Abstract
Introduction
Guidelines recommend that patients with clinical stage IIIA non-small cell lung cancer (NSCLC) undergo histologic confirmation of pathologic lymph nodes. Studies have suggested that invasive mediastinal staging is underutilized, though practice patterns have not been rigorously evaluated.
Methods
We used the Surveillance, Epidemiology, and End Results-Medicare database to identify patients with stage IIIA NSCLC diagnosed from 1998 through 2005. Invasive staging and use of positron emission tomography (PET) scanning were assessed using Medicare claims. Multivariable logistic regression was used to identify patient characteristics associated with use of invasive staging.
Results
Of 7583 stage IIIA NSCLC patients, 1678 (22%) underwent invasive staging. Patients who received curative intent cancer treatment were more likely to undergo invasive staging than patients who did not receive cancer specific therapy (30% vs. 9.8%, adjusted odds ratio [OR} 3.31, 95% CI 2.78–3.95). The oldest patients (age 85–94) were less likely to receive invasive staging than the youngest ((age 67–69) (27.6 % vs. 11.9%, OR 0.46, 95% CI 0.34–0.61)). Sex, marital status, income and race were not associated with the use of the invasive staging. The use of invasive staging was stable throughout the study period, despite an increase in the use of PET scanning from less than 10% of patients prior to 2000 to almost 70% in 2005.
Conclusion
Nearly 80% of Medicare beneficiaries with stage IIIA NSCLC do not receive guideline adherent mediastinal staging; this failure cannot be entirely explained by patient factors or a reliance on PET imaging. Incentives to encourage use of invasive staging may improve care.
Keywords: Non-small cell lung cancer, mediastinal staging, mediastinoscopy
Introduction
Accurate staging of lung cancer is essential to the determination of appropriate treatment. Stage IIIA non-small cell lung cancer (NSCLC) is most commonly defined by cancer spread to ispsilateral mediastinal (N2) lymph nodes. Prior studies have indicated that CT and PET scanning lack sufficient sensitivity or specificity to serve as the sole staging modality.1–10 A 1997 statement from the ATS/ERS statement noted that invasive staging of enlarged lymph nodes is mandatory.11 The American Thoracic Society (ATS), European Respiratory Society (ERS), and American College of Chest Physicians (ACCP), have for many years endorsed invasive sampling of mediastinal lymph nodes suspected of containing malignant cells.1,11–13 Therefore, patients should not be given the diagnosis of clinical stage IIIA NSCLC based on PET scan findings without tissue confirmation.
Prior work has suggested that use of mediastinal staging is far lower than recommended by guidelines14–16. One analysis of trends in staging of Medicare patients diagnosed with NSCLC between 1998 and 2002 found that 65% of Stage IIIA patients were staged with CT scan only, 30% with CT in addition to either PET or invasive biopsy, and 5% with CT, PET and invasive biopsy17. This analysis also found a positive association between use of additional staging modalities and survival.
We examined the actual practice for mediastinal staging of Medicare patients with stage IIIA NSCLC to explore the reasons for its underutilization. The advantages of studying the Medicare population include ethnic, socioeconomic and geographic diversity as well as a stable single payer insurance coverage for the entire period of study. We identified which staging modalities were most frequently used during the years 1998–2005 and examined patient factors associated with the use of invasive staging.
Methods
Data Source and Study Sample
This study was deemed exempt by the Yale Human Investigations Committee. Data were obtained from the SEER-Medicare linked database, which contains tumor registry data linked to Medicare claims for patients representing 26% of the US population.18,19 Prior to 2000, only 11 of the current 16 registries participated in the SEER program; this subset of registries, which represented 14% of the population, is referred to in this study as the pre-expansion registries.
We selected subjects ages 67–94 who were diagnosed with Stage IIIA NSCLC between 1998 and 2005. Patients were identified as IIIA using the American Joint Committee on Cancer (AJCC) stage variable prior to 2004 or collaborative stage variable after 2004 provided by SEER. The collaborative stage variable uses all data available from both clinical staging techniques and surgical resection if performed. Exclusion criteria included the following: unknown month of diagnosis, diagnosis reported on death certificate or autopsy, prior lung cancer diagnosis, or any other cancer diagnosis in the 6 months before and after the stage IIIA NSCLC diagnosis. In order to ensure that we had complete data for the sample, patients had to have been continuously enrolled in fee-for-service Medicare Parts A and B beginning 24 months prior to diagnosis through the earliest of the following events: initiation of treatment, death, or 6 months after diagnosis.
We also analyzed a subgroup of Stage IIIA patients treated with both chemotherapy and radiation but not surgery within six months of diagnosis. This analysis allowed us to confirm our findings in a group of patients healthy enough for aggressive treatment and without the impact of unsuspected N2 disease found incidentally at the time of surgical resection.
Treatment Groups
Treatment was assessed using Medicare claims in the 6 months after diagnosis (Appendix 1). We divided patients into 3 groups: Patients who did not receive chemotherapy, surgery or radiation were classified as best supportive care. Patients who received chemotherapy or radiation alone were classified as cancer specific therapy. Patients who received combination chemotherapy and radiation therapy or any therapy that involved surgical resection were classified as curative intent therapy.
Outcome
The primary outcome was receipt of invasive mediastinal staging. We used the inpatient, outpatient, and physician Medicare claims to search for Current Procedural Terminology (CPT) codes for PET scan, mediastinoscopy, mediastinotomy, TBNA, EUS, or VATS biopsies (Appendix 1). For the majority of the study period, EBUS-TBNA and conventional TBNA were billed using the same CPT code, so we could not separate these two procedures. For analytic purposes, we combined mediastinoscopy and mediastinotomy into one group. We searched for mediastinal staging procedures performed 6 months before diagnosis through the initiation of treatment or for 6 months after diagnosis in the case of patients who were not treated with any cancer specific therapy.
As a secondary outcome, we calculated the 3-year survival of the subset of stage IIIA NSCLC patients who were diagnosed in 1998–2004 and received both chemotherapy and radiation within six months of diagnosis, but did not undergo surgery.
Co-variates
The following variables were selected a priori as factors that might influence whether a patient received invasive staging: age, sex, race, comorbidities, marital status, income, health care system access, treatment group as defined above, SEER registry, and year of diagnosis. Age was categorized as 67–69, 70–74, 75–79, 80–84, ≥85; race as white, black, or other; and marital status as married, unmarried, or unknown. Income was defined as the median household income at the zip code level, categorized into quintiles. We created a dichotomous variable indicating whether a claim had been submitted for influenza vaccination in the 18 months prior to the diagnosis, which has been used previously as marker for healthcare system access20.
