Abstract
Vaccines represent one of the greatest triumphs of modern medicine. Despite the common origins of vaccinology and immunology more than 200 years ago, the two disciplines have evolved along such different trajectories that most of the highly successful vaccines have been made empirically, with little or no immunological insight. Recent advances in innate immunity have offered new insights about the mechanisms of vaccine-induced immunity and have facilitated a more rational approach to vaccine design. Here we will discuss these advances and emerging themes on the immunology of vaccination.
The invention of vaccination was a turning point in the war between microbes and humans. Although improved sanitation and antibiotics may have saved more lives, vaccines represent the most cost-effective life-saving device in history. Despite their success, one of the great iro-nies of vaccinology is that the vast majority of vaccines have been developed empirically, with little or no understanding of the immunological mechanisms by which they induce protective immunity. However, the failure to develop vaccines against global pandemics such as infection with human immunodeficiency virus (HIV) despite decades of effort has underscored the need to understand the immunological mechanisms by which vaccines confer protective immunity. It is now clear that the immune system has evolved qualitatively different types of responses to protect against different pathogens. For example, distinct subsets of helper T cells, such as TH1, TH2 and TH17, are effective at protecting against different pathogens1 (Table 1). Follicular helper T cells (TFH cells) produce interleukin 21 (IL-21) and help with the differentiation of B cells and generation of memory B cells2. In addition, differentiating memory CD4+ and CD8+ T cells can be subcategorized into central memory and effector memory cell subsets, each with a distinct functionality3. This places a great premium on understanding and harnessing the mechanisms that stimulate such diverse responses in the context of vaccines against different pathogens. Research during the past decade has identified a fundamental role for the innate immune system in sensing vaccines and adjuvants and in programming protective immune responses. The innate immune system can sense microbes through pattern-recognition receptors (PRRs), such as the Toll-like receptors (TLRs), which are expressed by various cells, including dendritic cells (DCs)4,5. In addition to TLRs, other types of PRRs, including the C-type lectin-like receptors6 and the cytosolic Nod-like receptors7, sense a broad range of microbial stimuli, and the cytosolic RIG-I-like receptors sense viral nucleic acids8. There are many subsets of functionally distinct DCs, and it is now clear that the DC subset, as well as the nature of the PRR, have a key role in determining the magnitude and quality of adaptive immune responses9,10.
Table 1.
Programming T cell responses with innate immunity
| Stimulus | T cell response |
Transcription factor |
Differentiation factor(s) |
Effector molecule(s) |
APC subsets with an intrinsic ability to induce the T cell response |
PRRs | Signaling in DCs | Accessory cells |
|---|---|---|---|---|---|---|---|---|
| Bacteria, viruses | TH1 | T-bet | IL-12p70, IFN-γ |
IFN-γ | Mouse: splenic CD8α+ cells80,81,12; monocyte-derived DCs Human: Blood CD11c+ myeloid DCs11,94; skin CD14+ dermal DCs95 |
TLRs in general4,5,9 |
p38, Jnk4,5,9 | NK cells |
| Parasites, viruses, bacteria, allergens |
TH2 | GATA-3 | IL-4 | IL-4, IL-5, IL-13 |
Mouse: CD8α− DCs12,80,81; dermal DCs84 Human: Langerhans cells95; pDCs94 |
TLR2, TLR4, Nod1, Nod2, DC-SIGN (all ref. 12) |
Erk–c-Fos12,, ROS84 |
Basophils98–100 |
| Fungi, bacteria | TH17 | RORγt | IL-6, IL-1β, TGF-β |
IL-17, IL-22 | Mouse: Intestinal CD11b+ DCs17 Human: ? |
Dectin-1 (ref. 96), dectin-2 |
Syk96 | Neutrophils |
| Helminths | TH9 | Pu.1 | IL-4, TGF-β | IL-9 | ? | ? | ? | ? |
| Skin pathogens? | TH22 | AHR | IL-6, TNF | IL-22 | Mouse: ? Human: pDCs3 |
? | ? | ? |
| Viruses, bacteria, parasites |
TFH | Bcl-6 | (IL-21) | IL-21 | ? | TLR | ? | B cells2 |
| Pathogens, com- mensals |
Treg | Foxp3 | (TGF-β), retinoic acid |
TGF-β | Mouse: Intestinal CD103+ DCs101,102,, pDCs, macrophages17 Human: Intestinal macrophages12 |
TLR2, TLR6 | Erk-RALDH106,, WNT–β-catenin103 |
Macrophages12 |
| Pathogens, com- mensals |
Tr1 | ? | IL-10 | IL-10 | ? | TLR2, TLR6 (refs. 12,103) |
? | ? |
| Viruses | CTL | Eomes | IL-12 | Perforin | Mouse: CD8α+ DCs82,83,, CD103+ skin DCs Human: BDCA-3+ DCs91–93 |
TLR3, TLR7, TLR9 (refs. 4,5,9) |
? | CD4+ T cells |
Different microbial stimuli induce distinct classes of helper T cell responses. DCs have a central role in sensing microbial stimuli (as well as nonmicrobial stimuli such as allergens) and program distinct classes of helper T cell responses. Work has begun to identify the nature of the DC subsets, PRRs and signaling pathways in DCs that mediate such responses. In addition, DCs act together with other accessory cell types, such as basophils and natural killer (NK) cells to orchestrate a particular response.
The subject of how the innate immune system regulates adaptive immunity in general has been reviewed extensively4,5,9–12, so we will not provide a detailed description of this topic. Instead, we will discuss emerging themes on the immunology of vaccination. One such theme focuses on the immunological deconstruction of vaccines and describes new insights into the mechanisms by which vaccines and adjuvants are sensed by the innate immune system and stimulate adaptive immunity. These mechanistic insights will undoubtedly enable the rational design of vaccines against pandemics and emerging infections.
A second theme focuses on insights into how the innate immune system programs protective immune responses and regulates the magnitude, quality and persistence of vaccine-induced immunity. For example, in addition to its effect on modulating T cell differentiation, innate immunity controls the antibody response at critical checkpoints of antigen-driven B cell differentiation. Thus, recent work has highlighted the fact that TLR triggering can regulate the persistence of the germinal center–memory B cell differentiation pathway13 and the role of basophils in enhancing the survival of plasma cells in the bone marrow14. Furthermore, innate programming of DCs in the lymph nodes may provide instructive cues for the migration of activated T cells and B cells to mucosal tissues15, and various subsets of DCs and macrophages may regulate the differentiation of antigen-specific T cells and B cells at mucosal sites16–18.
