Abstract
Parkinson’s disease (PD) is a common, treatable movement disorder that often remains undiagnosed despite clinically manifest symptoms. Screening for parkinsonism could lead to improved detection and earlier treatment, and facilitate research studies of PD prevalence. In order to determine the feasibility of screening, this study evaluated the validity of previously developed screening questionnaires. We systematically searched online databases PubMed and EMBASE for English-language studies published between 1980 and 2009. In each database a “Parkinson(s) disease” or “parkinsonism” term was combined with a screening term (“screening instrument,” screening questionnaire,” “screen” or “prevalence survey”) and a validity term (“validation,” “sensitivity” and “specificity”). Included studies reported the psychometric properties of at least one self-report questionnaire for parkinsonism. Twenty-seven studies met the inclusion criteria. From these studies, 9 screening questionnaires were identified. Sensitivity and specificity estimates varied widely. Sensitivity estimates were as high as 100% when questionnaires were tested among previously diagnosed PD patients and included a high number of parkinsonism-specific items, but were as low as 48% when tested among early cases in a community-based sample. Specificity estimates were lower, ranging from 22–100%. An older sample, presence of multiple co-morbid conditions and lower literacy led to lower specificity estimates. Higher specificity estimates were seen when the screening questionnaires were administered by a physician. Screening questionnaires can detect symptomatic parkinsonism. However, the performance of these questionnaires varied based on the individual items, study sample, and method of administration. The performance of screening questionnaires in the detection of early or mild parkinsonism was modest.
Keywords: Parkinson’s disease, early detection, instruments, sensitivity, specificity
Introduction
Parkinsonism is a clinical syndrome that consists of four cardinal signs: bradykinesia, rigidity, tremor, and postural instability. Parkinson’s disease (PD), the most common cause of parkinsonism, is the second most prevalent neurodegenerative disease of aging, currently affecting over 4 million people worldwide, and expected to affect about 9 million people by the year 2030 [1]. However, individuals with PD may not be aware that they are affected. Population-based studies of PD prevalence demonstrate that from 12% to 83% of individuals with clinically manifest PD were previously undiagnosed, that is, they had not yet received a clinical diagnosis of PD at the time of the study despite presence of motor symptoms [2–9].
Epidemiologists have developed several screening instruments in an attempt to improve the detection of PD. An effective screening instrument for parkinsonism can lead to earlier detection of disease which can, in turn, allow for the initiation of appropriate therapy and improve health outcomes. Secondly, through the use of screening, we can carry out prevalence studies to evaluate the burden of neurological disease, especially in developing countries where access to neurological care may be limited. This will help policymakers estimate healthcare costs and allocate resources more efficiently. Lastly, screening can help to identify cases for population-based case-control studies that contribute to understanding of the genetic and environmental risk factors for PD [10].
To date, there has been no systematic comparison of the performance of existing parkinsonism screening questionnaires. It is unclear which screening methods are appropriate for different settings. Researchers have recommended a two-step screening process to identify prevalent cases of parkinsonism, that is, a screening questionnaire to identify potential cases followed by confirmatory neurological examination [11]. However, there are inherent limitations to this approach [12]. Some of these limitations are related to the quality of the screening instrument used. A high false negative rate will lead to underestimation of prevalence and concomitant misallocation of resources. A high false positive rate will increase the burden of follow-up neurological exams needed, will increase study time and costs, and can worsen a patient’s anxiety.
The goals of this systematic review were to provide a comprehensive overview of parkinsonism screening questionnaires and to evaluate the performance of these questionnaires in the detection of parkinsonism.
Methods
Search strategy
To identify relevant studies, we systematically searched online databases PubMed (Medline) and EMBASE for English-language articles published between January 1980 and December 2009. A manual search of the references lists from relevant retrieved articles was also completed. In each database, a “Parkinson(s) disease” or “parkinsonism” term was combined with a screening term (“screening instrument,” “screening questionnaire,” “screen,” “screening,” or “prevalence survey”) and a validity term (“validity,” “validation,” “sensitivity,” or “specificity”). After excluding duplicates, this search yielded 1587 articles. The titles and abstracts (or full articles if the titles and abstracts provided insufficient information) were then reviewed by authors ND and AA for possible inclusion. Peer-reviewed abstracts presented at national meetings were also included if they met the inclusion criteria described below.
Study Inclusion Criteria
Studies were selected if they met the following inclusion criteria: 1) English language, and 2) Reported both sensitivity and specificity estimates of a screening instrument that: a) assessed self-reported symptoms through a questionnaire, and b) intended to detect parkinsonism.
Data extraction
Abstracted data included the publication date, study setting, sample size, screening questionnaire components, method of screening questionnaire administration, sensitivity and specificity, criteria for parkinsonism, method of gold standard evaluation and any noted limitations or comments.
Analysis
Performance of each screening questionnaire was assessed both quantitatively through the reported sensitivity and specificity estimates, and qualitatively based on study methodology and limitations.
Results
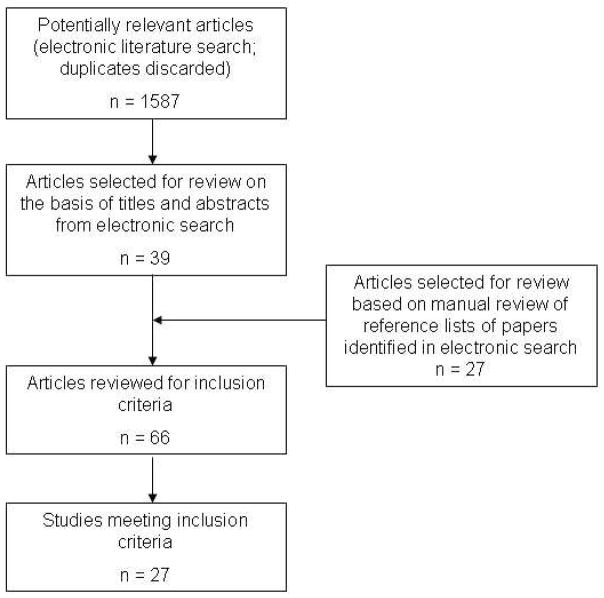
Twenty-seven articles were identified that reported the sensitivity and specificity of parkinsonism screening questionnaires (Figure). A total of 9 different parkinsonism screening questionnaires were evaluated in these articles (Table 1).
