Abstract
Background/Aim
Elevated serum immunoglobulin G4 (sIgG4) is a feature of autoimmune pancreatitis (AIP) and IgG4-associated cholangitis (IAC); a >2-fold increase in sIgG4 is considered highly specific for these disorders. Many patients with IAC present with biliary strictures and obstructive jaundice, making cholangiocarcinoma (CCA) an important differential diagnosis. We determined the value of sIgG4 in distinguishing IAC from CCA.
Methods
sIgG4 levels were measured in a test cohort of 126 CCA and 50 IAC patients. The results were confirmed in a validation cohort of 161 CCA and 47 IAC patients.
Results
Of the 126 CCA patients in the test cohort, 17 (13.5%) had elevated sIgG4 (>140 mg/dL) and 4 (3.2%) had >2-fold (>280 mg/dL) increase. Primary sclerosing cholangitis (PSC) was present in 31/126 CCA patients, of whom 7 (22.6%) had elevated sIgG4 and 2 (6.5%) had >2-fold elevation. Of the 50 IAC patients, 39 (78.0%) had elevated sIgG4 and 25 (50.0%) had >2-fold increase. The results in the validation cohort were consistent with those of the test cohort.
Conclusion
Although elevated sIgG4 levels are characteristic of IAC, some patients with CCA, particularly with PSC, have elevated IgG4 levels, including a small percentage with a more than 2-fold increase in IgG4. Therefore sIgG4 elevation alone does not exclude the diagnosis of CCA. Depending on the prevalence of the two diagnoses, the use of a 2-fold cut-off for sIgG4 may not reliably distinguish IAC from CCA. At a cut-off of 4 times the upper limit of normal, sIgG4 is 100% specific for IAC.
Keywords: Immunoglobulin G4, IgG4-associated cholangitis, IgG4-related systemic disease, Autoimmune pancreatitis, Primary sclerosing cholangitis
Introduction
IgG4-related systemic disease (ISD) is a multi-system fibroinflammatory syndrome characterized by elevated levels of serum immunoglobulin G subclass 4 (sIgG4) and a multifocal IgG4-rich lymphoplasmacytic infiltration of affected organs. The condition is generally associated with intense sclerosis and responds favorably to glucocorticoids (1–3). The prototype of ISD is autoimmune pancreatitis (AIP), which by virtue of its clinical and radiologic characteristics (pancreatic mass, painless jaundice, weight loss, and diabetes) can mimic pancreatic adenocarcinoma (4, 5). Other organs that can be involved in this condition include the biliary tree, salivary glands, retroperitoneum, lymph nodes, kidneys and aorta (2, 6, 7). Both the pancreatic and extrapancreatic variants of ISD respond well to steroid therapy (8). In 2001 it was reported that an elevated sIgG4 level is highly sensitive and specific for AIP (1, 9). Since then sIgG4 has seen wide use in cases of suspected AIP (or ISD) and has become part of criteria for the diagnosis of AIP, included in both the consensus Asian diagnostic criteria for autoimmune pancreatitis and the Mayo Clinic HISORt criteria (Histology, Imaging, Serum IgG4, Other organ involvement and Response to therapy) (3, 10, 11).
IgG4-associated cholangitis (IAC) is the biliary manifestation of ISD which is commonly found in association with AIP and presents with biliary strictures and obstructive jaundice that may mimic primary sclerosing cholangitis (PSC) or cholangiocarcinoma (CCA). IAC may also occur without the classic radiologic findings of pancreatic involvement seen in AIP, which can make it difficult to distinguish between IAC and PSC or CCA (12–18).
The reliability of sIgG4 to distinguish between IAC and other pancreaticobiliary diseases has recently been questioned. An elevated sIgG4 has been reported in 9% of patients with PSC (19). Histologic and immunohistochemical examination of explanted livers from patients who underwent liver transplantation for PSC showed the presence of elevated sIgG4 in 22% of cases and IgG4 positive plasma cell infiltrates in the hilar regions of 23% of the explanted livers. Further, the presence of IgG4 positive plasma cell infiltrates was associated with a more aggressive clinical course including a significantly shorter time to transplant, a lower likelihood of cirrhosis at the time of transplant, and a greater than 3-fold higher risk of PSC recurrence after transplant (20). These findings raise the possibility that IgG4 positive plasma cell infiltrates define a distinct subtype of PSC. Of particular interest, 17% of the PSC cases with IgG4 positive plasma cell infiltrates were associated with cholangiocarcinoma, and 18% of non-PSC related cholangiocarcinomas showed moderate IgG4 positive plasma cell infiltrates. It has also been shown that histologic examination reveals higher numbers of IgG4 positive cells in IAC than in PSC (6). Although the sIgG4 was not assessed, another recent study has shown positive tissue staining for IgG4 in 9 of 26 (35%) liver tissue specimens from patients with autoimmune hepatitis (21).
Regarding the utility of IgG4 in distinguishing ISD from cancer, between 7 to10% of pancreatic cancer patients have been found to have elevated sIgG4 (22, 23), but the utility of the sIgG4 in distinguishing IAC from CCA has not been examined to date. Several studies have reported cases of IAC (either isolated or in association with AIP) mimicking CCA on presentation. Unfortunately, a number of these patients were treated with surgical resections that could have been avoided if the correct diagnosis of IAC had been made (11, 13–18, 24). On the other hand, treatment of patients suspected of having IAC with glucocorticoids when the actual underlying condition is CCA may not only delay accurate diagnosis and timely intervention, but may result in fatal outcomes. It is therefore important to develop minimally invasive methods for distinguishing IAC from other pancreaticobiliary diseases, particularly CCA.
