Abstract
Fatty acids released from white adipose tissue ( WAT) provide important energy substrates during fasting. However, uncontrolled fatty acid release from WAT during non-fasting states causes lipotoxicity and promotes inflammation and insulin resistance, which can lead to and worsen type 2 diabetes (DM2). WAT is also a source for insulin sensitizing fatty acids such as palmitoleate produced during de novo lipogenesis. Insulin and leptin are two major hormonal adiposity signals that control energy homeostasis through signaling in the central nervous system. Both hormones have been implicated to regulate both WAT lipolysis and de novo lipogenesis through the mediobasal hypothalamus (MBH) in an opposing fashion independent of their respective peripheral receptors. Here, we review the current literature on brain leptin and insulin action in regulating WAT metabolism and discuss potential mechanisms and neuro-anatomical substrates that could explain the opposing effects of central leptin and insulin. Finally, we discuss the role of impaired hypothalamic control of WAT metabolism in the pathogenesis of insulin resistance, metabolic inflexibility and type 2 diabetes.
Keywords: Adipose tissue, Lipolysis, De novo lipogenesis, Sympathetic nervous system, Brain, Insulin, Leptin
1 Introduction
Access to high caloric food has become temptingly easy over the last decades, while our lifestyle has become more sedentary, resulting in an unprecedented epidemic of obesity [1]. The obese state promotes insulin resistance and markedly increases the risk for DM2. WAT plays a critical role in energy homeostasis both as an endocrine organ and as a storage organ of energy rich triglycerides (TGs). Obesity is commonly associated with dysfunctional WAT [2–4] which is the source of excess free fatty acids (FFAs) and inflammatory mediators that cause and worsen insulin resistance that sets the stage for DM2 [5].
In mammals surplus nutrients are converted mainly into TGs that can be most efficiently stored in WAT. In the fed state nutrients are absorbed in the gut and converted into TGs in the liver. The liver then secretes these TGs as very low density lipoproteins, that will either be utilized by the muscle or, when supply exceeds demand, are stored in WAT [6]. Conversely, during fasting or energy demanding states, such as exercise and infection, WAT breaks down its stored TGs during lipolysis which releases glycerol and FFAs into the bloodstream to provide energy substrates for processes such as β-oxidation and gluconeogenesis [7, 8]. The continuous transition between fasting and feeding requires WAT to dynamically switch from a fatty acid storing to a fatty acid releasing mode according to metabolic needs. This metabolic flexibility is critical for energy homeostasis. Thus, WAT dysfunction is characterized by the inability of WAT to store lipids or restrain lipolysis in the fed state. WAT dysfunction is observed in lipodystrophic [9], obese and DM2 patients [10–12] and results in elevated circulating FFAs. Excessive lipolysis in WAT causes accumulation of ectopic lipids and promotes a pro-inflammatory state [13–15], which can cause or worsen insulin resistance in muscle and liver [16–18].
WAT is also capable of synthesizing fatty acids during de novo lipogenesis. Although, quantitatively WAT de novo lipogenesis adds little to the whole body lipid pool [19], it may serve important metabolic functions that we are just beginning to explore. A recent study has shown that the fatty acid palmitoleate, which seems to be mainly released by WAT, bears systemic insulin sensitizing properties in mice [20]. Data on palmitoleate in humans are mixed. While high circulating palmitoleate is associated with improved cholesterol profiles in men, it is also linked to increased TG levels and insulin resistance [21]. However, these studies did not differentiate the source of palmitoleate, which is important since increased hepatic lipid production is associated with insulin resistance [22, 23] rendering liver derived palmitoleate a possible confounder. Indeed, palmitoleate from exogenous sources (non-liver-derived), such as dairy products, is associated with lower insulin resistance and incidence of DM2 in humans [24]. In rodents, whose lipogenic capacity seems to exceed that of humans [25], palmitoleate is implicated in improving glucose uptake in vitro and in vivo [20, 26] and to also reduce hepatosteatosis by blocking lipogenesis in the liver [20]. Yet, the exact molecular mechanism of how palmitoleate exerts its insulin sensitizing effects remains to be elucidated. Furthermore, there is emerging evidence that in human obesity WAT de novo lipogenesis is reduced [27–29], which may result in lower palmitoleate secretion from WAT contributing to insulin resistance, although this has not been stringently proven. Therefore, failure of WAT de novo lipogenesis may represent an additional feature of WAT dysfunction. Apart from lipid production, storage and release, WAT is a highly active endocrine organ that secretes adipokines such as adiponectin and leptin, that control energy homeostasis by regulating appetite and partitioning of glucose and lipids, which has been reviewed extensively elsewhere [30].