In the final theme of this review (humanity as a model), we discuss how such insights have been provided mainly by elegant studies in animal models, particularly mice. However, unlike laboratory mice, humans have considerable heterogeneity in terms of age, nutritional status and genetic diversity. The effect of malnutrition and obesity on the immune system is an area that clearly deserves intense study, particularly in the context of vaccination. In this context, there are now increasing efforts to understand immune regulation from the point of view of systems biology19–21.
Immunological deconstruction of vaccines
Vaccines can be classified into two broad groups. The first group, live attenuated vaccines, comprises weakened versions of the pathogens; these mimic the kind of protective immunity induced in people who survive live infection22. Examples of this group include vaccines against acute infections caused by invariant pathogens such as smallpox, yellow fever, measles, mumps, rubella and chicken pox. Live attenuated vaccines have been administered to billions of people worldwide and elicit strong cellular and antibody responses and often confer immunity that lasts for several decades, with even a single immunization22. However, in many acute infections, such as infection with respiratory syncytial virus and malaria, natural infection itself does not engender complete protection against reinfection, so any vaccine must improve on what nature has evolved. Furthermore, pathogens that mutate rapidly (such as HIV), those that exist as multiple serotypes (such as dengue virus) or those that cause persistent or latent infection (such as HIV and hepatitis C virus) pose formidable immunological challenges22.
The second group includes subunit vaccines (such as the vaccine against recombinant hepatitis B), toxoid vaccines that consist of inactivated toxins (such as vaccines against diphtheria and tetanus), carbohydrate vaccines (such as vaccines against pneumococcus) and conjugate vaccines (such as vaccines against Haemophilus influenzae type B or meningococcus)22. Such vaccines usually contain substances called adjuvants, which enhance the magnitude and modulate the quality of the immune response. Despite several decades of research, few adjuvants have been licensed for use around the world. These include alum (an aluminum salt–based adjuvant), AS04 (a combination adjuvant composed of monophosphoryl lipid A (a TLR4 ligand) adsorbed to alum)23,24 and oil-in-water emulsions (such as MF59 and AS03)23,24. The paucity of adjuvants licensed for clinical use reflects critical knowledge gaps about the mechanisms of action of adjuvants and, notably, about the mechanisms that mediate potential toxic effects. Live attenuated vaccines such as those against smallpox or yellow fever are the most successful vaccines ever made and can confer lifelong memory, whereas nonliving vaccines induce protection of much shorter duration and require booster vaccination to maintain protective immunity. Thus, a single dose of the smallpox vaccine maintains serum antibody titers for more than 50 years25,26 and cellular immunity is also maintained for decades. Such vaccines, therefore, serve as gold standards, and learning the mechanisms by which they induce protective immunity would be invaluable in the design of new vaccines against global pandemics and emerging infections27,28.
As attenuated vaccines consist of viruses (such as smallpox or yellow fever) or bacteria (such as bacillus Calmette-Guérin), it is very likely that they signal through several different PRRs, including TLRs. However, although several studies have examined the PRRs that sense pathogens, few studies have examined the PRRs that sense live vaccines. Notably, only a handful of studies have examined how these PRRs influence the adaptive immune responses to live attenuated vaccines. Bacillus Calmette-Guérin activates DCs via TLRs, but whether TLR signaling is required for adaptive immunity is unknown29. The yellow fever vaccine YF-17D activates multiple TLRs (TLR2, TLR3, TLR7, TLR8 and TLR9) on plasmacytoid and myeloid DCs30 (B.P., unpublished data; Table 2). The activation of multiple TLRs suggests that signaling via any single TLR may be redundant but, surprisingly, DCs from mice deficient in any single TLR are substantially impaired in their cytokine response to YF-17D, which suggests that there might be synergistic activation of multiple TLRs30. Signaling via particular combinations of TLRs results in synergistic activation of DCs31. Vaccination with YF-17D induces a mixed TH1-TH2 profile. Vaccination of mice deficient in the adaptor MyD88 results in a much lower frequency of antigen-specific interferon-γ (IFN-γ)-secreting CD4+ T cells and CD8+ T cells (TH1 and TC1 cells, respectively). In contrast, vaccination of TLR2-deficient mice results in a greatly enhanced TH1 and TC1 response, consistent with a regulatory role for TLR2 (ref. 12). Furthermore, YF-17D also activates the cytosolic receptors RIG-I and Mda5 (ref. 19), although the effect of this on adaptive immunity is unknown. Consistent with the triggering of multiple TLRs by YF-17D, synthetic nanoparticles that resemble viruses in size (~300 nm in diameter) and immune composition (containing combinations of TLR ligands) induce synergistic enhancement of antigen-specific T cell responses and high-affinity neutralizing antibody responses that last for the entire lifespan of a mouse13.
Table 2.
Innate immune activation by vaccines and adjuvants
| Innate immune mechanism |
Type of immune response |
|
|---|---|---|
| Licensed vaccine | ||
| Yellow fever (YF-17D) | Activates multiple DC subsets through TLR2, TLR3, TLR7, TLR8 and TLR9; activates RIG-I and Mda5 |
CTLs; TH1 and TH2; neutralizing antibody |
| Smallpox (vaccinia virus) | Inhibits DC activation and causes cell death; blocks TLR4 and TLR3 signaling |
CTLs; neutralizing antibody |
| Bacillus Calmette-Guérin | Activates TLR2, TLR4, TLR9 and DC-SIGN |
TH1 and TH2 |
|
Licensed adjuvant-
vaccine combinations |
||
| Alum | TLR signaling not critical for induction of antibody responses; induces caspase-1 and inflammasome activation in DCs |
TH2; antibody |
| MF59 | Mechanism unknown; enhanced uptake by antigen presenting cells probably important |
TH2; antibody |
| AS04 | TLR4 agonist | TH1; antibody |
| Emerging adjuvants | ||
| CpG DNA | TLR9 ligand | TH1, antibody |
| TLR7 and TLR8 ligands | TLR7 ligands | TH1, antibody |
| Flagellin-protein fusions | Activates TLR5 and the inflammasome components IPAF and NAIP5 |
TH1 and TH2 |
Innate immune activation by some licensed vaccines and vaccine-adjuvant combinations, and emerging adjuvants being used in combination with various vaccines (these have been shown to stimulate innate immunity in clinical trials in combination with a variety of vaccines). MF59 is licensed in Europe in combination with the Novartis vaccine against influenza; AS04, an MPL derivative, is licensed for use in Europe in combination with vaccine against hepatitis B.
