Abstract
OBJECTIVE
To determine if chlorhexidine can be used as an intervention to prolong the time to relapse of oral candidiasis.
SUBJECTS AND METHODS
A double-blinded randomized clinical trial was performed in 75 HIV/AIDS subjects with oral candidiasis. Clotrimazole troche was prescribed, and the subjects were re-examined every 2 weeks until the lesions were completely eradicated. The subjects were then randomly divided into two groups; 0.12% chlorhexidine (n = 37, aged 22–52 years, mean 34 years) and 0.9% normal saline (n = 38, aged 22–55 years, mean 38 years). They were re-examined every 2 weeks until the next episode was observed.
RESULTS
The time to recurrence of oral candidiasis between the chlorhexidine and the saline group was not statistically significant (P > 0.05). The following variables were significantly associated with the time of recurrence; frequency of antifungal therapy (P = 0.011), total lymphocyte (P = 0.017), alcohol consumption (P = 0.043), and candidiasis on gingiva (P = 0.048). The subjects with lower lymphocyte showed shorter oral candidiasis-free periods (P = 0.034).
CONCLUSIONS
Chlorhexidine showed a small but not statistically significant effect in maintenance of oral candidiasis-free period. This lack of significance may be due to the small sample size. Further study should be performed to better assess the size of the effect, or to confirm our findings.
Keywords: chlorhexidine, oral candidiasis, HIV/AIDS, intervention, recurrence
Introduction
Oral candidiasis is the most common oral lesion reported in HIV/AIDS patients in both developed and developing countries (Glick et al, 1994; Nittayananta and Chungpanich, 1997). The lesion is caused by Candida species, which are present as part of the natural flora of the oral cavity. Three distinct clinical features of oral candidiasis are commonly observed in HIV/AIDS subjects; pseudomembranous, erythematous, and angular cheilitis (EC-Clearinghouse, 1993). The disease may cause oral discomfort, pain, loss of taste, and affects quality of life. Moreover, without treatment, the lesion may spread to the esophagus, causing invasive esophageal candidiasis, which is categorized as an AIDS-defining illness (CDC, 1992). Different antifungal agents such as azoles, both topical (clotrimazole) and systemic (fluconazole, itraconazole), can be used in treating the lesions (Greenspan, 1994). However, due to the underlying immune deficiency, the relative ease with which oral candidiasis can be treated contrasts with the high rate of recurrence observed among HIV/AIDS subjects. Thus, interventions that prolong the time to recurrence of the disease are needed.
Chlorhexidine-containing mouth-rinse has been shown to possess antifungal activity both in vitro and in vivo (Bobichon and Bouchet, 1987; Epstein et al, 1992; Pizzo and Giuliana, 1998; Ellepola and Samaranayake, 1999). A previous study by Barasch et al (2004) reported that chlorhexidine mouth-rinse may be useful in treating as well as preventing oral candidiasis in HIV-infected children. Adhesion of Candida to the mucosal surfaces is a vital prerequisite for successful colonization and infection. Chlorhexidine is capable of inhibiting candidal adhesion to the surfaces (Ellepola and Samaranayake, 2001). Chlorhexidine mouthwash (0.12–0.2%) has been found to be useful in prevention and treatment of oral candidiasis as well as to reduce recurrence of the lesions (Barasch et al, 2004). As chlorhexidine does not induce resistance to azoles and does not produce serious side effects (Barasch et al, 2004), the mouth-rinse may be used as an adjunct in treating oral candidiasis among HIV/AIDS subjects (Ellepola and Samaranayake, 2001), or as an intervention to prolong the time to recurrence of the lesions. The primary objective of this study was to determine if chlorhexidine mouth-rinse can be used as an intervention after antifungal therapy to prolong the time to relapse of oral candidiasis among HIV/AIDS subjects. A secondary goal was to evaluate potential modifiers of this effect, including smoking, alcohol consumption, colony forming units (CFU) of Candida, and total lymphocyte cell counts.