Elevation of the sIgG4 remains an essential element in the HISORt criteria, but whether the serum (or tissue) IgG4 level can distinguish IAC from CCA (e.g. in a patient with an isolated biliary stricture) is not yet known. Therefore the principal aim of this study was to determine the utility of the sIgG4 in distinguishing IAC from CCA. The following questions were addressed: (1) Is the sIgG4 level discriminatory between IAC and CCA? (2) At what sIgG4 value can IAC be reliably distinguished from CCA (without the benefit or harm of an invasive histologic diagnosis)? (3) Is the ability of the sIgG4 to distinguish IAC from CCA affected by the concomitant existence of PSC? To answer these questions, we (1) compared sIgG4 levels in a test cohort of 126 patients with CCA and 50 patients with IAC as well as in a validation cohort of 161 patients with CCA and 47 patients with IAC; (2) compared the demographic and serologic characteristics of patients with CCA without PSC (CCA−PSC), CCA with concomitant PSC (CCA+PSC), and IAC; and (3) examined whether there is a sIgG4 threshold at which CCA (with or without PSC) could be distinguished from IAC with relatively high specificity and sensitivity. The secondary aim of this study is to determine the clinical significance of sIgG4 in CCA. The relationship between sIgG4 and CA19-9 levels and the association of sIgG4 with survival of CCA patients were investigated in both cohorts.
Materials and Methods
Study Subjects
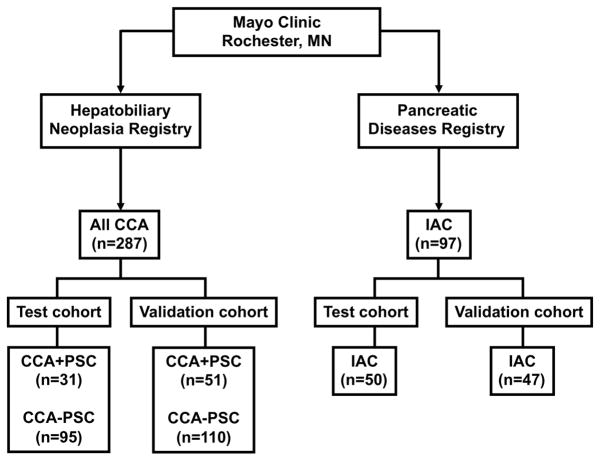
The protocol for this study was approved by the Mayo Clinic Institutional Review Board. Patients referred to the Mayo Clinic Hepatobiliary Neoplasia Clinic between March 2003 and February 2011 and subsequently diagnosed with CCA, were included (Figure 1). A total of 287 CCA patients were divided into 2 separate cohorts. The test cohort included 126 CCA patients enrolled between March 2003 and June 2006. An additional 161 CCA patients enrolled between July 2006 and February 2011 served as a validation cohort. The diagnosis of CCA was determined by histology, standard imaging criteria, or clinical course. The final diagnosis, age, gender, and clinical presentation, diagnosis and last follow-up dates, status at the last follow-up visit, serum IgG4 and CA 19-9 levels were abstracted from the clinical record.
Figure 1. Outline of the Study Groups.
All CCA = All cases of Cholangiocarcinoma; PSC = Primary Sclerosing Cholangitis; CCA+PSC (CCA with concomitant PSC); CCA−PSC (CCA without PSC); IAC (IgG4-Associated Cholangitis).
A total of 97 patients with AIC, as determined by the HISORt criteria, came from a prospective database of ISD cases maintained at Mayo Clinic Rochester (12). Of these, 50 patients who were seen at Mayo Clinic between January 1989 and October 2006 were included in the test cohort. At the time of last follow-up in March 2011, these 50 IAC patients had follow-up for a mean duration of 58.6 months (range 11.5–265.9 months) after initial presentation and a mean duration of 45.0 months (range 1.5–84.9 months) after initiation of treatment. None of the IAC patients in the test cohort developed clinical evidence of CCA during follow-up. For the validation cohort, 47 IAC patients who were seen at Mayo Clinic between November 2006 and February 2011 were included; none of these patients developed CCA during follow-up. The mean (range) follow-up was 20.6 (0.5–62.1) months after initial presentation and 19.3 (0.0–46.1) months after initiation of treatment.
Serology
Serum concentrations of IgG subclass 4 were measured by automated nephelometry (Behring Nephelometer II; Dade Behring, Inc, Newark, DE)(12).
Histology and Tissue IgG4 Immunostaining
Tissue immunostaining using monoclonal anti-human IgG4 antibody was performed as previously reported (3). The number of IgG4-positive plasma cells per high-power field (hpf) was counted in each specimen (Nikon E 600, field diameter 0.625 mm; Nikon, Tokyo, Japan). Moderate (11–30 cells/hpf) to severe (>30 cells/hpf) infiltration with IgG4-positive cells in the presence of characteristic histology was considered diagnostic of AIP. Scores were assigned as negative (0–1) or positive (2–3). All histologic specimens were reviewed by a single pathologist (L.Z). IAC was diagnosed histologically from a resection specimen or core biopsy if there was a lymphoplasmacytic infiltrate within and around bile ducts with associated obliterative phlebitis and storiform fibrosis leading to sclerosis of the bile duct (25–27).
Imaging
Available cholangiograms, computerized tomography, magnetic resonance imaging and magnetic resonance cholangiopancreatography scans from the 31 patients with CCA−PSC in the test cohort were reviewed by a single radiologist (N.T.) for features of AIP.
