Abstract
Eccrine sweat glands in the mouse are found only on the footpads and when mature, resemble human eccrine glands. Eccrine gland anlagen were first apparent at 16.5 days post-conception (DPC) in mouse embryos as small accumulations of cells in the mesenchymal tissue beneath the developing epidermis resembling hair follicle placodes. These cells extended into the dermis where significant cell organization, duct development, and evidence of the acrosyringium were observed in 6-7 postpartum day (PPD) mice. Mouse specific keratin 1 (K1) and 10 (K10) expression was confined to the strata spinosum and granulosum. In 16.5 and 18.5 DPC embryos, K14 and K17 were both expressed in the stratum basale and diffusely in the gland anlagen. K5 expression closely mimicked K17 throughout gland development. K6 expression was not observed in the developing glands of the embryo but was apparent in the luminal cell layer of the duct by 6-7 PPD. By 21 PPD the gland apertures appeared as depressions in the surface surrounded by cornified squames and the footpad surface lacked the organized ridge and crease system seen in human fingers. These data serve as a valuable reference for investigators who utilize genetically engineered mice for skin research.
Keywords: embryology, development, immunohistochemistry, and scanning electron microscopy
The eccrine sweat gland is an epidermal appendage that can be described as a long-branched tubular structure consisting of a highly coiled secretory portion and a relatively straight ductal portion that carries secretions to the skin surface (Fig. 1-3). The coiled secretory portion and proximal duct are located in the dermis while the distal duct ultimately traverses the epidermis as a tortuous structure referred to as the acrosyringium32,44 that terminates in a round aperture at the skin surface.7 The microscopic appearance of fully developed eccrine sweat glands in humans and mice is strikingly similar.15 Human eccrine glands exhibit basal clear cells and superficial dark cells lining the secretory portion of the gland. The clear cells secrete the major portion of sweat, which is water and electrolytes, while the dark cells secrete periodic acid-Schiff (PAS)-positive glycoproteins.7 By contrast, studies of the mouse eccrine gland reveal only one type of cell lining the secretory region,28 exhibiting characteristics most consistent with clear cells. Mouse sweat is higher in potassium when compared with other species,31 but whether this correlates to the presence of only one cell type is not known. A third type of cell found in the secretory region that exists in humans and mice is the myoepithelial cell, whose function is not completely understood. There is some speculation that myoepithelial cells might protect the secretory cells from over-distention.7 They might also serve to extrude secreted contents of the glands.22
Fig. 1.
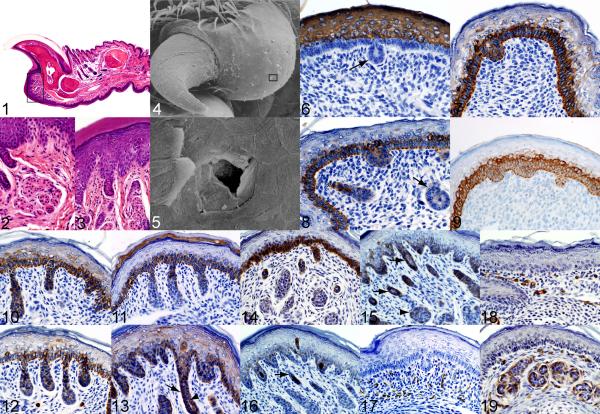
Adult C57BL/6J mouse, case No.1. Saggital section of P3 illustrates the normal nail unit. Below the hyponychium (flat, slightly concave area below nail and bone) is the convex foot pad (boxed area). HE. Fig. 2. The boxed area in Fig. 1 is enlarged showing the tightly coiled, fully developed eccrine gland. HE. Fig. 3. The overlying epidermis with the coiled acrosyringium extends into the underlying dermis. HE. Fig. 4. Adult C57BL/6J mouse, case No. 2. The foot pad is a series of domed structures proximal to the nail unit and along the length of the volar surface of the digit and metacarpal/metarsal areas of the foot itself. No ridges and troughs are present indicating that mice do not have the equivalent of finger prints found on human and other primate digits. Gold sputter coat, SEM. Fig. 5. Higher magnification of the boxed area in Fig. 4. The eccrine gland duct osteum is a small opening lined by squames. Gold sputter coat, SEM. Fig. 6. 18.5 DPC C57BL/6J mouse, case No. 3. Mouse keratin 1 expression is limited to the strata corneum and spinosum of the epidermis with no labeling of gland anlagen (arrow). K1 with hematoxylin counterstain. Fig. 7. 16.5 DPC C57BL/6J mouse, case No. 4. Mouse keratin 14 labels the stratum basale of the epidermis as well as eccrine gland anlagen. K14 with hematoxylin counterstain. Fig. 8. 18.5 DPC C57BL/6J mouse, case No. 3. Mouse keratin 17 expression is prominent in the stratum basale of the epidermis. Within the gland anlagen, keratin 17 expression appears to be present in the distal portion of what is possibly the developing duct region. The group of cells deep in the dermal mesenchyme, possibly representing early secretory region, shows little labeling (arrow). K17 with hematoxylin counterstain. Fig. 9. 18.5 DPC C57BL/6J mouse, case No. 3. Mouse keratin 5 labels the stratum basale and eccrine anlagen similar to keratin 14. K5 with hematoxylin counterstain. Fig. 10. 1 day old C57BL/6J mouse, case No. 5. Developing eccrine glands in the foot pads of neonatal mice express mouse specific keratin K14 as well as basal cells. K14 with hematoxylin counterstain. Fig. 11. 1 day old C57BL/6J mouse, case No. 5. Developing eccrine glands and basal cells in the foot pads of neonatal mice express keratin 5. K5 with hematoxylin counterstain. Fig. 12. 1 day old C57BL/6J mouse, case No. 5. Developing eccrine glands and basal cells in the foot pads of neonatal mice express keratin17. K17 with hematoxylin counterstain. Fig. 13. 6 day old C57BL/6J mouse, case No. 6. Foot pad epidermis and eccrine gland and duct labeled for mouse specific keratin 14. K6 with hematoxylin counterstain. Fig. 14. 6 day old C57BL/6J mouse, case No. 6. Foot pad epidermis and eccrine gland and duct labeled for mouse specific keratin 5. K5 with hematoxylin counterstain. Fig. 15. 6 day old C57BL/6J mouse, case No. 6. Foot pad epidermis and eccrine gland and duct labeled for mouse specific keratin17. Cells are organizing into ducts (arrows) and immature secretory units and associated acini (arrowhead). K17 with hematoxylin counterstain. Fig. 16. 6 day old mouse, case No. 6. Eccrine gland duct lining epithelia labeled for mouse specific keratin 6. K6 with hematoxylin counterstain. Fig. 17. 18.5 DPC C57BL/6J mouse, case No. 3. Smooth muscle actin labels vascular smooth muscle walls in the dermis but not the eccrine gland anlagen. Smooth muscle actin with hematoxylin counterstain. Fig. 18. 6 day old C57BL/6J mouse, case No. 6. Smooth muscle actin is expressed by myoepithelial cells and appears as a continuous band encircling the acini of the secretory units in the eccrine gland (arrows). Smooth muscle actin with hematoxylin counterstain. Fig. 19. 21 day old C57BL/6J mouse, case No. 7. Smooth muscle actin labels a thin, distinct band of cells, the myoepithelial cells, which encompass individual acini of the secretory region of the eccrine gland (arrow). Smooth muscle actin with hematoxylin counterstain.