Comorbidity was assessed by searching all Medicare claims in the 2 years prior to diagnosis. We used the comorbid conditions recommended by Elixhauser et al21 that we had previously determined were significantly associated with survival (Appendix 2). Only codes that appeared on at least 1 inpatient claim or 2 or more outpatient/physician claims occurring more than 30 days apart were used. We created a sum score of the number of comorbidities each patient had and then stratified patients into 3 groups: 0, 1–2, or ≥3 comorbidities.
Statistical analysis
We determined the percent of patients receiving each type of invasive staging procedure for each year during the study period (1998–2005). Bivariate and multivariate logistic regression was used to identify patient factors associated with receipt of invasive staging. For the secondary analysis, we conducted a logistic regression analysis using 3-year survival as the outcome. SAS statistical software, version 9.2 (SAS Institute Inc., Cary, North Carolina), was used for all analysis
Results
Our sample consisted of 7,583 patients (Table 1). Of the 7,583 patients, 1678 (22%) underwent at least one invasive staging procedure. Of these, 88% received a single invasive staging procedure such as mediastinoscopy alone, while 12% received 2 or more invasive staging procedure such as TBNA followed by mediastinoscopy.
Table 1.
Baseline Characteristics of Cohort and Use of Invasive Staging Techniques in Stage IIIA NSCLC N=7583
| Age | |
| 67–69 | 1118 (15%) |
| 70–74 | 2305 (30%) |
| 75–79 | 2197 (29%) |
| 80–84 | 1331 (18%) |
| 85–94 | 632 (8%) |
| Sex | |
| Male | 4308 (57%) |
| Female | 3275 (43%) |
| Race | |
| White | 6653 (88%) |
| Black | 635 (8%) |
| Other | 295 (4%) |
| Marital Status | |
| Married | 3972 (52%) |
| Unmarried | 3349 (44%) |
| Unknown | 262 (4%) |
| Income | |
| 1st quintile | 1449 (19%) |
| 2nd quintile | 1451 (19%) |
| 3rd quintile | 1446 (19%) |
| 4th quintile | 1450 (19%) |
| 5th quintile | 1447 (19%) |
| Unknown | 340 (4%) |
| Influenza Vaccination | |
| Influenza Vaccination in last 18 months | 4013 (53%) |
| No Influenza Vaccination | 3570 (47%) |
| Treatment Group* | |
| Best supportive care | 1834 (24%) |
| Cancer specific therapy | 2051 (27%) |
| Curative intent therapy | 3698 (49%) |
| Invasive Staging | |
| Any Invasive Staging Technique | 1678 (22%) |
| No Invasive Staging | 5905 (78%) |
Patients classified as best supportive care did not receive any cancer specific therapy (chemotherapy, radiation, or surgery). Patients who received chemotherapy or radiation alone were classified as cancer specific therapy. Patients who received combination chemotherapy and radiation therapy or any therapy that involved surgical resection were classified as curative intent therapy.
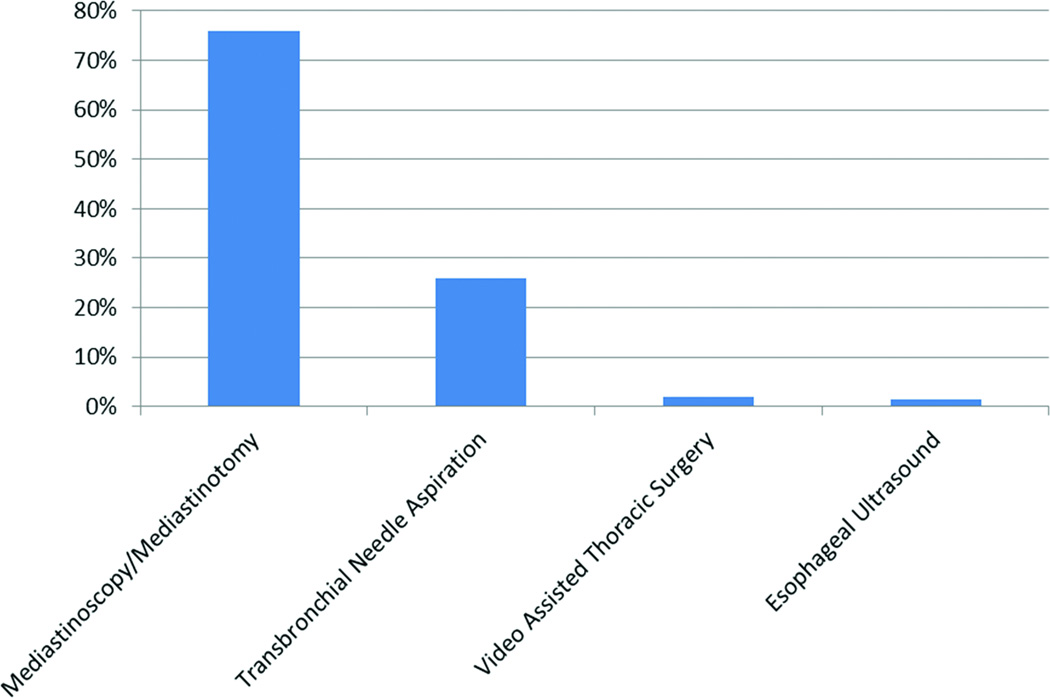
As shown in Figure 1, mediastinoscopy (or mediastinotomy) was the most commonly used invasive procedure (76% of invasively staged patients) followed by TBNA with or without ultrasound guidance (26% of invasively staged patients). VATS and EUS were rarely used.
Figure 1.
Invasive Staging Techniques
1678 patients underwent invasive staging. Mediastinoscopy/Mediastinotomy was used in 1270 patients (76%), Transbronchial Needle Aspiration in 451 (26%), Video Assisted Thoracic Surgery in 35 (2%) and Esophageal Ultrasound in 28 (1.6%). Since 12.5% of invasively staged patients underwent more than one procedure, the numbers sum to > 100%.