Materials and methods
Subjects
A double-blinded randomized clinical trial was performed in HIV-infected heterosexual adults previously diagnosed as seropositive for antibody to HIV, using a particle agglutination test for antibodies to HIV (SERODIA®-HIV; Fujirebio Inc., Tokyo, Japan) and an enzyme-linked immunosorbent assay (ELISA) (Enzygnost® Anti-HIV1/2 Plus; Behring, Behringwerke AG, Marburg, Germany), and who presented with oral candidiasis. All subjects were those who lived at Wiwekwanasom temple, or were outpatients at an internal medicine unit at Songklanagarind Hospital, a university hospital in Songkhla province in the South, or at Bamratnaradoon Institute in Nonthaburi, Thailand. Other inclusion criteria were (i) no current use or history of antifungal therapy within the last 3 months; (ii) able to use a mouth-rinse properly; (iii) able to come for follow up visits for at least a 3-month period after complete treatment of oral candidiasis; and (iv) willingness to provide informed consent. The exclusion criteria were (i) HIV-seropositive subjects without oral candidiasis or with diabetes, history of organ transplantation, or any other immunosuppressive disease and (ii) any current treatment or history of taking antifungals for the last 3 months.
History taking and study procedure
At the first visit, a health history and oral examination were performed by a dentist examiner. Subjects with any form of oral candidiasis, clinically diagnosed following the criteria classified by the EC-Clearinghouse (1993), and confirmed by culture (Samaranayake and Holmstrup, 1989), were asked to participate in the study. The nature of the study was explained, and informed consent was obtained from the patients. The study protocol was approved by the research and ethics committee of Prince of Songkla University, Thailand.
The type and location of oral candidiasis were recorded. An oral rinse technique (Samaranayake and Holmstrup, 1989) to determine the CFU of Candida was also performed at the first visit. Total lymphocyte cell counts were recorded as baseline data for the immune status of the subjects (WHO, 1990). Clotrimazole troches (10 mg) taken five times per day were provided for all subjects to treat oral candidiasis, and the subjects were re-examined every 2 weeks until the candidiasis was completely eradicated as determined by the dentist on clinical examination. The subjects were then randomly divided into two groups to receive either 0.12% chlorhexidine mouth-rinse or 0.9% normal saline. The patients were encouraged to use the mouth-rinse three times a day by swishing it in the mouth for 1 min before spitting out. All of the subjects were followed up every 2 weeks by a second blinded dentist examiner until the next episode of oral candidiasis was observed and recorded.
Data management and analysis
Data entry was performed using SPSS for Windows version 6.1 (SPSS Inc., Chicago, Illinois, USA). Data were analyzed by multiple regression analysis and Kaplan–Meier survival estimates using STATA computer package version 6.0 (STATA Corp LP, College Station, Texas, USA).
Results
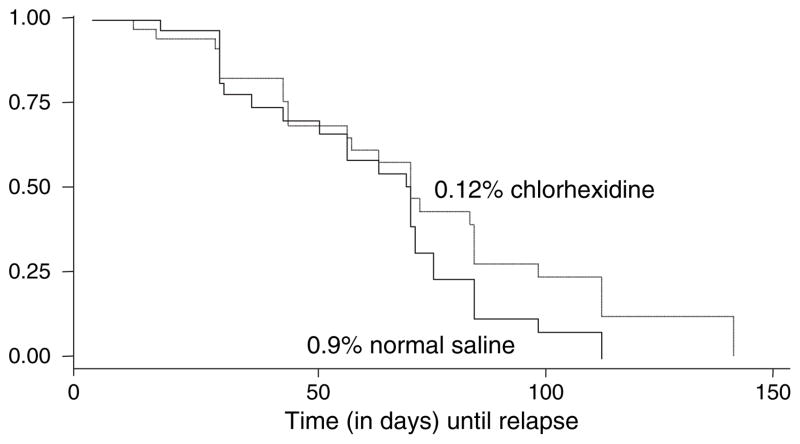
