameron RG, Porlier N, Sandhu J, Hill RP, Vallis K, Hendler A, Gariepy J. 111In-labeled EGF is selectively radiotoxic to human breast cancer cells overexpressing EGFR. J Nucl Med. 2000;41:429–438. [PubMed] [Google Scholar]
- [22].Walker RA, Dearing SJ. Expression of epidermal growth factor receptor mRNA and protein in primary breast carcinomas. Breast Cancer Res Treat. 1999;53:167–176. doi: 10.1023/a:1006194700667. [DOI] [PubMed] [Google Scholar]
- [23].Sloat BR, Sandoval MA, Hau AM, He Y, Cui Z. Strong antibody responses induced by protein antigens conjugated onto the surface of lecithin-based nanoparticles. J Control Release. 2010;141:93–100. doi: 10.1016/j.jconrel.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yanasarn N, Sloat BR, Cui Z. Nanoparticles engineered from lecithin-in-water emulsions as a potential delivery system for docetaxel. Int J Pharm. 2009;379:174–180. doi: 10.1016/j.ijpharm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- [26].French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J Biol Chem. 1995;270:4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- [27].Spitzer E, de Los Angeles M, Perez R, Grosse R. Binding properties of biotinylated epidermal growth factor to its receptor on cultured cells and tissue sections. J Cell Biochem. 1989;41:47–56. doi: 10.1002/jcb.240410202. [DOI] [PubMed] [Google Scholar]
- [28].Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- [29].Kurihara K, Miyashita S, Sazaki G, Nakada T, Durbin SD, Komatsu H, Ohba T, Ohki K. Incorporation of impurity to a tetragonal lysozyme crystal. Journal of Crystal Growth. 1999;196:285–290. [Google Scholar]
- [30].Blessing T, Kursa M, Holzhauser R, Kircheis R, Wagner E. Different strategies for formation of pegylated EGF-conjugated PEI/DNA complexes for targeted gene delivery. Bioconjug Chem. 2001;12:529–537. doi: 10.1021/bc0001488. [DOI] [PubMed] [Google Scholar]
- [31].Gao J, Chen K, Xie R, Xie J, Lee S, Cheng Z, Peng X, Chen X. Ultrasmall near-infrared non-cadmium quantum dots for in vivo tumor imaging. Small. 2010;6:256–261. doi: 10.1002/smll.200901672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Francis GE, Delgado C, Fisher D, Malik F, Agrawal AK. Polyethylene glycol modification: relevance of improved methodology to tumour targeting. J Drug Target. 1996;3:321–340. doi: 10.3109/10611869608996824. [DOI] [PubMed] [Google Scholar]
- [33].Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- [34].Wade A, Weller PJ. Handbook of Pharmaceutical Excipients. 2nd Ed 1994. pp. 392–399. [Google Scholar]
- [35].Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593–1611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- [36].Singh M. Transferrin As A targeting ligand for liposomes and anticancer drugs. Curr Pharm Des. 1999;5:443–451. [PubMed] [Google Scholar]
- [37].Zhang Y, Zhang J. Surface modification of monodisperse magnetite nanoparticles for improved intracellular uptake to breast cancer cells. J Colloid Interface Sci. 2005;283:352–357. doi: 10.1016/j.jcis.2004.09.042. [DOI] [PubMed] [Google Scholar]
- [38].Dienst A, Grunow A, Unruh M, Rabausch B, Nor JE, Fries JW, Gottstein C. Specific occlusion of murine and human tumor vasculature by VCAM-1-targeted recombinant fusion proteins. J Natl Cancer Inst. 2005;97:733–747. doi: 10.1093/jnci/dji130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.