Abstract
Human papillomaviruses (HPVs), etiological agents of epithelial tumors and cancers, initiate infection of basal human keratinocytes (HKs) facilitated by wounding. Virions bind to HKs and their secreted extracellular matrix (ECM), but molecular roles for wounding or ECM binding during infection are unclear. Herein we demonstrate HPV31 activates signals promoting cytoskeletal rearrangements and virion transport required for internalization and infection. Activation of tyrosine and PI3 kinases precedes induction of filopodia whereon virions are transported toward the cell body. Coupled with loss of ECM bound virions this supports a model whereby virus activated filopodial transport contributes to increased and protracted virion uptake into susceptible cells.
Keywords: papillomavirus, filopodia, signaling, keratinocyte, cytoskeleton, extracellular matrix
INTRODUCTION
Virus-induced cytoskeletal rearrangements are commonly initiated via cellular signaling to prepare a cell for and facilitate infection. At the level of initial cellular interaction, cytoskeletal reorganization can contribute to virion confinement at the cell surface within endocytic centers (Ewers et al., 2005; Pelkmans, Puntener, and Helenius, 2002), delivery from attachment to internalization receptors (Coyne and Bergelson, 2006), or particle transport towards the cell body along filopodia (Lehmann et al., 2005; Mercer and Helenius, 2008). In addition to travel along previously formed structures, recent evidence suggests viruses can activate filopodia formation to cause increased viral uptake (Mercer and Helenius, 2008; Sharma-Walia et al., 2004). Once a virion has accessed its internalization receptor, endocytosis occurs, and vesicles must cross the thick cortical actin cytoskeleton. Pre-existing pathways involved in endocytosis usually promote passage of endocytic vesicles through this barrier (Apodaca, 2001); however, rearrangement of cortical actin filaments is observed in virus infected cells and contributes to infection efficiency (Li et al., 1998; Pelkmans, Puntener, and Helenius, 2002). Once internalized, numerous viruses have been demonstrated to utilize the microtubule cytoskeleton and the motor protein machinery for transport from the cell periphery towards the nucleus (reviewed by (Radtke, Dohner, and Sodeik, 2006). The HPV L2 minor capsid protein interacts with actin and the microtubule motor protein dynein to direct intracellular transport (Florin et al., 2006; Yang et al., 2003). Despite the implications for actin and microtubule networks and motor proteins in HPV entry and infection (reviewed by (Ozbun, Campos, and Smith, 2007), the precise role each plays remains to be elucidated. In this report, we investigated the ability of oncogenic HPV type 31 (HPV31) to modulate the cytoskeletal architecture. We found specific signaling events are required for infection and for actin rearrangements to promote virus particle uptake from the extracellular matrix (ECM) via filopodia.
RESULTS
HPV31 Exposure Induces Cytoskeletal Rearrangements that are Signal Dependent and Required for Infection
Rearrangements of the actin and microtubule cytoskeletal networks are frequently involved in endocytosis of ligands and viruses (reviewed by (Radtke, Dohner, and Sodeik, 2006). Visualization of the actin cytoskeleton in unexposed HKs revealed a strong cortical actin network, stress fibers, and few filopodia (Fig. 1A; mock). After HK exposure to low does of HPV31 for short periods of time, the cortical actin network and stress fibers began to break down, and by 10m and 30m, a strong induction of filopodia was observed (Fig. 1A). As seen in the XZ cross-sections, virion exposure caused extensive three-dimensional filopodial movements, rising microns out of the plane from the slide (Fig. 1A). The signaling pathways regulating cytoskeletal reorganization and filopodia induction by HPV31 were investigated using actin-GFP transfected cells (Fig. 1BC). Genistein and wortmannin suppressed filopodia induction by HPV31 suggesting that both tyrosine kinase and phosphoinositide 3 (PI3)-kinase activities regulate the reorganization of the actin cytoskeleton that leads to formation of these structures. Quantification of the number of filopodia per cell confirmed a significant requirement for tyrosine and PI3 kinases during HPV31 filopodia activation (Fig. 1C).
Figure 1. HK exposure to HPV31 causes cytoskeletal reorganization that is tyrosine and PI3 kinase dependent and required for infection.