Figure 1.
Flow chart of literature search and selection of papers for review
Table 1.
Screening questionnaires for parkinsonism
| Screen name or author | Type | # of articles that evaluated the screening instrumenta | Setting | Sensitivity | Specificity |
|---|---|---|---|---|---|
| WHO [13]/modified WHO | Questionnaire and physical tasks | 9 | Clinic/hospital | 93–100%b | 26–89%b |
| Tanner [30] | Questionnaire | 11 | Clinic | 48–100% | 77–100% |
| Community | 61–92% | 29–92% | |||
| Mutch [36] | Questionnaire | 2 | Clinic | 91% | 92% |
| Community | 87% | 28% | |||
| Anderson [26] | Questionnaire | 1 | Hospital | 100% | 80% |
| Chang [29] | Questionnaire | 1 | Clinic | 100% | 58% |
| ILSA [28] | Questionnaire and physical tasks | 1 | Unknown | 85–95%b | 90–100%b |
| Chan [40] | Questionnaire | 1 | Hospital | 100% | 45% |
| Community | 100% | 42% | |||
| Nicoletti [38] | Questionnaire and physical tasks | 1 | Hospital | 100% | 90% |
| Ishihara [39] | Questionnaire | 1 | Community | 96% | 56% |
One article evaluated two screening questionnaires;
Estimates include other conditions in addition to parkinsonism;
Abbreviations: ILSA – Italian Longitudinal Study on Aging; WHO – World Health Organization
Comprehensive screening questionnaires
The World Health Organization (WHO) developed the first protocol to test for neurological disorders [13]. This screening protocol aimed to identify a broad range of neurological disorders including parkinsonism, stroke, epilepsy, peripheral neuropathy, and headache. The screen included two items, not specific for parkinsonism, that asked about uncontrollable shaking and the inability to walk, and also items that assessed postural tremor, heel-toe walk, finger-nose-finger, and picking up a matchstick off the ground. Prevalence studies conducted in Nigeria [14], India [15], Spain [16], Saudi Arabia [17] and China[18] have utilized this screening protocol to identify potential parkinsonism cases, and then to perform clinical examinations of all those who screened positive. Some of these published studies report the sensitivity and specificity of the screen from pilot testing among previously diagnosed cases [11, 16, 17, 19]. However, details on the methods of these pilot tests, including diagnostic or gold standard criteria for parkinsonism and characteristics of the sample tested, are minimal. Furthermore, the reported sensitivity and specificity are not specific for the detection of parkinsonism, but pool all neurological conditions. Among these studies using the WHO screen, sensitivity estimates ranged from 93% to 100% (not specific for parkinsonism) and specificity estimates ranged from 78% to 89%.
Modified versions of the original WHO protocol have also been developed and tested [20–24]. With the addition of items related to difficulty using utensils/buttoning and unusual movements, as well as revised items that specifically asked about the presence of tremor, need for assistive devices with walking and falls, and assessment of finger tapping and four meter gait assessment, the overall sensitivity of the instrument improved from 98% to 100% (not specific for parkinsonism), and specificity improved from 29% to 61% [23]. However, Bergareche, et al [22] report that the 95% confidence interval for the sensitivity estimate, specific for parkinsonism, of their modified WHO screening questionnaire was wide (52%–100%) given the low number of detected cases. The National Institute of Mental Health and Neurosciences in India developed a modified WHO screening protocol that included two additional questions about changes in handwriting and abnormal postures to detect movement disorders [20]. Sensitivity and specificity was reported as 84% (not specific for parkinsonism) and 99.9%, respectively. Specificity for these modified WHO screening questionnaires ranged from 26% to 99.9%, and sensitivity ranged from 84% to 100% (not specific for parkinsonism).
There have been other comprehensive screening protocols developed to detect a wide range of health conditions that include parkinsonism. Anderson, et al [25, 26] described one comprehensive screening protocol to identify epilepsy, febrile seizures, stroke/transient ischemic attack and parkinsonism. Within the larger prevalence study, the authors report that a pilot test of the screening questionnaire among 24 cases of parkinsonism and 20 controls showed an estimated sensitivity to detect parkinsonism of 100% and a specificity of 80% [26]. However, they did not report how parkinsonism was defined. Another comprehensive screening protocol to detect parkinsonism, cardiovascular disease, diabetes, stroke, thyroid disease and neuropathy was designed and conducted by the Italian Longitudinal Study on Aging [27]. This screening protocol included an interview, nurse visit, and physician examination. Sensitivity with the inclusion of the physician examination was reported as 85–95% (not specific for parkinsonism) and specificity 90–100% [28]. Lastly, an instrument designed to detect parkinsonism, stroke and epilepsy was developed by Chang, et al [29]. This included 3 items that asked about prior symptoms, 3 items about prior diagnoses and 7 items that assessed physical function. When evaluating only the 3 items that asked about prior symptoms, the sensitivity to detect parkinsonism was 67%.
Table 2 provides of summary of these comprehensive screening questionnaires.
Table 2.