Statistical analysis
Data were analyzed using JMP version 8.0.0 (SAS Institute Inc., Cary, NC). Differences between groups were evaluated by using the Chi-square or Fisher’s exact test for qualitative variables and the rank sum test for quantitative variables. Receiver operator characteristic (ROC) curves were used to judge the diagnostic utility of sIgG4 levels. IgG4 values are represented as median as well as mean ± standard error of mean (SEM), and a two-tailed P value of less than 0.05 was considered significant.
Spearman’s Correlation coefficient analysis was used to determine the relationship between CA19-9 and sIgG4 level in CCA patients. Survival of CCA patients was defined as the time from diagnosis to death or last follow up visit date. Median survival of CCA patients with elevated IgG4 > ULN was compared to that of CCA patients with normal sIgG4 levels by the Kaplan Meier method.
Results
Elevated sIgG4 occurs in 13.5% of test cohort and 12.4% of validation cohort patients with cholangiocarcinoma
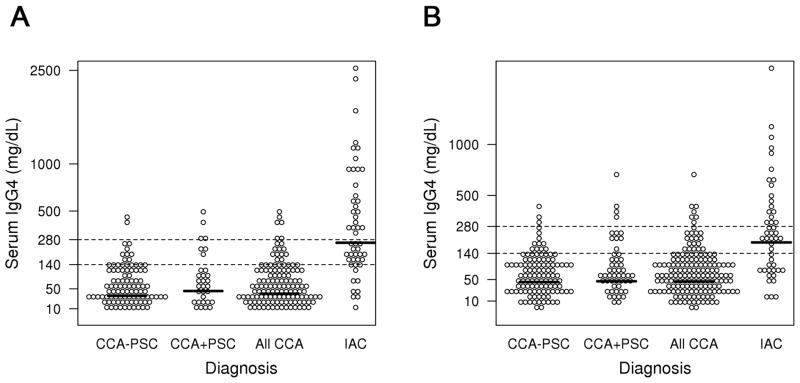
For the test cohort, we used frozen serum collected at the time of diagnosis from 126 patients with CCA (82 hilar or extrahepatic CCA and 44 intrahepatic CCA). We compared the sIgG4 level and other clinical and laboratory characteristics in these patients to those of 50 patients with known IAC. As expected, the mean ± SEM and the median sIgG4 levels in the 50 IAC patients (475.8 ± 77.2, median 261.0 mg/dL) were significantly higher than the levels in the 126 patients with CCA (irrespective of PSC status) (68.6 ± 7.4, median 37.5 mg/dL)(p<0.0001, rank sum test) (Table 1A). The individual sIgG4 levels in each group are shown in the scatter plot (Figure 2). In the test cohort of 126 CCA patients (54 females, 72 males), 17 (13.5%) had a sIgG4 greater than 1xULN (i.e. >140 mg/dL), while 4 (3.2%) had a value greater than 2xULN (>280 mg/dL). None of the CCA patients had a sIgG4 value greater than 4xULN (>560 mg/dL). Of the sera from the 50 IAC patients (9 females, 41 males), 39 (78.0%) had an IgG4 level greater than 1xULN and 25 (50.0%) had an IgG4 value over 2xULN. Table 1B shows the results of sIgG4 levels in the validation cohort of 161 CCA and 47 IAC patients. In summary, the mean ± SEM and median sIgG4 levels and the proportion of patients who had sIgG4 levels greater than 1xULN and 2xULN (140 and 280 mg/dL) in CCA and IAC patients were similar to the findings in the test cohort, except that 1 of the 51 (2.0%) CCA+PSC patients in the validation cohort had a sIgG4 over 4xULN (>560 mg/dL).
Table 1.
Serum IgG4 in the CCA and IAC groups in the test and validation cohorts
| A. Test Cohort | ||||
|---|---|---|---|---|
| CCA − PSC | CCA + PSC | All CCA | IAC | |
| Number | 95 | 31 | 126 | 50 |
| Age, Mean ± SD | 61.2 ± 13.5 | 46.5 ± 13.7 | 57.6 ± 14.4 | 60.3 ± 16.0 |
| Gender | 43F, 52M | 11F, 20M | 54F, 72M | 9F, 41M |
| Mean IgG4 ± SEM | 61.4 ± 7.2* | 90.7 ± 20.2* | 68.6 ± 7.4 | 475.8 ± 77.2 |
| Median IgG4 | 32.1 | 44.1 | 37.5 | 261.0 |
| Range of IgG4 | 3.4 – 421.0 | 3.9 – 450.0 | 3.4 – 450 | 6.0 – 2490.0 |
| % IgG4 >140mg/dl | 10 (10.5%) | 7 (22.6%) | 17 (13.5%) | 39 (78.0%) |
| % IgG4 >280mg/dl | 2 (2.1%) | 2 (6.5 %) | 4 (3.2%) | 25 (50.0%) |
| B. Validation Cohort | ||||
| CCA − PSC | CCA + PSC | All CCA | IAC | |
| Number | 110 | 51 | 161 | 47 |
| Age, Mean ± SD | 66.4 ± 11.7 | 52.1 ± 11.9 | 61.9 ± 13.5 | 55.3 ± 17.4 |
| Gender | 42F, 68M | 16F, 35M | 58F, 103M | 13F, 34M |
| Mean IgG4 ± SEM | 64.6 ± 6.3¶ | 92.5 ± 16.2¶ | 73.4 ± 6.7 | 277.1 ± 55.2 |
| Median IgG4 | 43.7 | 45.7 | 45.1 | 190.5 |
| Range of IgG4 | 0.3 – 383.0 | 3.0 – 620.0 | 0.3 – 620.0 | 7.0 – 905.0 |
| % IgG4 >140mg/dl | 10 (9.1%) | 10 (19.6%) | 20 (12.4%) | 30 (63.8%) |
| % IgG4 >280mg/dl | 3 (2.7%) | 4 (7.8%) | 7 (4.3%) | 16 (34.0%) |
P=0.18;
P=0.11
All CCA = All cases of Cholangiocarcinoma; PSC = Primary Sclerosing Cholangitis; CCA+PSC (CCA with concomitant PSC); CCA−PSC (CCA without PSC); IAC (IgG4-Associated Cholangitis)
Figure 2. Scatter plots of serum IgG4 in the different study groups in the test (Figure 2A) and validation cohort (Figure 2B).