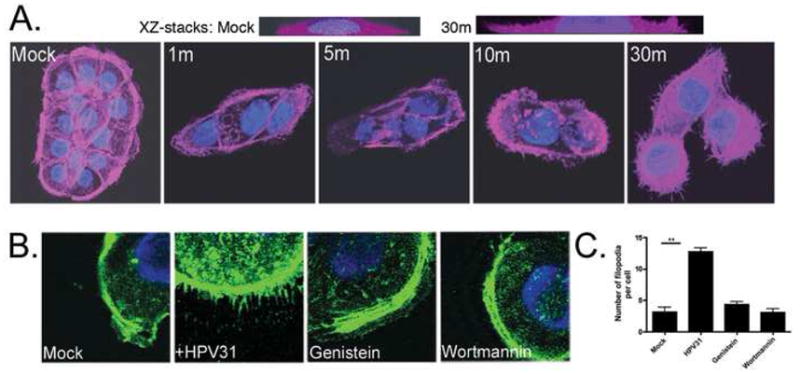
A, HaCaT cells were seeded and allowed to attach for 16h before exposure to HPV31 virions at 100 vge/cell for the indicated times (min). Cells were fixed, permeabilized and stained with AF680-phalloidin to visualize the actin network. Slides were mounted with Vectashield/DAPI and confocal photomicroscopy was performed with a 63X objective; the two upper panels show XZ-stacks from 0 to 5 microns above the slide plane. B, Actin-GFP transfected HaCaT cells were exposed to HPV31 in the presence or absence of inhibitors of tyrosine kinase (genistein; 100 μM), or PI3 kinase (wortmannin; 450 nM) for 30m at 37°C. Slides were mounted with Vectashield/DAPI and confocal photomicroscopy performed (63X objective). C, Slides shown in (B) were blinded and the number of filopodia in the plane of the ECM per cell was quantified. Error bars represent SEM and statistical significance was achieved with p<0.01(**).
To determine the requirement of signaling and the cytoskeletal reorganization during virion entry and infection, we employed an infectious entry assay, which measures the ability of virions to attach, enter, translocate, uncoat, and express early viral RNAs. HKs were exposed to low doses of HPV31 virions in the presence of well-characterized inhibitors for 16h. After removal of the chemicals, an HPV31-neutralizing antibody was added, and infection was allowed to proceed for an additional 32h. This experimental design was used to minimize chemical cytotoxic effects. Addition of the neutralizing antibody does not affect infection by successfully internalized virus (Smith, Campos, and Ozbun, 2007); thus, any infection reduction observed indicates that virions were either held at the cell surface during the chemical treatment or were internalized into a nonproductive entry route. Infections were blocked in the presence of inhibitors of tyrosine kinases (genistein), PI3 kinases (wortmannin), actin polymerization (cytochalasin D), and microtubule polymerization (colchicine) (Fig. 2A). However, no infection inhibition was observed upon treatment with inhibitors of protein kinase C (Calphostin C) and MEK1/2 (PD90859), neither of which is directly involved in cytoskeletal rearrangements. Confocal microscopy at 24h post-exposure confirmed that a majority of virions were held at the cell surface following inhibition of tyrosine kinases (genistein), PI3 kinases (wortmannin), and the actin motor protein myosin II (blebbistatin) (Fig. 2C-E). However, in untreated cells HPV31 virions were observed at perinuclear locations (Fig. 2B). Together these data indicate that tyrosine and PI3 kinase signaling lead to actin rearrangements required for filopodia formation as well as HPV31 infectious entry into HKs. The few virions that appear internalized in kinase inhibitor-treated cells (Fig. 2CD) likely reflect non-specific internalization and result in a non-infectious entry pathway (Fig. 2A).
Fig. 2. Cytoskeletal dynamics and signaling inhibitors block HPV31 entry and infection.
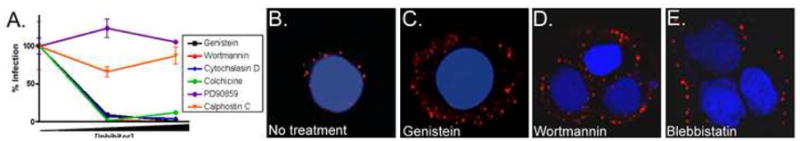
A, HaCaT cells were pretreated with inhibitor and infected with HPV31 at 100 vge/cell in the presence of inhibitors: 50 or 100 μM genistein (black boxes), 300 or 450 nM wortmannin (red triangles), 5 or 10 μM cytochalasin D (blue triangles), 50 or 100 nM colchicine (green circles), 20 or 30 μM PD90859 (purple circles), or 500 or 750 nM calphostin C (orange inverted triangles). Infections progressed for 16h in the presence of chemicals, inhibitors were removed, and cells were refed with media containing neutralizing antibody. Infections proceeded for 48h before RNA isolation. Total RNAs were subjected to RT-qPCR in triplicate to quantify spliced HPV31-E1^E4 transcripts indicative of infection; error bars represent SEM. B-E, Cells were pretreated with inhibitors for 30m (100 μM genistein, 450 nM wortmannin; 150 μM blebbistatin), then exposed to AF594-HPV31 at 10,000 vge/cell; the higher dose of fluorescent virions is required to visualize bulk cellular interactions. Infections were maintained for 16h with inhibitor present; inhibitors were washed out and infections proceeded 8h longer (total 24h) in the presence of neutralizing antibody before fixing and microscopy as in Fig. 1.