2 Regulation of lipid metabolism by insulin and leptin
Lipid partitioning is regulated by several circulating factors, such as hormones and cytokines. The focus of this review will be on insulin, secreted by the endocrine pancreas, and leptin, produced primarily by adipocytes. The two hormones circulate in levels proportional to body fat and are considered the main endocrine adiposity signals in mammals [31] that communicate current energy availability to the brain. Within the brain, and in particular the hypothalamus, leptin and insulin signaling are integrated with other signals such as neurotransmitters and nutrients. The hypothalamus, in turn, orchestrates nutrient partitioning and appetite [31, 32]. The prevailing paradigm of brain insulin’s and leptin’s role in the regulation of energy homeostasis is that they act synergisti-cally. Both hormones suppress food intake via signaling in the hypothalamus [33–39], although brain insulin’s anorectic effects have recently been challenged [40]. However, clinically their effects are quite different: Insulin has several anabolic properties and diabetic patients started on insulin tend to gain weight [41], while leptin administration reduces adiposity in leptin deficient rodents and humans [35, 42].
Insulin is considered the major anti-lipolytic [7] and prolipogenic regulator [43] in WAT and these effects are thought to be exclusively mediated via the insulin receptor expressed on adipocytes [44]. Humans with insulin receptor mutations exhibit lipodystrophy or even lipoatrophy, a severe reduction in WAT mass with increased circulating fatty acids [45, 46]. The classical explanation for this phenotype is that the lipodystrophy results from the loss of peripheral insulin receptor signaling in adipocytes. However, mice that lack the insulin receptor exclusively in fat tissue develop only a mild reduction in adipose tissue mass [47], indicating that the loss of the adipocyte insulin receptor cannot fully explain the lipodystrophic phenotype of patients with insulin receptor defects. Furthermore, the inducible deletion of the insulin receptor throughout the periphery but not the brain only moderately reduces adiposity, while inducible deletion of the insulin receptor in the whole body, that is brain and periphery, leads to severe lipodystrophy within 4 weeks [48, 49]. This indicates that brain insulin signaling plays a pivotal role in preserving fat mass and retaining FFAs in WAT.
While insulin suppresses lipolysis and induces lipogenesis in WAT, the acute effects of leptin on WAT metabolism oppose those of insulin by inducing lipolysis and inhibiting lipogenesis, which contribute in the long-term to leptin’s ability to reduce adiposity [50, 51]. These effects of leptin (as most metabolic effects) are mediated primarily via signaling in the brain. Re-constitution of neuronal leptin receptors completely reverses the lipotoxic, dysmetabolic phenotype of leptin receptor deficient db/db mice [52]. Conversely, deletion of the peripheral leptin receptor in mice but not the brain results in no obvious dysmetabolic phenotype or alterations in adiposity [53].
Further support for the concept that insulin and leptin have opposing effects on WAT metabolism has been provided by studies of leptin deficient humans where insulin action was studied with pancreatic clamp studies either with or without leptin replacement therapy. Interestingly, upon discontinuation of leptin therapy, baseline FFA levels decreased and were more effectively suppressible by insulin [54].
3 Brain regulation of WAT metabolism by leptin and insulin
The anti-adiposity effects of systemic leptin can be reproduced by infusing small amounts of leptin intracerebroventricular (ICV) and these anti-adiposity effects of leptin occur in part independent of its anorexic effects [55]. Conversely, reducing neuronal leptin receptor levels by 50% in mice results in increased fat mass, while food intake seems not to be affected [56]. Acutely, leptin infused into the MBH suppresses de novo lipogenesis by increasing protein expression and the activation state of key lipogenic enzymes such as fatty acid synthase and ATP citrate lyase [57]. Furthermore, MBH leptin suppresses fatty acid uptake into visceral WAT, while it stimulates lipolysis by increasing the activation state of hormone sensitive lipase (HSL) via phosphorylation of the serine residues 563 and 660 [57]. Since these HSL phosphorylation sites are targets of protein kinase A [58], an enzyme that is induced via the sympathetic nervous system, it is likely that MBH leptin stimulates lipolysis by increasing sympathetic nervous system outflow to WAT.