One-hundred and two HIV-seropositive subjects with oral candidiasis were enrolled, and received the 10 mg clotrimazole troche regimen for treating the lesions. Of these, 37 HIV-subjects were randomly assigned to receive 0.12% chlorhexidine mouth-rinse (aged 22–52 years, mean 34 years), and 38 subjects to receive 0.9% normal saline solution (aged 22–55 years, mean 38 years). Twenty-seven subjects were dropped from the study due to their severely ill status or death. The demographic data for all subjects with complete followup are shown in Table 1. There was no statistically significant difference between the two groups with respect to smoking habit, alcohol consumption, plaque score, CFU of Candida, and total lymphocyte cell counts. Table 2 shows the type of oral candidiasis in both groups prior to randomization. The pseudomembranous type was the most common found among the chlorhexidine-treated subjects (n = 17, 46%), followed by its combination with the erythematous type (n = 9, 24%), and erythematous type alone (n = 5, 14%). Locations of oral candidiasis prior to randomization found among both treatment and control groups are shown in Table 3. The most common sites of the prerandomization lesions in both groups were tongue (84% vs 89%), followed by labial/buccal mucosa (43% vs 39%), hard palate (32% vs 34%), and soft palate (24% vs 34%), respectively. Factors associated with the time to recurrence of the lesions are shown in Table 4. Duration of pre-enrollment antifungal therapy (number of visits; visits were every 2 weeks) (range 1–6), total lymphocyte cell counts, alcohol consumption, and the presence of oral candidiasis on gingiva were found to be significant factors associated with the time to recurrence of oral candidiasis (each P < 0.05). Figure 1 shows Kaplan–Meier survival estimates by type of mouthrinse. Chlorhexidine mouth-rinse showed a small but not statistically significant effect in maintenance of an oral candidiasis-free period among the subjects compared to normal saline solution. The time to recurrence of oral candidiasis counted by number of visits ranged from 1 to 15 (median 3) in the chlorhexidine-treated group, and 1–8 (median 2) in the control group, respectively.
Table 1.
Demographic data and characteristics for the two groups
| Variables | Chlorhexidine group (n = 37) | Normal saline group (n = 38) |
|---|---|---|
| Sex, n (%) | ||
| Male | 20 (54) | 16 (42) |
| Female | 17 (46) | 22 (58) |
| Marital status, n (%) | ||
| Single | 20 (54) | 12 (32) |
| Married | 15 (41) | 15 (39) |
| Divorce | 1 (3) | 3 (8) |
| Widow | 1 (3) | 8 (21) |
| Religion, n (%) | ||
| Buddhism | 37 (100) | 36 (95) |
| Others | 0 | 2 (5) |
| Highest education, n (%) | ||
| Never attended school | 1 (3) | 2 (5) |
| Primary school | 17 (46) | 20 (53) |
| Secondary school | 10 (27) | 15 (39) |
| Higher than secondary school | 9 (24) | 1 (3) |
| Stage of HIV infection, n (%) | ||
| Symptomatic | 20 (54) | 21 (55) |
| AIDS | 17 (46) | 17 (45) |
| Smoking habit, n (%) | ||
| Smoker | 19 (51) | 23 (61) |
| Non-smoker | 18 (49) | 15 (39) |
| Alcohol consumption, n (%) | ||
| Drinker | 18 (49) | 20 (53) |
| Non-drinker | 19 (51) | 18 (47) |
| Plaque score (mean) | 17.9 | 17.6 |
| Presence of denture | ||
| None | 33 | 30 |
| Fixed and/or removable denture | 4 | 8 |
| Colony forming units (CFU) of Candida | ||
| Range | 5 × 103–1.1 × 106 | 6.2 × 103–1.1 × 106 |
| Median | 5.1 × 105 | 5.3 × 105 |
| Total lymphocyte cell counts(cell mm−3), n (%) | ||
| <1000 | 9 (24) | 10 (26) |
| 1000–2000 | 15 (41) | 15 (39) |
| >2000 | 14 (38) | 13 (34) |
Table 2.
Types of oral candidiasis
| Oral candidiasis | Chlorhexidine group (n = 37) | Normal saline group (n = 38) |
|---|---|---|
| Pseudomembranous | 17 (46%) | 23 (61%) |
| Erythematous | 5 (14%) | 5 (13%) |
| Pseudoa + Erythb | 9 (24%) | 8 (21%) |
| Pseudoa + Angular cheilitis | 1 (3%) | 0 |
| Erythb + Angular cheilitis | 2 (5%) | 0 |
| Pseudoa + Erythb + Angular cheilitis | 3 (8%) | 2 (5%) |
Pseudomembranous.
Erythematous.
Table 3.