HPV31 Transport on Filopodia Facilitates Virion Uptake From the Extracellular Matrix
Upon exposure of cultured cells to HPV particles, a clear majority of capsids attach to the ECM deposited under and around the cell (Culp, Budgeon, and Christensen, 2006; Day et al., 2007) (our observations). It has been suggested that attachment to ECM factors may represent a mechanism of concentrating virions near target cells, the basal cells of the epithelia (Culp, Budgeon, and Christensen, 2006). For ECM binding to promote cell infection some function must increase exposure of these bound virions to the cell plasma membrane, and we reasoned that filopodial projections could promote this activity of particle uptake as shown for other viruses (Lehmann et al., 2005; Mercer and Helenius, 2008). Supporting this idea, we found that virions increasingly disappear from the ECM over several hours (Fig. 3A). To determine if this disappearance was related to virus particle mobility on the ECM as a means of accessing the cell plasma membrane, fluorescence recovery after photobleaching (FRAP) was performed. Fluorescently labeled HPV31 pseudovirions encapsidating a plasmid, rather than the oncogenic viral genome, were used due to the biohazard associated with these live cell manipulations. The results demonstrated that ECM-bound fluorescent-HPV31 particles are essentially immobile and unable to diffuse toward cells (Fig. 3B), indicating the cell must interact with the ECM to engage these particles.
Figure 3. Virus particles disappear from the ECM over time but are not mobile.
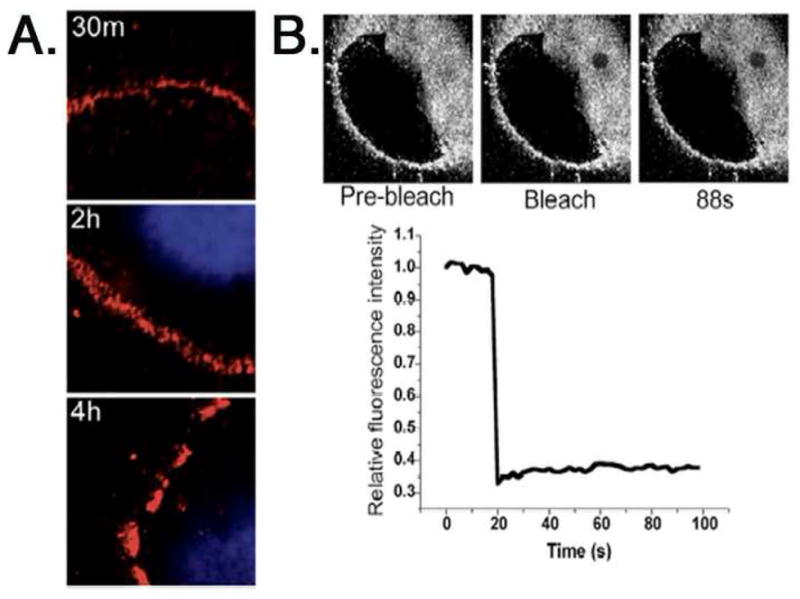
A, Saturating doses of AF594-HPV31 virions (red; ≈10,000 vge/cell) were bound to the ECM of HaCaT cells and incubated at 37°C for indicated times. Cells were fixed and mounted with Vectashield/DAPI. B, FRAP using AF647-HPV31 pseudovirus bound to the ECM of A431 HK. The laser was turned to full power to cause photobleaching, and the affected area was monitored for fluorescence recovery. Quantification is represented as fluorescence within bleached area versus time and normalized to pre-bleach intensity. HaCaT cell ECM also showed no fluorescence recovery in similar assays (not shown).