Studies of screening questionnaires that were designed to detect other health conditions in addition to parkinsonism
| Author | Date | Sample | Survey details | Parkinsonism definition | Gold standard | Findings | Comments |
|---|---|---|---|---|---|---|---|
| WHO screen [13] – 15 general neurology items and 7 physical tasks | |||||||
| Osuntokun[11] | 1982 | Subjects with and without parkinsonism | Administered by trained interviewer | Not reported | Prior diagnosis by unspecified provider | SEN 95% and SPEC 80% | Parkinsonism specific results not reported |
| Bharucha[19] | 1987 | Subjects with and without parkinsonism | Additional 2 items to assess self-reported symptoms; administered by trained interviewer | 3 of 4 cardinal signs | Prior diagnosis by unspecified provider | SEN 100% and SPEC 89% | Parkinsonism specific results not reported |
| Gutierrez-del-Olmo[16] | 1989 | Not reported | Administered by medical student | Not reported | Not reported | SEN 93% and SPEC 78% | SEN estimate is for parkinsonism and transient ischemic attack combined |
| Al Rajeh[17] | 1993 | Subjects with and without parkinsonism | Administered by trained interviewer | 2 of 4 cardinal signs | Not reported | SEN 98% and SPEC 89% | Parkinsonism specific results not reported |
| Gourie-Devi[24] (modified) | 1996 | 173 random sample of community residents | 12 items to assess for self-reported symptoms | 2 of 4 cardinal signs | Not reported | SEN 95% and SPEC 98% | Parkinsonism specific results not reported |
| Das[20] (modified) | 2006 | 3041 stratified random sample of community residents | 14 items to assess self-report symptoms | 2 of 4 cardinal signs | Not reported | SEN 84% and SPEC 99.9% | Parkinsonism specific results not reported |
| Bower[23] (modified ) | 2009 | 63 patients with various neurological diseases, 21 patients with pain, and 44 controls. Final instrument tested on 78 of these 128 | Original 15 self-report items and 7 physical task WHO screen AND revised 24 self-report items and 16 physical task screen administered by nonmedical interviewer | Not reported | Prior diagnosis by attending neurologist | Original WHO screen: SEN 98% and SPEC 29% for cut-off score of 1 Revised WHO screen: SEN 100% and SPEC 61% for cut-off score of 1 |
Parkinsonism specific results not reported |
| Anderson, et al[26] screen – Sections on symptoms, prior diagnoses, ancillary tests and medications | |||||||
| Anderson[26] | 1995 | 24 patients with known PD and 20 controls | Administered by trained interviewer | Not reported | Not reported | SEN 100% and SPEC 80% | Head of household completed screen for all household members |
| Chang, et al[29] screen – 3 general neurology items, 7 physical tasks and prior diagnoses | |||||||
| Chang[29] | 1996 | 6 patients with known PD, 34 with mix of tremor, stroke and epilepsy, and 60 controls from neurology clinic | Administered by trained interviewer | 2 of 4 cardinal signs | Review of medical records | SEN 100% and SPEC 58% for cut-off score of 1 (all items and exam); SEN 67% for cut-off score of 1 for symptom items; SEN 50% for prior diagnosis of parkinsonism item | Tremor item had highest individual sensitivity to detect PD |
| Italian Longitudinal Study on Aging[28] – Section on symptoms, prior diagnoses and physical examination of gait and tone | |||||||
| Italian Longitudinal Study on Aging[28] | 1997 | Not reported | Examination of gait and tone by physician | Definite = [2 of 4 cardinal signs] OR [1 of 4 cardinal signs on PD treatment] Possible = 1 of 4 cardinal signs | Not reported | SEN 85–95% and SPEC 90–100% Positive predictive value 60% and negative predictive value 90% for parkinsonism |
Parkinsonism specific results not reported for SEN and SPEC estimates |
Abbreviations: SEN – sensitivity; SPEC – specificity; WHO – World Health Organization
Parkinsonism-specific screening questionnaires
Subsequent screening instruments specifically designed to detect parkinsonism have been developed. Of these, the most commonly tested and used is the 9-item screening questionnaire developed by Tanner and colleagues in 1990 [30], and subsequently validated in a larger community sample [31]. Questions ask about difficulty getting out of a chair, tremor, difficulty buttoning, smaller handwriting, softer voice, change in facial expression, lack of balance, “freezing”, and shuffling gait. It has been translated from English and tested in multiple other languages including Spanish [32], German [33], Italian [33], Kannada (India) [34], and Portugese [35]. The screening questionnaire has been tested in clinic (Table 3) and community-based samples (Table 4). It can be used as a self-administered questionnaire or administered by a trained interviewer or physician. Performance of this screening questionnaire ranges widely with sensitivity estimates ranging from 48% to 100% and specificity estimates ranging from 29% to 100%. The variation can largely be explained by different study sample characteristics (discussed below).
Table 3.
Studies of parkinsonism-specific screening questionnaires tested among sample of only previously diagnosed parkinsonism cases
| Author | Date | Sample | Survey details | Parkinsonism definition | Gold standard | SEN | SPEC | Cut-off value (range) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Tanner, et al[30] screen – 9 parkinsonism specific items | |||||||||
| Tanner*[30] | 1990 | 178 patients with mix of neurological disorders, 5 with arthritis and 53 normal older adults | Self-administered | Not reported | Exam by movement-disorder neurologist | 91% 84% |
81% 90% |
4 (0–9) 5 (0–9) |
Lower SPEC for patients when only included group with movement disorders (78%) and motor deficits (72%) |
| Duarte[32] | 1995 | 40 patients with known PD and 100 patients from ophthalmology clinic | Self-administered (mailed), translated to Spanish | Not reported | Exam by neurologist | 92% 100% |
95% 100% |
4.4 (0–9) 42 (weighted range 0 – 67) |
Of the PD cases, 5% were stage 1, 28% were stage 2 and 67% were stage 3 or 4. High SEN to detect moderate to advanced disease |
| Rocca[41] | 1998 | 37 patients with PD and 16 unaffected controls | Telephone interview by trained, blinded interviewer | [3 of 4 cardinal signs] OR [2 cardinal signs with neurologist diagnosis of PD and/or response to levodopa] | Review of medical records | 97% 89% |
44% 88% |
2 (0–9) 4 (0–9) |
Proxy could respond for patient including deceased subjects Individual items with highest SEN were poor balance and trouble buttoning; tremor had highest SPEC |
| Pramstaller[33]0000000000 | 1999 | 40 patients with PD or atypical parkinsonism and 36 unaffected controls | Additional 2 items related to prior diagnosis and treatment; translated to German and Italian (mailed) | Bradykinesia + at least 1 of rigidity, rest tremor or postural instability | Exam by movement disorder neurologist | 95% 90% 95% |
89% 94% 90% |
3 (not reported) 4 (not reported) 24 (weighted range 0–67) |
Higher frequency of false positive responses in subjects with depression, osteoporosis and older age Higher frequency of false negative responses in subjects with earlier stages, tremor as main or only symptom and younger age |
| Tan*[43] | 2002 | 20 patients with PD, 33 with stroke/TIA, 13 with epilepsy and 31 from general neurology | Additional item that asked about previous diagnosis of PD; administered by trained interviewer | Not reported | Exam by neurologist | 100% 85% |
37% 77% |
1 (0–9) 3 (0–9) |
Low specificity in sample with neurological co-morbidity Not specified if screen was translated (81 subjects Chinese, 9 Indian and 7 Malay in Singapore) |
| Mutch, et al[36] screen – 8 parkinsonism specific items | |||||||||
| Mutch[36] | 1991 | 35 patients with known PD and 88 controls from general practice | Self-administered | 2 of 4 cardinal signs | Exam | 91% 97% |
92% 66% |