In the developing human fetus the eccrine sweat gland anlagen is evident as early as 15 weeks of development with the most mature structures observed on the toes and fingers and the least mature on the trunk.21 At 22-24 weeks of development the eccrine glands have a structure similar to that seen in adults;21 they are functional by the end of the second trimester.9 The full complement of eccrine glands is present in the human at birth and there is no new development afterward.5 The density of eccrine sweat glands in human skin varies throughout the body. They are most numerous on the soles and the forehead and least numerous on the trunk and extremities.5 Eccrine glands serve a very important thermoregulatory function in humans but also respond to emotional and gustatory stimuli.7,14 In other mammals that have been studied such as cats, mice, and rats, eccrine glands are found only on the footpads. Because of the limited distribution of the glands in these species, it is unlikely that they serve any thermoregulatory function. It appears instead that they secrete sweat to increase traction for improved mobility and greater tactile sensation in the foot.11,37 Eccrine sweat glands are innervated by sudomotor nerve fibers which are sympathetic nerve fibers utilizing acetylcholine as the principle neurotransmitter.7 In mice, it was found that a single sweat gland can receive multiple innervations.28
In humans dysfunction of the eccrine glands is placed into one of the following three general categories: 1) excessive sweat production (hyperhidrosis), 2) decreased sweat production (hypohidrosis), and 3) absence of sweat production (anhidrosis).4 There is evidence in mice and rats that eccrine gland function is dependent upon aquaporin 5 (Aqp5) expression25 and mis-expression might be one mechanism for eccrine gland dysfunction. These disorders typically arise secondary to other disease processes.4,44 Eccrine gland morphology is altered in human patients with chronic renal disease, Down syndrome, and tetralogy of Fallot.34 There is also a class of diseases termed the anhidrotic ectodermal dysplasias, some of which result in a complete absence of eccrine glands in affected individuals.10 Cystic fibrosis (CF) causes an alteration in the electrolyte composition of eccrine gland sweat that has become one diagnostic criterion for the disease.44 There are also many types of benign and malignant neoplasms arising from eccrine gland tissue in humans.43 These neoplasms are believed to be relatively rare, although many feel the current classification system is inadequate, making true incidence obscure.41
The advent of genetically engineered mice (GEM) and their widespread use in biomedical research has prompted great interest in the phenotypes arising from induced genetic mutations. It is therefore important that investigators examine as many anatomical structures as possible when new GEMs are discovered or created. Many anatomical tissues that might exhibit abnormalities in structure or function resulting from genetic manipulation often go undocumented. Examples are the eccrine glands in the feet of GEMs, which are often neglected unless nails are severely deformed. Consequently, abnormalities in eccrine sweat gland structure are often incidental findings described only when investigators evaluate nails. Although these glands are documented and analysis is described in routine necropsy protocols,29,33 eccrine glands are seldom evaluated when searching for phenotypic deviations resulting from genetic manipulation. Classic mouse models where this gland is affected include the phenotypic mimics crinkled (Edaraddcr-2J), downless (Edardl), sleek (Edarsl), and tabby (EdaTa), the latter of which is homologous to the human gene encoding ectodysplasin-A (EDA).36 These mutant mice all have the same phenotype due to mutations in the cascade of receptor-ligand interactions resulting in complete absence of eccrine glands which phenocopies some types of anhidrotic ectodermal dysplasia in humans.2,39 Mice that are deficient in the gene encoding for the cytoplasmic adaptor protein tumor necrosis factor associated factor 6 (Traf6tm1Jino) fail to develop eccrine sweat glands and exhibit a phenotype consistent with hypohidrotic ectodermal dysplasia.24 Transgenic mice expressing the simian virus 40 large T-antigen develop mixed tumors of the eccrine glands and might serve as a useful model of heterotopic bone disease affecting humans.16 Even considering these reports, there is little in the literature addressing eccrine gland development and structure in either GEMs or wild-type mice, thus it is important that these structures be examined when characterizing the phenotypes arising from new genetic mutations.
The objective of this study was to characterize the anatomical structure and normal expression patterns of selected proteins in the mouse eccrine gland during the embryonic, neonatal, juvenile, and young adult stages.