Influenza virus (a single-stranded RNA virus from which the live attenuated vaccine against influenza is derived) activates plasmacytoid DCs (pDCs) via TLR7 and myeloid DCs through the adaptor IPS-1, which signals downstream of RIG-I32. The early innate response to influenza virus is dependent on MyD88 and IPS-1, and mice deficient in both of these molecules failed to launch innate responses. However, antigen-specific antibody responses and CD4+ T cell responses are MyD88 dependent but IPS-1 independent. In contrast, induction of antigen-specific CD8+ T cell responses is not impaired in MyD88- or IPS-1-deficient mice32. To what extent the live attenuated influenza vaccine triggers the same PRRs that influenza virus itself triggers remains to be determined. Similarly, vaccination with the inactivated whole virus requires TLR7-mediated production of type I interferons by pDCs for its immunogenicity33. Consistent with that, the immunogenicity of the whole inactivated vaccine against H5N1 influenza is dependent on MyD88-dependent TLR7 signaling34. Adenovirus vectors, which are being developed as vaccines against infection with HIV, as well as malaria and other diseases, are among the most potent for inducing CD8+ T cell responses. Antigen-specific CD8+ T cell responses elicited by recombinant adenovirus vectors are diminished in Myd88−/− mice but not in mice deficient in the adaptor TRIF or Tlr3−/− mice35,36. However, the absence of individual TLRs does not have an appreciable effect on the CD8+ T cell response. Moreover, responses are not diminished in mice deficient in the IL-1 receptor or Il18−/− mice. These data suggest that adenovirus vectors engage multiple redundant MyD88-dependent signaling pathways. However, the nature of the PRRs involved remain to be identified.
Until recently alum was the only licensed adjuvant in the USA; however, oil-in-water emulsions such as MF59 and AS03 are licensed for adjuvant-plus-influenza vaccines in Europe. AS04 is approved for vaccines against hepatitis B virus and human papillomavirus in Europe and has been licensed in the USA23,24 (Table 2). A central issue is how to design adjuvants that stimulate the relevant class of immune response required for protection, such as a specific helper T cell subset, cytotoxic T cells (CTLs) or long-term memory T cells or B cells, or adjuvants that induce the mucosal homing and persistence of antigen-specific lymphocytes. This issue requires a mechanistic understanding of how successful adjuvants mediate their immunogenicity; that is, the nature of the DC subsets and PRRs and other cell types targeted by the adjuvants.
Despite the widespread use of alum in several vaccines over the past 70 years (in vaccines against hepatitis B virus, human papillomavirus, diphtheria and tetanus and H. influenzae type B and the conjugate vaccine against pneumococcus), its mechanism of action remained a ‘black box’ until recently. Alum consists of aluminum salts that can be emulsified with the antigen to generate a gel-like substance and generally induces a TH2-biased response. A wide-spread belief has been that alum exerts a depot effect whereby the emulsion retains antigen at the site of injection and releases it slowly to promote sustained antigen presentation23,24. Recently it has been shown that alum induces antibody responses independently of TLR signaling37. Furthermore, alum exerts a direct effect on IL-4-producing Gr-1+ cells that are essential for priming and clonal expansion and optimal antibody production by B cells in vivo38. Several groups have demonstrated that alum signals through the NLRP3 inflammasome39–41 (Table 2). Thus, DCs or macrophages stimulated in vitro with alum plus lipopolysaccharide induce IL-1β and IL-18 in a manner dependent on caspase-1 and NLRP3 (refs. 39–41). However, whether NLRP3 is required for the adjuvant characteristics of alum remains controversial; some studies have demonstrated abrogation of antibody responses in Nlrp3−/− mice39,41, but others have shown only partial or no effects40. Furthermore, the mechanisms by which alum induces TH2 responses remain poorly understood.
MF59, a squalene-based oil-in-water emulsion, has been licensed for use with the influenza vaccines in the elderly since 1997. Squalene is an intermediate in the human steroid hormone biosynthetic pathway and is a precursor to cholesterol. MF59 enhances the immunity elicited by and cross-protection of the vaccine against seasonal influenza in children42. MF59 and the similar squalene-based oil-in-water emulsion AS03 have been licensed in Europe for vaccines against pandemic influenza and were widely used for the 2009 H1N1 influenza pandemic42. A comparison of MF59, CpG DNA and alum after vaccination has demonstrated that although all adjuvants promote the recruitment of antigen-presenting cells, MF59 triggers more rapid influx of CD11b+ blood cells. Furthermore, MF59 is the most potent inducer of genes encoding cytokines, cytokine receptors and adhesion molecules involved in leukocyte migration. Finally, microarray analysis of muscle cells at the site of injection has shown that all three adjuvants induce a core set of 168 genes, with MF59 inducing the most genes. The efficient adjuvant activity of MF59 might therefore be mediated by strong innate responses at the site of immunization, including in muscle cells42. The nature of the PRR, target cells and signaling networks that mediate the immunogenicity remain unknown.
An emerging class of adjuvants is those that signal via TLRs. A clear rationale for this is that many successful vaccines contain their own endogenous adjuvants that seem essential for their immunogenicity. The adjuvant AS04 consists of monophosphoryl lipid A (MPL), which is a lipopolysaccharide derivative and a TLR4 ligand. AS04 is licensed for use, in combination with alum, in GlaxoSmithKline’s Cervarix vaccine against human papillomavirus and the vaccine against hepatitis B virus43. Although both lipopolysaccharide and MPL signal via TLR4, the latter signals mainly via TRIF44, whereas the former signals via both MyD88 and TRIF, which results in enhanced proinflammatory cytokines and possible toxicity. Unlike the TH2-biased response elicited by alum, AS04 induces a TH1 response. A head-to-head comparison of Cervarix and Gardasil, a vaccine from Merck that includes only alum as its adjuvant45,46, has shown that both vaccines (which contain virus-like particles from human papillomavirus type 16 or 18) efficiently induce protective immunity and diminish the associated cervical intraepithelial neoplasia in young women (who are seronegative for the relevant vaccine at baseline)45. However, 1 month after three doses, Cervarix produces significantly higher titers (~3.7-fold) neutralizing antibody to human papillomavirus type 16 than does Gardasil, as well as more memory B cells47. Whether the vaccine that includes MPL as its adjuvant induces a response of longer duration remains to be determined.
Innate programming of protective immunity
Most vaccines are believed to confer protection through neutralizing antibodies48. Antibodies are thought to be the correlate of protection against blood-borne viruses such as hepatitis49 and yellow fever50,51; toxin-secreting bacteria, such as diphtheria52 and tetanus53; viruses that infect via mucosal routes, such as influenza54,55 and rotaviruses56; rabies virus57, which infects neuronal axons; and pneumococcal and meningococcal bacteria, which are leading causes of pneumonia and meningitis58,59. The antigen-specific antibody responses to such vaccines are measured by assays such as enzyme-linked immunosorbent assays (which measure the titer of binding antibody), as well as assays that measure functional antibody activity, including the inhibition and neutralization of hemagglutination and opsonophagocytosic capacity. Understanding the precise mechanisms by which antibody molecules confer protection against pathogens and learning how to induce such protective responses with adjuvants that target the innate immune system represent key areas of research.