Next, we investigated whether movement of filopodial structures over ECM-bound virions might represent a mechanism for increasing viral uptake from extracellular regions distal to the cell. Time-lapse confocal microscopy was performed on live HaCaT HKs and on A431 HKs expressing GFP-tagged ErbB1, which we previously used to demonstrate the retrograde transport of activated epidermal growth factor receptors (EGFR, ErbB1) along filopodia toward the cell body (Lidke et al., 2005). We observed retrograde viral transport of individual viral particles (i.e., “surfing”) along filopodia towards the cell body (Fig. 4AB, supplemental videos 1–3), supporting a model in which filopodia facilitate increased uptake of HPV31 from the ECM. Calculation of virion transport velocity along filopodia revealed a speed of 40 nm/s ± 15 nm/s, a velocity higher than observed for EGFR retrograde transport on the same cell line (Lidke et al., 2005). This suggests that virus binding increased the rate of actin flow as compared to EGFR activation or that the virus is transported via a motor protein (i.e., myosin VI).
Figure 4. Transport of HPV31 via filopodia increases viral uptake from the ECM.
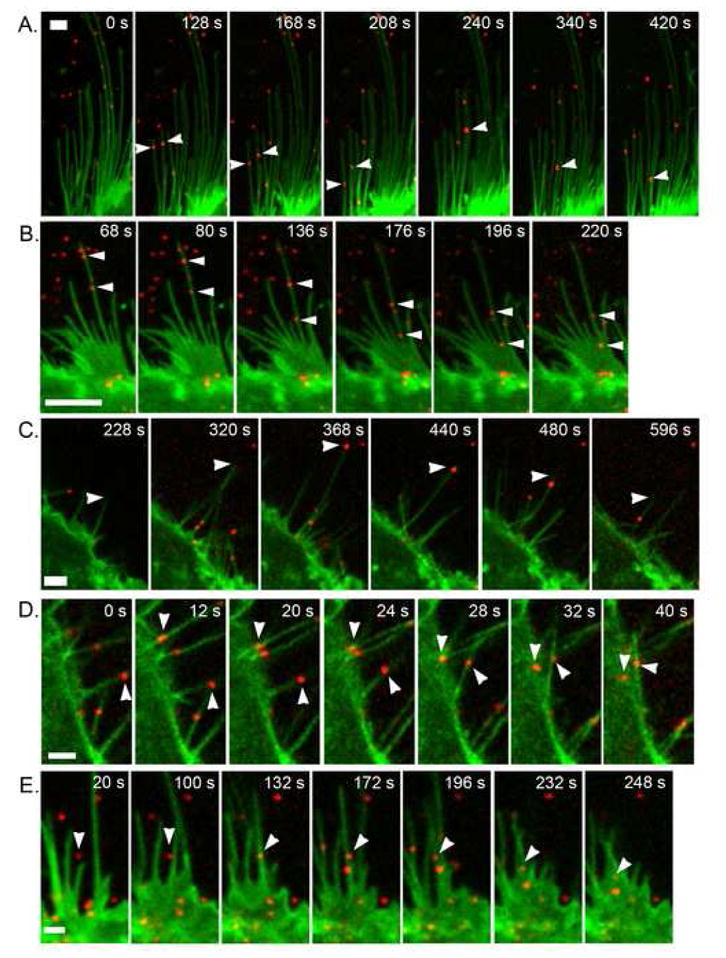
AF647-HPV31 pseuodvirions (red; ≈10,000 vge/cell) were added to A431 cells stably expressing ErbB1-eEGF (green) and immediately monitored over time by confocal fluorescence microscopy. A-B, Arrowheads mark the movement of single virus particles via retrograde transport from the filopodium periphery toward the cell body. C, An example of filopodial retraction where the filopodial tip is marked by arrowheads. D-E, Examples of filopodial lateral curling to bring particles to the cell body; arrowheads denote the movement of single virus particles. The scale bars represent 2 μm in A, D, E and 5 μm in B, C. These data are based upon time lapse videos provided as supplemental videos.
In addition to active transport, we observed two other modes of distal particle transfer to the cell body via filopodia: namely filopodial retraction (Fig. 4C; supplemental video 4) and cases where the filopodial tip with attached virion was bent back toward the cell body, which we term lateral curling (Fig. 4DE; supplemental videos 5–7). Lateral curling was found to occur upon virus binding directly to filopodia (Fig. 4D), or in some cases involved capture of virus particles bound to the ECM (Fig. 4E). In many instances when particles reached the cell body, virion motion transitioned from active transport to rapid diffusion, indicating either internalization at the base of the filopodia or transfer to the adherent (basolateral) cell surface. While virus particles were seen diffusing rapidly on the cellular filopodia, typically virions initiated active transport within 8 s (2 frames) of binding (Fig. 4A: supplemental video 1). The latter demonstrates rapid engagement of the transport machinery.