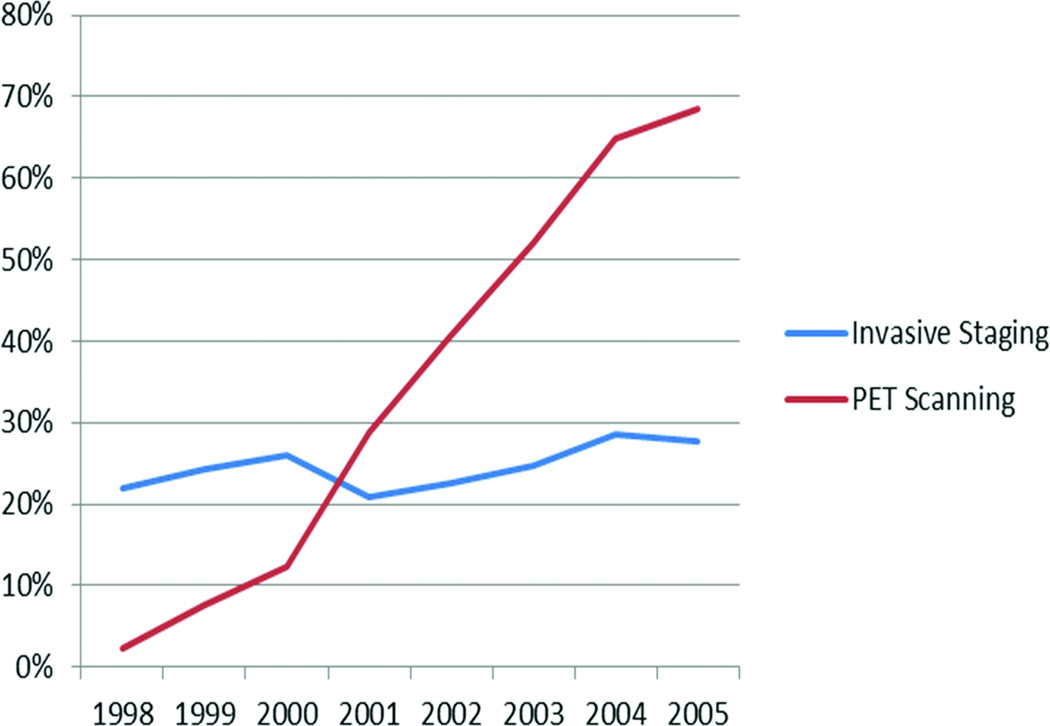
The use of invasive staging did not change significantly during the study period. However, the use of PET scanning increased from 2.4% in 1998 to 68.4% in 2005 (Figure 2).
Figure 2.
Use of PET scanning and invasive staging, 1998–2005 in pre-expansion registries
In the unadjusted analysis, older age, black race, higher comorbidity, or being unmarried significantly decreased the likelihood of receiving invasive staging (Table 2). Patients who received aggressive cancer treatment were significantly more likely to have received invasive staging. However, even among these patients, only a minority (30%) underwent invasive staging. Furthermore, even in the “high likelihood” subgroups (no comorbidities, white, married) less than 30% underwent invasive staging.
Table 2.
Unadjusted and adjusted odds ratios for receipt of invasive staging
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| N | % staged | OR | 95% CI | OR | 95% CI | |
| Age group | ||||||
| 67–69 | 1118 | 27.6 | 1.00 | -- | 1.00 | -- |
| 70–74 | 2305 | 25.4 | 0.90 | 0.76–1.05 | 0.92 | 0.78–1.09 |
| 75–79 | 2197 | 22.4 | 0.76 | 0.64–0.90 | 0.81 | 0.68–0.96 |
| 80–84 | 1331 | 16.3 | 0.51 | 0.42–0.62 | 0.57 | 0.46–0.70 |
| 85–94 | 632 | 11.9 | 0.35 | 0.27–0.47 | 0.46 | 0.34–0.61 |
| Sex | ||||||
| Male | 4308 | 22.1 | 1.00 | -- | n/a | n/a |
| Female | 3275 | 22.2 | 1.01 | 0.91–1.13 | n/a | n/a |
| Race | ||||||
| White | 6653 | 22.5 | 1.00 | -- | 1.00 | -- |
| Black | 635 | 17.3 | 0.72 | 0.58–0.89 | 0.88 | 0.69–1.12 |
| Other | 295 | 24.4 | 1.11 | 0.85–1.46 | 1.33 | 0.97–1.83 |
| Comorbidities | ||||||
| 0 | 2747 | 24.1 | 1.00 | -- | 1.00 | -- |
| 1–2 | 3169 | 22.6 | 0.92 | 0.82–1.04 | 0.93 | 0.82–1.05 |
| ≥3 | 1667 | 18.1 | 0.70 | 0.60–0.81 | 0.81 | 0.69–0.95 |
| Marital Status | ||||||
| Married | 3972 | 24.3 | 1.00 | -- | 1.00 | -- |
| Unmarried | 3349 | 19.7 | 0.82 | 0.72–0.92 | 0.93 | 0.83–1.05 |
| Unknown | 262 | 19.9 | 0.78 | 0.55–1.10 | 1.01 | 0.73–1.40 |
| Income | ||||||
| 1st quintile | 1449 | 17.7 | 1.00 | -- | 1.00 | -- |
| 2nd quintile | 1451 | 21.4 | 1.26 | 1.05–1.52 | 1.07 | 0.87–1.30 |
| 3rd quintile | 1446 | 22.4 | 1.34 | 1.12–1.61 | 0.99 | 0.81–1.22 |
| 4th quintile | 1450 | 24.3 | 1.49 | 1.24–1.78 | 1.09 | 0.88–1.34 |
| 5th quintile | 1447 | 24.9 | 1.54 | 1.28–1.84 | 1.12 | 0.90–1.39 |
| Unknown | 340 | 22.1 | 1.31 | 0.98–1.75 | 1.13 | 0.83–1.54 |
| Flu shot in prior 18 months | ||||||
| No | 3570 | 19.7 | 1.00 | -- | 1.00 | -- |
| Yes | 4013 | 24.3 | 1.31 | 1.17–1.46 | 1.23 | 1.09–1.39 |
| Year of diagnosis | ||||||
| 1998 | 502 | 22.7 | 1.00 | -- | n/a | n/a |
| 1999 | 461 | 24.3 | 1.09 | 0.81–1.47 | n/a | n/a |
| 2000 | 1033 | 22.6 | 0.99 | 0.77–1.28 | n/a | n/a |
| 2001 | 983 | 19.8 | 0.84 | 0.65–1.09 | n/a | n/a |
| 2002 | 1038 | 18.8 | 0.79 | 0.61–1.02 | n/a | n/a |
| 2003 | 1130 | 21.2 | 0.92 | 0.71–1.18 | n/a | n/a |
| 2004 | 1247 | 23.7 | 1.06 | 0.83–1.36 | n/a | n/a |
| 2005 | 1189 | 24.6 | 1.11 | 0.87–1.43 | n/a | n/a |
| Treatment type | ||||||
| Best supportive care | 1834 | 9.8 | 1.00 | -- | 1.00 | -- |
| Cancer specific therapy | 2051 | 19.1 | 2.18 | 1.80–2.63 | 2.18 | 1.80–2.66 |
| Curative intent therapy | 3698 | 30.0 | 3.96 | 3.34–4.69 | 3.31 | 2.78–3.95 |
| SEER region | ||||||
| San Francisco | 253 | 13.8 | 1.00 | -- | 1.00 | -- |
| Connecticut | 558 | 26.5 | 2.25 | 1.50–3.37 | 2.07 | 1.36–3.14 |
| Detroit | 866 | 25.5 | 2.13 | 1.45–3.15 | 2.04 | 1.36–3.06 |
| Hawaii | 131 | 13.7 | 0.99 | 0.54–1.83 | 0.68 | 0.35–1.30 |
| Iowa | 602 | 24.6 | 2.03 | 1.36–3.04 | 1.87 | 1.21–2.88 |
| New Mexico | 140 | 17.1 | 1.29 | 0.73–2.27 | 1.29 | 0.71–2.34 |
| Seattle | 486 | 32.5 | 3.00 | 2.00–4.50 | 3.05 | 1.99–4.66 |
| Utah | 94 | 22.3 | 1.79 | 0.98–3.27 | 1.69 | 0.91–3.17 |
| Atlanta | 258 | 21.3 | 1.69 | 1.06–2.69 | 1.55 | 0.96–2.50 |
| San Jose | 153 | 20.3 | 1.58 | 0.93–2.69 | 1.33 | 0.77–2.30 |
| Los Angeles | 496 | 30.0 | 2.68 | 1.78–4.01 | 2.48 | 1.63–3.78 |
| Rural Georgia | ** | ** | 0.86 | 0.29–2.59 | 0.91 | 0.29–2.80 |
| Greater California | 1181 | 20.1 | 1.56 | 1.07–2.30 | 1.51 | 1.01–2.26 |
| Kentucky | 840 | 15.5 | 1.14 | 0.76–1.71 | 1.05 | 0.69–1.63 |
| Louisiana | 562 | 15.1 | 1.11 | 0.73–1.70 | 1.14 | 0.72–1.79 |
| New Jersey | 930 | 23.0 | 1.86 | 1.26–2.75 | 1.70 | 1.13–2.54 |
