Abstract
Background
Prostate cancer screening using prostate-specific antigen (PSA) testing remains controversial. Trade-offs between the potential benefits and downsides of screening must be weighed by men deciding whether to participate in prostate cancer screening; little is known about benefit:harm trade-offs men are willing to accept.
Methods/Design
The Community Preferences for Prostate Cancer Screening (COMPASs) Study examines Australian men's preferences for prostate cancer screening using PSA testing. The aims are to (1) determine which factors influence men's decision to participate in prostate cancer screening or not and (2) determine the extent of trade-offs between benefits and harms that men are willing to accept in making these decisions. Quantitative methods will be used to assess men's preferences for PSA screening. Using data on the quantitative outcomes of PSA testing from the published literature, a discrete choice study will be designed to quantitatively assess men's preferences. A web-based survey will be conducted in approximately 1000 community respondents aged 40–69 years, stratified by family history of prostate cancer, to assess men's preferences for PSA testing. A mixed logit model will be used; model results will be expressed as parameter estimates (β) and the odds of choosing screening over no screening. Trade-offs between attributes will also be calculated.
Ethics and Dissemination
The COMPASs study has been approved by the University of Sydney, Human Research Ethics committee (Protocol number 13186). The results will be published in internal reports, in peer-reviewed scientific journals as well as via conference presentations.
Article summary
Article focus
To assess men's preferences for prostate cancer screening and determine the relative importance of various factors that influence men's decision to participate in prostate cancer screening or not.
To determine the extent of trade-offs between benefits and harms that men are willing to accept in making decisions about participation in screening.
Key messages
Prostate cancer screening may offer some benefit in terms of a reduction in prostate cancer-specific mortality. However, there is also evidence of substantial harms: screened men have a higher likelihood of being diagnosed as having prostate cancer, including the diagnosis of cancers that would not have become clinically apparent within the man's lifetime, meaning more men experiencing the attendant harms of diagnosis and treatment such as unnecessary biopsies from false-positive prostate-specific antigen tests or impotence and/or incontinence from treatments.
Trade-offs between the potential benefits and downsides of screening must be weighed by men deciding whether to participate in prostate cancer screening; little is known about benefit:harm trade-offs men are willing to make.
This study will use best practice quantitative methods for preference elicitation (discrete choice experiments) to assess men's preferences for prostate-specific antigen screening and the trade-offs they are willing to make decision whether to participate in screening.
Strengths and limitations of this study
The strengths of the study are that it is the first study to use discrete choice methods to examine men's preferences for prostate cancer screening, and the benefit:harm trade-offs men may be willing to make; it will consider the influence of age and family history on preferences in a large cohort of men, broadly representative of the Australian population aged 40 to 69 years.
The limitation is that it is conducted in one country, Australia, and thus its generalisability may be limited by the prevailing screening environment.
Background
Screening for prostate cancer using prostate-specific antigen (PSA) testing remains controversial. Recently published evidence suggests that prostate cancer screening using PSA testing may offer some benefits in terms of reducing prostate cancer-specific mortality; no trials have demonstrated a reduction in overall mortality associated with screening.1 2 However, these same trials also report evidence of substantial harms: men who participate in screening have a significantly higher likelihood of being diagnosed as having prostate cancer than those not screened, including the diagnosis of cancers that would not have become clinically apparent within the man's lifetime, meaning that more men experiencing the attendant harms of diagnosis and treatment such as unnecessary biopsies from false-positive PSA tests or impotence and/or incontinence from treatments.1–3 In deciding whether to undergo prostate cancer screening, men therefore need to weigh up these potential benefits with the potential risks, harms and costs associated with screening.