Materials and Methods
Mice
Inbred C57BL/6J (JR# 664) mice were obtained from production colonies of The Jackson Laboratory (Bar Harbor, ME). Females were observed each morning for the formation of a copulatory plug (0.5 days post conception (DPC)), then housed with other pregnant females at the same stage. At days 13.5, 14.5, 15.5, 16.5, 17.5, 18.5, 19.5 DPC embryos were collected for tissue processing. Embryonic time points studied correspond to Theiler stages (TS) 22, 23, 24, 25, 26, and then 2 days beyond as TSs are not defined beyond 17.5 DPC (TS 26).12 Parturition typically occurred between 18.5 and 19.5 days of gestation. Newborn mice were designated as “day 0” individuals. To examine gland development in the postnatal period, tissues from 0 and 1 day-old mice (designated the “neonatal” group), 2, 3, 4, 5, 6, 7, and 21 day-old mice were also studied. For all time points, tissues from a minimum of 2 animals were collected and processed for examination. All mice and methods utilized in this study were included in a protocol approved by The Jackson Laboratory’s Institutional Animal Care and Use Committee (IACUC).
Mouse Tissue collection and preparation for histology
Embryos were fixed in Fekete’s acid alcohol formalin and then transferred to 70% ethanol. Tissues were embedded in paraffin and serial sagittal sections were made with every fifth section stained with hematoxylin and eosin (H&E) to identify optimal sections. A subset was stained using the periodic acid-Schiff (PAS) reaction.
Mouse Tissue Immunohistochemistry
Unstained tissue sections cut from paraffin blocks were processed routinely30 using mouse specific affinity purified rabbit polyclonal antibodies directed against terminal differentiation proteins well defined in normal adult mouse skin (mouse keratins K1, K5, K6 (KRT6A, KRT6B, KRT71-KRT75), K10, K14, K17, filaggrin (FLG), loricrin (LOR), Involucrin (IVL), and smooth muscle actin (actin, alpha 2, smooth muscle, aorta ACTA2; hereafter referred to as smooth muscle actin or SMA). Sources and specific methods are described in detail elsewhere (http://tumor.informatics.jax.org).20 Diaminobenzidine was used as the substrate.
Tissue preparation for scanning electron microscopy
Tissues were fixed overnight at 4°C in 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB, pH 7.2). After rinsing the tissues several times in PB, they were postfixed in 1% osmium tetroxide in PB for 48 hours at 4°C, dehydrated in a graded series of ethanol and dried. Samples were mounted and sputter-coated with a 4 nm layer of gold. The volar surfaces of foot pads were examined at 20kV at a working distance of approximately 15 mm on a Hitachi S3000N VP Scanning Electron Microscope (Hitachi Science Systems, Ltd., Japan).1
Results
Mouse Eccrine gland embryological development and protein expression (13.5 DPC-18.5 DPC)
The eccrine gland anlagen were not found in 13.5-15.5 DPC embryos. Evidence of eccrine gland anlagen was first observed in 16.5 DPC embryos (Fig 7). These appeared as small, well-organized accumulations or invaginations of cuboidal cells just beneath the developing basal cell layer of the epidermis (Fig 6-9) similar to placodes seen in developing hair follicles.26 At this early stage, anlagen were difficult to find consistently in all tissue sections analyzed. By 18.5 DPC, these areas became progressively more organized and appeared to extend deeper in the mesenchymal tissue of the foot pad. Some cell accumulations appeared to be separate from the epidermal basal layer, possibly representing early development of the secretory region (Fig 8). There was some evidence of duct development at 16.5 DPC, although this was not consistently found. Duct regions were more readily observed in 18.5 DPC tissues (Fig 8). The glands could be observed consistently in all 18.5 DPC tissue sections analyzed, which possibly reflects an increasing density with age.
The overall keratin expression patterns were similar in foot pads from both the 16.5 DPC and the 18.5 DPC embryos. Antibody to K1 strongly labeled the stratum spinosum and stratum granulosum layers of the epidermis in both the 16.5 and 18.5 DPC embryo but did not label the eccrine gland anlagen to any degree (Fig 6). Patterns for K10 were similar to K1 (not shown). K5 and K14 are commonly co-expressed in the skin. This was also the case for the eccrine gland anlagen. The patterns for K5 and K14 were similar at both 16.5 DPC (Fig 7) and 18.5 DPC (not shown) time points. K14 and K5 expression was prominent in the stratum basale of the epidermis and was equally prominent in the gland anlagen (Fig 6-9). There was light expression of K17 in the stratum basale and in the central area of the gland anlagen in the 16. 5 DPC embryo (not shown). The pattern for K17 was similar at 18.5 DPC (Fig 8), but was significantly higher than levels at 16.5 DPC. Within the gland anlagen the K17 signal was stronger in the more distal portion of the presumptive duct (Fig 8) than it was in the proximal portion, the region that might represent the developing secretory units of the gland. K6 was detected in neither the epidermal layers nor in the developing gland. Smooth muscle actin which labels myoepithelial cells and vascular smooth muscle,19 was evident in small vessels but not the eccrine gland anlagen (Fig 17). Loricrin (LOR), filaggrin (FLG), and involucrin (IVL) expression was limited to the epidermis of the footpad and skin but were not expressed in eccrine glands at any stage of development (not shown). This pattern of expression for LOR, FIL, and IVL (i.e. solely in the epidermis) remained constant throughout development, therefore, data pertaining to these proteins is not presented. Smooth muscle actin (SMA) expression at this stage was limited to vascular smooth muscle (Fig 18).
Eccrine gland structure and protein expression in the Neonate (0-2 PPD)
At birth, the eccrine glands were significantly more advanced in structural development when compared to 18.5 DPC embryos, indicating that rapid organization had occurred during a relatively short period of time. At this stage, there was clear evidence of early duct formation (Fig 10-12). Many of these regions appeared as chords of poorly organized, primordial cells extending from the proximal termination of the forming gland in the dermis to the basal layer of the epidermis. In most cases, these duct-like structures were 2-4 cells in thickness, and there was no evidence of lumen formation (Fig. 10-12). Also, the proximal extent of the developing gland expanded into a bulbous formation that is likely the earliest development of the secretory coils (Fig 10-12).