To the contrary, chronic ICV insulin infusion can increase fat mass in mice without affecting food intake [49]. As pointed out earlier systemic insulin exerts strong anti-lipolytic and pro-lipogenic effects in WAT within minutes or at most hours. Insulin infusion into the MBH acutely restrains lipolysis in rats, as assessed by WAT triglyceride hydrolase activity and HSL activation state, by reducing sympathetic nervous system outflow to WAT [59]. The role of the parasympathetic nervous system in the central regulation of WAT by insulin and leptin is untested at present, however parasympathetic innervation of WAT remains controversial [60, 61].
In contrast to leptin, MBH insulin increases expression of lipogenic proteins such as fatty acid synthase and acetyl-CoA carboxylase in rats thereby stimulating WAT de novo lipogenesis [59]. Furthermore, deletion of the insulin receptor in neurons of mice (Nirko) results in increased lipolytic rates in the fasted state and altered suppression of FFA release after re-feeding. Conversely, Nirko mice exhibit suppressed de novo lipogenesis in WAT, which coincides with a decrease in WAT palmitoleate levels [59]. Therefore, the loss of insulin signaling in the brain causes WAT dysfunction. In summary, these studies provide evidence that the opposing effects of leptin and insulin on WAT metabolism are at least in part mediated via the brain.
4 Lipolytic flux from WAT drives hepatic gluconeogenesis
FFA levels closely correlate with hepatic glucose production, independent of systemic insulin or glucose levels [67]. Furthermore, insulin’s anti-lipolytic properties are essential for the suppression of hepatic glucose production [66], because glycerol acts as a gluconeogenic precursor, while FFAs provide important energy substrates to the liver to fuel gluconeogenesis [8]. Both brain leptin and insulin have the ability to alter hepatic glucose flux (reviewed in [62]). Brain insulin infusion in rodents suppresses hepatic glucose production by decreasing gluconeogenesis, while glycogenolysis in the liver is not affected [63, 64]. Central leptin infusion acutelely induces gluconeogenesis while suppressing glycogenolysis. Thus, while central leptin alters glucose partitioning in the liver, this does not change net hepatic glucose output [65]. Therefore, the opposing effects of brain insulin and leptin on WAT lipolysis are mirrored in the regulation of hepatic gluconeogenesis through brain signaling by both these hormones. Lipolytic flux closely correlated with hepatic glucose production in rats that received brain insulin infusions [59], suggesting that the brain control of hepatic glucose production occurs in part via the central regulation of WAT lipolysis.
5 The role of Agrp/NPYand Pomc neurons in regulating WAT metabolism
It is tempting to speculate that the effects of brain insulin and leptin are mediated by a single neuronal subpopulation within the hypothalamus. Two likely candidates are proopiomelanocortin (Pomc) and agouti–related peptide (Agrp) expressing neurons, as they play signal roles in controlling energy homeostasis and are both targets of insulin and leptin [32]. Deletion of leptin receptors in either Pomc or Agrp neurons leads to moderate obesity, while the combined knock-out of leptin receptors in Agrp and Pomc neurons has an additive effect resulting in an approximately 30% increase in adiposity despite equal food intake among all strains [68, 69]. These findings suggest that brain leptin signaling in both Pomc and Agrp neurons plays an important role in the regulation of adiposity. Mice that lack both insulin and leptin receptors in Pomc neurons are markedly insulin resistant. However, double knock-out of leptin and insulin receptors on Pomc neurons partially reverses the obesity phenotype of isolated leptin receptor Pomc knock-out mice [70]. This suggests that the opposing effects of insulin and leptin are integrated in Pomc neurons, where insulin signaling seems to preserve fat mass, whereas leptin signaling decreases it. Yet, deletion of the insulin receptor in either Pomc or Agrp neurons causes no change in adiposity, indicating that other redundant pathways compensate. Furthermore, only Agrp insulin receptor knock-out mice, but not Pomc, fail to suppress hepatic glucose production during hyperinsulinemia [71]. Lipid fluxes have not been comprehensively studied in these mouse models as of yet. However, judging from the energy balance and glucose homeostasis phenotype of the aforementioned mouse models, it is unlikely that a single neuronal subtype can explain both insulin and leptin’s effects on lipid metabolism, rather that it is integrated within a complex network of neurons that is maintained in a delicate balance. In some cases insulin signaling in one neuronal subtype can even antagonize the effects of insulin signaling in another [72]. Thus, lifelong Cre-lox knock-out models have several limitations in identifying first order neurons that mediate the acute effects of brain insulin and leptin in regulating energy metabolism: First, it is unclear which neuronal populations in the CNS express Pomc and Agrp during development. Thus, during development Cre recombinase potentially targets cells that are different from the classic Agrp/Pomc neurons in adult animals [73]. Secondly, even if a specific neuronal knock-out model blunts the ability of brain insulin and/or leptin to regulate WAT metabolism, this does not necessarily imply that the particular neuronal population is the main target, only that the balance within the neuronal network is disturbed. This imbalance renders the hypothalamus insensitive to the acute effects of leptin and/or insulin, but also possibly to other effectors such as nutrients.