Location of oral candidiasis
| Location | Chlorhexidine group (n = 37) | Normal saline group (n = 38) |
|---|---|---|
| Lip | 4 (11%) | 4 (11%) |
| Labial/buccal mucosa | 16 (43%) | 15 (39%) |
| Hard palate | 12 (32%) | 13 (34%) |
| Soft palate | 9 (24%) | 13 (34%) |
| Oropharynx | 3 (8%) | 5 (13%) |
| Gingiva | 1 (3%) | 3 (8%) |
| Tongue | 31 (84%) | 34 (89%) |
| Floor of the mouth | 1 (3%) | 6 (16%) |
| Commissure | 2 (5%) | 5 (13%) |
| Whole mouth | 1 (3%) | 4 (11%) |
Table 4.
Factors associated with the time to recurrence of oral candidiasis
| Factors | 95% Confidence interval | P-value |
|---|---|---|
| Duration of pre-enrollment antifungal therapy (no. 2-week visits) | 0.45–0.90 | 0.011 |
| Total lymphocyte cell counts | 0.42–0.92 | 0.017 |
| Alcohol consumption | 1.02–3.19 | 0.043 |
| Presence of oral candidiasis on gingiva | 1.01–8.77 | 0.048 |
Figure 1.
Kaplan–Meier survival estimates, by type of mouth-rinse
Discussion
It is well established that patients with HIV/AIDS usually develop oral candidiasis during the course of the disease (EC-Clearinghouse, 1993; Glick et al, 1994; Nittayananta and Chungpanich, 1997; Chidzonga, 2003). Due to the underlying immunodeficiency, the relapse of the lesion is common among patients after cessation of antifungal agents. However, no specific recommendation has so far been made to prolong the time to recurrence of oral candidiasis among this patient group. This is of particular importance for those in developing countries, where antifungal agents are not affordable for most patients. This strategy of intervention may help to reduce the frequent use of expensive antifungal drugs among the subjects, and to limit the emergence of azole-resistant strains of Candida.
Our study revealed that chlorhexidine mouth-rinse, which has been shown to possess antifungal activity may be useful in prolonging the time to relapse of oral candidiasis among HIV-infected adults, however, there was not a statistically significant difference from the use of normal saline control rinses in this study. This lack of a difference in effect may be due to an inadequate sample size. However, it might also indicate that the effect of mechanical cleansing rinses themselves may play a role in preventing the adhesion of oral Candida to the surface, which is the first critical step of the infection (Ellepola and Samaranayake, 1998), and partially masks any advantage of chlorhexidine over normal saline.
The high positive-charge density of chlorhexidine has been found to be responsible for its effectiveness as a broad spectrum antimicrobial agent. Chlorhexidine binds to negatively charged microbial cell surfaces leading to a disruption of the cell membrane of the microorganisms (Rolla and Melsen, 1975; Brown et al, 1987). Thus, antifungal activity of chlorhexidine in this study may be due to both its fungicidal activity and its mechanical effect that inhibits fungal adhesion to mucosal epithelial cells. Previous studies using chlorhexidine in combination with other antifungal agents have demonstrated varying degree of success in the management of oral candidiasis associated with denture stomatitis (Olsen, 1975; Kulak et al, 1994; Arikan et al, 1995) and in patients with neoplastic disease undergoing chemotherapy and/or head and neck radiation (Ferretti et al, 1990). Treatment with fluconazole plus chlorhexidine produces a better improvement of palatal inflammation in denture stomatitis than the single medication alone (Kulak et al, 1994; Arikan et al, 1995). A significantly decreased incidence of clinical oral candidiasis was observed in a group of neoplastic patients undergoing chemotherapy when chlorhexidine is used in conjunction with nystatin or clotrimazole (Ferretti et al, 1990). However, it has been shown that the minimum inhibitory concentration (MIC) of the combined effect of nystatin and chlorhexidine was significantly higher than the values for each of the drugs alone (Barkvoll and Attramadal, 1989). This may be due to the formation of a low solubility chlorhexidine–nystain salt which renders the drug complex virtually ineffective as an antifungal agent (Barkvoll and Attramadal, 1989). Thus, chlorhexidine should not be used in conjunction with nystatin.
Previous studies have demonstrated that after rinsing with 10 ml of a 0.2% aqueous solution of chlorhexidine for 1 min, most of the agent is removed from the mouth in the first hour after rinsing (Bonesvoll et al, 1974a). Only 30% of the drug may be retained in the mouth for up to 24 h (Hjeljord et al, 1973; Bonesvoll and Olsen, 1974; Bonesvoll et al, 1974a,b; Seymour et al, 1999). It has been proposed that chitosan, a partially deacetylated chitin with antifungal properties and biologically safe polymer, should be incorporated with chlorhexidine in the form of gel to prolong release of incorporated chlorhexidine (Senel et al, 2000). However, the associated anti-Candida effect of these formulations remains to be determined.