Adding to the complexity and uncertainty in this decision-making environment are the somewhat conflicting recommendations regarding the value of prostate cancer screening: the American Urological Association recommends PSA screening be offered to all men aged 40 years or older.4 Other US groups recommend discussion of the potential benefits and harms of PSA screening in the context of a clinical consultation, with an emphasis on informed decision making and consideration of patient preferences (the American Cancer Society,5 the American College of Physicians6). In Australia, the Cancer Council of Australia's position on prostate cancer indicates “there is no national screening program in place, with current evidence showing that the PSA test is not suitable for population screening as the harms outweigh the benefits. Whether or not to be tested for prostate cancer is a matter of individual choice…”.7 The most recent draft guidelines from the US Preventive Services Task Force (USPSTF) go one step further and assign a ‘D’ rating to PSA screening “recommends against prostate-specific antigen (PSA)-based screening for prostate cancer… [for] men in the US population that do not have symptoms that are highly suspicious for prostate cancer, regardless of age, race, or family history.”8 This revised recommendation will replace the 2008 recommendation,9 which had previously concluded that in men younger than 75 years, there was insufficient evidence to make a recommendation (‘I’ rating).
Consumer preferences for screening
Over recent years, there has been an increasing recognition of the role and importance of preferences and values in individual clinical decisions and in shaping public health policy. Preference sensitive care refers to care where there are significant potential trade-offs among possible positive and negative outcomes; decisions regarding these interventions should necessarily reflect an individual's personal values and preferences and should be made only after individuals have considered sufficient information to make an informed choice.10 It has recently been suggested that prostate cancer screening should be viewed as preference sensitive care.11
There is an extensive body of literature quantifying preferences and trade-offs for bowel cancer screening12–16; however, despite the clear balance between harms and benefits in the context of PSA screening for prostate cancer, there has, to date, been little exploration of these issues. With possible benefits to screening in terms of prostate cancer-specific mortality reduction, there is also evidence of significant and multiple potential downsides. A decision about whether to participate in prostate cancer screening therefore requires consideration of the balance between these benefits and downsides. Where that balance sits for an individual man is highly personal and driven by his own preferences about the extent of trade-offs between benefits and harms that he is willing to accept. For this reason, the preferences of the individual should be paramount.
The aims of the Community Preferences for Prostate Cancer Screening (COMPASs) Study are therefore to
determine the relative importance of various factors that influence men's decision to participate in prostate cancer screening or not and
determine the extent of trade-offs between benefits and harms that men are willing to accept in making decisions about participation in screening.
By providing a better understanding of how men value particular aspects of prostate cancer screening and the trade-offs between benefits and harms of PSA screening that they are willing to accept, the COMPASs Study will provide vital information (1) for clinicians and consumers to facilitate an informed discussion of the potential benefits and downsides of PSA testing, (2) to inform health policy regarding the development of any possible future public screening programme such that any programme can be closely aligned to community attitudes and preferences and (3) highlight future research directions such that research and subsequent policy development can be focused in areas of most importance to consumers.
Methods/design
Overview of approach and methods
The COMPASs Study will use quantitative discrete choice methods to assess Australian men's preferences for prostate cancer screening.
Discrete choice experiments
Discrete choice experiments (DCEs) involve surveys in which respondents are asked to choose between hypothetical alternatives defined by a set of differing attributes. This method is becoming more widely used in health as a means of quantifying patient and consumer preferences for healthcare policies and programmes.17–20 The method is based on the idea that goods and services, including healthcare services, can be described in terms of a number of separate attributes or factors. The levels of attributes are varied systematically in a series of questions and respondents choose the option that they prefer for each question. People are assumed to choose the option that is most preferred or has the highest ‘value’. From these choices, a mathematical function is estimated that describes numerically the value that respondents attach to different choice options. Other data collected in the survey, including attitudinal questions and socio-demographic information, may also enter the value functions as explanatory variables. Ultimately, DCE studies can determine which attributes are driving patient preferences, the trade-offs people make between attributes and how changes in attributes can lead to changes in preferences and likely service uptake.