Best supportive care = not treated with surgery, chemo or radiation, Cancer specific therapy = treated with chemotherapy or radiation alone, Curative intent therapy = treated with combination chemotherapy and radiation or any combination that included surgical resection. N=number of patients, %staged=percent of patients who underwent invasive clinical staging, Adjusted OR = adjusted odds ratio adjusted for age, race, SEER region, comorbidities, income and access to health care services as measured by receipt of influenza vaccination
Suppressed to protect confidentiality due to cell size
After adjusting for all significant variables, only age, comorbidity, receipt of influenza vaccination, and treatment type remained independently associated with use of invasive staging. Patients with ≥3 comorbidities were less likely to have received invasive staging compared to patients without any comorbidity (OR 0.81; 95% CI 0.69–0.95), while patients who had greater healthcare access, as measured by receipt of influenza vaccination, were more likely to have received invasive staging compared to patients who did not receive the vaccine (OR 1.23; 95% CI 1.09–1.39). However, in all subgroups, the use of invasive staging was the exception rather than the rule.
There was significant geographic variation in the use of invasive staging between SEER regions (Tables 2, 3).
Table 3.
Multivariable Analysis to predict use of invasive staging among subset of Stage IIIA patients Treated with Combined Chemotherapy and Radiation
| Variable | Odds Ratio | 95% CI | Adjusted Odds Ratio | 95% CI | |
|---|---|---|---|---|---|
| Age | |||||
| Age 67–69 | Ref | n/a | Ref | n/a | |
| Age 70–74 | 0.85 | 0.66–1.12 | 0.80 | 0.61–1.04 | |
| Age 75–79 | 0.63 | 0.47–0.83 | 0.58 | 0.43–0.77 | |
| Age 80–84 | 0.47 | 0.33–0.67 | 0.27 | 027–0.57 | |
| Over Age 85 | 0.41 | 0.21–0.80 | 0.36 | 0.18–0.71 | |
| Gender | |||||
| Male | Ref | n/a | n/a | n/a | |
| Female | 1.18 | 0.97–1.43 | Not significant | Not significant | |
| Race | |||||
| White | Ref | n/a | |||
| Black | 0.72 | 0.49–1.06 | Not significant | Not significant | |
| Other | 1.68 | 1.02–2.78 | Not significant | Not significant | |
| Comorbidities | |||||
| 0 | Ref | n/a | n/a | n/a | |
| 1–2 | 1.00 | 0.81–1.24 | Not significant | Not significant | |
| ≥3 | 0.80 | 0.60–1.06 | Not significant | Not significant | |
| Marital Status | |||||
| Married | Ref | n/a | n/a | n/a | |
| Unmarried | 0.98 | 0.80–1.20 | Not significant | Not significant | |
| Unknown | 1.13 | 0.65–1.95 | Not significant | Not significant | |
| Income | |||||
| 1st quintile | Ref | n/a | n/a | n/a | |
| 2nd quintile | 1.37 | 1.00–1.89 | 1.30 | 0.92–1.84 | |
| 3rd quintile | 1.32 | 0.96–1.83 | 1.06 | 0.74–1.52 | |
| 4th quintile | 1.45 | 1.05–2.00 | 1.11 | 076–1.61 | |
| 5th quintile | 1.58 | 1.14–2.18 | 1.28 | 0.86–1.88 | |
| Unknown | 1.14 | 0.67–1.94 | 0.86 | 0.49–1.52 | |
| Influenza Vaccination | |||||
| Negative Vaccination | Ref | n/a | n/a | n/a | |
| Positive Vaccination | 1.28 | 1.05–1.56 | 1.31 | 1.06–1.61 | |
| Year of Diagnosis | |||||
| 1998 | Ref | n/a | n/a | n/a | |
| 1999 | 1.03 | 0.58–1.84 | Not significant | Not significant | |
| 2000 | 1.04 | 0.64–1.70 | Not significant | Not significant | |
| 2001 | 0.62 | 0.37–1.05 | Not significant | Not significant | |
| 2002 | 0.67 | 0.40–1.10 | Not significant | Not significant | |
| 2003 | 0.75 | 0.46–1.22 | Not significant | Not significant | |
| 2004 | 1.07 | 0.67–1.70 | Not significant | Not significant | |
| 2005 | 0.97 | 0.61–1.55 | Not significant | Not significant | |
| SEER Registry | |||||
| San Francisco | Ref | n/a | |||
| Connecticut | 3.72 | 1.46–9.34 | 4.57 | 1.74–12.01 | |
| Detroit | 4.18 | 1.71–10.20 | 5.46 | 2.15–13.86 | |
| Hawaii | 1.19 | 0.31–4.65 | 0.64 | 0.15–2.68 | |
| Iowa | 3.71 | 1.49–9.24 | 4.33 | 1.65–11.38 | |
| New Mexico | 2.63 | 0.88–7.88 | 3.31 | 1.05–10.40 | |
| Seattle | 5.53 | 2.21–13.84 | 6.52 | 2.51–16.92 | |
| Utah | 2.87 | 0.80–10.26 | 3.07 | 0.83–11.44 | |
| Atlanta | 3.18 | 1.20–8.49 | 4.19 | 1.52–11.58 | |
| San Jose | 2.24 | 0.74–6.80 | 2.51 | 0.81–7.80 | |
| Los Angeles | 5.47 | 2.13–14.06 | 6.82 | 2.57–18.10 | |
| Rural Georgia | 1.02 | 0.11–9.84 | 1.71 | 0.17–16.94 | |
| Greater California | 2.26 | 0.93–5.51 | 2.71 | 1.07–6.86 | |
| Kentucky | 2.03 | 0.81–5.06 | 2.43 | 0.93–6.38 | |
| Louisiana | 2.14 | 0.84–5.42 | 2.78 | 1.04–7.43 | |
| New Jersey | 2.90 | 1.18–7.09 | 3.58 | 1.42–9.06 | |