1 (0–2; tremor + shuffling gait) 1 (0–8; full screen) |
Only controls who screened positive were examined, but if the exam confirmed parkinsonism they were not included as true positives |
| Meneghini, et al (modified WHO) screen[21] – 2 parkinsonism specific items, 7 general neurology items and 7 physical tasks | |||||||||
| Meneghini[21] (modified WHO) | 1992 | 21 patients with known parkinsonism, 66 with mix of other neurologic disease and 21 controls | Administered by neurologist | [2 of 4 cardinal signs if not on PD therapy] OR [1 of 4 cardinal signs if on PD therapy] | Exam by senior neurologist | 100% 95% |
86% 100% |
1 (0–16) 1 (0–7) |
Included idiopathic PD, drug-induced, vascular, postencephalitic and atypical parkinsonism cases Measurement of specificity was with group completely free of disease |
| Bergareche[22] (modified WHO) | 2004 | 6 patients with known PD, 91 controls | Administered by trained interviewer | [2 of 4 cardinal signs] OR [1 of 4 signs if on PD treatment] | Not reported | 100% [51.7–100] | 25.8% [17.7–36.6] | Not reported | Medical and neurological co-morbidities of control population not reported |
| Nicoletti, et al[38] screen -- 4 parkinsonism specific items and 5 physical tasks | |||||||||
| Nicoletti[38] | 2004 | 40 patients with known PD (20 stage 1–2; 20 stage 3–5), 20 with ET and 20 controls | Administered by trained interviewer | [2 of 4 cardinal signs] OR [1 of 4 signs if on PD treatment] | Not reported | 100% [80–100] | 90% [67–98] | 1 (0–9) | Controls completely free of disease |
| Ishihara, et al[39] screen – 6 parkinsonism specific items | |||||||||
| Ishihara[39] | 2005 | 18,645 (subcohort of larger community-based study); 11,539 answered all screen items | Self-administered | Not reported | Self-reported PD, administrative record review (hospital discharge and death certificates) | 96% 91% |
56% 76% |
1 (0–6) 1 (0–5; exclusion of gait item) |
Physical examinations of cases/controls not conducted to verify PD diagnosis Could not ascertain previously undiagnosed PD |
Abbreviations: ET – essential tremor; SEN – sensitivity; SPEC – specificity; WHO – World Health Organization;
abstract presented at national/international meeting; values in brackets represent 95% confidence interval
Table 4.
Studies of parkinsonism-specific screening questionnaires tested among samples of undiagnosed PD cases.
| Author (Screen) | Date | Sample | Survey details | Parkinsonism definition | Gold standard | SEN | SPEC | Cut-off value (range) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Tanner, et al[30] screen – 9 parkinsonism specific items | |||||||||
| Tanner*[31] | 1994 | 592 community residents | Additional 2 items that asked about prior PD diagnosis and treatment | Not reported | Exam by unspecified provider | 91% - all 100% - diagnosed PD 75% - undiagnosed PD |
81% | Not reported | Items related to prior diagnosis and five symptoms (micrographia, soft speech, facial expression, tremor and trouble buttoning) most sensitive |
| Racette[42] | 1999 | 124 patients with PD, 78 asymptomatic subjects with ≥ 2 family members with PD (53 normal, 25 previously undiagnosed) | Administered by trained interviewer | Definite = [3 of 4 cardinal signs] OR [2 signs + asymmetry and supplemental features]; Probable = [2 of 4 signs] OR [asymmetric onset of 1 sign]; Possible = 1 sign | Exam by neurologist | 100% - diagnosed PD 48% - undiagnosed PD 66% - definite cases only |
63% | 1 (0–9) | Potential bias since subjects aware of family history of PD and may be more aware of symptoms or tend to deny symptoms Examiner was not blinded |
| Höglinger[44] | 2004 | 1030 adults over 50 who attended clinics of GPs screened, but only 124 of these examined by neurologist | Self-administered; translated to German | [Bradykinesia + at least one of rigidity, rest tremor or postural instability] AND, for PD diagnosis, nigrostriatal denervation by SPECT | Exam by movement-disorder neurologist, and second exam by independent neurologist | 92% 100% |
84% 97% |
3 (0–9) GP clinical impression only |
Only subjects who screened positive by questionnaire, GP exam, clinical impression or prior diagnosis from records examined by neurologist (N=74) and additional 50 who screened negative – may falsely elevate SEN |
| Sarangmath [34] | 2005 | Part 1: 57 nursing home patients; Part 2: 24 patients with PD and 27 controls | Part 1: Administered by research assistant; Part 2: Administered by neurologist; translated to Kannada (India) | [2 of 4 cardinal signs] OR [1 sign and treatment with PD medications] | Exam by movement disorder neurologist | Part 1: 61–75% Part 2: 100% |
25–61% 89% |
2 (0–9; with at least one bradykinesia item and one other item positive for Part 1 and 2) | Similar SEN between literate and illiterate population, but SPEC lower among illiterate population; medical experience of interviewer affects performance of screen |
| Taylor[37] | 2006 | 30 newly diagnosed PD patients from incidence study and 314 subjects from GP practices (72% who screened positive to two items on gait and tremor; 28% who screened negative) | Self-administered (mailed) | 2 of 4 cardinal signs | Exam by neurologist | 90% 95% [ 83–99] |
33% 28% [23–33] |
2 (0–9; Tanner screen) 1 (0–2; Modified 2-item screen) |
Non-responders to study were older and not all responders gave consent to have confirmatory exam (response bias) |
| Barbosa[35] | 2006 | 667 community members who screened positive (94% of total positive) and 197 who screened negative (42% of total negative) | Additional item that asked about PD treatment or dopamine blocker use; translated to Portuguese | Bradykinesia + at least one of rest tremor, rigidity or postural instability | Exam by 2 physicians (movement disorder neurologist and geriatrician) | 100% | 29% | 2 (0–9 if untreated) OR 1 (0–9 if on PD medication or dopamine blocker) | Not all participants who completed screening questionnaire were examined (response bias) |
| Chan, et al [40] screen – 11 parkinsonism specific items | |||||||||
| Chan[40] | 2000 | 16 patients with known PD, 40 hospital-based controls and 136 randomly selected community controls | 11 items; administered by trained research assistant | 2 of 4 cardinal signs | Exam by geriatrician or neurologist | 100% 84% |
45% - hospital 42% - comm. 86% |
1 (0–11) 2 (0–3; reduced screen) |
10% of sample had parkinsonism, half of these cases were newly diagnosed (5 with PD and 2 with vascular parkinsonism) |
| Mutch, et al[36] screen – 8 parkinsonism specific items | |||||||||
| Taylor[37] | 2006 | 30 newly diagnosed PD patients from incidence study and 314 subjects from GP practices (72% who screened positive to two items on gait and tremor; 28% who screened negative) | Self-administered (mailed) | 2 of 4 cardinal signs | Exam by neurologist | 87% 95% [83–99] |
28% 28% [23–33] |
2 (0–8; Mutch Screen) 1 (0–2; Modified 2-item screen) |
Non-responders to study were older and not all responders gave consent to have confirmatory exam (response bias) |
Abbreviations: GP – general practitioner; SEN – sensitivity; SPEC – specificity; SPECT – single photon emission computed tomography;
abstract presented at national meeting; values in brackets represent 95% confidence interval
Other screening questionnaires to identify parkinsonism have had variable accuracy. Mutch, et al [36] developed an 8-item screening questionnaire specific for parkinsonism. They found that the inclusion of two items that inquired about the presence of tremor and shuffling gait had a sensitivity of 91% and specificity of 92% when tested among a clinic-based sample of previously diagnosed cases of PD (Table 3). When this same screening questionnaire was subsequently tested among a community-based sample that included previously undiagnosed cases, the sensitivity decreased slightly to 87%, and the specificity dropped to 28% [37] (Table 4).