6 The neurophysiologic effects of insulin and leptin signaling in Pomc and Agrp neurons
Insulin and leptin affect Pomc and Agrp neurons in a distinct manner. While leptin increases the frequency of action potentials in some Pomc expressing neurons [74, 75], insulin hyperpolarizes a subset of Pomc neurons [70, 71, 75]. Both hormones seem to depend on intact PI3K signaling to exert these effects [75] and, surprisingly, both insulin and leptin stimulate PI3K activity in Pomc cells [76]. Yet, how can two hormones affect neuronal activity in an opposing fashion and at the same time activate the same intracellular signaling cascade? This apparent paradox was solved by the demonstration that the opposing neuronal responses to insulin and leptin are integrated in two distinct subpopulations of Pomc neurons that reside in different areas of the hypothalamus, rather than in the same exact neurons [77]. However, other reports suggest that insulin is able to hyperpolarize a small number of Pomc neurons, which were pre-stimulated with leptin [75], suggesting that in rare cases, insulin and leptin signals can be integrated in single Pomc neurons. Taken together, leptin and insulin seem to induce opposing electrophysiological responses in subpopulations of Pomc neurons in the hypothalamus (also reviewed in [78, 79]).
In Agrp neurons insulin and leptin induce different signaling cascades. Insulin increases while leptin suppresses PI3K in a process that requires synaptic transmission from Pomc and other inhibitory presynaptic neurons [76]. Insulin’s electrophysiological effects in Agrp neurons are heterogeneous; only a small subset of Agrp neurons is insulin responsive and insulin has either depolarizing [75, 80] or hyperpolarizing effects [71]. Leptin seems to not affect Agrp neuron spike frequency in studies performed in mice [75, 80], but to hyperpolarize pacemaker neurons in the rat arcuate nucleus of the hypothalamus (ARC)[81]. Thus, insulin and leptin affect subsets of Agrp and Pomc neurons in an opposing fashion. The finding that in Agrp neurons insulin activates while leptin suppresses PI3K, as well as the opposing electrical responses evoked in Pomc neurons, could represent potential mechanisms through which brain insulin and leptin exert opposing effects on WAT metabolism.
7 Future directions
Pomc and Agrp neurons, while important in the regulation of energy homeostasis, are only two of many neuronal subtypes that reside within the MBH. As leptin and insulin receptors are expressed widely within the CNS, other neuronal populations besides Agrp and Pomc are likely to participate in the regulation of WAT metabolism through brain leptin and insulin and await further characterization. Further, the electrophysiology as well as the intracellular signaling events that are triggered by insulin and leptin, require further study. This knowledge could prove critical in understanding the molecular events that lead to the diverging effects of brain insulin and leptin signaling in regulating WAT metabolism. Furthermore, it remains largely unclear how insulin and/or leptin signaling impact upon other signaling pathways, such as the GABA-ergic or dopamine-ergic system, and how these signaling events are integrated with nutrient sensing in the MBH.
