Abstract
The mitochondria-associated membrane (MAM) is a sub-region of the endoplasmic reticulum (ER) that facilitates crosstalk between the ER and mitochondria. The MAM actively influences vital cellular processes including Ca2+ signaling and protein folding. Detergent-resistant microdomains (DRMs) may localize proteins to the mitochondria/MAM interface to coordinate these events. However, the protein composition of DRMs isolated from this region is not known. Lipid-raft enriched DRMs were isolated from a combined mitochondria/MAM sample and analyzed using two-dimensional reversed-phased tandem mass spectrometry. Strict post-acquisition filtering of the acquired data led to the confident identification 250 DRM proteins. The majority (58%) of the identified proteins are bona fide mitochondrial or ER proteins according to Gene Ontology annotation. Additionally, 74% of the proteins have previously been noted as MAM-resident or -associated proteins. Furthermore, ~20% of the identified proteins have a documented association with lipid rafts. Most importantly, known internal LR marker proteins (inositol 1,4,5-trisphosphate receptor type 3, erlin-2, and voltage-dependent anion channel 1) were detected as well as most of the components of the mitochondrial/MAM-localized Ca2+ signaling complex. Our study provides the basis for future work probing how the protein activities at the mitochondrion/MAM interface are dependent upon the integrity of these internal lipid-raft-like domains.
Keywords: Ca2+ signaling, detergent-resistant membranes, mitochondria, mitochondria-associated ER membranes, protein folding
1. Introduction
Lipid rafts (LRs) are classically defined as cholesterol- and sphingolipid-enriched microdomains found in plasma membranes (PMs) [1]. They are thought to provide a more ordered environment within the cell membrane due to the presence of lipid species that, together with cholesterol, stabilize the structure through close packing [2]. Rafts are characteristically insoluble in cold, non-ionic detergents and can be experimentally isolated as detergent-resistant membranes (DRMs) [3]. A key characteristic of LR-enriched microdomains is that they sequester select proteins and act as organizing scaffolds for numerous processes (e.g. molecule trafficking) [1,4,5,6].
Although LRs were initially thought to reside solely in the plasma membrane, increasing evidence suggests the presence of raft-like regions in internal organelles [7,8,9,10,11]. Membrane rafts derived from within the cell would most likely exhibit different biochemical properties and contain a unique set of proteins since these regions have distinctive lipid compositions and decreased levels of cholesterol [12]. Although rafts from these internal structures have not yet been characterized in a global way, significant progress is evident. Membrane rafts isolated from mitochondria contain the voltage-dependent anion channel 1 (VDAC1) and the fission protein hFis and can recruit other proteins when the cell death program is initiated [10,11]. Raft-like domains in the endoplasmic reticulum (ER) are characterized by the presence of two proteins that only localize to the ER, the prohibitins erlin-1 and erlin-2 [9]. In addition, DRMs isolated from the ER sub-structure known as the mitochondria-associated membrane (MAM) were recently shown to contain the novel ligand-responsive sigma-1 receptor molecular chaperone and the type 3 inositol 1,4,5-trisphosphate receptor (IP3R3) [13].
The MAM has garnered much attention recently as a signaling focal point because it specifically interacts with the mitochondrial membrane in order to integrate and coordinate Ca2+ signaling [13,14,15]. Deregulation of Ca2+ trafficking can result in improper protein folding, metabolic disruption, and apoptosis [16,17,18,19] and recent studies suggest that membrane rafts are involved in coordinating the protein interactions required for proper Ca2+ exchange between the MAM and mitochondria [13,20]. For this reason, we comprehensively characterized a combined mitochondria/MAM DRM sample in order to determine the identity of the proteins localized at this interface within this specialized subdomain. Fortuitously, the MAM is physically distinct from “normal” ER and is tethered to the mitochondrion by protein filaments [21], a feature that allows for its co-purification with mitochondria during enrichment by centrifugation. DRMs were then rapidly isolated from crude mitochondria samples by differential detergent solubility, solubilized and trypsinized using gel-assisted digestion [22], and analyzed via two-dimensional reversed-phased (RP/RP) tandem mass spectrometry (MS/MS). Our analysis allowed for the confident identification of proteins known to reside in the mitochondrion and/or the MAM and that facilitate crosstalk between the two organelles. This new knowledge provides further insight into the biological processes that may be regulated by intracellular LR-enriched DRMs.