Nicoletti, et al [38] also developed a screening questionnaire to detect parkinsonism that included 4 items to assess symptoms and 5 physical tasks. This screening questionnaire, only tested once among a clinic-based sample of previously diagnosed PD and essential tremor cases and normal controls, demonstrated a sensitivity of 100% and specificity of 90%. Ishihara, et al [39] tested the performance of 6 items that assessed for symptoms of parkinsonism. This study used self-reported PD and medical records to establish a diagnosis of PD. Using any positive response as an indicator of a positive test, the screening questionnaire had a sensitivity of 96% and specificity of 56%. Exclusion of an item related to slower walking increased specificity to 76%, but decreased sensitivity slightly to 91%. All parkinsonism-specific screening questionnaires that were tested among a sample of previously diagnosed cases of PD are summarized in Table 3.
Table 4 summarizes the studies that evaluated the performance of parkinsonism screening questionnaires among a sample that included previously undiagnosed PD cases. This includes only three of the nine screening questionnaires identified in the review [30, 36, 40]. Both sensitivity and specificity estimates were, on average, lower than equivalent estimates for screening questionnaires tested among previously diagnosed cases.
Item Characteristics
One of the important characteristics that affected the sensitivity and specificity of the screening questionnaires was the number and content of items intended to detect symptoms of parkinsonism.
When only 3 items, not specific to parkinsonism, were included in a screening questionnaire sensitivity was moderate at 67% [29]. On the other hand, Chan, et al [40] tested a screening questionnaire with 11 parkinsonism-specific items, the most of any screen tested, and had a sensitivity of 100% in a sample of both previously diagnosed and undiagnosed PD cases. It is expected that increasing the item number will identify more cases of disease. However, specificity was low at 45% for this screening questionnaire, which will decrease its positive predictive value and utility.
Moreover, several of these screening questionnaires included between 5 and 16 physical assessments (e.g. checking for tone at elbow, evaluating gait) in addition to items that assessed for symptoms [13, 21, 23, 27, 29, 38]. These physical assessments were performed by both physician and non-medical examiners. Sensitivity for these screening questionnaires was high (98–100%). Specificity estimates for these screening questionnaires ranged from 58–90%. However, these screening questionnaires were all tested among individuals with previously diagnosed Parkinson’s disease which is easier to detect.
Sample Characteristics
The characteristics of the sample chosen for testing of the screening questionnaires also contributed to how well they performed. This was best illustrated by the 11 studies that evaluated the Tanner screen in multiple settings and demonstrated a wide range of sensitivities and specificities [30–35, 37, 41–44]. When the sample tested included only cases of previously diagnosed PD, sensitivity estimates were higher, ranging from 90–100% [30, 32–34, 37, 41, 43]. The three studies of community-based samples that included previously undiagnosed cases had lower sensitivity estimates of 48%, 61% and 75% [31, 34, 42]. Undiagnosed PD cases in the community are expected to be milder and at earlier PD stages which is harder to detect. Three other studies tested the Tanner screen in a community-based sample. However, these were limited by the fact that not all participants were examined to verify the diagnosis, which could falsely elevate sensitivity estimates [35, 37, 44]; most (72–94%) who screened positive were examined, but only between 5–42% of those who screened negative were examined. The inclusion of patients with moderate to advanced disease also resulted in higher sensitivity estimates than when only patients with mild disease were included [32].
When tested among people with co-morbid conditions, particularly other movement disorders or motor deficits, specificity estimates were low to moderate at 37, 58 and 78% [29, 30, 43]. Specificity was also lower in an older sample [33] and with lower literacy [34]. Specificity of the screening questionnaires was lowest when tested among a community sample, with four studies reporting specificity estimates less than 50% [34, 35, 37, 40]. However, three additional studies that were done among a community sample found specificities slightly higher at 63, 81 and 84% [31, 42, 44].
Administration of screening questionnaire
Lastly, the methods of screening questionnaire administration affected performance. Studies where a physician administered the screening questionnaire had comparatively higher sensitivities and specificities regardless of the screening questionnaire used [21, 28, 34]. Furthermore, one study showed that a general practitioner’s overall impression of PD demonstrated higher sensitivity and specificity than a screening questionnaire [44].
Discussion
Over the past 30 years, researchers have employed 9 different screening questionnaires to detect parkinsonism. The performance of these screening questionnaires varied based on the item characteristics of the questionnaire, the sample chosen to test the questionnaires, and method of questionnaire administration. There are several methodological considerations of these validation studies that will be discussed including the definition of a gold standard for parkinsonism diagnosis, adequacy of sample size and response bias.