Finally, retrograde viral tracer studies in rodents revealed multiple CNS regions that are involved in autonomic innervation of WAT. The paraventricular nucleus (PVN) of the hypothalamus, which is known to project directly to spinal sympathetic neurons [82], stained positive at an early stage after virus injection into WAT [83, 84]. At later time points the infection spread to the ARC and lateral hypothalamus [83], suggesting that these brain regions are upstream of the PVN. Interestingly, only Pomc expressing, but not NPY expressing neurons in the ARC co-localized with viral infection [83]. Pomc expressing neurons release α-melanocyte stimulating hormone, a cleavage product of Pomc, which activates melanocortin receptors. Central melanocortin agonists increase lipolysis and SNS outflow to WAT in siberian hamsters [85] and melanocortin 4 receptor mRNA expression highly co-localizes with pseu-dorabies virus infected neurons [84], making Pomc neurons a likely integration site of MBH insulin and leptin signals to WAT. Given the distinct localization of the insulin and leptin reactive Pomc subpopulations within the MBH, it may be that these neuronal subsets project to different 2nd order neurons that then activate or block SNS outflow to WAT integrating the opposing effects of insulin and leptin on a topographic basis. There is evidence for this in rats, since neurons of the anterior half of the ARC (insulin responsive neurons) were found to project to autonomic areas such as the dorsal vagal complex [86], whereas neurons of the caudal portion (leptin responsive Pomc neurons) primarily connect to the PVN [77, 87].
Adiposity is regulated by many factors and the direct regulation of WAT lipolysis and lipogenesis represents only two among these. The metabolic phenotyping of currently available mouse models of neuron specific insulin and leptin receptor deletions has been mostly limited to the study of body composition, glucose fluxes and serum lipid profiles, parameters that are only indirectly affected through alterations in lipolytic flux from WAT. A more comprehensive assessment of lipid fluxes during fasting to re-feeding transitions or hyperinsulinemic clamps as well as in vivo determinations of WAT de novo lipogenesis in these mouse models will further our understanding of the regulation of WAT metabolism through the CNS.
It is important to point out that although long-term leptin treatment improves energy homeostasis in leptin deficient rodents and humans, the acute effects of leptin in non-leptin deficient rodents are conceivably detrimental if for example increased lipolysis is not counterbalanced by increased fatty acid utilization resulting in lipotoxicity. Under physiologic circumstances plasma levels of insulin and leptin change in parallel—both hormones are increased in the fed state and low during fasting although these changes during the fasting to re-feeding transition are much more