Receipt of cancer specific therapy and curative intent therapy were associated with use of invasive staging. However, even in the subset of patients who received combined radiation therapy and chemotherapy, only 30% underwent invasive staging. Only age, SEER region, and receipt of influenza vaccination were significant covariates (Table 3).
In patients treated with both chemotherapy and radiation but not surgical resection, 3-year survival was 21%. In multivariate analysis of this group, invasive staging was associated with improved 3-year survival (adjusted HR 1.61, 95% CI 1.21–2.12). Other factors associated positively with survival included younger age and fewer comorbidities (Table 4).
Table 4.
Results of Multivariable Analysis to predict 3-year survival for Patients Treated with Chemotherapy and Radiation*
| Variable | Odds Ratio | 95% CI | Adjusted Odd Ratio | 95% CI | |
|---|---|---|---|---|---|
| Age | |||||
| Age 67–69 | Ref | n/a | n/a | n/a | |
| Age 70–74 | 0.60 | 0.42–0.85 | 0.61 | 0.43–0.90 | |
| Age 75–79 | 0.70 | 0.49–0.99 | 0.75 | 0.52–1.08 | |
| Age 80–84 | 0.47 | 0.30–0.75 | 0.533 | 0.33–0.86 | |
| Age >85 | 0.22 | 0.07–0.73 | 0.26 | 0.08–0.86 | |
| Gender | |||||
| Male | Ref | n/a | n/a | n/a | |
| Female | 1.57 | 1.21–2.03 | 1.52 | 1.17–1.99 | |
| Race | |||||
| White | Ref | n/a | n/a | n/a | |
| Black | 0.98 | 0.60–1.6 | 1.18 | 0.70–2.0 | |
| Other | 0.93 | 0.43–2.00 | 0.85 | 0.39–1.85 | |
| Comorbidities | |||||
| 0 | Ref | n/a | n/a | n/a | |
| 1–2 | 0.96 | 0.73–1.27 | 1.02 | 0.77–1.35 | |
| ≥3 | 0.43 | 0.27–0.68 | 0.49 | 0.31–0.78 | |
| Marital Status | |||||
| Married | Ref | n/a | n/a | n/a | |
| Unmarried | 0.86 | 0.65–1.3 | Not significant | Not significant | |
| Unknown | 0.75 | 0.33–1.68 | Not significant | Not significant | |
| Income | |||||
| 1st quintile | Ref | n/a | n/a | n/a | |
| 2nd quintile | 1.21 | 0.72–1.74 | 1.11 | 0.71–1.75 | |
| 3rd quintile | 1.20 | 0.78–1.86 | 1.21 | 0.76–1.91 | |
| 4th quintile | 1.54 | 1.01–2.35 | 1.51 | 0.97–2.37 | |
| 5th quintile | 1.42 | 0.92–2.18 | 1.41 | 0.89–2.34 | |
| Unknown | 0.78 | 0.33–1.82 | 0.83 | 0.35–1.97 | |
| Influenza Vaccination | |||||
| Negative Vaccination | Ref | n/a | n/a | n/a | |
| Positive Vaccination | 1.21 | 0.86–1.46 | Not significant | Not significant | |
| Year of Diagnosis | |||||
| 1998 | Ref | n/a | n/a | n/a | |
| 1999 | 1.31 | 0.6–2.85 | Not significant | Not significant | |
| 2000 | 1.01 | 0.51–2.01 | Not significant | Not significant | |
| 2001 | 1.53 | 0.58–2.30 | Not significant | Not significant | |
| 2002 | 1.59 | 0.82–3.08 | Not significant | Not significant | |
| 2003 | 1.49 | 0.78–2.87 | Not significant | Not significant | |
| 2004 | 1.81 | 0.96–3.41 | Not significant | Not significant | |
| SEER Region | |||||
| San Francisco | Ref | n/a | n/a | n/a | |
| Connecticut | 2.26 | 0.72–7.08 | Not significant | Not significant | |
| Detroit | 2.55 | 0.86–7.55 | Not significant | Not significant | |
| Hawaii | 2.00 | 0.45–9.00 | Not significant | Not significant | |
| Iowa | 2.11 | 0.69–6.43 | Not significant | Not significant | |
| New Mexico | 0.85 | 0.18–4.11 | Not significant | Not significant | |
| Seattle | 2.06 | 0.66–6.38 | Not significant | Not significant | |
| Utah | 2.27 | 0.50–10.29 | Not significant | Not significant | |
| Atlanta | 1.47 | 0.43–5.04 | Not significant | Not significant | |
| San Jose | 1.76 | 0.45–6.84 | Not significant | Not significant | |
| Los Angeles | 2.33 | 0.73–7.49 | Not significant | Not significant | |
| Rural Georgia | 1.70 | 0.16–18.44 | Not significant | Not significant | |
| Greater California | 1.52 | 0.51–4.54 | Not significant | Not significant | |
| Kentucky | 1.45 | 0.47–4.43 | Not significant | Not significant | |
| Louisiana | 1.13 | 0.35–3.63 | Not significant | Not significant | |
| New Jersey | 1.38 | 0.45–4.18 | Not significant | Not significant | |
Discussion
We found an underutilization of histologic confirmation in clinical staging during the years 1998–2005. This practice was inconsistent with evidence based guidelines. The failure of physicians to follow clinical practice guidelines is well documented across different specialties. A review by Cabana et al. described reasons that guidelines are not followed which are discussed below.22
Physicians might be unaware of evidence supporting recommendations for invasive staging in IIIA lung cancer patients. The extensive evidence base supporting the guidelines and the lack of an obvious change following publication of the American College of Chest Physician guidelines in 2003 suggest that this is not the case.