There were no significant changes in keratin expression patterns in the epidermis or in the developing gland for the neonate when compared to the embryonic time points. K1 and K10 expression remained confined to the stratum spinosum and granulosum (not shown). K14 was diffusely and strongly expressed throughout the stratum basale and developing gland, which was similar to the pattern for K17 (Fig 10-12). K17 expression intensity, however, appeared to be slightly less than K14. The expression pattern for K5 closely resembles that of K14 and K17 with respect to the epidermis (Fig 10-12). In the developing eccrine gland, K5 expression appears to be lesser than K14 and K17 and is restricted to approximately the distal one third of the duct as it enters the epidermal layer (Fig 11). Low levels of SMA expression were evident in the 1 day-old neonate, but the pattern was inconsistent and sparse within the dermal layer (not shown) suggesting the earliest differentiation of myoepithelial cells associated with secretory units was occurring. There was no evidence of K6 expression in the neonate (not shown).
Mouse Eccrine gland structure and protein expression at 6-7 PPD
Eccrine gland structure at 6-7 days of age was significantly more mature in appearance than the previous time points examined. The duct regions appeared to be organizing into basal and lumenal cell layers (Fig 15, 16). In some tissues examined, there was evidence of duct lumen formation (Fig 13). There was also evidence of the developing acrosyringium in some tissue sections (Fig 16). It was still not possible based on structural appearance to accurately and consistently discern the coiled secretory portions of the gland from the duct portions at this age.
As was expected, K1 and K10 continue to show expression solely in the stratum spinosum and granulosum of the epidermis (not shown). The pattern of K14 expression was similar to that seen in the neonatal mouse. K14 was prominent in the stratum basale of the epidermis and in the developing duct; within the entire length of developing duct, it appeared to be diffusely expressed in basal and lumenal cell layers (Fig 13). K14 was also expressed in structures that appear to be differentiating into secretory units of the glands, although the cellular organization was not advanced enough to accurately make that determination (Fig 13).
K5 and K17 expression patterns were similar to K14 with respect to the epidermis, with both being expressed in the stratum basale. In the developing duct, both K5 and K17 started to show a more intense expression pattern in the basal cell layers when compared to the lumenal cells, particularly in the distal duct region. This was especially true for K17 (Fig 15). Cell aggregates in the dermis that likely represented the developing secretory regions also showed a basal cell labeling pattern with the K5 and K17 antibodies (Fig 14, 15). These findings in the eccrine glands were in contrast to K14, which showed a more diffuse expression pattern in the ducts. This indicates advanced cellular differentiation in the duct and secretory coil is occurring at this age.
One significant change at this age is that K6 expression was evident in the developing gland duct (Fig 16). K6 expression was limited to the lumenal cell layer of the duct and it shows very nicely a distinction between basal and lumen cell layers at this age (Fig 16). Similar to K5 and K17, K6 expression at this age provided some evidence that cell layers within the duct are becoming mature and achieving their final fate as either basal or lumenal cells. Smooth muscle actin expression was apparent as a continuous band in the basal region of what are presumably the developing secretory portions of the glands (Fig 19) because myoepithelial cells in the secretory acini are the only cells of the eccrine glands known to express SMA. This is further evidence of cellular differentiation and organization in the eccrine sweat gland at this age.
Mouse Eccrine gland structure and protein expression at 21 PPD
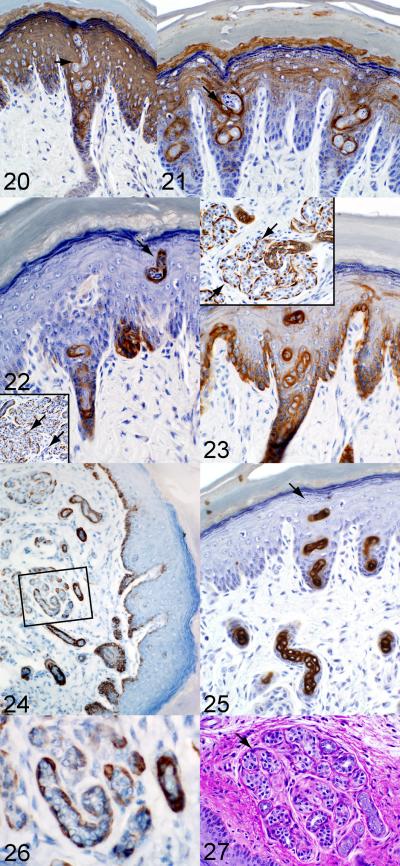
By 21 days of age, the eccrine sweat glands have all the structural characteristics of adult glands (see Figure 1-5 for adult structural features). Coiled secretory regions were evident and the ducts were well organized into the basal and lumenal cell layers (Fig 20-27). There is a well-developed basement membrane in the coiled portion of the duct within the dermis, and the secretory loops could be distinguished from the ductal loops based on cell morphology (Fig 20-26). Similar to the findings of others,28 only light cells appeared to be present in the secretory portion of the gland based on PAS reaction (Fig 27). Cells of the secretory coil therefore appeared as a distinct cuboidal monolayer sitting the basement membrane in most sections (Fig 27). In addition, the acrosyringium was readily identified as it traversed the epidermal layer of the skin (Fig 20-23, 25). 67 day old mice were also examined (not shown), but no significant differences from 21 day old mice were observed indicating that eccrine glands are fully developed anatomically by 21 days of age.
Fig. 20.