2. Material and Methods
2.1 Materials
Standard laboratory chemicals were obtained from Thermo Fisher Scientific (Rockfold, IL). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) with the exception of the following: FBS was from Atlanta Biologicals (Lawrenceville, GA); protease inhibitor cocktail was from Roche Applied Science (Indianapolis, IN); sequencing-grade modified-trypsin was from Promega (Madison, WI). Primary antibodies (anti-flotillin-2 and anti- histone H3) and all secondary antibodies were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA) except for the anti-IP3R3 primary antibody, which was obtained from BD Sciences (Franklin Lakes, NJ).
2.2 Cell Culture
NG108-15 cells (ATCC, Manassass, VA) were cultured at 37 °C, 5% CO2 in MEM (pH 7.4) containing 10% FBS, 0.1 mM hypoxanthine, 400 nM aminopterin, and 0.016 mM thymidine. Cells were considered confluent at 80% flask coverage.
2.3 Whole Cell Lysate Preparation
Attached cells were dissociated with 1.5 mM EDTA solution, washed twice with PBS, and resuspended in 0.32 M sucrose and 10 mM Tris-HCl (pH 7.4) containing a cocktail of protease inhibitors (Roche). Cells were manually lysed by 40 strokes of a Dounce homogenizer. Nuclear debris was removed from the whole cell lysate by low speed centrifugation (500 × g, 5 min, 4 °C).
2.4 DRM Enrichment
Bulk DRMs from the crude mitochondrial fraction (P2, Fig. 1) were isolated using a modified version of a published protocol [3,13]. Care was taken to maintain a homogeneous solution of Triton X-114 throughout the isolation procedure by keeping the temperature at 4°C at all times [23]. Briefly, mitochondria/MAMs were obtained from whole cell lysates by centrifugation at 16,000 × g for 45 min. The pellet was resuspended in 150 mM NaCl, 0.5% TritonX-114 in 10 mM Tris (pH 7.4). After a 1 h end over end rotation period, the sample was centrifuged at 12,000 × g for 30 min to reduce plasma membrane contamination. The supernatant was removed and then centrifuged at 100,000 × g for 1 h. The resulting pellet was taken as the DRM fraction. DRM proteins were solubilized in a mixture of 2% SDS and 10 mM Tris (pH 7.4). Aliquots of each fraction from each stage of the enrichment procedure were analyzed by Western blotting.
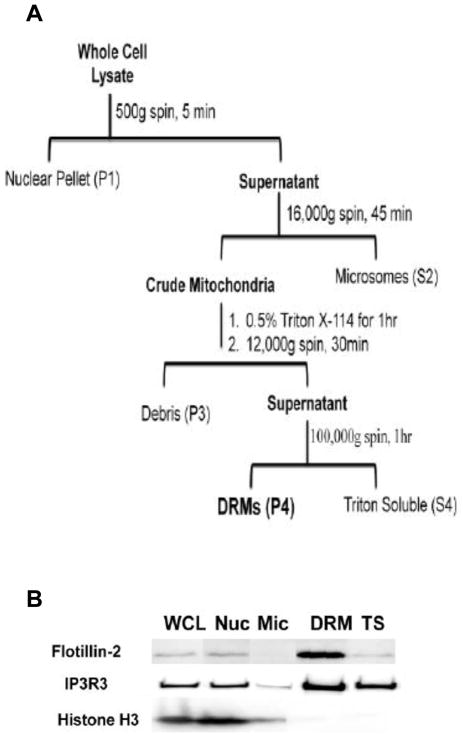
Figure 1. Flowchart and validation of enrichment procedure.
(A) Overview of the isolation procedure. A crude mitochondrial pellet was obtained by differential centrifugation from NG 108-15 whole cell lysates. MAM/Mitochondrial DRMs were isolated from crude mitochondria by differential detergent solubility using Triton X-114. (B) Western blot analysis of protein markers. Select fractions from the DRM enrichment procedure were assayed for biomarkers for lipid rafts (flotillin-2), MAM (IP3R3), and nuclei (histone H3). An equal amount of protein, as determined by BCA assay, was loaded in each lane. The experiment was performed at least 3 times with similar results. Abbreviations used: WCL, whole cell lysate; Nuc, nucleus; Mic, microsomes; DRM, detergent-resistant membranes; TS, triton X-114 soluble.
2.5 SDS-PAGE and Western Blot Analysis
Protein samples separated by SDS-PAGE (NuPAGE Novex 10% Bis/Tris gels; Invitrogen Corp., Calsbad, CA) were electrophoretically transferred to a PVDF membrane (0.2 μm, Millipore, Billerica, MA) at 4 °C for 90 min (45 V) using the Invitrogen XCell II Blot Module. HRP -conjugated secondary antibodies allowed for detection and visualization of specific antigens by ECL (SuperSignal Chemiluminescent Substrate; Thermo Fisher Scientific). Blots were imaged using a Syngene GeneGnome (Frederick, MD) bio-imaging system.