Despite the importance of antibodies, emerging evidence also points to a key role for T cells. For example, persistent varicella-specific T cells induced by vaccination against varicella virus are useful correlates of protection from infection and reactivation (shingles) in children and the elderly60,61. Furthermore, antibody titers after vaccination against influenza are unreliable for predicting risk of influenza in the elderly62. Instead, an inverse correlation between the magnitude of influenza-specific T cell responses and risk of influenza acquisition has been demonstrated62. In addition, patients with high frequencies of cytomegalovirus-specific T cells are less likely to have reactivation of cytomegalovirus when they are given immunosuppressive drugs to prevent rejection after transplantation63,64. Finally, humans with particular mutations in the genes encoding IL-17 or its receptor have chronic mucocutaneous immunity to Candida albicans65, which suggests a role for TH17 cells in immunity to C. albicans. In fact, many pandemics that need effective vaccines, such as infection with HIV, or tuberculosis and malaria, are believed to require strong T cell responses for protection66–68.
The goal of any T cell–based vaccine is to induce antigen-specific memory T cells that persist long after the antigen has been eliminated and confer protection against subsequent infection. Vaccine-driven T cell differentiation can result in phenotypically and functionally diverse populations of cells. For example, naive CD4+ T cells can differentiate into any of several subsets of helper T cells (TH1, TH2, TH17, TH21, TFH, TH22 or TH9) with distinct effector functions that mediate protection against different pathogens (Table 1). Thus, intracellular pathogens require TH1-driven CTLs, whereas infections with helminths and fungi are best controlled by TH2 and TH17 responses, respectively. Naive CD8+ T cells can differentiate into effector cells that circulate or reside in tissues and provide immediate protection against infection at the portals of entry, including mucosal tissues. In contrast, central memory T cells reside in the T cell–rich areas of lymphoid organs and provide a pool of precursor cells that undergo rapid clonal expansion in response to antigenic challenge and differentiate into effector cells.
Programming T cell responses with innate immunity
The magnitude, quality and persistence of memory T cell responses can conceivably be regulated at many steps via enhancing the clonal expansion phase, decreasing the contraction phase, stabilizing the memory phase or some combination of these strategies. The rate of clonal expansion is dependent on several variables, including the recruitment of naive, antigen-specific T cells to DCs, cycling time and rate of cell death. A major challenge during the initiation of the immune response is that the antigen-bearing DCs and the rare, antigen-specific T cells must find each other. The precursor frequency of naive epitope-specific CD8+ T cells has been estimated to be in the order of 1 cell in 2 × 105 cells. Thus, in an uninfected mouse containing ~2 × 107 to 4 × 107 naive CD8+ T cells, there are estimated to be 100–1,200 epitope-specific cells69–71. Therefore, a single lymph node probably contains only 10 or 120 antigen epitope–specific cells. The main difficulty in observing these rare antigen-specific cells has been addressed by the application of sophisticated microscopic techniques. Initial calculations based on the rate of T cell migration and the volume of space swept by the dendrites of a DC suggested a 95% likelihood that a single antigen-specific T cell will encounter at least one DC during a single flow through the lymph node. Thus, random movements of T cells and DCs are alone sufficient to initiate an immune response. However, naive T cells traffic along a well-ordered network of fibroblastic reticular cells (FRCs) during their migration from high endothelial venules to lymphatic exits72. DCs are also positioned along the same FRC network, thus enhancing the probability of interaction with T cells73. However, even with the constraints posed by migration along the FRC network, the chance that a second T cell will find an antigen-bearing DC already in contact with a T cell (as would be needed for antigen-specific CD4+ T cells and CD8+ T cells to recognize antigen on the same DC) is exceedingly low. One adaptation that seems to mitigate this problem is that interactions between a DC and CD4+ T cells on FRCs lead to local production of the chemokines CCL3 and CCL4, which attract naive CD8+ T cells that have upregulated the chemokine receptor CCR5 and quickly migrate toward a DC ‘licensed’ by a CD4+ T cell74. T cells undergo three phases of interactions with antigen-bearing DCs. During phase 1, T cells migrate along the FRCs searching for antigen-bearing DCs and undergo very transient ‘fly-by’ interactions. In the second phase, when the T cells have found their antigen-bearing DCs, stable contacts (24–30 hours in duration) are formed. In the third phase, the activated T cells separate from the DCs, rapidly divide and undergo transient interaction with further DCs75–79. Despite such impressive insights, it should be noted that most of these studies have involved adoptive transfer of peptide-pulsed DCs and thus the relevance of such mechanisms to DC–T cell interactions in situ during vaccination or infection needs further study. Notably, whether distinct subsets of DCs act differently remains to be determined. This is a critical point, because distinct DC subsets can prime different types of immune responses in different ways. For example, splenic CD11c+CD11b+CD8α− DCs (located in the T cell–rich areas) and CD11c+CD11b−CD8α+ DCs (located in the marginal zones) induce TH2 responses and TH1 responses, respectively80,81. CD11c+CD11b−CD8α+ DCs seem more efficient at cross-presenting antigens to CD8+ T cells82,83. Microbial stimuli or allergens that induce TH1 or TH2 responses seem to target CD11c+CD8α+ DCs or CD11c+CD11b+ migrating dermal DCs, respectively, in different ways84. So, if distinct, geographically segregated DC subsets are adapted to prime CD8+ T cells versus CD4+ T cells, the question of how (and where) the three-cell interaction among a DC, CD4+ T cell and CD8+ T cell occurs remains an enigma. Finally, whether the lessons learned from such imaging studies are universally applicable to different vaccines and adjuvants that target and trigger distinct DC subsets and elicit distinct innate responses is not known.
Once a productive interaction between a DC and T cell is established, the clonal expansion of T cells can be regulated by several variables, including innate cytokines such as IL-12 and IL-18, which are secreted by DCs and induce IFN-γ production by T cells; IFN-α secreted by pDCs85 or other cell types, which can act directly on T cells to induce robust CD8+ T cell population expansion86; or proinflammatory cytokines such as IL-6, which may overcome the suppressive effects of regulatory T cells (Treg cells)87. Thus, adjuvants that enhance DC survival, the induction of costimulatory molecules and controlled release of type I interferons (such as IFN-α) and proinflammatory cytokines may be particularly effective in inducing T cell differentiation. However, such factors must be strictly regulated, as prolonged antigenic stimulation88, chronic stimulation with DCs89 or inappropriate timing of exposure to IFN-α90 can inhibit T cell differentiation.