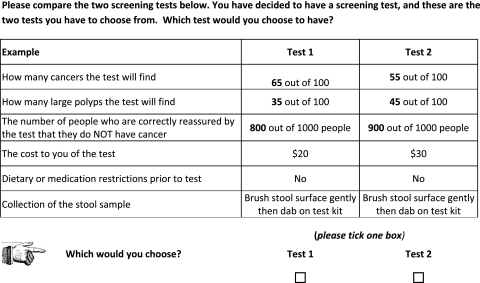
Figure 1 illustrates an example from an Australian survey of consumer preferences for colorectal cancer screening tests.16 The example involves two unlabelled alternative tests (figure 1) described using five different attributes (how many cancers the test will find, how many large polyps the test will find, the number of people correctly reassured that they do NOT have cancer, cost, dietary and medication restrictions, stool sample collection), each set at specific levels. By presenting respondents with a series of choices where the levels of the attributes are varied, researchers are able to quantify how these attributes influence choice. In this example, the analysis indicates consumer preferences for immunochemical faecal occult blood testing as a screening test for colorectal cancer.
Figure 1.
Example of a discrete choice question.
Given a sufficient number of choices to allow variation across all attributes, this approach enables estimates of the marginal effect of each attribute on choice and the marginal rate of substitution or trade-offs between attributes. In principle, this can be done by offering respondents choices using every combination of attributes, a ‘full factorial’ design. In practice, such a design is rarely feasible; efficient designs are therefore paramount, particularly when considering multiple choice options and interactions between attributes and socio-demographic characteristics on choice.
The following section details the specific methods that will be applied in the COMPASs Study; we will follow the ISPOR Guidelines for Good Research Practices for conjoint analysis in health.20
Study methods
Stage 1: deciding attributes and levels
A systematic review of the literature will be conducted to ascertain attributes for inclusion. These will include PSA test performance characteristics, such as potential mortality benefits from screening, number of diagnoses of prostate cancer as well as harms such as the number of men experiencing false-positive PSA results and subsequent unnecessary biopsies, potential harms associated with downstream treatment of prostate cancer, such as impotence and urinary or faecal incontinence21 and out of pocket costs. Our existing published model3 will be used to estimate these outcomes in men who screen and who do not screen, over a 10-year time frame. Model outputs, and therefore attribute levels, will be stratified by age and risk based upon family history.
Stage 2: design of discrete choice questionnaires
Once the attributes have been decided based on stage 1, a design for the discrete choice studies will be created. Statistically efficient designs will be used. This approach to design links statistical efficiency to the likely econometric model that is to be estimated from choice data using the design.22 23 This approach relaxes the orthogonality constraint and attempts to minimise the expected asymptotic variance–covariance (AVC) matrix of the design. Efficient choice designs therefore attempt to maximise the likely asymptotic t ratios obtained from choice data collected. As such, they attempt to minimise the correlation in the data for estimation purposes and collect data such that parameter estimates have as small as possible SEs. These designs make use of the fact that the AVC matrix (the roots of the diagonal of this matrix are the asymptotic SEs) of the parameters can be derived if the parameters are known. Since the objective of the DCE is to estimate these parameters, they are unknown at the time of design. However, if some prior information about these parameters is available (eg, parameter estimates available in the literature from similar studies or parameter estimates from pilot studies), then this AVC matrix can be determined, assuming that the priors are correct.
An initial efficient choice design will be created, based on the likely a priori sign of parameters. This initial design will be piloted in a sample of 100 respondents and preliminary models estimated. Parameter estimates from the models will be used to generate the final efficient designs for the main discrete choice study.
In addition to the discrete choice questions, information on socio-demographic characteristics of respondents will also be collected for each survey.
Stage 3: DCE survey
Respondents
Men aged 40–69 years of low, moderate and high risk of prostate cancer, based upon family history of prostate cancer, will be recruited to complete the DCE survey. Low-risk men are those with no first-degree relatives (FDR) affected by prostate cancer. Men with one affected FDR are considered at moderate risk and men with either two or more affected FDR or one FDR diagnosed at a young age (<60 years) are considered at high risk. Based on their age and family history of prostate cancer, they will be allocated to a version of the survey with attribute levels relevant to their age and risk. No additional exclusion criteria will be applied.