2.6 Gel-assisted Digestion
DRM proteins were digested with trypsin using a gel-assisted method [24]. Briefly, a polyacrylamide gel was created by adding 18.5 μL of a 19:1 mixture of 40% (v/v) acrylamide solution/bis-acrylamide, 2.5 μL of 10% (w/v) ammonium persulfate, and 1 μL of 100% TEMED to 50 μg of protein in a Eppendorf tube. After polymerization, the gel/protein matrix was cut into small pieces and washed twice with 50% (v/v) acetonitrile in 25 mM ammonium bicarbonate (pH 8.0), followed by a single wash with neat acetonitrile. Dried gel pieces were rehydrated with trypsin solution and incubated at 37 °C for 18 h. Peptides were extracted using 0.1% acetic acid, 50% acetonitrile in 0.1% acetic acid, and neat acetonitrile sequentially. Extracts were pooled and concentrated by vacuum centrifugation to ~2 μL.
2.7 High pH Reversed-phase (RP) HPLC
Peptide digests were reconstituted in 0.1% acetic acid and loaded onto an Agilent ZORBAX 300Extend-C18 column (150 × 2.1 mm i.d., 3.5 μm) using an Agilent 1200 binary HPLC system. Peptides were separated by gradient elution from 0–80% mobile phase B in 20 min with a constant flow rate of 0.1 mL/min. Mobile phase A was composed of 1% methanol in 20 mM ammonia (pH 10.5) while mobile phase B consisted of a 90/10 mix of acetonitrile/1% methanol in 20 mM ammonia. UV absorbance was monitored at 214 nm. Fractions were collected every minute and concentrated by vacuum centrifugation to ~2 μL.
2.8 LC-MS/MS and Data Analysis
Each offline HPLC fraction was reconstituted in 50 μL of 0.1% acetic acid and analyzed by nanoLC-MS/MS using a Tempo MDLC system coupled to a QSTAR Elite hybrid quadrupole time-of-flight mass spectrometer (ABSciex, Foster City, CA) with an ionization voltage setting of 1800 V. The peptides were eluted at a flow rate of 70–80 nl/min onto analytical columns (50 μm ID, 10 cm length of Monitor 100Å-Spherical Silica C18; Column Engineering Inc., Ontario, CA) using the following gradient: 0–30% solvent B in 40 minutes; 30–60% B in 40 minutes; and 60–95% B in 20 minutes. Solvent A consisted of 0.1% formic acid and 2% acetonitrile in water and solvent B contained 0.1% formic acid and 2% water in acetonitrile. MS data were acquired in information-dependent acquisition mode with Analyst QS 2.0 (ABSciex) with Smart IDA enabled. MS cycles were comprised of one full scan (m/z range = 300–2000, 1 sec accumulation) followed by sequential MS/MS scans of the four most abundant ions (+2 to +4 charge state, minimum ion count = 100, collision energy = 40.0 V, exclusion time = 15 sec, maximum accumulation time = 2 sec). The Paragon algorithm within ProteinPilot 2.0.1 (ABSciex) and a combined mouse (ver. 3.84) and rat (ver. 3.84) International Protein Index (IPI) database was used to search the MS/MS files. A 95% confidence threshold for protein matches was used to filter the data, which corresponded to an unused protein score ≥1.3.
3. Results and Discussion
3.1 Isolation and analysis of DRM proteins from a combined Mitochondria/MAM Sample
Novel lipid microdomains differ from classically-defined LRs in that they can localize within internal organelles [9,10,11,25], be cholesterol-independent [26,27], remain stable over extended timeframes [28,29], and/or exhibit a higher density on sucrose gradients [30]. Because of this anticipated variability, we chose to isolate a bulk DRM sample that contains LRs. Our rapid method to enrich for internal organelle DRMs used differential centrifugation in combination with differential detergent extraction (Fig. 1A). We specifically chose to use Triton X-114 because DRMs derived from internal organelles are preserved in its presence [13,31]. Initially, a series of centrifugation steps were performed to deplete the cell lysate of the nucleus (P1 pellet) and microsomes (S2 supernatant) [13]. The crude mitochondrial pellet (P2 pellet) was then solubilized with Triton X-114, cleared of debris, and centrifuged to obtain DRMs (P4 pellet). As a final step, the DRM pellet was resuspended in 2% SDS to ensure complete dissolution.
