A defining moment in the global AIDS response has been reached. The discourse is no longer about HIV prevention or HIV treatment; it is now about HIV control through the implementation of antiretrovirals as key components of combination interventions. Barely a year ago, visions of HIV control would have been considered far-fetched. Impetus for this mindset change, which has been building since the XVIII International AIDS Conference in Vienna last year, emanates from the compelling evidence that antiretroviral drugs prevent HIV infection in the general heterosexual population, released this week and presented at the 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention in Rome by the PartnersPrEP1 and Botswana TDF22 trials.
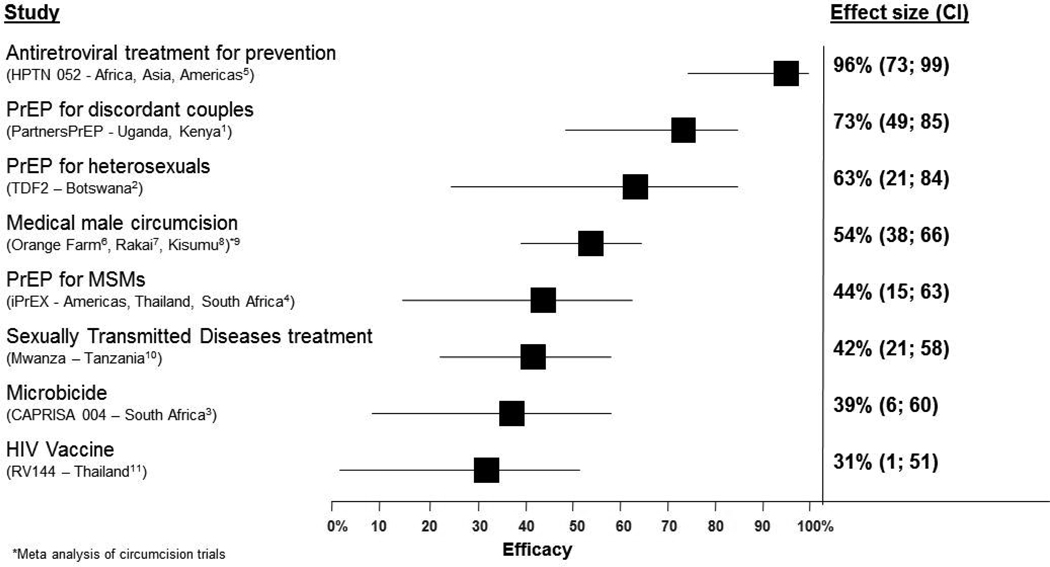
The PartnersPrEP trial1, involving 4758 HIV discordant couples from Kenya and Uganda, found that daily oral tenofovir disoproxil fumarate (TDF) and TDF-emtricitabine reduced HIV transmission by 62% and 73%, respectively. The Bostwana TDF2 trial2, in 1200 heterosexual men and women from the general population, found that daily oral TDF-emtricitabine reduced HIV transmission by 63%. These findings follow close on the heels of the CAPRISA 004 trial3 of tenofovir gel, the iPrEX trial4 of oral TDF-emtricitabine in men who have sex with men and the HPTN 052 trial5 of early antiretroviral treatment as HIV prevention. Importantly, these new findings fill a critical gap in HIV prevention with a readily available antiretroviral approach to prevent heterosexual transmission in both men and women(figure). Women benefit from a new prevention option under their control, which is particularly important for those not assured of their partner’s fidelity or willingness to use a condom. The hope these studies add to HIV prevention is further bolstered by the recent step taken by the pharmaceutical company, Gilead Sciences Inc, to lodge the drugs, TDF and emtricitabine with the UNITAID patent pool12 thus enabling lower cost versions of the drugs to be manufactured and thereby facilitating wider access in poor countries.
Figure 1.
HIV prevention technologies shown to be effective in reducing HIV incidence in randomised controlled trials1-11
PrEP = Pre-Exposure Prophylaxis. *Meta analysis of circumcision trials
There is now no doubt that antiretroviral drugs prevent HIV infection. However, important scientific questions remain: whether the inclusion of emtricitabine in preexposure prophylaxis (PrEP) formulations provides sufficient additional benefit to warrant the additional costs and side-effects; whether daily use and use-with-sex of PrEP have similar levels of effectiveness and safety; whether the safety, effectiveness, cost, and acceptability profiles of oral and topical PrEP merit implementation of both formulations; whether PrEP leads to masking of HIV acquisition that is then revealed once PrEP is withdrawn; and whether the new results can be generalised to the type of hyper-endemic settings (HIV incidence exceeding 5% per annum) where the FEMPrEP trial13 was done. Since inadequate drug levels may not have been responsible for the lack of effectiveness observed in the FEMPrEP study14, the search for an explanation for this intriguing and contrary result needs to be pursued with vigour.