DISCUSSION
Epithelial wounding facilitates PV infection in vivo (Roberts et al., 2007), but the molecular aspects of this process remain poorly understood. Arguably, access to the mitotically active basal cells is a primary wounding facet important for establishing persistent PV infections. Two additional consequences of epithelial wound healing appear to have considerable roles in infection. The ECM secreted by keratinocytes into the wound bed is found to bind high numbers of HPV particles, and may act as a reservoir for virus exposure to susceptible cells (Culp, Budgeon, and Christensen, 2006). We show that these ECM bound particles are immobile, and thus unable to access susceptible cells unaided. However, we find that filopodia, which can be activated by wounding (reviewed by (Mattila and Lappalainen, 2008), are also induced by low dose HPV31 exposure and act to increase virion acquisition from the ECM to cells over many hours. Early gene expression as a measure of HPV31 infection of HKs is detected as early as 4h post exposure (Ozbun, 2002), likely resulting from direct plasma membrane binding of virions. Therefore, we propose that the process of active and protracted virion uptake from the ECM by filopodia is the primary reason that the bulk of HPV31 virions, most of which bind to the ECM, appear to have an unusually long internalization half-time (≈14h) as we reported (Smith, Campos, and Ozbun, 2007).
Transport of viruses along filopodia during the attachment and entry process has been documented for a number of enveloped viruses (Lehmann et al., 2005; Mercer and Helenius, 2008; Sharma-Walia et al., 2004). Ours is the first report of filopodial translocation by a nonenveloped virus: HPV31 particles bind to filopodia and transport to the cell body using retrograde transport, retraction, and lateral curling mechanisms before particle entry into the cytosol. Although the effect of blebbistatin as a myosin II and retrograde F-actin flow inhibitor was cytotoxic over our infection time course, the finding that blebbistain blocks HPV31 entry is consistent with similar experiments with murine leukemia virus and vesicular stomatitis virus where filopodial movement and infection are inhibited (Lehmann et al., 2005). In all, these characteristics of retrograde filopodial movement and HPV31 entry are comparable to those seen for murine leukemia virus and vesicular stomatitis virus infections in vitro (Lehmann et al., 2005).
Activation of filopodia by HPV31 is dependent upon intracellular signaling, a path of investigation prompted by our observation that HPV31 localizes to lipid rafts and enters via a caveolar route, both of which are associated with increased signaling (Smith, Campos, and Ozbun, 2007). Filopodial structures and HPV31 infection are inhibited by agents that interfere with tyrosine and PI3 kinase activity and actin polymerization, as well as microtubules, which can mediate filopodia movement (Schober et al., 2007). However, we cannot conclude that these agents block infection solely by inhibiting filopodia. Actin and microtubule rearrangements are likely required at multiple stages during early HPV infection and highly specific inhibitors of filopodia are not available. Thus, there is no current means to directly test whether filopodia induction is required for infection. We found that a few virions bind directly to the plasma membrane where they are able to enter prior to filopodia activation in HKs (Smith, Campos, and Ozbun, 2007), and in the absence of signaling (Fig. 2CD). Collectively, these observations argue against a strict requirement for filopodia in HPV31 infection of HKs, implying rather that filopodial transport of virions from the ECM functions to increase cell exposure to virions and particle uptake as seen for other viruses (Lehmann et al., 2005; Mercer and Helenius, 2008).
This work provides an additional explanation as to why wounding promotes HPV infection of stratified epithelium. Virions can gain access by direct binding to the plasma membrane of susceptible (basal) cells, which promotes a more rapid infection (Ozbun, 2002), or by binding the ECM where HPV exposure and/or wounding have activated signaling and filopodia to result in increased virus uptake from the surrounding environment. Each of these increases the likelihood of establishing infection in susceptible cells. With a particle-to-infectious unit ratio for PVs of ≈104:1 but only 1-2×104 receptors per cell (reviewed by (Ozbun, Campos, and Smith, 2007), it is logical that these viruses would employ supplementary means for increasing their exposure to host cells. One could postulate that a basal cell need not be fully exposed via a wound to be HPV infected, but that mere filopodial activation might conceivably stimulate susceptible cells to reach actin-rich fibers up through the epithelial layers where virions could attach and be translocated to the cell body. This study reveals yet another example of normal cellular processes hijacked by viruses for their advantage.