DRM samples generated using similar approaches to ours are enriched in lipid microdomains as verified in published studies [3,32,33,34]. Our Western blot data indicated that the DRM fraction we obtained is enriched in flotillin-2, a LR protein marker, and IP3R3, a protein that is an MAM DRM component [13] (Fig. 1B). Histone H3, a biomarker for the nucleus, is essentially absent in the insoluble fraction suggesting minimal contamination of DRMs by nuclear material (Fig. 1B). Collectively, these data confirm that LR-enriched DRMs were isolated from the P4 pellet.
Solubilized DRM proteins were subjected to our multiplexed analysis strategy that includes gel-assisted digestion [22] and RP/RP-MS/MS (Y. Cao, H. M. Johnson, and C. R. Bazemore-Walker, under review). During sample processing, detergent is removed from the sample, proteins are in-gel digested with trypsin, peptides are fractionated offline by RP-HPLC at pH 10 and peptide fractions are analyzed by RP-LC-MS/MS at pH 2. A total of 4 analyses were conducted: two technical replicates for each of two biological replicates. The MS/MS spectra were searched using ProteinPilot software and a combined rat/mouse IPI database and 2,033 unique peptides representing 447 proteins were identified. We required that each protein: be substantiated by the detection of 2 or more unique peptides at the 95% confidence level; have a protein unused score of 2.6 or greater; and have a protein confidence level of >95%. Spectra were also searched against a decoy database and an FDR of 1% was determined. Applying these filters resulted in the identification of 250 high-confidence proteins.
3.2 Identification and categorization of Mitochondria/MAM DRM Proteins
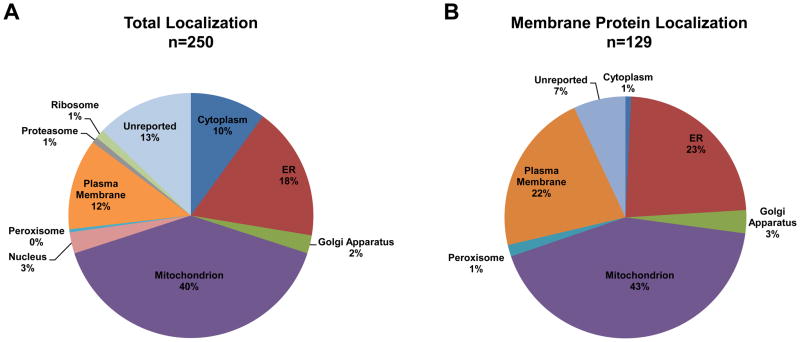
The majority of our 250 high-confidence proteins (146 proteins) are bona fide mitochondrial or ER proteins (Fig. 2A; Table S1). Proteins without a reported location comprise the third largest group followed closely by PM proteins. The proteins annotated as residing in the PM may actually have multiple subcellular locations that are not yet described in the GO database, a problem noted in other publications [35,36]. The low percentage of protein identifications from the Golgi, nucleus and other subcellular locations suggests that these proteins are residual contamination. Almost 52% of the 250 high confidence identifications are membrane proteins, mostly of mitochondrial, ER, or PM origin (Fig. 2B; Table S1). Our manual review of the literature indicates that ~20% (49 proteins) of the 250 proteins have been described as components of LRs or DRMs previously (Table S1). Importantly, we detected the known internal LR marker proteins IP3R3 (specific for the MAM) [13], erlin-2 (specific for the ER) [9], and VDAC1 (specific for the mitochondria) [10]. Additionally, 74% (184 proteins) of the 250 proteins have previously been noted as MAM-resident or -associated proteins (Table S1). In sum, these results provide strong evidence that our isolation method preferentially recovered DRM proteins from mitochondria and MAMs and that the sample was enriched in LR proteins.
Figure 2. Subcellular localization of the identified proteins based on GO annotation.
(A) Proteins were distributed according to their annotated subcellular location based on the number of proteins identifications per category. The total number of protein identifications (n) is shown above each pie chart. (B) Nearly 52% of the identified proteins are known membrane proteins. The organelle association is shown based on protein identifications per organelle.