It has been shown that pH of the oral cavity significantly affects both the binding and the release of chlorhexidine (Seymour et al, 1999). Drug retention is greatly reduced by reducing the pH of the rinsing solution. The availability of negatively charged receptor sites for chlorhexidine may be diminished when the environment becomes acidic. However, an increase in the pH does not seem to affect retention of the drug (Ellepola and Samaranayake, 2001). In addition, free calcium ions have also been shown to reduce the oral binding of chlorhexidine and increase its release from protein binding sites (Seymour et al, 1999). This may be due to the competition between the ions and the drug for available carboxyl groups on oral tissues. Because most toothpaste contains calcium salts as filler agents, the use of chlorhexidine as a mouth-rinse or an ingredient in toothpaste should take the potential calcium–chlorhexidine interaction into consideration. Patients should be advised to use chlorhexidine at least 30 min after toothbrushing to obtain the greatest benefit of the mouth-rinse (Seymour et al, 1999).
To the best of our knowledge, the use of normal saline rinse as a mechanical intervention to reduce the adherent of oral Candida in HIV/AIDS subjects has never been assessed. The results of our study revealed that saline rinses alone may have a beneficial effect due to mechanical cleansing. However, due to lack of a no-treatment control group, it is not possible to prove the usefulness of normal saline as another effective alternative intervention in some developing countries where chlorhexidine mouth-rinse is still relatively expensive or even not available.
Our study showed that both prior frequency of antifungal therapy and total lymphocyte cell counts were significantly associated with the time to recurrence of oral candidiasis among the subjects. This may reflect the degree of immune deficiency of the individuals, as those with the low levels of lymphocyte cell counts required a longer period of time in treating oral candidiasis before the lesions disappeared than those with higher levels of lymphocyte cell counts. In this study, alcohol consumption was also found to have a statistically significant association with the time to recurrence of oral candidiasis. These findings are in agreement with our previous study focusing on risk factors associated with oral lesions in HIV/AIDS patients in Thailand (Nittayananta et al, 2001a). The association of alcohol with atrophy and disruption of the stratification pattern of the oral mucosa has been reported (Valentine et al, 1985; Maier et al, 1994). This may facilitate the adhesion of oral Candida to the mucosal surfaces leading to infection. Interestingly, the location of oral candidiasis on gingiva also showed a statistically significant association to the time to recurrence of the lesions. This may reflect the degree of immune deficiency of the subjects, as oral candidiasis is rarely found on gingiva unless patients are severely ill, with extremely low level of lymphocyte cell counts (Nittayananta et al, 2001a).
Oral Candida was found to be a good indicator of immune defects among individuals at high risk for developing AIDS (Brodt et al, 1986), and the level of Candida determined by the number of CFU among the subjects with AIDS was found to be higher than that of the asymptomatic or symptomatic subjects (Nittayananta et al, 2001b). However, in our study the number of CFU of oral Candida was not found to be statistically significantly associated with the time to recurrence of oral candidiasis. Further study with a greater number of subjects should be performed to clarify this finding.
There are several limitations of this study including a limited sample size, unknown levels of compliance with the rinsing regimen, and lack of a no-treatment control group to determine the mechanical cleansing effect of chlorhexidine and normal saline rinses.
In conclusion, our study revealed that chlorhexidine mouth-rinse showed a small, but not statistically significant, effect in maintenance of an oral candidiasis-free period among HIV/AIDS subjects compared to normal saline solution rinses. This lack of significance may be due to the small sample size. Further study with a larger number of subjects should be performed to better assess the size of the effect, and the value of a preventive rinsing protocol in maintaining a candidiasis-free status.
Acknowledgments
This study was supported by the International AIDS Research and Training Program (IARTP) at the University of Washington through a grant from the Fogarty International Center, National Institutes of Health (D43 TW00007). The authors wish to thank staff and patients at Wiwekwanasom Temple, and Songklanagarind Hospital in Songkla province, and Bamratnaradoon Institute in Nonthaburi, Thailand for their kind helps and cooperation.