As antigen-specific T cells proliferate, they are faced with a range of developmental fates, such as whether they will become a TH1, TH2, TH17, TH21, TFH, TH9 or Treg cell, whether they will become a short-lived effector cell or central memory cell, or whether or not to home to mucosal tissues. Understanding the mechanisms that determine this ‘decision-making’ process is an intense area of research. What role does the innate immune system have in decoding pathogen-encoded information and providing instructive cues during T cell differentiation? The differentiation of effector CD4+ T cells is directed mainly by cytokines that induce specific transcription factors in the T cells to specify their differentiation fates. For example, IL-12 activates the transcription factor STAT4 and induces the transcription factor T-bet, which specifies TH1 cells. The innate control of T cell and B cell responses can be considered in terms of various hierarchies of organization, in which DCs, their innate receptors and signaling networks, and their interactions with other cells and local microenvironments represent different levels of the hierarchy12. At the cellular level, the type of DC subset and the nature of the PRRs triggered by the vaccine, as well as local environmental signals, all provide instructive cues that guide the differentiation of T cells. In mice, CD8α+ DCs and CD8α− DCs can induce TH1 and TH2 responses in different ways80,81, and CD8α+ DCs are particularly efficient at cross-presenting exogenous antigens to cytotoxic T cells82. A putative human counterpart of CD8α+ DCs, the so-called BDCA-3+ DCs, has been discovered, with a similar phenotype and superior cross-presenting ability91–93. Furthermore, in humans, pDCs in the blood94 and Langerhans cells in the skin95 can ‘preferentially’ induce TH2 responses and in some cases also induce Treg cells. Whether there is a subset with a propensity to induce TFH cells is not known, although antigen presentation by B cells is known to be important in this2. At the receptor level, TLRs in general induce DCs to prime TH1 responses, although certain TLR ligands (such as low-dose TLR 4 ligands or TLR2 ligands) induce DCs to prime TH2 or Treg cell responses12. Furthermore, signaling through the C-type lectin dectin-1 induces TH17 responses96 (Table 1). Therefore, adjuvants that target particular DC subsets or specific PRRs may be useful in tailoring the appropriate class of response97. At a higher level of the hierarchy, cell-cell interactions seem to be key in orchestrating the appropriate response. Published work has highlighted the importance of cooperation between basophils and DCs84,98,99 and between other innate cell populations and DCs in the induction of TH2 responses100 (Table 1). Such observations suggest the use of adjuvants that trigger multiple cell types. At the highest level of the hierarchy, cytokines and chemokines in the local microenvironment ‘instruct’ DC function. For example, intestinal DCs seem programmed to induce Treg cells101–103, whereas total spleen DCs prime TH1 and undifferentiated TH0 responses104. Furthermore, human epithelial cells trigger DC-mediated allergic inflammation by producing thymic stromal lymphopoietin105. These results are consistent with the observation that DCs in distinct microenvironments induce different TH responses. Therefore, adjuvants delivered via different routes must be able to reprogram the intrinsic tissue-specific bias of DCs. Finally, the intracellular signaling networks and transcription factors that control the ‘decision-making’ process in DCs are just beginning to be appreciated. Activation of the transcription factor NF-κB and the mitogen-activated protein kinases p38 and Jnk program DCs to produce IL-12p70 and induce TH1 responses. In contrast, activation of the mitogen-activated protein kinase Erk–c-Fos pathway seems to favor a TH2 bias12, and activation of the Erk-RALDH enzymes106 or β-catenin103 programs DCs to induce T regulatory responses (Table 1).
A second ‘decision-making’ process concerns whether a cell will become a central memory or effector memory cell; this topic has been reviewed extensively3,107–109. Finally, innate signals can program activated T cells to migrate to mucosal tissues. For example, the vitamin A (retinol) metabolite retinoic acid enhances expression of the integrin α4β7 and the chemokine receptor CCR9 (which mediate homing to the gut) on T cells after activation and ‘imprints’ gut tropism on them110. Furthermore, intestinal antigen-presenting cell subsets constitutively express retinoic acid–metabolizing enzymes17,101,102, and activation of DCs with certain stimuli, such as certain TLR2 ligands, can induce retinoic acid–metabolizing enzymes in splenic DCs106. As many infections, such as infection with HIV, occur almost exclusively via mucosal transmission, a protective CD8+ T cell–based vaccine must elicit memory CD8+ T cells that can promptly migrate to the sites of virus entry or that exist at such sites before infection. In this context, an adenoviral vector that can induce retinoic acid–metabolizing enzymes in lymph node DCs has been shown to program the priming of α4β7- and CCR9-expressing T cells15.
Programming antibody responses with innate immunity
Although the regulation of T cell responses by innate immunity has been appreciated for some time, evidence is emerging for key roles of the innate immune system in regulating the magnitude, quality and persistence of antibody responses. For example, protection against diphtheria, tetanus, lyme disease, hepatitis A, polio, rabies, yellow fever, meningococcal and pneumococcal bacteria is dependent on the magnitude of the antibody response22. However, for many vaccines it is the quality of the antibody response that matters. Thus, of all the detectable antigen-binding antibodies, only a subset may be able to neutralize the pathogen in vitro (Fig. 1). However, another subset might be considered to have high affinity (because, for example, they have an affinity of over 1 × 10−9 M), and another subset might have certain effector functions, such as opsonophagoyctic capacity or antibody-dependent cell-mediated cytotoxicity, dependent on the Fc portion of the antibody (Fig. 1). Ultimately, of course, what matters is whether the antibodies are able to protect against infection in vivo, and this may or may not have something to do with the various aforementioned properties of antibodies. There are many examples in which quality does matter. For example, lack of affinity maturation because of poor TLR stimulation leads to enhanced respiratory syncytial virus disease111. Furthermore, vaccination of infants with the polysaccharide vaccine against meningococcus usually does not stimulate bactericidal antibodies despite high antibody titers112. Similarly, the polysaccharide vaccine pneumococcus provides some protection against invasive pneumococcal disease in a healthy elderly population, but there is evidence that vaccine efficacy decreases with age because of impaired induction of opsonophagoyctic antibodies113. Finally, the antibody isotype influences the efficacy of toxin neutralization through a mechanism that requires engagement of the Fc receptor (FcγR)114. Therefore, better understanding of the mechanisms by which the innate immune system regulates the quality of antibody responses will facilitate the design of adjuvants that target the right DC subsets or PRRs to induce the appropriate quality of antibody response.
Figure 1.