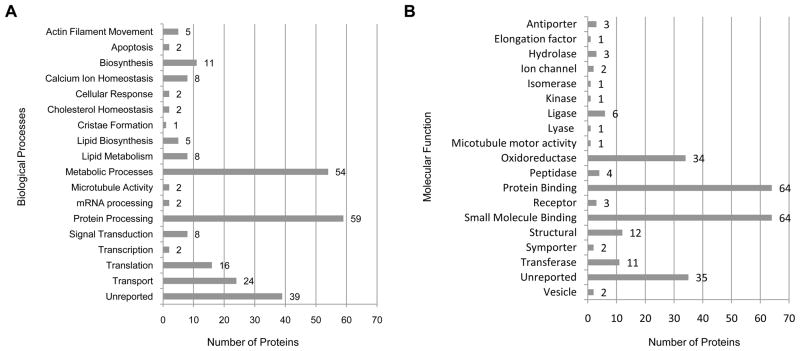
Further classification of the 250 proteins according to biological process and molecular function revealed that the proteins segregate into categories congruous with the central role of mitochondria in metabolism and the transport/protein processing activity of the MAM (Fig. 3; Table 1). Protein processing (24%), metabolic processes (22%), and transport (10%) are the top three biological activities represented (Fig. 3A). Proteins localized to the MAM that participate in different aspects of protein processing (Table 1) as well as components of the mitochondrial electron transport chain (ETC) and the tricarboxylic acid (TCA) cycle were detected (Table S1). In addition, enzymes tasked with cholesterol, glucose, lipid, and glycerophospholipid metabolism were found (Table S1). Particularly noteworthy is the detection of members of the Ca2+ macromolecular complex that resides at the ER-mitochondrion interface and exerts control over Ca2+ signaling and transport between the two organelles [14,37]. The proteins in this complex that were detected include IP3R3 (gene symbol Itpr3), VDAC1 (Vdac1), Grp75 (Hspa9), Bip/Grp78 (Hspa5), ERp57 (Pdia3), calnexin (Canx), calreticulin (Calr), and the adenine nucleotide transporters ANT1 (Slc25a4) and ANT2 (Slc25a5) (Table 1). Furthermore, the functional classification of the 250 proteins separate into roughly two categories – enzymes and binding proteins (Fig. 3B). This ‘big picture’ view of molecular function supports the roles of the identified proteins in the biological processes that occur in the mitochondrion and the MAM regions, respectively.
Figure 3. Classification of identified proteins according to biological process and molecular function.
(A) Biological processes and (B) molecular functions associated with identified proteins.
Table 1.
Select listing of identified proteins involved in relevant biological processes
| UniProt | Name | Gene | MAMa | Lipid Raftb | Biological Processc |
|---|---|---|---|---|---|
| P05141 | ADP/ATP translocase 2 | Slc25a5 | Yes | Yes | Calcium signaling |
| P21796 | Isoform Mt-VDAC1 of Voltage-dependent anion-selective channel protein 1 | Vdac1 | Yes | Yes | |
| P48962 | ADP/ATP translocase 1 | Slc25a4 | Yes | Yes | |
| Q14573 | Inositol 1,4,5-trisphosphate receptor type 3 | Itpr3 | Yes | Yes | |
| O00116 | Alkyldihydroxyacetonephosphate synthase, peroxisomal precursor | Agps | Yes | No | Lipid Biosynthesis |
| P28288 | Golgi resident protein GCP60 | Acbd3 | Yes | No | |
| P49327 | Fatty acid synthase | Fasn | Yes | No | |
| Q9DB73 | NADH-cytochrome b5 reductase 1 | Cyb5r1 | Yes | No | |
| E7EQR6 | Isoform 1 of T-complex protein 1 subunit alpha | Tcp1 | No | No | Protein Folding |
| O14967 | Calmegin | Clgn | No | Yes | |
| O60613 | 15 kDa Selenoprotein | Sep15 | No | No | |
| O60884 | DnaJ (Hsp40) homolog, subfamily A, member 2, isoform CRA_b | Dnaja2 | Yes | Yes | |
| P07237 | Protein disulfide-isomerase | P4hb | Yes | Yes | |
| P08238 | Heat shock protein HSP 90-beta | Hsp90ab1 | Yes | Yes | |
| P27824 | Calnexin | Canx | Yes | No | |
| P30101 | ERp57 | Pdia3 | Yes | Yes | |
| P38646 | Stress-70 protein, mitochondrial | Hspa9 | Yes | Yes | |
| P48643 | T-complex protein 1 subunit epsilon | Cct5 | Yes | No | |
| P50990 | T-complex protein 1 subunit theta | Cct8 | Yes | No | |
| P80317 | Chaperonin subunit 6a | Cct6a | Yes | Yes | |
| Q12931 | 80 kDa protein | Trap1 | Yes | No | |
| Q13724 | ManNosyl-oligosaccharide glucosidase | Mogs | Yes | No | |
| Q14318 | Isoform 1 of Peptidyl-prolyl cis-trans isomerase FKBP8 | Fkbp8 | Yes | No | |
| Q14697 | Isoform 1 of Neutral alpha-glucosidase AB | Ganab | Yes | No | |
| Q9NVH1 | 59 kDa protein | DNAJC11 | Yes | No | |
| Q9NYU2 | UDP-glucose:glycoprotein glucosylrransferase 1 | Uggt | No | No | |
| Q9UBS4 | DnaJ homolog subfamily B member 11 | DNAJB11 | Yes | No | |