Footnotes
Author contributions
W Nittayananta and TA DeRouen designed the study and researched grant application. W Nittayananta, P Arirachakaran, T Laothumthut, K Pangsomboon and S Petsantad performed the clinical examination and collected the data. W Nittayananta prepared and revised the paper. V Vuddhakul performed the laboratory investigation of Candida. H Sriplung analysed the data and prepared the figures. S Jaruratanasirikul conducted the medical assessment of subjects. MD Martin reviewed and edited the paper.
References
- Arikan A, Kulak Y, Kadir T. Comparison of different treatment methods for localized and generalized simple denture stomatitis. J Oral Rehabil. 1995;22:365–369. doi: 10.1111/j.1365-2842.1995.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Barasch A, Safford MM, Dapkute-Marcus I, Fine DH. Efficacy of chlorhexidine gluconate rinse for treatment and prevention of oral candidiasis in HIV-infected children: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:204–207. doi: 10.1016/j.tripleo.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Barkvoll P, Attramadal A. Effect of nystatin and chlorhexidine digluconate on Candida albicans. Oral Surg Oral Med Oral Pathol. 1989;67:279–281. doi: 10.1016/0030-4220(89)90354-x. [DOI] [PubMed] [Google Scholar]
- Bobichon H, Bouchet P. Action of chlorhexidine on budding Candida albicans: scanning and transmission electron microscopic study. Mycopathologia. 1987;100:27–35. doi: 10.1007/BF00769565. [DOI] [PubMed] [Google Scholar]
- Bonesvoll P, Olsen I. Influence of teeth, plaque and dentures on the retention of chlorhexidine in the human oral cavity. J Clin Periodontol. 1974;1:214–221. doi: 10.1111/j.1600-051x.1974.tb01260.x. [DOI] [PubMed] [Google Scholar]
- Bonesvoll P, Lokken P, Rolla G, et al. Retention of chlorhexidine in the human oral cavity after mouth rinses. Arch Oral Biol. 1974a;19:209–212. doi: 10.1016/0003-9969(74)90263-5. [DOI] [PubMed] [Google Scholar]
- Bonesvoll P, Lokken P, Rolla G. Influence of concentration, time, temperature and pH on the retention of chlorhexidine in the human oral cavity after mouth rinses. Arch Oral Biol. 1974b;19:1025–1029. doi: 10.1016/0003-9969(74)90089-2. [DOI] [PubMed] [Google Scholar]
- Brodt HR, Helm EB, Werner A, et al. Spontaneous course of LAV/HTLV-III infection. Follow-up of persons from AIDS-risk groups. Dtsch Med Wochenschr. 1986;111:1175–1178. doi: 10.1055/s-2008-1068603. [DOI] [PubMed] [Google Scholar]
- Brown AT, Largent BA, Ferretti GA, et al. Chemical control of plaque-dependent oral diseases: the use of chlorhexidine. Compend Contin Educ Dent. 1987;7:719–724. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) . 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41:1–19. [PubMed] [Google Scholar]
- Chidzonga MM. HIV/AIDS orofacial lesions in 156 Zimbabwean patients at referral oral and maxillofacial surgical clinics. Oral Dis. 2003;9:317–322. doi: 10.1034/j.1601-0825.2003.00962.x. [DOI] [PubMed] [Google Scholar]
- EC-Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Human Immunodeficiency Virus. Classsification and diagnostic criteria for oral lesions in HIV infection. J Oral Pathol Med. 1993;22:289–291. [PubMed] [Google Scholar]
- Ellepola AN, Samaranayake LP. Adhesion of oral Candida albicans isolates to denture acrylic following limited exposure to antifungal agents. Arch Oral Biol. 1998;43:999–1007. doi: 10.1016/s0003-9969(98)00075-2. [DOI] [PubMed] [Google Scholar]
- Ellepola AN, Samaranayake LP. The in vitro post-antifungal effect of nystatin on Candida species of oral origin. J Oral Pathol Med. 1999;28:112–116. doi: 10.1111/j.1600-0714.1999.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Ellepola AN, Samaranayake LP. Adjunctive use of chlorhexidine in oral candidoses: a review. Oral Dis. 2001;7:11–17. [PubMed] [Google Scholar]