21 day old C57BL/6J mouse, case No. 7. Mouse specific keratin 1 is expressed in the foot pad epidermis but not the eccrine gland duct lining epithelia. K1 with hematoxylin counterstain. Fig. 21. 21 day old C57BL/6J mouse, case No. 7. Mouse specific keratin 10 is expressed in the foot pad epidermis and outlines the eccrine gland duct lining but not luminal epithelia. K10 with hematoxylin counterstain. Fig. 22. 21 day old C57BL/6J mouse, case No. 7. Antibodies against mouse specific keratin 17 label basal cells of the ducts (arrows) as well as the myoepithelial cells of the secretory region (inset, arrow). K17 with hematoxylin counterstain. Fig. 23. 21 day old C57BL/6J mouse, case No. 7. Mouse specific keratin 14 is expressed in the basal cells of the foot pad epidermis, the cuboidal epithelium of the ducts, and myoepithelial cells surrounding acini (insert, arrow). K14 with hematoxylin counterstain. Fig. 24. 21 day old C57BL/6J mouse, case No. 7. Mouse specific keratin 5 labels basal cells of the foot pad epidermis and myoepithelial cells of the eccrine gland acini and ducts. K5 with hematoxylin counterstain. Fig. 26. This is an enlargement of the boxed area in Figure 24 to illustrate expression of keratin 5 within myoepithelial cells. K5 with hematoxylin counterstain. Fig. 25. 21 day old C57BL/6J mouse, case No. 7. Mouse specific keratin 6 labels only the duct luminal epithelium. K6 with hematoxylin counterstain. Fig. 27. 21 day old C57BL/6J mouse, case No. 7. The secretory units within the dermis appear as well-structured cuboidal cell monolayers situated on a basement membrane. There is no evidence of PAS-positive staining cells in the secretory acini, the ‘dark cells’ as would be seen in humans. Periodic Acid Schiff reaction.
Keratin protein expression patterns in the glands of the 21 day old mouse were generally similar to those of mice at the 6-7 day time point. K1 and K10 were again expressed in the stratum spinosum and granulosum and did not appear to be expressed in any portion of the eccrine gland (Fig 20, 21). K14 expression was still prominent in the stratum basale of the epidermis and in the ducts of the eccrine gland. The K14 antibody labeled both the basal and lumenal cell layers of the duct, and there is no clear distinction between these two cell layers based on K14 expression pattern in this region. By contrast, within the secretory portion of the gland, there is distinct K14 expression in the basal area that quite probably represents the myoepithelial cells based on their orientation between the basement membrane and mouse cuboidal cells (Fig 23). K14 expression by myoepithelial in salivary glands cells further supports the conclusion that the cells in this area of this type.40
K17 expression is confined to the basal cells of both the secretory region and the duct (Fig 22). This is comparable to the pattern observed at 6-7 days. The most notable difference was that the pattern was more distinct at 21 days, particularly in the secretory region because the gland has matured significantly and has achieved adult architecture. This was also the case for K6 and SMA expression patterns, which showed a similar but more easily defined pattern of expression compared to that seen at 6-7 days of age (Fig 19, 25). K6 expression was distinctly seen in the lumenal cells of the ducts (Fig 25). Compared to the previous age, the antibody to SMA labeled a narrower band between secretory cells and the basement membrane that still encircles entire secretory acinar structures (Fig 19). This would indicate that as myoepithelial cells mature they flatten into a nearly spindle-shaped cell (in 2-dimensions and stellate-shaped in 3-dimensions) and position themselves between secretory cells and the basement membrane.
SEM of gland aperature
Evaluation of the footpad surface of adult mice showed that it is generally smooth. The footpad lacked an organized ridge and crease system observed on human fingertips, and mice had nothing resembling a finger print at this level (Fig 4). Cornified cells form polyhedral plates (squames) around depressions that represent the aperature of the eccrine gland exocrine duct at the epidermal surface (Fig 5). The density was not measured in this study, but when compared with images from studies of human skin surface and eccrine gland aperatures,32 it appears that the density in mice is much less. Sulfur analyses by X-ray microanalysis revealed levels consistent with those of interfollicular skin but not hair fibers or nails, which were over twice as high (Not shown).17
Discussion
The eccrine sweat glands are widely distributed in the human body and found only in the foot pads of mice as well as many other mammals. We report here the developmental pattern, structure, and keratin expression patterns of the eccrine sweat gland in the mouse using IHC and light microscopy as well as SEM.
The tactile pads in the mouse foot, which will eventually develop into the foot pads in the neonatal mouse, begin developing at about stage 14.5 DPC.12 Webbing in the digital interzone on both front and rear limbs recedes dramatically revealing definable digits at approximately this same time (14.5-15 DPC). Nails begin to develop on the medial digits by about 15.5 DPC, but not on the most medial digit until 17.5 DPC. These corresponding developmental events as well as the development of eccrine gland anlagen occur in the human at approximately 8-9 weeks of gestation.9 In the mouse, we identified eccrine gland structures in foot pads as early as 16.5 DPC, which is very close to embryonal day 16 (E16) when glands are reported to develop in the rat.23 This is perhaps not surprising because this is a time in mouse fetal development when early terminal differentiation of the epidermis is occurring.3 In addition, at 14 DPC, just prior to our observation of initiation of eccrine gland anlagen development, there is the first evidence of the expression of genes such as Eda, which are known to be important regulators of eccrine gland formation.36 Other epidermal appendages, such as hair follicles, are also developing at this time at 15.5 DPC (TS 24).13
At 16.5 DPC, the glands were more consistently found in pads in the proximal region of the toes examined. We hypothesize that because the distal portion of the developing digit is “younger” than the proximal portion, gland density would be less in the distal portion and thus might develop glands later in development. Our finding is in contrast; however, to findings reported in one study of human embryos where the glands were more abundant in distal finger sections in 15-week old embryos.21 By about 17.5-18.5 DPC, gland anlagen became more apparent and were more consistently identified on labeled tissue sections (Fig 6-9). While we could have missed glandular structures at earlier time points, this is unlikely as we used large numbers of serial sections (3 sections per protein studied) and multiple specimen replicates were examined. Furthermore, nothing in literature contradicts our findings.