| Q9UGP8 | Translocation protein SEC63 homolog | Sec63 | Yes | No | |
| P04843 | Dolichyl-diphosphooligosaccharide--protein glycosylTransferase subunit 1 | Rpn1 | Yes | Yes | Protein Processing in the ER |
| P04844 | Dolichyl-diphosphooligosaccharide--protein glycosylTransferase subunit 2 | Rpn2 | Yes | No | |
| P05198 | Eukaryotic translation initiation factor 2 subunit 1 | Eif2s1 | Yes | No | |
| P11021 | 78 kDa glucose-regulated protein | Hspa5 | Yes | No | |
| P27797 | Calreticulin | Calr | Yes | Yes | |
| Dolichyl-diphosphooligosaccharide--protein glycosylTransferase 48 | |||||
| P39656 | kDa subunit | Ddost | Yes | Yes | |
| P51571 | Translocon-associated protein subunit delta | Ssr4 | Yes | No | |
| P55072 | Transitional endoplasmic reticulum ATPase | Vcp | Yes | No | |
| Q07065 | Cytoskeleton-associated protein 4 | Ckap4 | Yes | No | |
| Q9Y4L1 | Hypoxia up-regulated protein 1 | Hyou1 | Yes | No |
Proteins associated with or localized in the MAM based on manual literature review;
Proteins previously identified in lipid rafts based on manual literature review;
Biological processes based on Gene Ontology annotation. Proteins shown in bold print are known members of the MAM Ca2+ macromolecular signaling complex.
The significance of DRMs in processes, such as signal transduction and small molecule transport, is an emerging theme in the literature [4,5]. Here, we describe biologically relevant proteins isolated from LR-enriched DRM samples obtained from crude mitochondria membranes. Our gel-assisted shotgun proteomic method identified MAM residents that assist protein processing at the ER and regulate Ca2+ trafficking from the ER to the mitochondrion. Our method also revealed that Triton X-114-resistant DRMs are present in mitochondria and contain proteins that facilitate ATP production and export from the mitochondrion. Interestingly, glycolytic and TCA enzymes as well as components of the ETC and ATP synthases have been detected in the MAM recently [38]. This fact combined with their presence in our data suggests that it is plausible that DRMs exert influence on these disparate processes as well.
We detected 250 proteins that were identified across four replicate analyses with at least 2 unique peptides at the 95% confidence level, making our identifications robust. Based on our findings, we postulate that several activities specific to the mitochondria and ER are (at least partially) dependent on DRMs. This study provides the basis for further investigation probing the activity and association of the Ca2+ signaling complex with LRs after physiological perturbation. We also note that we did not detect other well known proteins involved in regulating the physical contact between the mitochondrion and the ER such as PACS-2 and mitofusin-2. It is possible that our proteomic analysis was limited to the more highly abundant mitochondrial proteins found in the sample. However, the detection of numerous MAM marker proteins argues against this. Consequently, it is feasible that the ‘physical contact’ proteins, and the interaction that they create between the mitochondrion and ER, are not modulated by lipid rafts.
Supplementary Material
Highlights.
Identified 250 proteins from mitochondria/MAM detergent-resistant membranes.
Identified proteins primarily involved in metabolic and protein processing activities.
Detected known ER/MAM/mitochondria lipid-raft markers (erlin-2, IP3R3, and VDAC1).
Detected most of the members of the MAM-localized Ca2+ macromolecular complex.
Acknowledgments
We thank Professors Wayne Bowen (Brown University, Department of Molecular Pharmacology, Physiology and Biotechnology) and Jay Tang (Brown University, Department of Physics) for use of their equipment and facilities. This study was funded in part by NIH grant R03 DA027182 and by a RI-INBRE Pilot Project Grant (under NIH NCRR #P20RR016457) to C. R. B.-W.