MATERIALS AND METHODS
Cell Culture, Virion Production, Infections
Cell culture, virion production and purification, infections, virion labeling with Alexa Fluor (AF) 594 or AF647 carboxylic acid, succinimidyl ester (Molecular Probes), and RT-qPCR infectivity assays have been previously described (Smith, Campos, and Ozbun, 2007); HPV31 pseudovirus was produced as previously reported, encapsidating pClNeo- GFP plasmid (Pyeon, Lambert, and Ahlquist, 2005). Biochemicals were purchased at Sigma or Calbiochem, and cells were preincubated with inhibitors (genistein, wortmannin, cytochalasin D, colchicine, blebbistatin, PD90859, calphostin C) for 30m prior to viral attachment. Infections were allowed to proceed for 16h in the presence of inhibitors, at which point media/inhibitor was removed and replaced with media containing an HPV31-neutralizing antibody (H31.A6) diluted 1:1000. Cells were harvested at 48h for total RNA, which was subjected to RT-qPCR to quantify infections using primers, probes, and conditions as previously described (Smith, Campos, and Ozbun, 2007).
Visualization of Actin Cytoskeleton
HaCaT cells were seeded onto coverslips and allowed to attach overnight. HPV31 was diluted to 100 vge/cell in media and added to cells for various amounts of time; mock samples were exposed to an equal volume of virion dilution buffer in media for 30 min). Cells were washed and fixed with 3.7% paraformaldehyde, permeabilized with 0.1% TX-100, and stained with AF680 phalloidin (Molecular Probes). Coverslips were mounted with Vectashield/DAPI (Vector Labs). Alternatively, HaCaT cells were transfected with pGFP-actin (Invitrogen); 2 days post-transfection cells were pretreated with inhibitors for 30m prior to and during a 30m HPV31 exposure. Cells were fixed with 3.7% paraformaldehyde and mounted with Vectashield/DAPI. Microscopy was performed using a Zeiss LSM510 META confocal microscope with a 63X objective. The number of filopodia per cell was assessed at the adherent surface (in the plane of the ECM) for 20 random cells per slide in a blinded fashion.
FRAP and Live Cell Microscopy
Live cell imaging was performed using the Zeiss META system equipped with a 63x 1.4 NA oil objective and FRAP was performed at room temperature; single virus particle tracking was at 30°C. Cells were allowed to attach ≥16 h at 37°C then exposed to AF647-HPV31 PsV (≈10,000 vge/cell) for 1 h. With a focal point at the plane of the ECM, AF647-PsV was excited using the 633 nm diode laser and photobleaching performed by turning laser to full power. Emission was collected with a 650 longpass filter, and images were acquired using 2x line averaging at intervals of one image every 2 sec. AF647-HPV31 PsV (red) was added to eGFP-ErbB1-A431 or HaCaT cells, and immediately monitored over time as reported (Lidke et al., 2005). GFP and AF647 were simultaneously excited using the 488 nm argon line and the 633nm diode laser. GFP emission was collected through 505–530 bandpass filter and the AF647 with a 650 longpass filter. Images were acquired using 2x line averaging at intervals of one image every 4 sec. Time series were Gaussan filtered and corrected for GFP photobleaching. PsV particles undergoing transport were tracked using routines written in Matlab (The Mathworks, Inc) and fit to the equation describing active transport MSD = 2DΔt + v^2Δt, where MSD is the mean square displacement, D is the diffusion coefficient, v is velocity and Δt is the time interval (Lidke et al., 2005). HaCaT cells were monitored by fluorescence and differential interference contrast (DIC) microscopy.
Supplementary Material
AF647-HPV31 pseudovirions (red) bind to filopodia of A431 cells expressing erbB1-eGFP (green). Corresponding time series to Fig. 4A. Upon binding, individual pseudovirions undergo retrograde transport towards the cell body. Note that transport is often initiated within seconds of pseudovirus binding. Images acquired at 30°C using 2x line averaging at intervals of one image every 4 sec; playback is 15 frames/s. Green channel has been Gaussian filtered with σx,y=1 and σt=2, red channel Gaussian filtered with σx,y=1. Scale bar represents 1 μm.
Same details as in Video 1; corresponding time series is 90° clockwise in Fig. 4B. Playback is 15 frames/s. Scale bar represents 1 μm. Note that upon reaching the cell body, the motion of the pseudovirus transitions from active transport to rapid diffusion.
AF647-HPV31 pseudovirions (red) bind to filopodia of HaCaT cells (DIC image, grey). Note pseudoviron on center filopodia undergoing transport that transitions from active transport to rapid diffusion at the cell body. Images acquired at 30°C using 2x line averaging at intervals of one image every 2 sec; playback is 5 frames/s. Red and green channels have been Gaussian filtered with σx,y=1. Scale bar represents 2 μm.