pronounced in the case of insulin and subtle with leptin [88]. In the fed state FFA release from WAT should be restrained while nutrient storage in WAT should increase, which are both a function of brain insulin signaling. To the contrary, leptin increases lipolysis and lowers de novo lipogenesis in WAT, both signs of WAT dysfunction. The fact that leptin administration in leptin deficient animals is not detrimental is likely due to the coordinated regulation of a number of metabolic effects such as an induction in β-oxidation. Thus, the balance between brain insulin and leptin action are a critical determinant of metabolic flexibility. The obese state is characterized through both leptin and insulin resistance. One can speculate that in the obese state, which is characterized by hyperleptinemia, the effects of leptin on WAT metabolism are preserved and not decreased as a consequence of leptin resistance. If this would turn out to be true, then leptin should drive WAT lipolysis and hamper de novo lipogenesis in obesity and DM2 and thus contribute to WAT dysfunction.
8 Summary
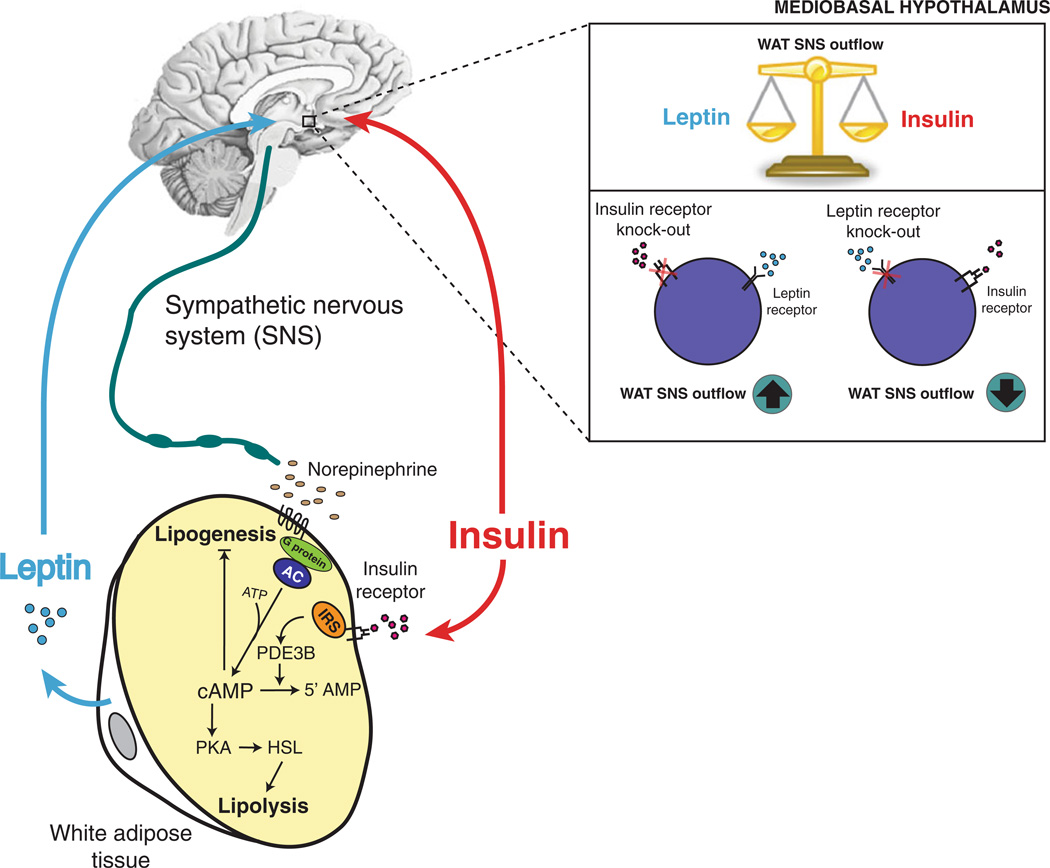
The acute effects of insulin and leptin on WAT lipolysis and de novo lipogenesis oppose each other and can in part be explained by hypothalamic signaling (See proposed model in Fig. 1). Leptin and insulin seem to activate or dampen sympathetic outflow to WAT, respectively. Impaired leptin and/or insulin signaling in the brain disrupt the brain control of lipolysis and de novo lipogenesis in WAT. This is mirrored by mouse models of either brain insulin and leptin receptor deficiency, which reproduce key components of WAT dysfunction in the obese and diabetic state. WAT is a driver of hepatic gluconeogenesis through its control of lipolytic flux, yet WAT is also an important source of insulin sensitizing fatty acid species. Thus, the control of WAT metabolism through brain insulin and leptin is likely to play an important role in lipid and glucose homeostasis.
Fig. 1.
Proposed model of MBH insulin and leptin regulation of WAT metabolism. The acute effects of insulin and leptin signaling in hypothalamic neurons regulate WAT de novo lipogenesis and lipolysis in an opposing fashion. MBH leptin infusion decreases de novo lipogenic protein expression and induces Hsl activation, whereas MBH insulin increases de novo lipogenesis and inhibits WAT lipolysis. These effects seem to be mediated through either stimulation or inhibition of sympathetic outflow to WAT. In addition, insulin exerts direct effects on WAT by binding to the adipocyte insulin receptor, which leads to the inhibition of phosphodiesterase 3B resulting in degradation of cyclic-AMP [89, 90]
Acknowledgements
This work was supported by NIH Grants DK074873, DK083568 and DK082724 and an ADA basic research award to C.B. and a European Foundation for the Study of Diabetes grant to T.S.. C.B. is the recipient of a Hirschl Award.