Clinicians might disagree with guidelines even at a population level. This seems unlikely given the lack of debate in the literature regarding the value of staging IIIA NSCLC. However, diagnostic and therapeutic nihilism related to the perception that little can be done for patients with lung cancer may be pertinent23,24.
Clinicians might agree with guidelines on a population level but feel they were not relevant to an individual patient. For example, a physician might believe that the positive predictive value of CT and PET in an individual patient is sufficiently reliable to obviate the need for histologic confirmation while acknowledging that this position is not supported by evidence. Many clinicians may not feel confident in their ability to perform invasive staging techniques specified by guidelines. This may subconsciously increase the likelihood of a physician recommending guideline discordant care for a particular patient.
Limited access to invasive staging procedures may discourage adherence to guidelines. Only approximately 12% of pulmonologists perform TBNA25–27 and less than 10% of lung cancer surgery is performed by dedicated thoracic surgeons28. General surgeons or cardiac surgeons performing thoracic surgery are less likely to truly be comfortable with mediastinoscopy. However, shifting all NSCLC care to specialized expertise is anything but simple. Even if all NSCLC cancer treatment was centralized at large centers, it is not clear if there are sufficient physicians trained to meet the needs of this large group of patients. Moreover, in the United States, such centralization would require a major cultural shift and many elderly patients would likely be unwilling to travel for this care.
The data suggests that some physicians routinely performed invasive staging and did so throughout the study period; while another larger group, routinely did not. Simply publishing guidelines and evidence supporting them is not sufficient to change practice. The rate of invasive staging is likely to reflect the availability of physicians with the skills and training to routinely perform invasive mediastinal staging. To actually improve patient care, leaders need to ensure that physicians have the resources needed to provide the recommended care and that incentives are aligned to encourage best practices.
Training physicians who currently care for lung cancer patients in invasive staging techniques and providing institutional resources for them may be the key to achieving guideline adherent care. For example, both practicing pulmonologists and practicing surgeons have successfully adopted EBUS-TBNA.29 However, the process of becoming an expert in a new procedure is arduous and the profession’s experience with the introduction of laparoscopy taught us to be cautious.30 Medical simulation is expensive but can reduce the learning curve for a new procedure and has been used in thoracic surgery.31 Even after use of simulation training, many physicians still want mentoring during their initial procedures. Unfortunately, there are many regulatory barriers to obtaining this mentoring including lack of reciprocity for licensing and credentialing. Addressing the need for effective continuing medical education should be a priority for medical leaders who desire to increase the rate of invasive staging of NSCLC.
Physicians and institutions also need incentives to pursue the difficult and expensive process of safely introducing invasive staging into their lung cancer practices. One policy based approach that may be effective is using the rate of invasive staging as quality indicator for the care of lung cancer patients. The recent past has shown us examples of how selection of quality indicators can dramatically impact practice in areas such as management of myocardial infarction.
The rate of use of invasive staging was not impacted by the increased use of PET. This shows that at least in stage IIIA patients PET was not replacing invasive staging, because this would have led to a decrease in invasive staging. Moreover, identification of PET avid lymph nodes did not prompt invasive staging for confirmation as recommended by guidelines since an increase in invasive would have accompanied this later scenario. This again suggests that guidelines alone are insufficient to change practice.
Our analysis is consistent with and expands on previous work.17 By separating patients invasively staged from those staged with a combination of CT scan and PET, we can appreciate how actual practice is differing from guidelines and expert opinion. We noted even higher rates of utilization of PET scanning than reported by Farjah et al17. This may be due to our inclusion of additional CPT codes for PET scanning not utilized in their study as well as our extended study period. Additionally, we did not observe the decline in utilization of invasive staging procedures that they reported. This is likely related to our inclusion of TBNA as an invasive staging procedure and our focus on patients with stage IIIA NSCLC who may be more likely to receive invasive staging than patients with either earlier or more advanced stages.
Our study has several limitations. First, data are only available on patients diagnosed through 2005. The impact of the dissemination of technologies such as EBUS and EUS over the last 5 years cannot be assessed. Second, we specifically evaluated an older Medicare population, and the results may not be generalizable to younger patients with other insurance. However, while age and insurance status are known to impact cancer therapies, most lung cancer patients are older than 65 years of age. Third, the use of SEER regions to examine geographic variability does not reflect geographic distribution of healthcare resources. However, our point in including this variation is only to provide additional evidence that variability in the use of staging techniques is due to factors other than patient characteristics. Further, due to the fact that patients may have diagnostic and treatment procedures at multiple institutions, assigning the responsibility for their care to single institution for research purposes is difficult. Therefore, we do not have data on the providers that treated any particular patient. Fourth, the SEER-Medicare database does not allow the determination of the results of an individual staging procedure in any given patient. Additionally, we are subject to the limitations of using an administrative database. For example, if a TBNA were to be performed but not billed, we would classify the patient incorrectly as not having had a TBNA. However, since this billing database is how providers are reimbursed, we are likely to capture the majority of procedures. The presence of patients in whom the absence of invasive staging would be considered medically acceptable is a potential confounder in our study.