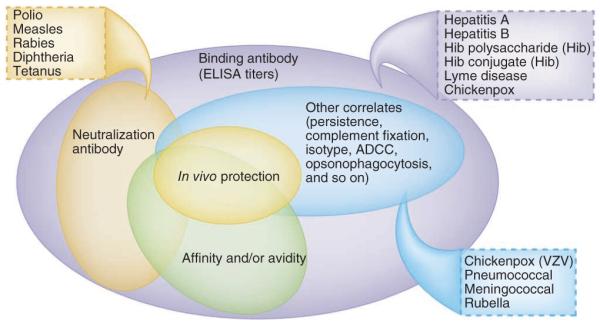
Varieties of antibody responses and their correlates of protection. Antibody responses to vaccination or infection can be qualitatively distinct and can be measured in different ways. Thus, all the antigen-specific binding antibodies to a given antigen can be measured by enzyme-linked immunosorbent assay (ELISA), and the affinity or avidity of antibodies can be assessed by surface plasmon resonance, with those antibodies having an affinity above a certain threshold being deemed high-affinity antibodies. The ability to neutralize the pathogen in vitro is one measure of antibody function. Other correlates include the ability to kill a pathogen by opsonophagocytosis, complement fixation, antibody-dependent cytotoxic cell killing, isotype and persistence. The correlates of protection provided by different vaccines differ in the aforementioned parameters; for example, correlates for polio, measles, rabies, diphtheria or tetanus are considered to be binding antibody titers assessed by enzyme-linked immunosorbent assay, and so on. Hib, H. influenzae type B; VZV, varicella zoster virus.
Another key variable is the persistence of the antibody response. For example, carbohydrate vaccines, such as the vaccine against meningococcus in children or the vaccine against pneumococcus in the elderly, induce immunity that is short lived. The results of an HIV vaccine trial in Thailand evaluating priming with recombinant canarypox-HIV vector and boosting with recombinant HIV-1 envelope gp120 subunit protein plus alum has demonstrated that vaccinees acquired HIV at a lower rate (31%) than people given placebo but that the vaccine had no effect on viral load or CD4+ T cell counts in vaccinees once they became infected15. Interestingly, the vaccine efficacy dropped over time. Although most vaccinees had binding antibodies, titers ‘collapsed’ after 24 weeks. The precise correlate of protection is under intense study, and the immune responses most consistently detected in this trial were CD4+ T cell proliferation, antibody-dependent cell-mediated cytotoxicity, and binding of antibodies to HIV-1 gp120 (ref. 115). Learning how to enhance the persistence of such responses with relevant adjuvants is critical (Fig. 2), and this might potentially be achieved by adjuvants, vectors and cytokines that stimulate natural killer ‘memory’ cells that traffic to the mucosal tissues and facilitate antibody-dependent cell-mediated cytotoxicity116–118. In addition, adjuvants and vectors that enhance the persistence of plasma cells will be useful (Fig. 2). As many live attenuated vaccines (such as those against smallpox and yellow fever) induce antibody responses that last several decades, nanoparticle-based vaccines that resemble viruses in size and immunological composition could be useful19,30. Immunization of mice with nanoparticles containing antigens plus MPL (a TLR4 ligand) with or without R-837 (a TLR7 ligand) induces synergistically more antigen-specific, neutralizing antibodies than does immunization with nanoparticles containing antigens plus a single TLR ligand13. In addition, there is much greater persistence of plasma cell responses lasting over 1.5 years. In principle, this could be achieved at multiple points in the cascade of events involved in the differentiation of antigen-specific B cells to memory B cells and long-lived plasma cells (Fig. 2). Microarray analysis of activated B cells early in the immune response (day 7) indicates that there is early programming toward B cell memory. The mechanism underlying this TLR4-TLR7 synergy is dependent on signaling via MyD88 and TRIF in DCs, but there is also a requirement for TLR signaling in B cells and, notably, both TLRs must be triggered on the same B cell. These results highlight the fact that the magnitude, quality and persistence of antibody responses can be regulated by innate immunity and that the germinal center reaction may be a useful target for adjuvants that promote memory B cell development and persistent plasma cells. The precise mechanisms by which DCs, TLRs and other PRRs might regulate germinal center differentiation, affinity maturation and lon-gevity of the response are still poorly understood, but several genetargeting studies have identified critical roles for molecules such as CD40, ICOS, IL-21, PD-1, CD95, IRF4 and Bcl-6 in this process. Furthermore, enhanced expression of BAFF (B cell–activation factor of the tumor necrosis factor family) or the proliferation-inducing ligand APRIL or their receptors, including BCMA (TNFRSF17), is known to enhance the survival of plasma cells119 (Fig. 2). Indeed, BCMA expression has been found to be the best predictor of neutralizing antibody responses to YF-17D19.
Figure 2.
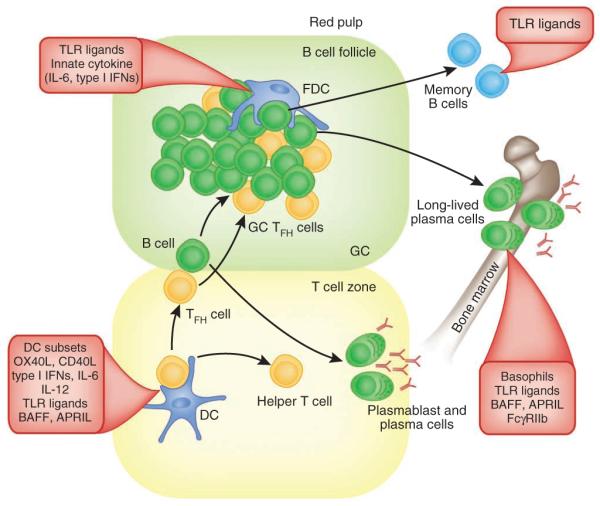
Programming antibody responses with innate immunity. Antigen-specific antibody responses to T cell–dependent antigens develop along two anatomically and functionally distinct pathways132–135. DC-mediated stimulation of antigen-specific TH cells in the T cell–rich areas is regulated by the DC subset and the PRRs triggered. Activated antigen-specific B cells migrate to the interface between the B cell follicle and T cell area132–135. Here, they interact with helper T cells, which results in the clonal expansion of B cells; these migrate to the bridging channels at the edges of the lymphoid areas of the spleen or the medullary cords in the lymph nodes and differentiate into short-lived plasma cells132–135. Other activated B cells migrate into B cell follicles and proliferate rapidly and form germinal centers (GC). In addition, some helper T cells (TFH cells) express CXCR5 and migrate into the follicles. Induction of TFH cells is controlled by the nature of the DC subset, innate cytokines, TLR ligands, costimulatory molecules, TLR ligands and so on. In the early phase of GC development, the dividing B cells (centroblasts) downregulate cell surface expression of immunoglobulin and undergo somatic hypermutation of their immunoglobulin genes132–135. Then, the centroblasts cease to divide and re-express their mutated immunoglobulin receptor, and cells (centrocytes) with heightened affinity for the antigen are thought to be selected for enhanced affinity of binding to antigen-antibody complexes on the follicular DCs, as well as by helper T cells in the light zone136. Positively selected centrocytes differentiate into long-lived plasma cells that migrate to the bone marrow or differentiate into recirculating memory B cells. The survival of long lived plasma cells or memory B cells is controlled by innate parameters such as TLR ligands, basophils14, BAFF or APRIL.