Abbreviations
- ARC
Arcuate nucleus of the hypothalamus
- DM2
Diabetes mellitus type 2
- WAT
White adipose tissue
- MBH
Mediobasal hypothalamus
- FFAs
Free fatty acids
- TGs
Triglycerides
- Agrp
Agouti-related peptide
- NPY
Neuropeptide Y
- Pomc
Proopiomelanocortin
- SD
Sprague Dawley
- Nirko
Neuronal insulin receptor knock-out
- PI3K
Phosphoinositide 3 kinase
- STAT
Signal transducer and activator of transcription
- HSL
Hormone sensitive lipase
- PVN
Paraventricular nucleus
- ICV
Intracerebroventricular
References
- 1.WHO. WHO; 2006. http://www.who.int/mediacentre/factsheets/fs311/en/index.html. [Google Scholar]
- 2.Gordon ES. Non-esterified fatty acids in the blood of obese and lean subjects. Am J Clin Nutr. 1960;8(5):740–747. [Google Scholar]
- 3.Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009;17(10):1872–1877. doi: 10.1038/oby.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298(4):C961–C971. doi: 10.1152/ajpcell.00547.2009. [DOI] [PubMed] [Google Scholar]
- 5.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006;6(3):177–181. doi: 10.1007/s11892-006-0031-x. [DOI] [PubMed] [Google Scholar]
- 6.Pond C. The fats of life. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 7.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48(5):275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Hers HG, Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- 9.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 10.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72(1):96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- 11.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes. 1993;42(11):1567–1573. doi: 10.2337/diab.42.11.1567. [DOI] [PubMed] [Google Scholar]
- 12.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, et al. Glucose, and free fatty acid metabolism in non-insulin-dependent diabetes mellitus Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120(10):3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54(12):3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 15.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 16.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97(12):2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden G, Cheung P, Stein TP, Kresge K, Mozzoli M. FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am J Physiol Endocrinol Metab. 2002;283(1):E12–E19. doi: 10.1152/ajpendo.00429.2001. [DOI] [PubMed] [Google Scholar]
- 19.Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004;30(4):294–309. doi: 10.1016/s1262-3636(07)70121-0. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, et al. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr. 2010;92(6):1350–1358. doi: 10.3945/ajcn.110.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133(2):496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 23.D’Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33(8):1817–1822. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, et al. Trans-palmitoleic acid metabolic risk factors new-onset diabetes in U.S adults: a cohort study. Ann Intern Med. 2010;153(12):790–799. doi: 10.1059/0003-4819-153-12-201012210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swierczynski J, Goyke E, Wach L, Pankiewicz A, Kochan Z, Adamonis W, et al. Comparative study of the lipogenic potential of human and rat adipose tissue. Metabolism. 2000;49(5):594–599. doi: 10.1016/s0026-0495(00)80033-5. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya Y, Hatakeyama H, Emoto N, Wagatsuma F, Matsushita S, Kanzaki M. Palmitate-induced down-regulation of sortilin and impaired GLUT4 trafficking in C2C12 myotubes. J Biol Chem. 2010;285(45):34371–34381. doi: 10.1074/jbc.M110.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002;282(1):E46–E51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- 28.Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52(5):882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- 29.Mayas MD, Ortega FJ, Macias-Gonzalez M, Bernal R, Gomez-Huelgas R, Fernandez-Real JM, et al. Inverse relation between FASN expression in human adipose tissue and the insulin resistance level. Nutr Metab (Lond) 2010;7(3) doi: 10.1186/1743-7075-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307(5708):375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 33.Weigle DS, Bukowski TR, Foster DC, Holderman S, Kramer JM, Lasser G, et al. Recombinant ob protein reduces feeding and body weight in the ob/ob mouse. J Clin Invest. 1995;96(4):2065–2070. doi: 10.1172/JCI118254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 35.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 36.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombi-nant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 37.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. PharmacolBiochemBehav. 2002;72(1–2):423–429. doi: 10.1016/s0091-3057(01)00780-8. [DOI] [PubMed] [Google Scholar]
- 38.Woods SC, Lotter EC, McKay LD, Porte D. Chronic intra-cerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282(5738):503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 39.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci. 1995;109(3):528–531. doi: 10.1037//0735-7044.109.3.528. [DOI] [PubMed] [Google Scholar]
- 40.Jessen L, Clegg DJ, Bouman SD. Evaluation of the lack of anorectic effect of intracerebroventricular insulin in rats. Am J Physiol Regul Integr Comp Physiol. 2010;298(1):R43–R50. doi: 10.1152/ajpregu.90736.2008. [DOI] [PubMed] [Google Scholar]
- 41.Franssila-Kallunki A, Groop L. Factors associated with basal metabolic rate in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35(10):962–966. doi: 10.1007/BF00401426. [DOI] [PubMed] [Google Scholar]
- 42.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, et al. Beneficial effects of leptin on obesity, T cell hypores-ponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assimacopoulos-Jeannet F, Brichard S, Rencurel F, Cusin I, Jeanrenaud B. In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism. 1995;44(2):228–233. doi: 10.1016/0026-0495(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 44.Degerman E, Landström TR, Holst LS, Göransson O, Härndahl L, Ahmad F, et al. Role for Phosphodiesterase 3B in Regulation of Lipolysis and Insulin Secretion. In: LeRoith D, Olefsky JM, Taylor SI, editors. Diabetes mellitus: A fundamental and clinical text. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 374–381. [Google Scholar]