Some patients may have been classified as IIIA in the SEER database but were only found postoperatively to have N2 involvment (“incidental N2”). However, one can argue these patient should have had invasive staging to prevent this situation, and studies indicate the rate of incidental N2 should be small. Another group for whom invasive staging can be questioned is those with mediastinal infiltration of tumor to the extent that individual nodes can no longer be discerned. However, in clinical practice this group would clearly be a minority of patients with stage IIIA disease. Finally, comorbidities may preclude considering curative intent treatment. Data from this study suggest that over 36% had no co-mordbidities.
In the end, the lack of invasive staging cannot be explained away as having been appropriate due to tumor extent or comorbidities. Even in the most favorable subgroups and youngest patients without comorbidities the rate of invasive staging was remarkably low (<30%). Furthermore, our analyses excluding surgical patients (and thus any incidental N2 patients) did not affect the results. Although the exact rate of invasive staging that should be performed cannot be determined, there is little doubt it should be substantially higher than <25%.
Conclusion
The majority of patients with stage IIIA NSCLC did not receive invasive mediastinal staging as recommended by guidelines and associated with improved survival. This was evident for patients of all races and socioeconomic strata. Patient related factors such as age and comorbidity do not fully explain this practice variation. This combined with the observed geographic variation in rates of invasive staging suggest that provider, not patient, factors are responsible. Incentives to encourage use of invasive staging may be useful in improving quality of care.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc., and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare Database. This study used the SEER-Medicare linked database. The interpretation and reporting of this data are the sole responsibility of the authors. The authors also acknowledge the National Cancer Institute (5R01CA149045).
Appendix 1
Codes for identification of Staging techniques and treatments
| CPT Codes for Invasive Staging Techniques | Endobronchial ultrasound | 31620 | |
| Bronchoscopy with TBNA | 31629 (additional lobes 31633) | ||
| Thoracoscopy of mediastinal space without bx | 32605 | ||
| VATS mediastinal biopsy | 32606 | ||
| Mediastinotomy | 39000 or 39010 | ||
| Mediastinoscopy | 39400 | ||
| Esophageal ultrasound | 43231, 43242, 43259, 76975 | ||
| Esophageal ultrasound guided aspiration | 43232 | ||
| Codes for Surgical Resection | Carinal reconstruction | ||
| Open pneumonectomy | 32440 | ||
| Removal of lung, total pneumonectomy; with resection of segment of trachea followed by broncho-tracheal anastomosis (sleeve pneumonectomy) | 32442 | ||
| Removal of lung, total pneumonectomy; extrapleural | 32445 | ||
| Open lobectomy | 32480 | ||
| Open Bilobectomy | 32482 | ||
| Open segmentectomy | 32484 | ||
| Open sleeve lobectomy | 32486 | ||
| Open completion pneumonectomy | 32488 | ||
| Open apical resection | 32503 | ||
| Resection of lung; with resection of chest wall | 32520 | ||
| Resection of lung; with reconstruction of chest wall, without prosthesis | 32522 | ||
| Resection of lung; with major reconstruction of chest wall, with prosthesis | 32525 | ||
| VATS segmentectomy/lobectomy | 32663 | ||
| Codes for Radiation Treatment | Brachytherapy | 77750–77799, 0182T | |
| Any External Beam (3-D Conformal) | 77402–416 | ||
| Any IMRT | (77301 and 77427), 77418, 0073T, G0174 | ||
| Stereotactic Surgery (“Radiosurgery/cyberknife”) | G0173, G0242, G0243, G0251, G0338, G0339, G0340, 0082T-0083T | ||
| Any Proton Beam | 77520–77525 | ||
| Any IGRT | 77421 | ||
| Codes for Chemotherapy Treatment (any chemo drug) | HCPCS Codes for Chemotherapy | 96400–96549, Q0083–Q0085, J9000–J9999, G0355–62 | |
| ICD9 Code for Chemotherapy | V58.1 | ||
| PET Scan Codes | G Codes | G0211, G0212, G0125, G0126, G0210, G0212, G0234 | |
| CPT Codes | 78811, 78812, 78813, 78815, 78816 | ||
Appendix 2
Comorbid Conditions
| Comorbid condition | N | % |
|---|---|---|
| Chronic Pulmonary Disease | 2401 | 31.66 |
| Diabetes Uncomplicated | 1284 | 16.93 |
| Cardiac Arrhythmia | 1257 | 16.58 |
| Peripheral Vascular Disorders | 1027 | 13.54 |
| Congestive Heart Failure | 978 | 12.9 |
| Solid Tumor without Metastasis | 827 | 10.91 |
| Fluid and Electrolyte Disorders | 592 | 7.81 |
| Valvular Disease | 503 | 6.63 |
| Depression | 433 | 5.71 |
| Diabetes Complicated | 329 | 4.34 |
| Deficiency Anemia | 294 | 3.88 |
| Rheumatoid Arthritis/collagen disease | 241 | 3.18 |
| Other Neurological Disorders | 224 | 2.95 |
| Renal Failure | 202 | 2.66 |
| Weight Loss | 161 | 2.12 |
| Pulmonary Circulation Disorders | 146 | 1.93 |
| Alcohol Abuse | 105 | 1.38 |
| Coagulopathy | 101 | 1.33 |
| Liver Disease | 72 | 0.95 |
| Metastatic Cancer | 72 | 0.95 |
| Psychoses | 71 | 0.94 |
| Paralysis | 68 | 0.9 |
| Lymphoma | 60 | 0.79 |
| Drug Abuse | 33 | 0.44 |
| AIDS/HIV | * | * |
Suppressed due to small cell size
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silvestri GA, Tanoue LT, Margolis ML, et al. The noninvasive staging of non-small cell lung cancer: the guidelines. Chest. 2003;123:147S–156S. doi: 10.1378/chest.123.1_suppl.147s. [DOI] [PubMed] [Google Scholar]
- 2.Shields TW. General thoracic surgery. 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 3.Gupta NC, Graeber GM, Bishop HA. Comparative efficacy of positron emission tomography with fluorodeoxyglucose in evaluation of small (<1 cm), intermediate (1 to 3 cm), and large (>3 cm) lymph node lesions. Chest. 2000;117:773–778. doi: 10.1378/chest.117.3.773. [DOI] [PubMed] [Google Scholar]