The DCE will be conducted using a web-based survey. Respondents will be recruited through a market research company with an existing online panel of respondents willing to complete online surveys and experience in administering online choice-based surveys. Recruitment will continue until the proposed sample size is reached. Upon consent, the potential respondent will be connected directly to the online site to complete the discrete choice survey. Respondents will be asked to choose between three labelled alternatives, two screening options and a no screening option (opt-out).
Sample size
The current theory of sampling for these experiments does not directly address the issue of minimum sample size requirements in terms of the reliability of the parameter estimates produced in the design of stated choice experiments.24 25 Rather, sampling theory as applied to choice modelling is designed to minimise the error in the choice proportions of the alternatives under study. This means that the final sample size required is based upon the characteristics of the design itself such as the number of attributes included, the attribute level range, the number of choice scenarios presented, the number of alternatives in each choice set and the size and direction of prior parameters obtained from the pilot study. Taking into account the Australian population distribution of men aged 40–69 years with different levels of family history of prostate cancer (low, no FDR ∼94% of the population aged 40–69 years; moderate, one FDR ∼5% to 6%; high, two FDR or one FDR diagnosed <60 years ∼0.5%) and to ensure sufficient numbers in risk subgroup for robust parameter estimates and that we are able to explore interactions between attributes and between attributes and socio-demographic factors and present subgroup analyses, we anticipate a sample size of approximately 1000 respondents (550 (low), 350 (moderate) and 100 (high)).
Stage 4: analysis
A mixed multinomial logit (MMNL) (also known as random parameters logit) model using a panel size specification will be used. A panel specification of the model allows for non-independence of observations provided by the same respondent; that is, it can account for correlations among the multiple choices made by the same individual. MMNL models relax certain statistical assumptions of more commonly used multinomial logit models and often lead to models that better explain choice behaviour.24 In multinomial logit choice models, commonly used in health economics, parameters associated with each attribute are treated as fixed. These fixed values are the average (or point estimates) associated with a population-level distribution; other information in the distribution is not considered. An MMNL allows consideration of the full distribution of a parameter estimate, and the fixed parameter becomes a random parameter. ‘Random parameter’ simply implies that each individual has an associated parameter estimate on that specified distribution. While the exact location of each individual's preferences on the distribution may not be known, estimates of ‘individual-specific preferences’ can be accommodated by deriving the individual's conditional distribution, based—within sample—on their choices (ie, prior knowledge).26 Interactions between attributes in the discrete choice surveys and between attributes and population characteristics (eg, age, family history of prostate cancer, prior PSA testing, prior prostate biopsy, marital status, education, income) will be explored in the mixed logit analysis for both studies.
Model results will expressed as parameter estimates (β), the odds of choosing screening over no screening (and 95% CIs of the ORs) and p values. Acceptable trade-offs between attributes will also be calculated.
Ethical approval
The COMPASs Study has been approved by the University of Sydney, Human Research Ethics Committee (protocol number 13186).
Confidentiality and anonymity of the data will be strictly maintained; only study staff will have access to de-identified respondent data. As respondents are being recruited by an external organisation, no individual identifying information will be ever provided to the study investigators; all respondents will be assigned a unique study ID. In addition, participants will not be identifiable in any publications. It will be made clear to all participants that they have the right to withdraw from the research at any point in time. No data monitoring committee will be required, and no interim analyses will be conducted.
As the DCE survey will be conducted as an online survey, written consent is not possible. As such, participant information for the online survey includes the following statement “Being in this study is completely voluntary—you are not under any obligation to consent and—if you do consent—you can withdraw at any time without affecting your relationship with The University of Sydney. By completing the survey you have consented to be part of the study. You may stop completing the online survey at any point if you do not wish to continue, and we will not use your answers”. As the survey is administered by an external organisation and is completely online, the study team will not have access to any information that could be used to identify respondents. Following study completion, only study investigators will have access to the de-identified respondent data.
Dissemination
The results will be published in internal reports, in peer-reviewed scientific journals as well as via conference presentations.