There are also many practical questions about implementation: how to increase uptake of HIV testing;15 how often to monitor HIV status in people on PrEP; how to achieve high coverage in those at highest risk; how to maintain high levels of adherence; how to reduce the risk of migration away from condoms (behavioural disinhibition); and how to monitor the risk of drug resistance. While attempts are being made to obtain data to address these questions and to generate the data to guide effective implementation, the development of normative guidance by WHO/UNAIDS and submissions for regulatory approvals of TDF and TDF-emtricitabine as PrEP for HIV infection are key next steps.
As antiretroviral drugs take a key role in the global effort to control the HIV epidemic, there is much to be learned from the contraceptive field where multiple technologies, approaches, formulations, and dosing options were developed to enable and maximise user choice and increase levels of uptake, coverage, and adherence and thereby ultimately to higher public health impact. [Beyond the questions of implementation, the future scientific challenge looming large is finding a drug or class of drugs with a resistance profile does not interfere with existing first and second AIDS treatment.
Treatment of HIV-positive people with HIV prevention and PrEP/microbicides for HIV-negative people are two sides of the same coin and cannot be viewed in isolation from each other. Although research on treatment for prevention, PrEP, and microbicides has mostly occurred in separate silos, their findings converge into a single focus in HIV prevention and necessitate guidance on how to use all three strategies synergistically for maximum benefit depending on the nature of the HIV epidemic. There is no magic bullet for HIV epidemic. Treatment for prevention will be dependent on the extent to which couples establish their HIV status, whether the HIV-positive partner in a discordant couple adheres to therapy, and whether the HIV-negative partner maintains fidelity within the partnership. PrEP will be dependent on the extent to which people seek to establish and regularly monitor their HIV status and those on PrEP adhere to their regimen and clinical monitoring. Hyper-endemic communities, such as those in South Africa where HIV prevalence in the community is high, may require both interventions jointly and synergistically; treatment of people infected with HIV to reduce risk of transmission within the discordant couple and PrEP to reduce the HIV-negative partner’s risk of HIV acquisition from outside partners.
Therein lie the three most complex policy, implementation, fiscal, and ethical challenges generated by these new findings; first, how to scale-up HIV testing, a key prerequisite in settings with stigma and discrimination. Second, how to extend antiretrovirals for both treatment and prevention when many of Africa’s health systems are already struggling to cope with patients with AIDS and are not able to initiate antiretroviral therapy in everyone who currently needs it for their survival. Third, in the context of limited resources how best to ration and prioritise the limited available implementation capacity.
In this defining moment in the response to HIV, a global commitment to increased financial resources for implementation, health systems strengthening, and greater implementation efficiency is imperative. Anything less will crush the hope and promise that antiretroviral drugs can change the course of the HIV epidemic.
Footnotes
Conflict of interest statement:
We were the co-Principal Investigators of the CAPRISA 004 trial of tenofovir gel. QAK is co-Principal Investigator of the HIV Prevention Trials Network, which is undertaking HPTN 052 trial of treatment for prevention. SSAK is an executive committee member of the Microbicide Trials Network, which is undertaking VOICE trial of oral and topical PrEP.
References
- 1.Partners PrEP study press release. Pivotal study finds that HIV medications are highly effective as prophyalxis against HIV infection in men and women in Africa. 2011. [accessed July 13, 2011]. http://depts.washington.edu/uwicrc/research/studies/files/PrEP_PressRelease-UW_13Jul2011.pdf. [Google Scholar]
- 2.Centers for Diseases Control and Prevention. [accessed July 13, 2011];CDC trial and another major study find PrEP can reduce risk of HIV infection among heterosexuals. 2011 July 13; http://www.cdc.gov/nchhstp/newsroom/PrEPHeterosexuals.html.
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N EnglJMed. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HPTN 052 press release. [accessed May 12, 2011];Initiation of antiretroviral treatment protects uninfected sexual partners from HIV infection (HPTN Study 052) 2011 http://www.hptn.org/web%20documents/PressReleases/HPTN052PressReleaseFINAL5_12_118am.pdf.
- 6.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2009;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 8.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 9.Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009;15:CD003362. doi: 10.1002/14651858.CD003362.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 11.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 12.Gilead Sciences Inc. [accessed July 15, 2011];Gilead Expands Access Program for Medications in Developing World. 2011 July 12; http://www.gilead.com/pr_1584101.
- 13.FEMPrEP study press release. [accessed April 18, 2011];FHI Statement on the FEM PrEP HIV Prevention Study. 2011 http://minilicious.wordpress.com/2011/04/18/fhi-statement-on-the-fem-prep-hiv-prevention-study.
- 14.Abdool Karim SS, Kashuba A, Werner L, Abdool Karim Q. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdool Karim SS. Stigma impedes AIDS prevention. Nature. 2011;474:29–31. doi: 10.1038/474029a. [DOI] [PubMed] [Google Scholar]