The purpose of the screening questionnaires is to identify patients who would be clinically diagnosed with parkinsonism. Almost all studies (96%) used a clinical diagnosis based on physician examination, either at the time of study entry or through review of medical records, as the gold standard for parkinsonism diagnosis. However, it should be noted that the sensitivity of physician diagnosis for PD is not 100%. Among a group of movement disorder specialists who have followed patients for a number of years with the ability to revise their initial diagnosis, the sensitivity of a clinical diagnosis compared to pathological confirmation was 91% [45]. When less strict clinical criteria are used for diagnosis (presence of cardinal features without review of exclusionary criteria or supportive features), sensitivity is lower (76%) [46]. An imperfect gold standard can lead to either under or over-estimates of the performance of the screening questionnaire [47].
The majority of studies in this review (17/26) used the presence of 2 of 4 cardinal signs as the clinical criterion for the gold standard diagnosis of parkinsonism. Of these studies, 3 specified that one of the cardinal signs must be bradykinesia. Another 6 studies required additional supportive criteria (exclusionary or inclusionary). The remaining 9 studies did not specify the parkinsonism criteria that were used.
The ideal gold standard for screening studies of parkinsonism depends on the study’s intent. Although broad definitions for parkinsonism may include individuals with atypical parkinsonism or secondary parkinsonism, researchers have argued that for community-based cross-sectional PD studies the presence of at least two of resting tremor, bradykinesia or rigidity should be used as the case definition to capture mild and early cases of PD [48].
Another methodological concern is sample size. An adequate number of parkinsonism cases is necessary to provide precise estimates of sensitivity; this may be a particular challenge in community studies where the frequency of parkinsonism is low [27]. However, few reports specified the rationale for the sample size selected, and only four (14%) indicated the 95% confidence interval for the sensitivity estimates [22, 23, 37, 38]. Finally, bias due to non-response may have affected the results. In general, response rates were high (70–95%). However, some studies, particularly those that were not clinic-based, reported response rates of 46–64% [34, 37, 39]. If the characteristics of the non-responders were very different from responders, the calculated sensitivity and specificity may not be accurate estimates.
The number of studies that report very high sensitivity estimates of their respective screening questionnaires is high – 14 of 27 report sensitivities of 100%. These estimates need to be interpreted in the context of the above limitations. Twelve of these studies tested the screening questionnaire among previously diagnosed PD which will be easier to detect given the subjects’ knowledge of PD and associated symptoms. The remaining two studies, although tested among community-based samples, were limited due to the lack of examination of all negative cases [35] and lack of report of the 95% confidence interval for the sensitivity estimates [35, 40].
This systematic review has some limitations to consider. First, only English-language studies were included in the review. Also, as in any review of the medical literature, there is the potential for publication bias. That is, studies with low sensitivity or specificity estimates of new or previously developed screening instruments may not have been published; thus, the results from published studies may not adequately represent the results of all studies done on a particular instrument. Additionally, many of the screening questionnaires were only tested in one sample making interpretation of the results more difficult. While three of the included studies were only presented in abstract form [30, 31, 43], these studies reported the performance of a screening questionnaire developed by Tanner and colleagues that was also assessed in eight other published reports with similar findings.
In summary, several questionnaire-based screens have been developed and tested to identify parkinsonism. These screening questionnaires had high sensitivity when more parkinsonism-specific items and physical tasks were included, when administered by a physician, and when tested among older, more advanced and previously diagnosed PD subjects. These screens had high specificity when administered by a physician, and among individuals with few co-morbid conditions. However, in the detection of early parkinsonism, sensitivity estimates were moderate and specificity estimates were low. Given the overall low pre-test probability of PD, a moderate to low specificity will lead to a low positive predictive value for the test making it less useful for population-based studies. Unfortunately, identification of early cases is often the primary goal of population screening.
The combination of a screening questionnaire with an additional test may augment screening performance. For example, video-taped examinations based on the Unified Parkinson’s Disease Rating Scale reviewed and scored later by neurologists is an additional method to assess for parkinsonism in large-scale population-based or field studies at reduced cost, time and travel (of the neurologist) compared to traditional in-person examinations [49–51]. Advanced neuroimaging (Single Photon Emission Computed Tomography and Positron Emission Tomography), genetic testing, and screening for non-motor symptoms associated with early PD (e.g. olfactory dysfunction, Rapid Eye Movement sleep behavior disorder) are additional tests that could aid in the detection of early PD although these enhancements would add cost [52].
While direct comparisons of the published parkinsonism screening questionnaires are not possible given the variability in study designs, study samples, parkinsonism criteria used and method of gold standard evaluation, based on the available data we make some conclusions. There were three screening questionnaires [30, 36, 40] that have been tested in high quality studies where the samples included both previously diagnosed and undiagnosed parkinsonism, the authors clearly defined the criteria for parkinsonism and neurologists performed the gold standard evaluation. They all demonstrated moderate to high sensitivity, but low to moderate specificity in the detection of parkinsonism. Ultimately, the decision on the best method to screen for parkinsonism will depend on the target populations, goals of the study and resources available.
Acknowledgments
Funding sources for study: This study was partly funded by NIH grant K23 AG034236 and the Parkinson Council, Inc.
This study was partly funded by NIH grant K23 AG034236 and the Parkinson Council, Inc.