Same details as in Video 1; corresponding time series is 90° clockwise in Fig. 4C. Filopodia extends, an AF647-HPV31 pseudovirions binds, and the filopodia retracts. Note that when the filopodia stops retracting, the pseudovirus begins transport towards the cell. Playback is 10 frames/s. Red and green channels has been Gaussian filtered with σx,y=1. Scale bar represents 5 μm.
Same details as in Video 1; corresponding time series is 90° clockwise in Fig. 4D. Filopodia at 6 o’clock obtains a particle and curls or whips counterclockwise to bring the bound pseudovirus to the cell body. Playback is 5 frames/s. Red and green channels have been Gaussian filtered with σx,y=1. Scale bar represents 2 μm.
Same details as in Video 1; corresponding time series is 90° clockwise in Fig. 4E. Filopodia picks-up ECM-bound pseudovirus and brings it to the cell body. Playback is 10 frames/s. Red and green channels have been Gaussian filtered with σx,y=1. Scale bar represents 2 μm.
AF647-HPV31 pseudovirions (red) bind to filopodia of HaCaT cells (DIC image, grey). Images acquired at 30°C using 2x line averaging at intervals of one image every 2 sec; playback is 5 frames/s. Scale bar represents 2 μm. Note the filopodia curling clockwise at 8 o’clock acquires a pseudovirus from the ECM.
Acknowledgments
We are grateful to N. Fusenig (DKFZ, Heidelberg, DE) for HaCaT cells, T. Kanda (National Institute of Infectious Diseases, Tokyo, JP) for the HPV31-L1/L2 expression plasmid, and N. Christensen (Penn State) for the H31.A6 monoclonal antibody. This work was supported by NIH Grant CA085747 (MAO), American Cancer Society IRG #192 (DSL), and NIH Training Grant T32 AI07538 (JLS). Anastacia Maldonado provided superb technical assistance. Fluorescent images in this paper were generated in the University of New Mexico Cancer Center Fluorescence Microscopy Facility, supported as detailed on the webpage: http://kugrserver.health.unm.edu:16080/microscopy/facility.html. We apologize to any investigators whose original articles could not be referenced due to space limitations.
Abbreviations
- AF
Alexa Fluor
- DAPI
4',6-diamidino-2-phenylindole
- ECM
extracellular matrix
- GFP
green fluorescent protein
- HK
human keratinocyte
- HPV
human papillomavirus
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- vge
viral genome equivalents
Footnotes
Author contributions: All authors designed the study, analyzed results, and wrote the paper; JLS and DSL performed the research.
Conflict of interest statement: No conflicts declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apodaca G. Endocytic Traffic in Polarized Epithelial Cells: Role of the Actin and Microtubule Cytoskeleton. Traffic. 2001;2(3):149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Bergelson JM. Virus-Induced Abl and Fyn Kinase Signals Permit Coxsackievirus Entry through Epithelial Tight Junctions. Cell. 2006;124(1):119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Culp TD, Budgeon LR, Christensen ND. Human papillomaviruses bind a basal extracellular matrix component secreted by keratinocytes which is distinct from a membrane-associated receptor. Virology. 2006;347(1):147–159. doi: 10.1016/j.virol.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Day PM, Thompson CD, Buck CB, Pang YY, Lowy DR, Schiller JT. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J Virol. 2007;81(16):8784–8792. doi: 10.1128/JVI.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers H, Smith AE, Sbalzarini IF, Lilie H, Koumoutsakos P, Helenius A. Single-particle tracking of murine polyoma virus-like particles on live cells and artificial membranes. Proc Natl Acad Sci USA. 2005;102(42):15110–15115. doi: 10.1073/pnas.0504407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin L, Becker KA, Lambert C, Nowak T, Sapp C, Strand D, Streeck RE, Sapp M. Identification of a Dynein Interacting Domain in the Papillomavirus Minor Capsid Protein L2. J Virol. 2006;80(13):6691–6696. doi: 10.1128/JVI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170(2):317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus Endocytosis Requires Actin Cytoskeleton Reorganization Mediated by Rho Family GTPases. J Virol. 1998;72(11):8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9(6):446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Vaccinia Virus Uses Macropinocytosis and Apoptotic Mimicry to Enter Host Cells. Science. 2008;320(5875):531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Ozbun MA. Human papillomavirus type 31b infection of human keratinocytes and the onset of early transcription. J Virol. 2002;76:11291–11300. doi: 10.1128/JVI.76.22.11291-11300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun MA, Campos SK, Smith JL. The Early Events of Human Papillomavirus Infections: Implications for Regulation of Cell Tropism and Host Range. In: Norrild B, editor. New Strategies for Human Papillomavirus Gene Regulation and Transformation. Research Signpost; Kerala, India: 2007. pp. 69–122. [Google Scholar]
- Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- Pyeon D, Lambert PF, Ahlquist P. Production of Infectious Human Papillomavirus Independently of Viral Replication and Epithelial Cell Differentiation. Proc Natl Acad Sci USA. 2005;102:9311–9316. doi: 10.1073/pnas.0504020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke K, Dohner K, Sodeik B. Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell. Cell Microbiol. 2006;8(3):387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13(7):857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Schober JM, Komarova YA, Chaga OY, Akhmanova A, Borisy GG. Microtubule-targeting-dependent reorganization of filopodia. J Cell Sci. 2007;120(7):1235–1244. doi: 10.1242/jcs.003913. [DOI] [PubMed] [Google Scholar]
- Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi's Sarcoma-Associated Herpesvirus/Human Herpesvirus 8 Envelope Glycoprotein gB Induces the Integrin-Dependent Focal Adhesion Kinase-Src-Phosphatidylinositol 3-Kinase-Rho GTPase Signal Pathways and Cytoskeletal Rearrangements. J Virol. 2004;78(8):4207–4223. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Campos SK, Ozbun MA. Human papillomavirus type 31 uses a caveolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes. J Virol. 2007;81(18):9922–9931. doi: 10.1128/JVI.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RC, Yutzy WH, Viscidi RP, Roden RBS. Interaction of L2 with beta-actin directs intracellular transport of papillomavirus and infection. J Biol Chem. 2003;278(14):12546–12553. doi: 10.1074/jbc.M208691200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AF647-HPV31 pseudovirions (red) bind to filopodia of A431 cells expressing erbB1-eGFP (green). Corresponding time series to Fig. 4A. Upon binding, individual pseudovirions undergo retrograde transport towards the cell body. Note that transport is often initiated within seconds of pseudovirus binding. Images acquired at 30°C using 2x line averaging at intervals of one image every 4 sec; playback is 15 frames/s. Green channel has been Gaussian filtered with σx,y=1 and σt=2, red channel Gaussian filtered with σx,y=1. Scale bar represents 1 μm.
Same details as in Video 1; corresponding time series is 90° clockwise in Fig. 4B. Playback is 15 frames/s. Scale bar represents 1 μm. Note that upon reaching the cell body, the motion of the pseudovirus transitions from active transport to rapid diffusion.
AF647-HPV31 pseudovirions (red) bind to filopodia of HaCaT cells (DIC image, grey). Note pseudoviron on center filopodia undergoing transport that transitions from active transport to rapid diffusion at the cell body. Images acquired at 30°C using 2x line averaging at intervals of one image every 2 sec; playback is 5 frames/s. Red and green channels have been Gaussian filtered with σx,y=1. Scale bar represents 2 μm.
Same details as in Video 1; corresponding time series is 90° clockwise in Fig. 4C. Filopodia extends, an AF647-HPV31 pseudovirions binds, and the filopodia retracts. Note that when the filopodia stops retracting, the pseudovirus begins transport towards the cell. Playback is 10 frames/s. Red and green channels has been Gaussian filtered with σx,y=1. Scale bar represents 5 μm.
Same details as in Video 1; corresponding time series is 90° clockwise in Fig. 4D. Filopodia at 6 o’clock obtains a particle and curls or whips counterclockwise to bring the bound pseudovirus to the cell body. Playback is 5 frames/s. Red and green channels have been Gaussian filtered with σx,y=1. Scale bar represents 2 μm.
Same details as in Video 1; corresponding time series is 90° clockwise in Fig. 4E. Filopodia picks-up ECM-bound pseudovirus and brings it to the cell body. Playback is 10 frames/s. Red and green channels have been Gaussian filtered with σx,y=1. Scale bar represents 2 μm.
AF647-HPV31 pseudovirions (red) bind to filopodia of HaCaT cells (DIC image, grey). Images acquired at 30°C using 2x line averaging at intervals of one image every 2 sec; playback is 5 frames/s. Scale bar represents 2 μm. Note the filopodia curling clockwise at 8 o’clock acquires a pseudovirus from the ECM.