- 4.Primack SL, Lee KS, Logan PM, et al. Bronchogenic carcinoma: utility of CT in the evaluation of patients with suspected lesions. Radiology. 1994;193:795–800. doi: 10.1148/radiology.193.3.7972827. [DOI] [PubMed] [Google Scholar]
- 5.Pozo-Rodriguez F, Martin de Nicolas JL, Sanchez-Nistal MA, et al. Accuracy of helical computed tomography and [18F] fluorodeoxyglucose positron emission tomography for identifying lymph node mediastinal metastases in potentially resectable non-small-cell lung cancer. J Clin Oncol. 2005;23:8348–8356. doi: 10.1200/JCO.2004.00.6361. [DOI] [PubMed] [Google Scholar]
- 6.Takamochi K, Nagai K, Yoshida J, et al. The role of computed tomographic scanning in diagnosing mediastinal node involvement in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2000;119:1135–1140. doi: 10.1067/mtc.2000.105830. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Nagai K, Yoshida J, et al. Clinical predictors of N2 disease in the setting of a negative computed tomographic scan in patients with lung cancer. J Thorac Cardiovasc Surg. 1999;117:593–598. doi: 10.1016/s0022-5223(99)70340-5. [DOI] [PubMed] [Google Scholar]
- 8.Saunders CA, Dussek JE, O'Doherty MJ, et al. Evaluation of fluorine-18-fluorodeoxyglucose whole body positron emission tomography imaging in the staging of lung cancer. Ann Thorac Surg. 1999;67:790–797. doi: 10.1016/s0003-4975(98)01257-0. [DOI] [PubMed] [Google Scholar]
- 9.Webb WR, Gatsonis C, Zerhouni EA, et al. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology. 1991;178:705–713. doi: 10.1148/radiology.178.3.1847239. [DOI] [PubMed] [Google Scholar]
- 10.McLoud TC, Bourgouin PM, Greenberg RW, et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319–323. doi: 10.1148/radiology.182.2.1732943. [DOI] [PubMed] [Google Scholar]
- 11.Pretreatment evaluation of non-small-cell lung cancer. The American Thoracic Society and The European Respiratory Society. Am J Respir Crit Care Med. 1997;156:320–332. doi: 10.1164/ajrccm.156.1.ats156.1. [DOI] [PubMed] [Google Scholar]
- 12.Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132:178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 13.Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:202S–220S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 14.Detterbeck F, Puchalski J, Rubinowitz A, et al. Classification of the thoroughness of mediastinal staging of lung cancer. Chest. 2010;137:436–442. doi: 10.1378/chest.09-1378. [DOI] [PubMed] [Google Scholar]
- 15.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80:2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. discussion 2056. [DOI] [PubMed] [Google Scholar]
- 16.Little AG, Gay EG, Gaspar LE, et al. National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer. 2007;57:253–260. doi: 10.1016/j.lungcan.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Farjah F, Flum DR, Ramsey SD, et al. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol. 2009;4:355–363. doi: 10.1097/JTO.0b013e318197f4d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. [DOI] [PubMed] [Google Scholar]
- 19.Institute NC. National Cancer Institute Surveillance, Epidemiology and End Results Website. 2010 http://seer.cancer.gov/about/
- 20.Smith BD, Haffty BG, Smith GL, et al. Use of postmastectomy radiotherapy in older women. Int J Radiat Oncol Biol Phys. 2008;71:98–106. doi: 10.1016/j.ijrobp.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 23.Jennens RR, de Boer R, Irving L, et al. Differences of opinion: a survey of knowledge and bias among clinicians regarding the role of chemotherapy in metastatic non-small cell lung cancer. Chest. 2004;126:1985–1993. doi: 10.1378/chest.126.6.1985. [DOI] [PubMed] [Google Scholar]
- 24.Langer CJ. Elderly patients with lung cancer: biases and evidence. Curr Treat Options Oncol. 2002;3:85–102. doi: 10.1007/s11864-002-0045-9. [DOI] [PubMed] [Google Scholar]
- 25.Dasgupta A, Mehta AC. Transbronchial needle aspiration. An underused diagnostic technique. Clin Chest Med. 1999;20:39–51. doi: 10.1016/s0272-5231(05)70125-8. [DOI] [PubMed] [Google Scholar]
- 26.Haponik EF, Shure D. Underutilization of transbronchial needle aspiration: experiences of current pulmonary fellows. Chest. 1997;112:251–253. doi: 10.1378/chest.112.1.251. [DOI] [PubMed] [Google Scholar]
- 27.Haponik EF, Russell GB, Beamis JF, Jr, et al. Bronchoscopy training: current fellows' experiences and some concerns for the future. Chest. 2000;118:625–630. doi: 10.1378/chest.118.3.625. [DOI] [PubMed] [Google Scholar]
- 28.Schipper PH, Diggs BS, Ungerleider RM, et al. The influence of surgeon specialty on outcomes in general thoracic surgery: a national sample 1996 to 2005. Ann Thorac Surg. 2009;88:1566–1572. doi: 10.1016/j.athoracsur.2009.08.055. discussion 1572-1563. [DOI] [PubMed] [Google Scholar]
- 29.Groth SS, Whitson BA, D'Cunha J, et al. Endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes: a single institution's early learning curve. Ann Thorac Surg. 2008;86:1104–1109. doi: 10.1016/j.athoracsur.2008.06.042. discussion 1109–1110. [DOI] [PubMed] [Google Scholar]
- 30.Rogers DA, Elstein AS, Bordage G. Improving continuing medical education for surgical techniques: applying the lessons learned in the first decade of minimal access surgery. Ann Surg. 2001;233:159–166. doi: 10.1097/00000658-200102000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter YM, Wilson BM, Hall E, et al. Multipurpose simulator for technical skill development in thoracic surgery. J Surg Res. 2010;163:186–191. doi: 10.1016/j.jss.2010.04.051. [DOI] [PubMed] [Google Scholar]