Footnotes
Financial disclosure/Conflict of interest related to manuscript: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–6. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.deRijk MC, Breteler MMB, Graveland GA, Ott A, Grobbee DE, vanderMeche FGA, et al. Prevalence of Parkinson’s disease in the elderly: The Rotterdam study. Neurology. 1995;45:2143–6. doi: 10.1212/wnl.45.12.2143. [DOI] [PubMed] [Google Scholar]
- 3.Kis B, Schrag A, Ben-Shlomo Y, Klein C, Gasperi A, Spoegler F, et al. Novel three-stage ascertainment method: prevalence of PD and parkinsonism in South Tyrol, Italy. Neurology. 2002;58:1820–5. doi: 10.1212/wnl.58.12.1820. [DOI] [PubMed] [Google Scholar]
- 4.Benito-Leon J, Bermejo-Pareja F, Rodriguez J, Molina JA, Gabriel R, Morales JM. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord. 2003;18:267–74. doi: 10.1002/mds.10362. [DOI] [PubMed] [Google Scholar]
- 5.Tison F, Dartigues JF, Dubes L, Zuber M, Alperovitch A, Henry P. Prevalence of Parkinson’s disease in the elderly: a population study in Gironde, France. Acta Neurol Scand. 1994;90:111–5. doi: 10.1111/j.1600-0404.1994.tb02689.x. [DOI] [PubMed] [Google Scholar]
- 6.Melcon MO, Anderson DW, Vergara RH, Rocca WA. Prevalence of Parkinson’s disease in Junin, Buenos Aires Province, Argentina. Mov Disord. 1997;12:197–205. doi: 10.1002/mds.870120210. [DOI] [PubMed] [Google Scholar]
- 7.Schoenberg BS, Anderson DW, Haerer AF. Prevalence of Parkinson’s disease in the biracial population of Copiah County, Mississippi. Neurology. 1985;35:841–5. doi: 10.1212/wnl.35.6.841. [DOI] [PubMed] [Google Scholar]
- 8.Morgante L, Rocca WA, Dirosa AE, Dedomenico P, Grigoletto F, Meneghini F, et al. Prevalence of Parkinson’s disease and other types of parkinsonism - a door-to-door survey in 3 Sicilian municipalities. Neurology. 1992;42:1901–7. doi: 10.1212/wnl.42.10.1901. [DOI] [PubMed] [Google Scholar]
- 9.Dotchin C, Msuya O, Kissima J, Massawe J, Mhina A, Moshy A, et al. The prevalence of Parkinson’s disease in rural Tanzania. Mov Disord. 2008;23:1567–672. doi: 10.1002/mds.21898. [DOI] [PubMed] [Google Scholar]
- 10.Schoenberg BS. Clinical neuroepidemiology in developing countries. Neuroepidemiology. 1982;1:137–42. [Google Scholar]
- 11.Osuntokun BO, Schoenberg BS, Nottidge VA, Adeuja A, Kale O, Adeyefa A, et al. Research protocol for measuring the prevalence of neurologic disorders in developing countries. Neuroepidemiology. 1982;1:143–53. [Google Scholar]
- 12.Bermejo F, Gabriel R, Vega S, Morales JM, Rocca WA, Anderson DW. Problems and issues with door-to-door, two-phase surveys: an illustration from central Spain. Neuroepidemiology. 2001;20:225–31. doi: 10.1159/000054794. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Protocol for measuring the prevalence of neurological disorders in developing countries. World Health Organization; 1981. [Google Scholar]
- 14.Osuntokun BO, Adeuja A, Schoenberg BS, Bademosi O, Nottidge VA, Olumide A, et al. Neurological disorders in Nigerian Africans: a community-based study. Acta Neurol Scand. 1987;75:13–21. doi: 10.1111/j.1600-0404.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- 15.Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of Parkinson’s disease in the Parsi community of Bombay, India. Arch Neurol. 1988;45:1321–3. doi: 10.1001/archneur.1988.00520360039008. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez-del-Olmo MC, Schoenberg BS, Portera-Sanchez A. Prevalence of neurological diseases in Madrid, Spain. Neuroepidemiology. 1989;8:43–7. doi: 10.1159/000110164. [DOI] [PubMed] [Google Scholar]
- 17.Al Rajeh S, Bademosi O, Ismail H, Awada A, Dawodu A, Al-Freihi H, et al. A community survey of neurological disorders in Saudi Arabia: The Thugbah Study. Neuroepidemiology. 1993;12:164–78. doi: 10.1159/000110316. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Schoenberg BS, Wang C, Cheng X, Rui D, Bolis CL, et al. A prevalence survey of Parkinson’s disease and other movement disorders in the People’s Republic of China. Arch Neurol. 1985;42:655–7. doi: 10.1001/archneur.1985.04060070045013. [DOI] [PubMed] [Google Scholar]
- 19.Bharucha NE, Bharucha EP, Dastur HD, Schoenberg BS. Pilot survey of the prevalence of neurologic disorders in the Parsi community of Bombay. Am J Prev Med. 1987;3:293–9. [PubMed] [Google Scholar]
- 20.Das SK, Biswas A, Roy T, Banerjee TK, Mukherjee CS, Raut DK, et al. A random sample survey for prevalence of major neurological disorders in Kolkata. Indian J Med Res. 2006;124:163–72. [PubMed] [Google Scholar]
- 21.Meneghini F, Rocca WA, Anderson DW, Grigoletto F, Morgante L, Reggio A, et al. Validating screening instruments for neuroepidemiologic surveys: experience in Sicily. J Clin Epidemiol. 1992;45:319–31. doi: 10.1016/0895-4356(92)90033-j. [DOI] [PubMed] [Google Scholar]
- 22.Bergareche A, De la Puente E, Lopez de Munain A, Sarasqueta C, de Arce A, Poza JJ, et al. Prevalence of Parkinson’s disease and other types of parkinsonism. J Neurol. 2004;251:340–5. doi: 10.1007/s00415-004-0333-3. [DOI] [PubMed] [Google Scholar]
- 23.Bower JH, Howlett W, Maro VP, Wangai H, Sirima N, Reyburn H. A screening instrument to measure the prevalence of neurological disability in resource-poor settings. Neuroepidemiology. 2009;32:313–20. doi: 10.1159/000209265. [DOI] [PubMed] [Google Scholar]
- 24.Gourie-Devi M, Gururaj G, Satishchandra P, Subbakrishna DK. Neuroepidemiological pilot survey of an urban population in a developing country. Neuroepidemiology. 1996;15:313–20. doi: 10.1159/000109921. [DOI] [PubMed] [Google Scholar]
- 25.Anderson DW, Schoenberg BS, Haerer AF. Racial differences in the prevalence of major neurological disorders. Neuroepidemiology. 1982;1:17–30. [Google Scholar]