The first evidence of gland formation at 16.5 DPC was the accumulation of cells into placode-like structures just beneath the basal epidermal layer. As the embryo develops further, these areas appear to extend into the dermis forming will eventually become the duct and gland secretory acini. This is similar to what is reported in humans during the third fetal month, when “organized local proliferations of basal cells”9 and “crowding of basal cells” were described.18 Our observations of gland anlagen in human tissue was similar to that reported with respect to structure and the time when anlagen were first observed.15 The cellular proliferations forming presumptive eccrine glands are likely the result of interactions between molecular signals known to regulate skin appendage morphogenesis in the developing embryo originating from the mesenchyme and the overlying ectoderm.3,27 The keratin expression patterns at this age indicate that the gland is still quite immature. The absence of K6 expression, which shows a sharp distinction between basal and lumenal cells in the more mature duct, indicates a lack of cellular differentiation at this time. Although antibodies against K14 and K17 label the gland anlagen, it is possible that because the glands are developing as extensions from the basal layer of the epidermis, which also expresses K14 and K17, that these K14 and K17 expressing cells represent the basal extension, not true differentiation of the gland structure itself. Studies of human eccrine gland embryonic development indicate that the glands resemble adult structure by approximately mid-term of gestation.9,21 We showed that eccrine gland anlagen in the mouse first appear late in gestation (assuming a normal 19-21 day gestation) (Fig 6-9), and mature structure is not achieved until 21 days of age (Fig 20-27).
The eccrine glands in the neonatal mouse exhibited significant advancements in development and cell proliferation compared to those in embryonic time points, but the most dramatic structural changes were observed in 6 and 7 day old mice. In the neonate, cellular organization revealed some early lumen development in the ducts and expansion of proximal duct regions in the dermis that will ultimately differentiate into the secretory region and coiled duct. The keratin expression profile in the neonate remained largely unchanged from the embryo. By 6-7 days of age, presumptive gland acini have formed, and acrosyringium is apparent. K6 expression was detectable, which seems to represent a major step forward in cellular differentiation within the duct. K17 expression also showed some gradients from proximal to distal regions of the duct. Myoepithelial cells expressing SMA were present indicating maturation of the secretory units of the gland but cellular organization of these regions still appear somewhat immature. Myoepithelial cells are reported to express keratins 5, 14, and SMA in the mouse40 and keratins 5, 17, 18, 19 in the human.21,42 At this age, it is difficult to determine accurately if myoepithelial cells are expressing the keratin proteins we examined. There is expression of K5, K14, and K17 in areas maturing into secretory units, but the loose cellular organization in the secretory region makes accurate histological interpretation difficult. It can be said with certainty that by 7 days of age, keratin expression patterns in eccrine glands closely resemble that which is seen in terminally mature glands, but overall architecture and cellular organization have not achieved what is seen in the adult gland.
Eccrine glands in the 21 day old mouse were structurally the same as glands in older adult mice 67 days of age, and there were no differences in protein expression profiles between these two ages. For these reasons, we conclude that the glands at 21 days of age, the time when mice are typically weaned from their mothers, are fully mature. As mentioned previously, this is considerably later than humans where the glands on the finger and toe tips appear to have essentially adult structure as early as 22 weeks of gestation, or approximately mid-term.21 This could be explained by the fact that in humans, eccrine sweat glands perform a vital thermoregulatory function7 and need to be abundant and functional at birth. In mice, as well as many other species, they presumably serve only to increase tactile sensation and traction on the foot pads, which are not functions necessary for survival of the animal at birth11,38 and therefore do not need to be as numerous and mature in early development. Furthermore, mice are altrical and relatively undeveloped at birth as exemplified by the fact that hair fibers do not emerge from the skin until 5 days PPD and eyelids do not open until 12 days PPD.6,35
The levels and patterns of keratin and SMA expression are essentially the same at 7 days as they were at 21 days, while overall structure of the glands is significantly less mature at 7 days. This suggests that terminal protein expression patterns develop at a stage earlier than the actual maturation of the gland structure. This is in agreement with studies of human eccrine glands where it appears that major terminal differentiation protein expression in the ducts precedes complete structural maturation.8,21 In the present study, the most distinctive changes in protein expression patterns with increasing age were observed with K6 and K17. K6 was expressed later than the other keratins studied (days 6-7) and also appeared to serve as a reliable means to differentiate the duct region from secretory region. K5 and K17 expression allowed some distinction between basal and lumenal cell layers in the ducts to be made, but K6 expression defined the lumenal cell layer in the duct more precisely. As such, K6 expression appears to serve as a good measure of gland structural maturity.
The appearance of the skin and eccrine gland aperatures on the mouse footpad as studied by SEM is rather simple. There were no epidermal ridges nor creases in the mouse foot pad as would be observed in the human.32 The gland aperature in mice appear as irregular openings in the epidermis surrounded by squames. Compared to humans, they appear to be less numerous, which likely reflects their comparatively limited function in the mouse.
We describe here for the first time the normal development of the mouse foot pad eccrine sweat glands and the expression patterns of selected keratin proteins, as well as characteristics of the aperature at the level of the epidermis. In many realms of biology, it is always important to first characterize normal structures so that one can identify abnormalities. As the world of GEMs continues to expand, the information presented here will serve as the basis for investigators to identify abnormalities in eccrine glands arising from the generation of novel genetic mutations in the laboratory mouse.
Acknowledgements
The authors thank J. Miller for his technical assistance, L.S. Bechtold for assistance with the scanning electron microscopy, and J. Hammer for graphics support.
Financial Disclosure/Funding This work was supported in part by grants from the Council for Nail Disorders, North American Hair Research Society, and the National Institutes of Health (CA34196, RR173).