Abbreviations
- DRMs
detergent-resistant membranes
- ETC
electron transport chain
- ER
endoplasmic reticulum
- IP3R3
inositol 1,4,5-trisphosphate receptor type 3
- LRs
lipid rafts
- MAM
mitochondria-associated ER membrane
- MS/MS
tandem mass spectrometry
- PM
plasma membrane
- RP/RP
reversed-phase reversed-phase
- TCA
tricarboxylic acid
- VDAC1
voltage-dependent anion channel 1
Footnotes
Supplementary data is available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Veatch SL, Keller SL. Lateral organization in model lipid membranes containing cholesterol. Molecular Biology of the Cell. 2002;13:359A–359A. [Google Scholar]
- 3.Adam RM, Yang W, Di Vizio D, Mukhopadhyay NK, Steen H. Rapid preparation of nuclei-depleted detergent-resistant membrane fractions suitable for proteomics analysis. BMC Cell Biol. 2008;9:30. doi: 10.1186/1471-2121-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagnat M, Keranen S, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci U S A. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 6.van Deurs B, Roepstorff K, Thomsen P, Sandvig K. Sequestration of epidermal growth factor receptors in non-caveolar lipid rafts inhibits ligand binding. Journal of Biological Chemistry. 2002;277:18954–18960. doi: 10.1074/jbc.M201422200. [DOI] [PubMed] [Google Scholar]
- 7.Alfalah M, Jacob R, Wetzel G, Busche R, Fischer I, Zimmer KP, Sallmann HP, Naim HY. A novel type of lipid microdomains implicated in early protein sorting in epithelial cells. European Journal of Cell Biology. 2005;84:122–122. [Google Scholar]
- 8.Muniz M, Morsomme P, Riezman H. Protein sorting upon exit from the endoplasmic reticulum. Cell. 2001;104:313–320. doi: 10.1016/s0092-8674(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 9.Browman DT, Resek ME, Zajchowski LD, Robbins SM. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. Journal of Cell Science. 2006;119:3149–3160. doi: 10.1242/jcs.03060. [DOI] [PubMed] [Google Scholar]
- 10.Ciarlo L, Manganelli V, Garofalo T, Matarrese P, Tinari A, Misasi R, Malorni W, Sorice M. Association of fission proteins with mitochondrial raft-like domains. Cell Death and Differentiation. 2010;17:1047–1058. doi: 10.1038/cdd.2009.208. [DOI] [PubMed] [Google Scholar]
- 11.Garofalo T, Giammarioli AM, Misasi R, Tinari A, Manganelli V, Gambardella L, Pavan A, Malorni W, Sorice M. Lipid microdomains contribute to apoptosis-associated modifications of mitochondria in T cells. Cell Death and Differentiation. 2005;12:1378–1389. doi: 10.1038/sj.cdd.4401672. [DOI] [PubMed] [Google Scholar]
- 12.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nature Reviews Molecular Cell Biology. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol. 2010;77:517–528. doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends in Cell Biology. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron S, Vangheluwe P, Sepulveda MR, Wuytack F, Raeymaekers L, Vanoevelen J. The secretory pathway Ca2+-ATPase 1 is associated with cholesterol-rich microdomains of human colon adenocarcinoma cells. Biochimica Et Biophysica Acta-Biomembranes. 2010;1798:1512–1521. doi: 10.1016/j.bbamem.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Chen JC, Wu ML, Huang KC, Lin WW. HMG-CoA reductase inhibitors activate the unfolded protein response and induce cytoprotective GRP78 expression. Cardiovasc Res. 2008;80:138–150. doi: 10.1093/cvr/cvn160. [DOI] [PubMed] [Google Scholar]
- 17.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: Calcium and ROS. Biochimica Et Biophysica Acta-Bioenergetics. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang WM, Chen KD, Chen LY, Lai MT, Lai YK. Mitochondrial calcium-mediated reactive oxygen species are essential for the rapid induction of the grp78 gene in 9L rat brain tumour cells. Cell Signal. 2003;15:57–64. doi: 10.1016/s0898-6568(02)00055-4. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer I. Altered mitochondria, energy metabolism, voltage-dependent anion channel, and lipid rafts converge to exhaust neurons in Alzheimer’s disease. Journal of Bioenergetics and Biomembranes. 2009;41:425–431. doi: 10.1007/s10863-009-9243-5. [DOI] [PubMed] [Google Scholar]