TFH cells have emerged as critical regulators of B cell memory and long-lived plasma cells2. Chemokine receptor CXCR5 expression120,121 and downregulation of the chemokine receptor CCR7 enables CD4+ T cells to home to the follicles, where they help the differentiation of germinal center B cells2,122 (Fig. 2). TFH cells produce large amounts of IL-21 and express the transcription factor Bcl-6. Given their central importance in regulating B cell memory and persistence, the DC subsets and PRRs that induce and maintain TFH cells must be understood. DC-associated molecules such as OX40L and CD40L, as well as cytokines (such as type I interferons, IL-6, IL-12 and IL-23), seem to have important roles in the generation of TFH cells2,122. The lower abundance of TFH cells in B cell–deficient mice or in mice whose B cells lack specific molecules (CD19, CD40, major histocompatibility complex class II or ICOS-L) suggests that B cells are also important for the generation of TFH cells.
Finally, many pathogens pose unique challenges to the induction of protective antibody responses. For example, in dengue infection, non-neutralizing antibodies induced by natural infection with one serotype of the dengue virus may enhance infection with a different serotype by the process of antibody-dependent enhancement123. Poorly neutralizing, cross-reactive antibodies are thought to contribute to enhancing the entry of virus into myeloid cells123. Therefore, a major challenge for those studying and developing vaccines is to induce a balanced immune response to all four of the dengue serotypes and diminish the risk of antibody-dependent enhancement.
Humanity as a model
Many fundamental insights in immunology have emerged from the study of animal models, such as inbred strains of mice, including knockout and transgenic mice. However, it is now clear that despite their many similarities, the immune systems of mice and humans differ in many important details (such as TLR expression on DC subsets)21,124,125. These differences pose an obstacle to the rapid translation of discoveries from mice to the clinic. There has been heightened interest in studying immune responses in humans. For example, studies have focused on immune responses to vaccination in humans using the approach of systems biology19,20. Indeed, in 2010 the National Institutes of Allergy and Infectious Diseases established a consortium focused on understanding the human immune system in its steady state and in response to vaccinations and infections. This consortium, which comprises several institutions throughout the USA, will receive more than $100 million funding over 5 years to analyze human immune responses to vaccination and infection by high-throughput approaches. This effort to systematically characterize immune responses in humans is likely to revitalize human immunology21,124 and accelerate the pace of vaccine development.
Finally, the age distribution of the world’s population is in the midst of an unprecedented change, resulting from a transition from high mortality and high fertility to one of low mortality and low fertility. Indeed, according to the Population Division of the United Nations Secretariat, by 2050 more than 21% of the world’s population will be over 65 years of age and another 20% will be under the age of 14. Therefore, more than 40% of the population will either be elderly or pediatric, yet understanding of how the immune systems in these populations sense and respond to vaccines is at best fragmentary. Furthermore, nearly 850 million people are malnourished and over a billion people are overweight. Therefore, understanding how age and nutritional status influence vaccine-induced immunity in humans is critical. The relationship between nutritional status and immunity or between immunosenescence and vaccine efficacy is well documented126, and it is known that the neonatal innate immune system, which is biased against the production of proinflammatory cytokines, impairs responses to many vaccines127. However, the molecular mechanisms that underlie the impairment of vaccine-induced immunity in the very young and very old are poorly defined.
Vitamin A deficiency is associated with enhanced susceptibility to almost all types of infections, with defects in both the innate and adaptive immune systems128,129. However, the evidence is conflicting, and it is now clear that the nutritional status of children influences immune responses in poorly understood and complex ways130. Similarly, the link between obesity and weak immunity is still being determined, but the chronic inflammation associated with obesity131 might negatively affect vaccine-induced immunity. Therefore, there is a need to critically reexamine the relationship between malnutrition or obesity and immune responses through clinical trials in which multiple parameters of innate and adaptive responses can be evaluated by cutting-edge technologies, including the tools of systems biology19–21.
Acknowledgments
We thank S. Plotkin for discussions; F. Sallusto for sharing the table on which Figure 1 is based; and M. Kwissa and H. Nakaya for helping with the formatting and art work for the figures. Supported by the US National Institutes of Health and the Bill & Melinda Gates Foundation.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotty S. Follicular helper CD4 T cells (TFH) Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting JP, Duncan JA, Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2010;327:286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins C, Gale M., Jr. Recognition of viruses by cytoplasmic sensors. Curr. Opin. Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Ueno H, et al. Harnessing human dendritic cell subsets for medicine. Immunol. Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce TH2 and tolerogenic responses. Nat. Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 13.Kasturi SP, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez M. Rodriguez, et al. Basophils support the survival of plasma cells in mice. J. immunol. 2010;185:7180–7185. doi: 10.4049/jimmunol.1002319. [DOI] [PubMed] [Google Scholar]
- 15.Ganguly S, Manicassamy S, Blackwell J, Pulendran B, Amara RR. Adenovirus type 5 induces vitamin A-metabolizing enzymes in dendritic cells and enhances priming of gut-homing CD8 T cells. Mucosal Immunol. 2011 February 2; doi: 10.1038/mi.2011.1. advance online publication, doi:10.1038/mi.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 17.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33:479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Querec TD, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaucher D, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33:516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotkin SA, Orenstein WA, Offit PA. Vaccines. 5th edn Saunders/Elsevier; Philadelphia: 2008. [Google Scholar]
- 23.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S, et al. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 26.Hammarlund E, et al. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 27.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat. Rev. Immunol. 2009;9:741–747. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed R, Akondy RS. Insights into human CD8+ T-cell memory using the yellow fever and smallpox vaccines. Immunol. Cell Biol. 2011;89:340–345. doi: 10.1038/icb.2010.155. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji S, et al. Maturation of human dendritic cells by cell wall skeleton of mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect. Immun. 2000;68:6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Querec T, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyama S, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 33.Koyama S, et al. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci. Transl. Med. 2010;2:25ra24. doi: 10.1126/scitranslmed.3000759. [DOI] [PubMed] [Google Scholar]
- 34.Geeraedts F, et al. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PloS Pathog. 2008;4:e1000138. doi: 10.1371/journal.ppat.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee EG, et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J. Virol. 2011;85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsay RW, et al. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J. Immunol. 2010;185:1513–1521. doi: 10.4049/jimmunol.1000338. [DOI] [PubMed] [Google Scholar]