Abbreviations
- DPC
days post conception
- EGA
estimated gestational age
- GEMS
Genetically Engineered Mice
- IHC
immunohistochemistry
- K1, K5, K6, K10, K14, and K17
(keratin) KRT1, KRT5, KRT6, KRT10, KRT14, and KRT 17, respectively
- PAS
periodic acid-Schiff
- PPD
postpartum days
- SEM
scanning electron microscopy
- SMA
Smooth Muscle Actin (ACTA2)
Footnotes
Declaration of Conflicting Interests The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- 1.Bechtold LS. Ultrastructural evaluation of mouse mutations. In: Sundberg JP, Boggess D, editors. Systematic characterization of mouse mutations. CRC Press; Boca Raton: 2000. pp. 121–129. [Google Scholar]
- 2.Blecher SR. Anhidrosis and absence of sweat glands in mice hemizygous for the Tabby gene: supportive evidence for the hypothesis of homology between Tabby and human anhidrotic (hypohidrotic) ectodermal dysplasia (Christ-Siemens-Touraine syndrome) J Invest Dermatol. 1986;87:720–722. doi: 10.1111/1523-1747.ep12456718. [DOI] [PubMed] [Google Scholar]
- 3.Byrne C, Hardman M, Nield K. Covering the limb--formation of the integument. J Anat. 2003;202:113–123. doi: 10.1046/j.1469-7580.2003.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheshire WP, Freeman R. Disorders of sweating. Semin Neurol. 2003;23:399–406. doi: 10.1055/s-2004-817724. [DOI] [PubMed] [Google Scholar]
- 5.Dobson RL. Eccrine Sweat Glands. In: Montagna W, Parakkal PF, editors. The Structure and Function of Skin. Third ed Academic Press, Inc.; New York, NY: 1974. pp. 366–411. [Google Scholar]
- 6.Feldman S, Gografe S, Kohn D, Swindle M. Laboratory mouse handbook. American Association for Laboratory Animal Science; Memphis, TN: 2006. [Google Scholar]
- 7.Groscurth P. Anatomy of sweat glands. Curr Probl Dermatol. 2002;30:1–9. doi: 10.1159/000060678. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K, Gross BG, Lever WF. The ultrastructure of the skin of human embryos. I. The intraepidermal eccrine sweat duct. J Invest Dermatol. 1965;45:139–151. doi: 10.1038/jid.1965.110. [DOI] [PubMed] [Google Scholar]
- 9.Holbrook KA. Human epidermal embryogenesis. Int J Dermatol. 1979;18:329–356. doi: 10.1111/ijd.1979.18.5.329. [DOI] [PubMed] [Google Scholar]
- 10.Holbrook KA. Structural abnormalities of the epidermally derived appendages in skin from patients with ectodermal dysplasia: Insight into developmental errors. In: Salinas CF, Opitz JM, Paul NW, editors. Recent advances in ectodermal dysplasias. Alan R. Liss; New York: 1988. pp. 15–44. [PubMed] [Google Scholar]
- 11.Johnson GS, Elizondo RS. Eccrine sweat gland in Macaca mulatta: physiology, histochemistry, and distribution. J Appl Physiol. 1974;37:814–820. doi: 10.1152/jappl.1974.37.6.814. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman MH. The atlas of mouse development. Academic Press; London ; San Diego: 1992. p. xvi.p. 512. [Google Scholar]
- 13.Kaufman MH, Bard JBL. The Anatomical Basis of Mouse Development. Academic Press; San Diego, California: 1999. p. 291. [Google Scholar]
- 14.Kreyden OP, Scheidegger EP. Anatomy of the sweat glands, pharmacology of botulinum toxin, and distinctive syndromes associated with hyperhidrosis. Clin Dermatol. 2004;22:40–44. doi: 10.1016/j.clindermatol.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Li HH, Zhou G, Fu XB, Zhang L. Antigen expression of human eccrine sweat glands. J Cutan Pathol. 2009;36:318–324. doi: 10.1111/j.1600-0560.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- 16.Maroulakou IG, Shibata MA, Anver M, Jorcyk CL, Liu M, Roche N, Roberts AB, Tsarfaty I, Reseau J, Ward J, Green JE. Heterotopic endochondrial ossification with mixed tumor formation in C3(1)/Tag transgenic mice is associated with elevated TGF-beta1 and BMP-2 expression. Oncogene. 1999;18:5435–5447. doi: 10.1038/sj.onc.1202926. [DOI] [PubMed] [Google Scholar]
- 17.Mecklenburg L, Paus R, Halata Z, Bechtold LS, Fleckman P, Sundberg JP. FOXN1 is critical for onycholemmal terminal differentiation in nude (Foxn1) mice. J Invest Dermatol. 2004;123:1001–1011. doi: 10.1111/j.0022-202X.2004.23442.x. [DOI] [PubMed] [Google Scholar]
- 18.Mehregan AH, Hashimoto K, Mehregan DA, Mehregan DR. Pinkus’ Guide to Dermatohistopathology. Sixth ed Appleton & Lange; Norwalk, CT: 1995. Normal Structure of Skin; pp. 40–42. [Google Scholar]
- 19.Mikaelian I, Hovick M, Silva KA, Burzenski LM, Shultz LD, Ackert-Bicknell CL, Cox GA, Sundberg JP. Expression of terminal differentiation proteins defines stages of mouse mammary gland development. Vet Pathol. 2006;43:36–49. doi: 10.1354/vp.43-1-36. [DOI] [PubMed] [Google Scholar]
- 20.Mikaelian I, Nanney LB, Parman KS, Kusewitt D, Ward JM, Naf D, Krupke DM, Eppig JT, Bult CJ, Seymour R, Ichiki T, Sundberg JP. Antibodies that label paraffin-embedded mouse tissues: a collaborative endeavor. Toxicol Pathol. 2004;32:1–11. doi: 10.1080/01926230490274335. [DOI] [PubMed] [Google Scholar]
- 21.Moll I, Moll R. Changes of expression of intermediate filament proteins during ontogenesis of eccrine sweat glands. J Invest Dermatol. 1992;98:777–785. doi: 10.1111/1523-1747.ep12499950. [DOI] [PubMed] [Google Scholar]