- 21.Hajnoczky G, Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA. Structural and functional features and significance of the physical linkage between ER and mitochondria. Journal of Cell Biology. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han CL, Chien CW, Chen WC, Chen YR, Wu CP, Li H, Chen YJ. A Multiplexed Quantitative Strategy for Membrane Proteomics OPPORTUNITIES FOR MINING THERAPEUTIC TARGETS FOR AUTOSOMAL DOMINANT POLYCYSTIC KIDNEY DISEASE. Molecular & Cellular Proteomics. 2008;7:1983–1997. doi: 10.1074/mcp.M800068-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Bordier C. Phase-Separation of Integral Membrane-Proteins in Triton X-114 Solution. Journal of Biological Chemistry. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 24.Lu XN, Zhu HN. Tube-gel digestion - A novel proteomic approach for high throughput analysis of membrane proteins. Molecular & Cellular Proteomics. 2005;4:1948–1958. doi: 10.1074/mcp.M500138-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghannam A, Hammache D, Matias C, Louwagie M, Garin J, Gerlier D. High-density rafts preferentially host the complement activator measles virus F glycoprotein but not the regulators of complement activation. Mol Immunol. 2008;45:3036–3044. doi: 10.1016/j.molimm.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Hansen GH, Immerdal L, Thorsen E, Niels-Christiansen LL, Nystrom BT, Demant EJ, Danielsen EM. Lipid rafts exist as stable cholesterol-independent microdomains in the brush border membrane of enterocytes. Journal of Biological Chemistry. 2001;276:32338–32344. doi: 10.1074/jbc.M102667200. [DOI] [PubMed] [Google Scholar]
- 27.Delmas O, Breton M, Sapin C, Le Bivic A, Colard O, Trugnan G. Heterogeneity of Raft-type membrane microdomains associated with VP4, the rotavirus spike protein, in Caco-2 and MA 104 cells. J Virol. 2007;81:1610–1618. doi: 10.1128/JVI.01433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knorr R, Karacsonyi C, Lindner R. Endocytosis of MHC molecules by distinct membrane rafts. Journal of Cell Science. 2009;122:1584–1594. doi: 10.1242/jcs.039727. [DOI] [PubMed] [Google Scholar]
- 29.Lindner R, Naim HY. Domains in biological membranes. Experimental Cell Research. 2009;315:2871–2878. doi: 10.1016/j.yexcr.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Otahal P, Angelisova P, Hrdinka M, Brdicka T, Novak P, Drbal K, Horejsi V. A new type of membrane raft-like microdomains and their possible involvement in TCR signaling. J Immunol. 2010;184:3689–3696. doi: 10.4049/jimmunol.0902075. [DOI] [PubMed] [Google Scholar]
- 31.Williamson CD, Zhang A, Colberg-Poley AM. The Human Cytomegalovirus Ul37 Exon 1 Protein Associates with Internal Lipid Rafts. J Virol. 2010 doi: 10.1128/JVI.01830-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang W, Di Vizio D, Kirchner M, Steen H, Freeman MR. Proteome Scale Characterization of Human S-Acylated Proteins in Lipid Raft-enriched and Non-raft Membranes. Molecular & Cellular Proteomics. 2010;9:54–70. doi: 10.1074/mcp.M800448-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang LY, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. Journal of Clinical Investigation. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh HY, Lee EJ, Yoon S, Chung BH, Cho KS, Hong SJ. Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. Prostate. 2007;67:1061–1069. doi: 10.1002/pros.20593. [DOI] [PubMed] [Google Scholar]
- 35.Qattan AT, Mulvey C, Crawford M, Natale DA, Godovac-Zimmermann J. Quantitative organelle proteomics of MCF-7 breast cancer cells reveals multiple subcellular locations for proteins in cellular functional processes. Journal of Proteome Research. 2010;9:495–508. doi: 10.1021/pr9008332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Li X, Mueller M, Wang Y, Zong C, Deng N, Vondriska TM, Liem DA, Yang JI, Korge P, Honda H, Weiss JN, Apweiler R, Ping P. Systematic characterization of the murine mitochondrial proteome using functionally validated cardiac mitochondria. Proteomics. 2008;8:1564–1575. doi: 10.1002/pmic.200700851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmen T, Lynes EM, Gesson K, Thomas G. Oxidative protein folding in the endoplasmic reticulum: Tight links to the mitochondria-associated membrane (MAM) Biochimica Et Biophysica Acta-Biomembranes. 2010;1798:1465–1473. doi: 10.1016/j.bbamem.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang A, Williamson CD, Wong DS, Bullough MD, Brown KJ, Hathout Y, Colberg-Poley AM. Quantitative Proteomic Analyses of Human Cytomegalovirus-Induced Restructuring of Endoplasmic Reticulum-Mitochondrial Contacts at Late Times of Infection. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M111.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.