- 37.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 39.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kool M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosca F, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. Usa. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2011;239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mata-Haro V, et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 45.Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev. Vaccines. 2009;8:1663–1679. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 46.Einstein MH, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum. Vaccin. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 47.Giannini SL, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 49.Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J. Infect. Dis. 1999;179:489–492. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 50.Lang J, et al. Comparison of the immunogenicity and safety of two 17D yellow fever vaccines. Am. J. Trop. Med. Hyg. 1999;60:1045–1050. doi: 10.4269/ajtmh.1999.60.1045. [DOI] [PubMed] [Google Scholar]
- 51.Wheelock EF, Sibley WA. Circulating virus, interferon and antibody after vaccination with the 17-D strain of yellow-fever virus. N. Engl. J. Med. 1965;273:194–198. doi: 10.1056/NEJM196507222730404. [DOI] [PubMed] [Google Scholar]
- 52.Ipsen J. Circulating antitoxin at the onset of diphtheria in 425 patients. J. Immunol. 1946;54:325–347. [PubMed] [Google Scholar]
- 53.Looney JM, Edsall G, Ipsen J, Jr., Chasen WH. Persistence of antitoxin levels after tetanus-toxoid inoculation in adults, and effect of a booster dose after various intervals. N. Engl. J. Med. 1956;254:6–12. doi: 10.1056/NEJM195601052540102. [DOI] [PubMed] [Google Scholar]
- 54.Dowdle WR, Coleman MT, Mostow SR, Kaye HS, Schoenbaum SC. Inactivated influenza vaccines. 2. Laboratory indices of protection. Postgrad. Med. J. 1973;49:159–163. doi: 10.1136/pgmj.49.569.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad. Med. J. 1973;49:152–158. doi: 10.1136/pgmj.49.569.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine. 2008;26:6754–6758. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Nagarajan T, et al. Human monoclonal antibody and vaccine approaches to prevent human rabies. Curr. Top. Microbiol. Immunol. 2008;317:67–101. doi: 10.1007/978-3-540-72146-8_3. [DOI] [PubMed] [Google Scholar]
- 58.Romero-Steiner S, et al. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 2006;13:165–169. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andreoni J, Kayhty H, Densen P. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J. Infect. Dis. 1993;168:227–231. doi: 10.1093/infdis/168.1.227. [DOI] [PubMed] [Google Scholar]
- 60.Arvin AM. Humoral and cellular immunity to varicella-zoster virus: an overview. J. Infect. Dis. 2008;197(Suppl 2):S58–S60. doi: 10.1086/522123. [DOI] [PubMed] [Google Scholar]
- 61.Levin MJ, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect. Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McElhaney JE, et al. T cell responses are better correlates of vaccine protection in the elderly. J. immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 63.Sester M, et al. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation. 2001;71:1287–1294. doi: 10.1097/00007890-200105150-00018. [DOI] [PubMed] [Google Scholar]
- 64.Bunde T, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J. Exp. Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat. Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 67.Hoft DF. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet. 2008;372:164–175. doi: 10.1016/S0140-6736(08)61036-3. [DOI] [PubMed] [Google Scholar]
- 68.Reyes-Sandoval A, Pearson FE, Todryk S, Ewer K. Potency assays for novel T-cell-inducing vaccines against malaria. Curr. Opin. Mol. Ther. 2009;11:72–80. [PubMed] [Google Scholar]
- 69.Blattman JN, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badovinac VP, Haring SJ, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8+ T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bajenoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindquist RL, et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 74.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 75.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 76.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 77.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 78.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 79.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 80.Pulendran B, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. Usa. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maldonado-Lopez R, et al. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.den Haan JM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 84.Tang H, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat. Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 86.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 88.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 89.Menges M, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nagai T, et al. Timing of IFN-β exposure during human dendritic cell maturation and naive Th cell stimulation has contrasting effects on Th1 subset generation: a role for IFN-β-mediated regulation of IL-12 family cytokines and IL-18 in naive Th cell differentiation. J. Immunol. 2003;171:5233–5243. doi: 10.4049/jimmunol.171.10.5233. [DOI] [PubMed] [Google Scholar]
- 91.Poulin LF, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J. Exp. Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jongbloed SL, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bachem A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rissoan MC, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 95.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 97.Soares H, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohnmacht C, et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 99.Hammad H, et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 101.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manicassamy S, et al. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8α+, and double-negative Peyer’s patch dendritic cells. J. Immunol. 2001;166:4884–4890. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 105.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 106.Manicassamy S, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 110.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin. Immunol. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Delgado MF, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against neisseria meningitidis. N. Engl. J. Med. 2010;362:1511–1520. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- 113.Romero-Steiner S, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 114.Abboud N, et al. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J. Exp. Med. 2010;207:2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 116.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell– mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. Usa. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mackay F, Schneider P. Cracking the BAFF code. Nat. Rev. Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 120.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schaerli P, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deenick EK, Ma CS, Brink R, Tangye SG. Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr. Opin. Immunol. 2011;23:111–118. doi: 10.1016/j.coi.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 123.Ubol S, Halstead SB. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin. Vaccine Immunol. 2010;17:1829–1835. doi: 10.1128/CVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Germain RN. Vaccines and the future of human immunology. Immunity. 2010;33:441–450. doi: 10.1016/j.immuni.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 125.Davis MM. A prescription for human immunology. Immunity. 2008;29:835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen WH, et al. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 128.Bang BG, Bang FB, Foard MA. Lymphocyte depression induced in chickens on diets deficient in vitamin A and other components. Am. J. Pathol. 1972;68:147–162. [PMC free article] [PubMed] [Google Scholar]
- 129.Stephensen CB. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 130.Moore SE, Goldblatt D, Bates CJ, Prentice AM. Impact of nutritional status on antibody responses to different vaccines in undernourished Gambian children. Acta Paediatr. 2003;92:170–176. doi: 10.1111/j.1651-2227.2003.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 131.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu. Rev. Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 133.MacLennan IC, et al. Extrafollicular antibody responses. Immunol. Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 134.Goodnow CC, Sprent J, de St Groth B. Fazekas, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 135.Phan TG, Gray EE, Cyster JG. The microanatomy of B cell activation. Curr. Opin. Immunol. 2009;21:258–265. doi: 10.1016/j.coi.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Victoria GD, et al. Germinal center drynamics by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2011;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