- 22.Monteiro-Riviere NA, Dellmann HD, Eurall JA. Textbook of Veterinary Histology. 5th ed Lippincott, Williams & Wilkins; Philadelphia, PA: 1998. Integument. [Google Scholar]
- 23.Mori N, Tsugane MH, Yamashita K, Ikuta Y, Yasuda M. Pathogenesis of retinoic acid-induced abnormal pad patterns on mouse volar skin. Teratology. 2000;62:181–188. doi: 10.1002/1096-9926(200010)62:4<181::AID-TERA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Naito A, Yoshida H, Nishioka E, Satoh M, Azuma S, Yamamoto T, Nishikawa S, Inoue J. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci U S A. 2002;99:8766–8771. doi: 10.1073/pnas.132636999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nejsum LN, Kwon TH, Jensen UB, Fumagalli O, Frokiaer J, Krane CM, Menon AG, King LS, Agre PC, Nielsen S. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci U S A. 2002;99:511–516. doi: 10.1073/pnas.012588099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 27.Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong CM. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quick DC, Kennedy WR, Yoon KS. Ultrastructure of the secretory epithelium, nerve fibers, and capillaries in the mouse sweat gland. Anat Rec. 1984;208:491–499. doi: 10.1002/ar.1092080404. [DOI] [PubMed] [Google Scholar]
- 29.Relyea MJ, Miller J, Boggess D, Sundberg JP. Necropsy methods for laboratory mice: biological characterization of a new mutation. In: Sundberg JP, Boggess D, editors. Systematic approach to evaluation of mouse mutations. CRC Press; Boca Raton: 2000. pp. 57–90. [Google Scholar]
- 30.Relyea MJ, Sundberg JP, Ward JM. Immunohistochemical and immunofluorescence methods. In: Sundberg JP, Boggess D, editors. Systematic approach to evaluation of mouse mutations. CRC Press; Boca Raton, FL: 2000. pp. 131–144. [Google Scholar]
- 31.Sato K, Cavallin S, Sato KT, Sato F. Secretion of ions and pharmacological responsiveness in the mouse paw sweat gland. Clin Sci (Lond) 1994;86:133–139. doi: 10.1042/cs0860133. [DOI] [PubMed] [Google Scholar]
- 32.Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989;20:537–563. doi: 10.1016/s0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- 33.Seymour R, Ichiki T, Mikaelian I, Boggess D, Silva KA, Sundberg JP. Necropsy methods. In: Hedrich HJ, editor. Laboratory Mouse. Elsevier Science; London: 2004. pp. 495–515. [Google Scholar]
- 34.Shankle WR, Azen SP, Landing BH. Comparisons of eccrine sweat gland anatomy in genetic, chromosomal, and other diseases, and a suggested procedure for use of sweat gland measurements in differential diagnosis. Teratology. 1982;25:239–245. doi: 10.1002/tera.1420250213. [DOI] [PubMed] [Google Scholar]
- 35.Smith RS, Sundberg JP, John SWM. The Anterior Segment. In: Smith RS, John SWM, Nishina PM, Sundberg JP, editors. Systematic Evaluation of the Mouse Eye: Anatomy, Pathology and Biomethods. CRC Press; Boca Raton: 2002. p. 363. [Google Scholar]
- 36.Srivastava AK, Pispa J, Hartung AJ, Du Y, Ezer S, Jenks T, Shimada T, Pekkanen M, Mikkola ML, Ko MS, Thesleff I, Kere J, Schlessinger D. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci U S A. 1997;94:13069–13074. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stumpf P, Kunzle H, Welsch U. Cutaneous eccrine glands of the foot pads of the small Madagascan tenrec (Echinops telfairi, Insectivora, Tenrecidae): skin glands in a primitive mammal. Cell Tissue Res. 2004;315:59–70. doi: 10.1007/s00441-003-0815-0. [DOI] [PubMed] [Google Scholar]
- 38.Stumpf P, Welsch U. Cutaneous eccrine glands of the foot pads of the rock hyrax (Procavia capensis, Hyracoidea, Mammalia) Cells Tissues Organs. 2002;171:215–226. doi: 10.1159/000063714. [DOI] [PubMed] [Google Scholar]
- 39.Sundberg JP. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. CRC Press; Boca Raton: 1994. p. 544. [Google Scholar]
- 40.Sundberg JP, Hanson CA, Roop DR, Brown KS, Bedigian HG. Myoepitheliomas in inbred laboratory mice. Vet Pathol. 1991;28:313–323. doi: 10.1177/030098589102800408. [DOI] [PubMed] [Google Scholar]
- 41.Urso C, Bondi R, Paglierani M, Salvadori A, Anichini C, Giannini A. Carcinomas of sweat glands: report of 60 cases. Arch Pathol Lab Med. 2001;125:498–505. doi: 10.5858/2001-125-0498-COSG. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe S, Ichikawa E, Takanashi S, Takahashi H. Immunohistochemical localization of cytokeratins in normal eccrine glands, with monoclonal antibodies in routinely processed, formalin-fixed, paraffin-embedded sections. J Am Acad Dermatol. 1993;28:203–212. doi: 10.1016/0190-9622(93)70028-r. [DOI] [PubMed] [Google Scholar]
- 43.Weedon D. Eccrine tumors: a selective review. J Cutan Pathol. 1984;11:421–436. doi: 10.1111/j.1600-0560.1984.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 44.Wenzel FG, Horn TD. Nonneoplastic disorders of the eccrine glands. J Am Acad Dermatol. 1998;38:1–17. doi: 10.1016/s0190-9622(98)70532-8. quiz 18-20. [DOI] [PubMed] [Google Scholar]