Cut-offs of 1xULN (>140mg/dL) and 2xULN (>280mg/dL) are shown as dotted lines. The y-axis has a square root scale.
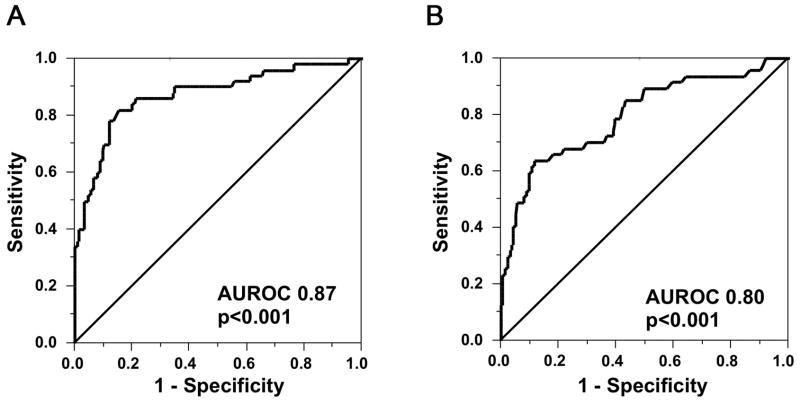
To help determine the ability of IgG4 to reliably distinguish IAC from CCA, we used receiver operating characteristic (ROC) curves to identify the appropriate sIgG4 cut offs for the two disease entities (Figure 3A). We found that in the test cohort a sIgG4 of over 2xULN (>280 mg/dL) yields a specificity of 97.0% in distinguishing IAC from CCA, while a sIgG4 of over 4xULN (>560 mg/dL) yields a specificity of 100%, albeit at a decreased sensitivity of 26%. Compared to the results of the test cohort, in the validation cohort the cut-off of sIgG4 of 2xULN was less sensitive (34% vs 50%) but still highly specific (96% vs 97%). The performance of sIgG4 in discriminating IAC from all CCA in the test (T) and validation (V) cohorts is summarized in Table 2.
Figure 3. Receiver operating characteristic (ROC) curves of IgG4 for diagnosis of IAC versus All CCA patients in the test and validation cohorts.
ROC curve of Sensitivity plotted against 1-Specificity of IgG4 in 50 IAC cases and All 126 CCA controls in the test cohort (A) and in 47 IAC and All 161 CCA controls in the validation cohort (B). The areas under the ROC curve are 0.87 and 0.80, respectively.
Table 2.
Performance of Serum IgG4 in discriminating IAC from All CCA in the Test (T) and Validation (V) cohorts
| IgG4 Cutoff | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Cohort | T | V | T | V | T | V | T | V |
| >140 (1x ULN) | 78 | 64 | 87 | 88 | 70 | 60 | 91 | 89 |
| >280 (2x ULN) | 50 | 34 | 97 | 96 | 86 | 70 | 83 | 83 |
| >560 (4x ULN) | 26 | 17 | 100 | 99 | 100 | 89 | 77 | 80 |
All CCA (All cases of Cholangiocarcinoma); IAC (IgG4- Associated Cholangitis); PPV (Positive Predictive Value); NPV (Negative Predictive Value); ULN (Upper Limit of Normal); IgG4 unit = mg/dL
Patients with cholangiocarcinoma secondary to PSC have a higher frequency of elevated sIgG4 than patients with cholangiocarcinoma without PSC
Of the 126 CCA patients in the test cohort, 31 had associated PSC (CCA+PSC) and the remaining 95 did not have PSC (CCA−PSC). Twenty-seven (87%) of the 31 CCA+PSC patients had hilar or extrahepatic CCA, while 4 (13%) had intrahepatic CCA. One of the CCA+PSC patients had an overlap syndrome of PSC with autoimmune hepatitis and a normal serum IgG4 of 7.7 mg/dL. Seven (22.6%) of the 31 CCA+PSC patients had sera with elevated IgG4 and 2 (6.5%) of these had a sIgG4 >2xULN; as compared with 10 (10.5%) and 2 (2.1%) respectively, for CCA−PSC patients (p = 0.13 and 0.25, Fisher’s exact test). The sIgG4 levels (mean ± SEM) were higher in the CCA+PSC group than in the CCA−PSC group, but the difference did not reach statistical significance (90.7 ± 20.2 vs 61.4 ± 7.2, P=0.18) (Table 1A). In the validation cohort of 161 CCA patients, the proportion of CCA+PSC patients with IgG4 levels >1xULN and >2xULN were consistent with those in the test cohort and are summarized in Table 1B. In this cohort also, CCA+PSC patients tended to have higher sIgG4 levels compared to CCA−PSC patients, but the difference did not reach statistical significance (92.5 ± 16.2 vs 64.6 ± 6.3, P=0.11).