- 26.Anderson DW, Melcon MO, Vergara RH. Methods for a prevalence survey of neurological disorders in Junin, Buenos Aires, Argentina. Neuroepidemiology. 1995;14:110–22. doi: 10.1159/000109786. [DOI] [PubMed] [Google Scholar]
- 27.Maggi S, Zucchetto M, Grigoletto F, Baldereschi M, Candelise L, Scarpini E, et al. The Italian Longitudinal Study on Aging (ILSA): design and methods. Aging Clin Exp Res. 1994;6:464–73. doi: 10.1007/BF03324279. [DOI] [PubMed] [Google Scholar]
- 28.The Italian Longitudinal Study on Aging Working Group. Prevalence of chronic disease in older Italians: comparing self-reported and clinical diagnoses. Int J Epidemiol. 1997;26:995–1002. doi: 10.1093/ije/26.5.995. [DOI] [PubMed] [Google Scholar]
- 29.Chang SF, Su CL, Chen ZY, Lee CS, Chen RC. Neuroepidemiological survey in Ilan, Taiwan (NESIT)(1): Validation of screening instrument in an out-patient department population. Acta Neurol Taiwan. 1996;5:105–10. [Google Scholar]
- 30.Tanner CM, Gilley DW, Goetz CG. A brief screening questionnaire for parkinsonism. Ann Neurol. 1990;28:267–8. [Google Scholar]
- 31.Tanner CM, Ellenberg JH, Mayeux R, Ottman R, Langston W. A sensitive and specific screening method for Parkinson’s disease (PD) Neurology. 1994;55(Suppl 2):A136. [Google Scholar]
- 32.Duarte J, Claveria LE, De Pedro-Cuesta J, Sempere AP, Coria F, Calne DB. Screening Parkinson’s disease: a validated questionnaire of high specificity and sensitivity. Mov Disord. 1995;10:643–9. doi: 10.1002/mds.870100518. [DOI] [PubMed] [Google Scholar]
- 33.Pramstaller PP, Falk M, Schoenhuber R, Poewe W. Validation of a mail questionnaire for parkinsonism in two languages (German and Italian) J Neurol. 1999;246:79–86. doi: 10.1007/s004150050312. [DOI] [PubMed] [Google Scholar]
- 34.Sarangmath N, Rattihalli R, Ragothaman M, Gopalkrishna G, Doddaballapur S, Louis ED, et al. Validity of a modified Parkinson’s disease screening questionnaire in India. Mov Disord. 2005;30:1550–6. doi: 10.1002/mds.20576. [DOI] [PubMed] [Google Scholar]
- 35.Barbosa MT, Caramelli P, Maia DP, Cunningham MCQ, Guerra HL, Lima-Costa MF, et al. Parkinsonism and Parkinson’s disease in the elderly: A community-based survey in Brazil (the Bambui study) Mov Disord. 2006;21:800–8. doi: 10.1002/mds.20806. [DOI] [PubMed] [Google Scholar]
- 36.Mutch W, Smith WC, Scott RF. A screening and alerting questionnaire for parkinsonism. Neuroepidemiology. 1991;10:150–6. doi: 10.1159/000110261. [DOI] [PubMed] [Google Scholar]
- 37.Taylor KSM, Counsell CE, Harris CE, Gordon JC. Screening for undiagnosed parkinsonism in people aged 65 years and over in the community. Parkinsonism Relat Disord. 2006;12:79–85. doi: 10.1016/j.parkreldis.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Nicoletti A, Salemia G, Morgante L, Le Pira F, Epifanio A, Reggio A, et al. A screening instrument for a Sicilian neuroepidemiological survey in the elderly. Arch Gerontol Geriatr. 2004;38:37–44. doi: 10.1016/s0167-4943(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 39.Ishihara LS, Khaw KT, Luben R, Bingham S, Welch A, Day N, et al. Self-reported parkinsonian symptoms in the EPIC-Norfolk cohort. BMC Neurol. 2005;5:15. doi: 10.1186/1471-2377-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan DKY, Hung WT, Wong A, Hu E, Beran RG. Validating a screening questionnaire for parkinsonism in Australia. J Neurol Neurosurg Psychiatry. 2000;69:117–20. doi: 10.1136/jnnp.69.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocca WA, Maraganore DM, McDonnell SK, Schaid DJ. Validation of a telephone questionnaire for Parkinson’s disease. J Clin Epidemiol. 1998;51:517–23. doi: 10.1016/s0895-4356(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 42.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Gen. 1999;88:539–43. [PubMed] [Google Scholar]
- 43.Tan LC, Tan AK, Saw SM, Hong CY, Lee WL, Venketasubramanian N. Validation of a screening questionnaire for Parkinson’s disease in Singapore. Mov Disord. 2002;17:S139–40. [Google Scholar]
- 44.Hoglinger GU, Rissling I, Metz A, Ries V, Heinermann A, Prinz H, et al. Enhancing recognition of early Parkinsonism in the community. Mov Disord. 2004;19:505–12. doi: 10.1002/mds.20033. [DOI] [PubMed] [Google Scholar]
- 45.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 46.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of the clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathologic study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyko EJ, Alderman BW, Baron AE. Reference test errors bias the evaluation of diagnostic tests for ischemic heart disease. J Gen Intern Med. 1988;3:476–81. doi: 10.1007/BF02595925. [DOI] [PubMed] [Google Scholar]
- 48.de Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MM, Maraganore DM, et al. A population perspective on diagnostic criteria for Parkinson’s disease. Neurology. 1997;48:1277–81. doi: 10.1212/wnl.48.5.1277. [DOI] [PubMed] [Google Scholar]
- 49.Racette BA, Tabbal SD, Jennings D, Good LM, Perlmutter JS, Evanoff BA. A rapid method for mass screening for parkinsonism. Neurotoxicology. 2006;27:357–61. doi: 10.1016/j.neuro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Louis ED, Levy G, Cote LJ, Mejia H, Fahn S, Marder K. Diagnosing Parkinson’s disease using videotaped neurological examinations: validity and factors that contribute to incorrect diagnoses. Mov Disord. 2002;17:513–7. doi: 10.1002/mds.10119. [DOI] [PubMed] [Google Scholar]
- 51.Camicioli R, Grossman SJ, Spencer PS, Hudnell K, Anger WK. Discriminiating mild parkinsonism: methods for epidemiological research. Mov Disord. 2001;16:33–40. doi: 10.1002/1531-8257(200101)16:1<33::aid-mds1014>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 52.Stephenson R, Siderowf A, Stern MB. Premotor Parkinson’s disease: clinical features and detection strategies. Mov Disord. 2009;24:S665–70. doi: 10.1002/mds.22403. [DOI] [PubMed] [Google Scholar]