- Epstein JB, Vickars L, Spinelli J, Reece D. Efficacy of chlorhexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surg Oral Med Oral Pathol. 1992;73:682–689. doi: 10.1016/0030-4220(92)90009-f. [DOI] [PubMed] [Google Scholar]
- Ferretti GA, Raybould TP, Brown AT, et al. Chlorhexidine prophylaxis for chemotherapy- and radiotherapy-induced stomatitis: a randomized double-blind trial. Oral Surg Oral Med Oral Pathol. 1990;69:331–338. doi: 10.1016/0030-4220(90)90295-4. [DOI] [PubMed] [Google Scholar]
- Glick M, Muzyka BC, Lurie D, Salkin LM. Oral manifestations associated with HIV-related diseases as markers for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol. 1994;77:344–349. doi: 10.1016/0030-4220(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Greenspan D. Treatment of oropharyngeal candidiasis in HIV-positive patients. J Am Acad Dermatol. 1994;31:S51–S55. doi: 10.1016/s0190-9622(08)81268-6. [DOI] [PubMed] [Google Scholar]
- Hjeljord LG, Rolla G, Bonesvoll P. Chlorhexidine-protein interactions. J Periodontal Res. 1973;12(Suppl):11–16. doi: 10.1111/j.1600-0765.1973.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Kulak Y, Arikan A, Delibalta N. Comparison of three different treatment methods for generalized denture stomatitis. J Prosthet Dent. 1994;72:283–288. doi: 10.1016/0022-3913(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Maier H, Weidauer H, Zoller J, et al. Effect of chronic alcohol consumption on the morphology of the oral mucosa. Alcohol Clin Exp Res. 1994;18:387–391. doi: 10.1111/j.1530-0277.1994.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Nittayananta W, Chungpanich S. Oral lesions in a group of Thai people with AIDS. Oral Dis. 1997;3(Suppl):S42–S45. doi: 10.1111/j.1601-0825.1997.tb00372.x. [DOI] [PubMed] [Google Scholar]
- Nittayananta W, Chanowanna N, Sripatanakul S, Winn T. Risk factors associated with oral lesions in HIV-infected heterosexual people and intravenous drug users in Thailand. J Oral Pathol Med. 2001a;30:224–230. doi: 10.1034/j.1600-0714.2001.300406.x. [DOI] [PubMed] [Google Scholar]
- Nittayananta W, Jealae S, Winn T. Oral Candida in HIV-infected heterosexuals and intravenous drug users in Thailand. J Oral Pathol Med. 2001b;30:347–354. doi: 10.1034/j.1600-0714.2001.300604.x. [DOI] [PubMed] [Google Scholar]
- Olsen I. Denture stomatitis The clinical effects of chlorhexidine and amphotericin B. Acta Odontol Scand. 1975;33:47–52. doi: 10.3109/00016357509004626. [DOI] [PubMed] [Google Scholar]
- Pizzo G, Giuliana G. Antifungal activity of chlorhexidine containing mouthrinses. An in vitro study. Minerva Stomatol. 1998;47:665–671. [PubMed] [Google Scholar]
- Rolla G, Melsen B. On the mechanism of the plaque inhibition by chlorhexidine. J Dent Res. 1975;54(Spec No B):B57–B62. doi: 10.1177/00220345750540022601. [DOI] [PubMed] [Google Scholar]
- Samaranayake LP, Holmstrup P. Oral candidiasis and human immunodeficiency virus infection. J Oral Pathol Med. 1989;18:554–564. doi: 10.1111/j.1600-0714.1989.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Senel S, Ikinci G, Kas S, et al. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm. 2000;193:197–203. doi: 10.1016/s0378-5173(99)00334-8. [DOI] [PubMed] [Google Scholar]
- Seymour RA, Meechan JG, Yates MS, editors. Pharmacology and dental therapeutics. Oxford University Press; Oxford: 1999. Pharmacological control of dental caries and periodontal disease; pp. 179–196. [Google Scholar]
- Valentine JA, Scott J, West CR, St Hill CA. A histological analysis of the early effects of alcohol and tobacco usage on human lingual epithelium. J Oral Pathol. 1985;14:654–665. doi: 10.1111/j.1600-0714.1985.tb00543.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Acquired Immune Deficiency Syndrome (AIDS): interim proposal for a WHO staging system for HIV infection and disease. Wkly Epidemiol Rec. 1990;65:221–228. [PubMed] [Google Scholar]