A proportion of cholangiocarcinomas, particularly from PSC patients, stain positive for IgG4, but none have the classic imaging features of AIP/IAC
Since biliary infiltration with IgG4-positive (IgG4+) plasma cells is one of the hallmarks of IAC, we examined the correlation between the sIgG4 level and the presence of biliary IgG4+ plasma cells in CCA patients in the test cohort with a sIgG4 above the upper limit of normal (17/126). Paraffin-embedded tissue was available from 13 of the 17 CCA patients (7 CCA+PSC, 6 CCA−PSC). Immunostaining for IgG4 showed IgG4 positive staining in 5 of the 13 tissues (41.7%) of which 4 of the 5 (80%) were CCA+PSC patients. We compared IgG4 tissue staining in this high-serum-IgG4 subgroup with tissue stains from 8 randomly selected low-serum-IgG4 CCA patients (all CCA+PSC). Only 1 out of the 8 (12.5%) low sIgG4 CCA patients had tissue IgG4 positivity. Finally, we evaluated the radiologic features of all 31 CCA+PSC patients in the test cohort against the classic imaging findings of AIP/AIC. While none of the cases had the typical imaging appearance, images from 3 of the patients were suspicious for AIP/IAC.
There is no significant difference in sIgG4 levels in patients with hilar, middle or distal extrahepatic CCA vs intrahepatic CCA
Of the 126 CCA patients in the test cohort, all 4 CCA patients with sIgG4 levels over 280 mg/dl had hilar CCA. Four of the 47 (8.5%) patients with intrahepatic CCA had sIgG4 levels over 140 mg/dl, compared to 11 of 62 (17.7%) patients with hilar CCA (P=0.26, Fisher’s Exact Test). Two of 17 (11.8%) patients with middle or distal extrahepatic CCAs had sIgG4 levels over 140 mg/dl (P=0.65 compared to patients with intrahepatic CCA and P=0.72 compared to patients with hilar CCA).
Correlation between sIgG4 and CA 19-9 level and survival in CCA patients
Table 3 summarizes the CA 19-9 levels and correlation coefficient of CA 19-9 and sIgG4 levels of CCA patients in the test and validation cohorts. The mean CA 19-9 levels were not significantly different between those with sIgG4 >1xULN and those with normal sIgG4 levels in both cohorts. Further, there was no correlation between sIgG4 and CA19-9 levels in both the All CCA patient group and the subgroup of CCA patients with elevated sIgG4 levels.
Table 3.
Level of CA 19-9 and correlation between sIgG4 and CA 19-9 level in CCA patients
| Test cohort (n=126*) | Validation cohort (n=161**) | Combined (n=287***) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CA 19-9¶ | sIgG4 >ULN (n=16) | sIgG4 ≤ULN (n=104) | All (n=120) | sIgG4 >ULN (n=20) | sIgG4 ≤ ULN (n=139) | All (n=159) | sIgG4 >ULN (n=36) | sIgG4 ≤ULN (n=243) | All (n=279) |
| Mean | 441 | 11,332† | 9880 | 8022 | 1980† | 2740 | 4653 | 5982† | 5811 |
| SEM | 191 | 4,850 | 4214 | 5408 | 669 | 890 | 3038 | 2126 | 1891 |
| Median | 53 | 89 | 88 | 161 | 188 | 185 | 97 | 111 | 111 |
| Range | 13-2302 | 1-344,850 | 1-344,850 | 7-101,309 | 0-74,618 | 0-101,309 | 7-101,309 | 0-344,850 | 0-344,850 |
| Correlation Coefficient | 0.194† | −0.016† | 0.004† | −0.307† | −0.093† | −0.049† | 0.006† | −0.056† | −0.027† |
Numbers of patients with missing data were 6, 2 and 8, respectively
normal value is less than 55 U/mL
P>0.05
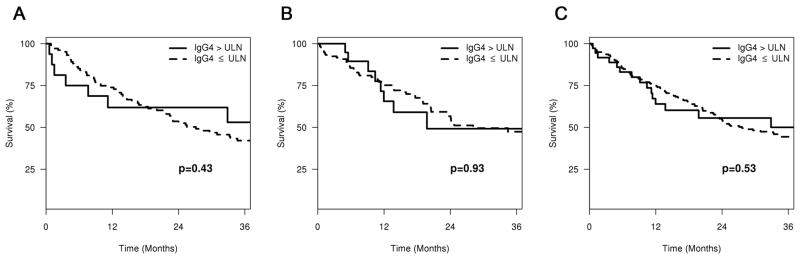
The median survival of all CCA patients with elevated sIgG4 over 1xULN was longer than for patients with normal sIgG4 levels, however, the difference did not reach statistical significance (97.1 vs 27.1 months, p=0.43, 19.8 vs 28.1 months, p=0.93 and 97.1 vs 27.6 months, p=0.53, for the test, validation and combined cohorts, respectively). Survival curve between the both groups were shown in Figure 4.
Figure 4.
Survival curves of CCA patients with normal and elevated sIgG4 levels in the test (Figure 4A), validation (Figure 4B), and combined (Figure 4C) cohorts
Discussion
Elevation of the sIgG4 is the best-known marker for AIP and IAC. Among the IgG4 subclasses, IgG4 makes up about 5% of the total IgG and is known for its low target antigen affinity and inability to bind C1q complement (28). Before high sIgG4 concentrations were associated with AIP, similar findings were made in a few pathological conditions, including atopic dermatitis, Bancroftian filariasis and in pemphigus vulgaris and foliaceus (9, 29–31). Since the discovery of high sIgG4 levels in AIP, several studies have explored the systemic ramifications of this disease to determine whether the presence of elevated sIgG4 is unique to only AIP in the gastrointestinal tract; or rather a characteristic shared by other pancreaticobiliary diseases. Through these efforts, it has become evident that AIP and its biliary variant IAC, are parts of a spectrum of a systemic IgG4-related fibro-inflammatory disease (ISD) that may involve other organs as well (1, 5, 12, 32). Indeed, recent work has shown that although the sIgG4 remains highly specific for AIP/IAC, some cases of pancreatic cancer (10%) and primary sclerosing cholangitis (9%) exhibit high levels of sIgG4 as well (19, 22, 23, 33). IAC can mimic CCA due to similarities in their radiologic and clinical features, but there is as yet no published work examining sIgG4 levels in CCA patients and the utility of sIgG4 for distinguishing IAC from CCA.