- 45.Hegele RA. Monogenic forms of insulin resistance: apertures that expose the common metabolic syndrome. Trends Endocrinol Metab. 2003;14(8):371–377. doi: 10.1016/s1043-2760(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 46.Donohue WL, Uchida I. Leprechaunism: a euphemism for a rare familial disorder. J Pediatr. 1954;45(5):505–519. doi: 10.1016/s0022-3476(54)80113-2. [DOI] [PubMed] [Google Scholar]
- 47.Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3(1):25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 48.Seibler J, Kleinridders A, Kuter-Luks B, Niehaves S, Bruning JC, Schwenk F. Reversible gene knockdown in mice using a tight, inducible shRNA expression system. Nucleic Acids Res. 2007;35(7):e54. doi: 10.1093/nar/gkm122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118(6):2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang MY, Lee Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem. 1999;274(25):17541–17544. doi: 10.1074/jbc.274.25.17541. [DOI] [PubMed] [Google Scholar]
- 51.Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, et al. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci USA. 1997;94(9):4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115(12):3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, et al. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148(8):3987–3997. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 54.Paz-Filho GJ, Ayala A, Esposito K, Erol HK, Delibasi T, Hurwitz BE, et al. Effects of leptin on lipid metabolism. Horm Metab Res. 2008;40(8):572–574. doi: 10.1055/s-0028-1082052. [DOI] [PubMed] [Google Scholar]
- 55.Gallardo N, Bonzon-Kulichenko E, Fernandez-Agullo T, Molto E, Gomez-Alonso S, Blanco P, et al. Tissue-specific effects of central leptin on the expression of genes involved in lipid metabolism in liver and white adipose tissue. Endocrinology. 2007;148(12):5604–5610. doi: 10.1210/en.2007-0933. [DOI] [PubMed] [Google Scholar]
- 56.McMinn JE, Liu SM, Liu H, Dragatsis I, Dietrich P, Ludwig T, et al. Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab. 2005;289(3):E403–E411. doi: 10.1152/ajpendo.00535.2004. [DOI] [PubMed] [Google Scholar]
- 57.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14(6):667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273(1):215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 59.Scherer T, O’Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13(2):183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 61.Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat-functional implications. J Clin Invest. 2002;110(9):1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buettner C, Camacho RC. Hypothalamic control of hepatic glucose production and its potential role in insulin resistance. Endocrinol Metab Clin North Am. 2008;37(4):825–840. doi: 10.1016/j.ecl.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434(7036):1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 64.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8(12):1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 65.Gutierrez-Juarez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem. 2004;279(48):49704–49715. doi: 10.1074/jbc.M408665200. [DOI] [PubMed] [Google Scholar]
- 66.Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest. 1996;98(3):741–749. doi: 10.1172/JCI118846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes. 1995;44(9):1038–1045. doi: 10.2337/diab.44.9.1038. [DOI] [PubMed] [Google Scholar]
- 68.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 69.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11(4):286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 72.Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in AgRP and POMC neurons. Diabetes. 2009 doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010;16(4):403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 75.Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10(5):343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115(4):951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci: The Official Journal of the Society for Neuroscience. 2010;30(7):2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belgardt BF, Okamura T, Bruning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol. 2009;587(Pt 22):5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belgardt BF, Bruning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N YAcad Sci. 2010;12(12):97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- 80.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117(8):2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7(5):493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 82.Yamashita H, Inenaga K, Koizumi K. Possible projections from regions of paraventricular and supraoptic nuclei to the spinal cord: electrophysiological studies. Brain Res. 1984;296(2):373–378. doi: 10.1016/0006-8993(84)90077-5. [DOI] [PubMed] [Google Scholar]
- 83.Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci USA. 2010;107(15):7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1467–R1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 85.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148(11):5339–5347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 86.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R247–R258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- 87.Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J Comp Neurol. 1995;358(4):518–530. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- 88.Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, et al. Expression of ob mRNA its encoded protein in odentsImpact of nutrition and obesity. J Clin Invest. 1995;96(3):1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Degerman E, Smith CJ, Tornqvist H, Vasta V, Belfrage P, Manganiello VC. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci USA. 1990;87(2):533–537. doi: 10.1073/pnas.87.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith CJ, Vasta V, Degerman E, Belfrage P, Manganiello VC. Hormone-sensitive cyclic GMP-inhibited cyclic AMP phosphodi-esterase in rat adipocytes Regulation of insulin- and cAMP-dependent activation by phosphorylation. J Biol Chem. 1991;266(20):13385–13390. [PubMed] [Google Scholar]