The HISORt criteria propose that in a patient with an unexplained biliary stricture, at least 2 of the following findings are required for a “probable IAC” diagnosis: a) elevated sIgG4, b) suggestive pancreatic imaging findings, c) another organ involvement or d) positive IgG4 staining on bile duct biopsy. Probable AIP/IAC becomes definite when the stricture responds to steroids (12). In the absence of suggestive pancreatic or other organ involvement on imaging, it is important to exclude CCA if possible before a trial of corticosteroids is administered for probable IAC. Hence, in patients with an isolated biliary stricture and an elevated sIgG4, before any invasive diagnostic procedures are performed to provide histologic confirmation, it is important to consider the following: (1) could this patient have CCA and a high sIgG4 concentration as well (such as is the case with previously published subsets of pancreatic cancer and PSC patients)? (2) Is the sIgG4 level alone sufficiently discriminatory between IAC and CCA? (3) If an elevated sIgG4 level by itself is capable of distinguishing IAC from CCA, then at what IgG4 value can it reliably do so? These questions are particularly important as there are clinical circumstances in which it is inappropriate to obtain a diagnostic needle biopsy that traverses the bile duct lining, particularly for protocols of liver transplantation for hilar cholangiocarcinoma, in which transbiliary biopsies have been associated with a high rate of post-transplant tumor recurrence.
Our results show that 17/126 (13.5%) and 20/161 (12.4%) of CCA patients had an elevated sIgG4 level, as compared to 39/50 (78.0%) and 30/47 (63.8%) of IAC patients in the test and validation cohorts, respectively. When the cut-point for an elevated IgG4 was considered to be twice the upper limit of normal sIgG4 level, the rate of elevated IgG4 decreases to 4/126 (3.2%) and 7/161 (4.3%) in all CCA patients in the test and validation cohorts, respectively. Relative to this, 25/50 (50.0%) and 16/47 (34.0%) of IAC patients in the test and validation cohorts had a sIgG4 greater than two times the upper limit of normal. Elevation of the sIgG4 to more than twice the upper limit of normal therefore substantially enhanced the specificity of the diagnosis, albeit with a penalty of an approximately 30% reduction in sensitivity. However, even with a cut-off of two times the upper limit of normal, because of the differences in the numbers of patients with IAC and CCA that are seen at a given institution, an elevated sIgG4 may be of limited utility in differentiating IAC from CCA. For example, at Mayo Clinic Rochester, approximately 10 cases of IAC are diagnosed per year, of whom about 5 cases (50% of 10, using the results from the test cohort) would have a sIgG4 greater than two times the upper limit of normal. Each year, in part because of the large CCA referral practice, approximately 250 cases of CCA are diagnosed per year, of whom 8 cases (3.2% of 250) would have a sIgG4 greater than two times the upper limit of normal. Therefore, a patient presenting with a biliary stricture and elevated sIgG4 greater than two times the upper limit of normal at our institution has a greater than 50% chance (8/(5+8) = 8/13 = 62%) of having CCA as the final diagnosis. While the exact proportions will be different at different institutions, this example illustrates the critical importance of our findings for the appropriate evaluation of patients presenting with biliary strictures and an elevated sIgG4. Among the subjects studied, the specificity for IAC (vs. CCA) is 100% at >450 mg/dL for the test cohort and >620 mg/dL for the validation cohort. Increasing the cut-off for diagnosis of IAC to a high specificity cut-off of four times the upper limit of normal (560 mg/dL) would allow more confidence in the diagnosis of IAC (vs. CCA) with specificities of 100% and 99% for the test and validation cohorts, but at the cost of a significantly decreased test sensitivity of 26% for the test cohort and 17% for the validation cohort.
Interestingly, a higher percentage (22.6% and 19.6%) of the subset of CCA patients with associated PSC (CCA+PSC) had an elevated sIgG4 than of the subset of CCA patients without PSC (CCA−PSC) (10.5% and 9.1%). With a cut-point of twice the upper limit of normal, 2/31 (6.5%) and 4/51 (7.8%) of CCA+PSC patients had IgG4 elevations above that level. There is therefore a trend towards a higher sIgG4 concentration in patients with CCA and concomitant PSC (CCA+PSC). In fact, the percentages of CCA+PSC patients with high sIgG4 levels (i.e. >140mg/dL) in both our cohorts is higher than those reported for pancreatic (10%) and non-CCA associated PSC (9%) (19, 22). In addition, CCA+PSC patients were more likely to have a positive tissue IgG4 by immunohistochemistry. This potential association of PSC with high serum and tissue IgG4 in CCA patients suggests that PSC patients with high IgG4 may be at increased risk of developing CCA. Considered together with the finding that PSC patients with elevated sIgG4 tend to have more severe liver disease and a shorter time to liver transplantation, our study suggests the possibility that IgG4 immunoreactivity may be one of the driving forces behind the malignant transformation from PSC to CCA or perhaps to other neoplastic processes such as non-Hodgkin lymphoma (11, 19, 34). It is also possible that those with PSC + CCA, especially those with sIgG4 greater than two times the upper limit of normal, could have had long standing IAC which was complicated by CCA. The fact that 3 patients with elevated sIgG4 had abnormal pancreatic imaging suggests that some patients could have had undiagnosed IAC. However, none of the resected bile ducts showed evidence of IAC despite staining for IgG4, which would argue against this hypothesis. Finally, we found no association of elevated sIgG4 levels with stricture distribution (intra- or extra-hepatic) and no correlation between sIgG4 and CA19-9 level in CCA patients. We also found no evidence of association of an overlap syndrome of autoimmune hepatitis in conjunction with PSC with elevated sIgG4 levels in CCA patients.
In summary, we have demonstrated the following findings relating to sIgG4 in cholangiocarcinoma: (1) the sIgG4 is elevated in a subset of patients with CCA; (2) CCA patients with concomitant PSC (CCA+PSC) are more likely to exhibit higher sIgG4 levels than those without PSC (CCA−PSC); (3) In order to more reliably distinguish IAC from CCA based on the sIgG4 alone, an IgG4 cut-point of at least twice the upper limit of normal is required. However, at approximately four times the upper limit of normal, sIgG4 is 99–100% specific for distinguishing IAC from CCA. In view of the similar clinical and radiologic features of CCA and IAC, CCA should be carefully ruled out in patients with suspected IAC whose sIgG4 is only mildly elevated, particularly when they do not fully meet the HISORt criteria required to diagnose IAC. This distinction is important in view of the use of steroids to manage IAC, which may result in delays in administering appropriate treatment of CCA.
Acknowledgments
Grant support: This work was supported by Grants CA100882, 3R56CA100882-06A1S1 and CA128633 from the National Institutes of Health; the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIDDK P30DK084567); the Mayo Clinic Cancer Center, and the Mayo Foundation (to LRR). Statistical support for the project described was provided by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Abbreviations used in this paper
- CCA
Cholangiocarcinoma
- PSC
Primary Sclerosing Cholangitis
- CCA+PSC
CCA with concomitant PSC
- CCA−PSC
CCA without PSC
- IAC
IgG4-Associated Cholangitis
- HCC
Hepatocellular Carcinoma
- ISD
IgG4-related Systemic Disease
- AIP
Autoimmune Pancreatitis
Footnotes
Conflicts of Interest: No conflicts of interest exist
Disclosures: None
Secretarial assistance: Victoria L. Campion and Erin Fairchild.
Author contributions:
Abdul M Oseini: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis
Roongruedee Chaiteerakij: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis
Abdirashid M. Shire: Acquisition of data; critical revision of the manuscript for important intellectual content
Amaar Ghazale: Study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content
Kaiya Joseph: Acquisition of data; critical revision of the manuscript for important intellectual content
Catherine D Moser: Acquisition of data; critical revision of the manuscript for important intellectual content
Ileana Aderca: Acquisition of data; critical revision of the manuscript for important intellectual content
Teresa A Mettler: Acquisition of data; critical revision of the manuscript for important intellectual content
Terry M Therneau: Statistical analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Lizhi Zhang: Tissue staining and pathological analysis; critical revision of the manuscript for important intellectual content
Naoki Takahashi: Radiologic imaging analysis and interpretation; critical revision of the manuscript for important intellectual content
Suresh T Chari: Study concept and design; analysis and interpretation of data; drafting of the manuscript; study supervision
Lewis R Roberts: Study concept and design; analysis and interpretation of data; drafting of the manuscript; obtained funding; study supervision
References
- 1.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T. IgG4-positive plasma cells specifically infiltrate various organs in autoimmune pancreatitis. Pancreas. 2004;29:167–168. doi: 10.1097/00006676-200408000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–1016. doi: 10.1016/j.cgh.2006.05.017. quiz 1934. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki K, Uchida K, Matsushita M, Takaoka M. Autoimmune pancreatitis. Intern Med. 2005;44:1215–1223. doi: 10.2169/internalmedicine.44.1215. [DOI] [PubMed] [Google Scholar]
- 5.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N, Funata N. Comparison of radiological and histological findings in autoimmune pancreatitis. Hepatogastroenterology. 2006;53:953–956. [PubMed] [Google Scholar]
- 6.Deshpande V, Chicano S, Finkelberg D, Selig MK, Mino-Kenudson M, Brugge WR, Colvin RB, et al. Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol. 2006;30:1537–1545. doi: 10.1097/01.pas.0000213331.09864.2c. [DOI] [PubMed] [Google Scholar]
- 7.Stone JH, Khosroshahi A, Deshpande V, Stone JR. IgG4-related systemic disease accounts for a significant proportion of thoracic lymphoplasmacytic aortitis cases. Arthritis Care Res (Hoboken) 62:316–322. doi: 10.1002/acr.20095. [DOI] [PubMed] [Google Scholar]
- 8.Tabata T, Kamisawa T, Takuma K, Anjiki H, Egawa N, Kurata M, Honda G, et al. Serum IgG4 concentrations and IgG4-related sclerosing disease. Clin Chim Acta. 2009;408:25–28. doi: 10.1016/j.cca.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 10.Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403–408. doi: 10.1007/s00535-008-2205-6. [DOI] [PubMed] [Google Scholar]
- 11.Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, et al. IgG4-related hepatic inflammatory pseudotumor with sclerosing cholangitis: a case report and review of the literature. Cases J. 2009;2:7029. doi: 10.4076/1757-1626-2-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–715. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Cheung MT, Lo IL. IgG4-related sclerosing lymphoplasmacytic pancreatitis and cholangitis mimicking carcinoma of pancreas and Klatskin tumour. ANZ J Surg. 2008;78:252–256. doi: 10.1111/j.1445-2197.2008.04430.x. [DOI] [PubMed] [Google Scholar]
- 14.Gamblin TC, Krasinskas AM, Slivka AS, Tublin ME, Demetris J, Shue E, Caro S, et al. Fibroinflammatory biliary stricture: a rare bile duct lesion masquerading as cholangiocarcinoma. J Gastrointest Surg. 2009;13:713–721. doi: 10.1007/s11605-008-0750-1. [DOI] [PubMed] [Google Scholar]
- 15.Hamano H, Kawa S, Uehara T, Ochi Y, Takayama M, Komatsu K, Muraki T, et al. Immunoglobulin G4-related lymphoplasmacytic sclerosing cholangitis that mimics infiltrating hilar cholangiocarcinoma: part of a spectrum of autoimmune pancreatitis? Gastrointest Endosc. 2005;62:152–157. doi: 10.1016/s0016-5107(05)00561-4. [DOI] [PubMed] [Google Scholar]
- 16.Oh HC, Kim MH, Lee KT, Lee JK, Moon SH, Song TJ, Eum J, et al. Clinical clues to suspicion of IgG4-associated sclerosing cholangitis disguised as primary sclerosing cholangitis or hilar cholangiocarcinoma. J Gastroenterol Hepatol. 25:1831–1837. doi: 10.1111/j.1440-1746.2010.06411.x. [DOI] [PubMed] [Google Scholar]
- 17.Chung DT, Tang CN, Lai EC, Yang GP, Li MK. Immunoglobulin G4-associated sclerosing cholangitis mimicking cholangiocarcinoma. Hong Kong Med J. 16:149–152. [PubMed] [Google Scholar]
- 18.Miglani RK, Murthy D, Bhat R, Kumar AK. Immunoglobulin G4-associated cholangitis mimicking cholangiocarcinoma in a young boy. J Postgrad Med. 56:140–142. doi: 10.4103/0022-3859.65294. [DOI] [PubMed] [Google Scholar]
- 19.Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, Chari S, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070–2075. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Lewis JT, Abraham SC, Smyrk TC, Leung S, Chari ST, Poterucha JJ, et al. IgG4+ plasma cell infiltrates in liver explants with primary sclerosing cholangitis. Am J Surg Pathol. 34:88–94. doi: 10.1097/PAS.0b013e3181c6c09a. [DOI] [PubMed] [Google Scholar]
- 21.Chung H, Watanabe T, Kudo M, Maenishi O, Wakatsuki Y, Chiba T. Identification and characterization of IgG4-associated autoimmune hepatitis. Liver Int. 30:222–231. doi: 10.1111/j.1478-3231.2009.02092.x. [DOI] [PubMed] [Google Scholar]
- 22.Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 23.Raina A, Krasinskas AM, Greer JB, Lamb J, Fink E, Moser AJ, Zeh HJ, 3rd, et al. Serum immunoglobulin G fraction 4 levels in pancreatic cancer: elevations not associated with autoimmune pancreatitis. Arch Pathol Lab Med. 2008;132:48–53. doi: 10.5858/2008-132-48-SIGFLI. [DOI] [PubMed] [Google Scholar]
- 24.Erdogan D, Kloek JJ, ten Kate FJ, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br J Surg. 2008;95:727–734. doi: 10.1002/bjs.6057. [DOI] [PubMed] [Google Scholar]
- 25.Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–1203. doi: 10.1097/01.pas.0000136449.37936.6c. [DOI] [PubMed] [Google Scholar]
- 26.Nishino T, Toki F, Oyama H, Oi I, Kobayashi M, Takasaki K, Shiratori K. Biliary tract involvement in autoimmune pancreatitis. Pancreas. 2005;30:76–82. [PubMed] [Google Scholar]
- 27.Uehara T, Hamano H, Kawa S, Sano K, Honda T, Ota H. Distinct clinicopathological entity ‘autoimmune pancreatitis-associated sclerosing cholangitis’. Pathol Int. 2005;55:405–411. doi: 10.1111/j.1440-1827.2005.01845.x. [DOI] [PubMed] [Google Scholar]
- 28.van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–422. [PMC free article] [PubMed] [Google Scholar]
- 29.Aalberse RC, Van Milligen F, Tan KY, Stapel SO. Allergen-specific IgG4 in atopic disease. Allergy. 1993;48:559–569. doi: 10.1111/j.1398-9995.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 30.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992;148:2731–2737. [PubMed] [Google Scholar]
- 31.Shirakata Y, Shiraishi S, Sayama K, Miki Y. Subclass characteristics of IgG autoantibodies in bullous pemphigoid and pemphigus. J Dermatol. 1990;17:661–666. doi: 10.1111/j.1346-8138.1990.tb03008.x. [DOI] [PubMed] [Google Scholar]
- 32.Alderlieste YA, van den Elzen BD, Rauws EA, Beuers U. Immunoglobulin G4-associated cholangitis: one variant of immunoglobulin G4-related systemic disease. Digestion. 2009;79:220–228. doi: 10.1159/000213364. [DOI] [PubMed] [Google Scholar]
- 33.Choi EK, Kim MH, Lee TY, Kwon S, Oh HC, Hwang CY, Seo DW, et al. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas. 2007;35:156–161. doi: 10.1097/MPA.0b013e318053eacc. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi N, Ghazale AH, Smyrk TC, Mandrekar JN, Chari ST. Possible association between IgG4-associated systemic disease with or without autoimmune pancreatitis and non-Hodgkin lymphoma. Pancreas. 2009;38:523–526. doi: 10.1097/MPA.0b013e31819d73ca. [DOI] [PubMed] [Google Scholar]