Discussion
The COMPASs Study is a comprehensive analysis of men's preferences for prostate cancer screening. Using best practice quantitative methods, COMPASs will provide an understanding of the preferences of Australian men on prostate cancer screening using PSA testing. Specifically, the aims of the COMPASs study are to (1) determine the relative importance of various factors that influence men's decision to choose prostate cancer screening or not and (2) determine the extent of trade-offs between benefits and harms that men are willing to accept in making decisions about participation in screening.
The analysis will provide:
Estimates of the marginal effect (importance) of each attribute on the decision to screen or not, for example, if a cost attribute is presented, the analysis will provide an estimate of relative importance of out of pocket cost on a man's decision to undergo prostate cancer screening.
Estimates of marginal rates of substitution between attributes based on the ratio of parameter estimates, giving an indication of the extent to which respondents are prepared to trade-off one attribute for another. For example, if the number of deaths due to prostate cancer and the number of men experiencing incontinence are offered as attributes in the survey, the marginal rate of substitution between these reflects the increase in the number of men experiencing incontinence that men are willing to accept as a trade-off to prevent one extra prostate cancer death.
An indication of the predicted uptake associated with different parameter levels within the estimated utility functions. This allows forecasting of, for instance, the likely level of uptake of screening given particular test characteristics, policy criteria and socio-demographic characteristics.
By providing a better understanding of how men value particular aspects of prostate cancer screening and the trade-offs between benefits and harms of PSA screening that they are willing to accept, the COMPASs Study will provide vital information (1) for clinicians and consumers to facilitate an informed discussion of the potential benefits and downsides of PSA testing, (2) to inform health policy regarding the development of any possible future public screening programme such that any programme can be closely aligned to community attitudes and preferences and (3) highlight future research directions such that research and subsequent policy development can be focused in areas of most importance to consumers.
Supplementary Material
Footnotes
To cite: Howard K, Salkeld GP, Mann GJ, et al. The COMPASs Study: Community Preferences for Prostate Cancer Screening. Protocol for a quantitative preference study. BMJ Open 2012;2:e000587. doi:10.1136/bmjopen-2011-000587
Funding: The COMPASs Study is funded under the National Health and Medical Research Council of Australia program grant 633003 (The Screening and Test Evaluation Program). The funders have no role in study design; collection, management, analysis and interpretation of data; nor in writing of any reports or the decision to submit the reports for publication.
Competing interests: None.
Ethics approval: The COMPASs study has been approved by the University of Sydney, Human Research Ethics Committee (protocol number 13186). The results will be published in internal reports, in peer-reviewed scientific journals as well as via conference presentations.
Contributors: KH conceived the original concept of this study. All authors contributed to discussion and revisions to the study design and approved the final study design. KH drafted the manuscript; all other authors were involved in overall revision of the manuscript. All authors are involved in the implementation of the project and have read and approved the final manuscript.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320–8 [DOI] [PubMed] [Google Scholar]
- 2.Andriole G, Grubb RL, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard K, Barratt A, Mann GJ, et al. A model of prostate-specific antigen screening outcomes for low- to high-risk men: information to support informed choices. Arch Intern Med 2009;169:1603–10 [DOI] [PubMed] [Google Scholar]
- 4.American Urological Association Can Prostate Cancer be found early? 2009. http://www.auanet.org/content/guidelines-and-quality-care/policy-statements/e/early-detection-of-prostate-cancer.cfm (accessed Oct 2011). [Google Scholar]
- 5.American Cancer Society Can Prostate Cancer be found early? 2010. http://www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_prostate_cancer_be_found_early_36.asp?sitearea (accessed Oct 2011). [Google Scholar]
- 6.American College of Physicians Screening for prostate cancer. Ann Intern Med 1997;126:484. [PubMed] [Google Scholar]
- 7.Cancer Council Australia Position Statement - Prostate Cancer. 2010. http://www.cancer.org.au/policy/positionstatements/prostatecancer.htm (accessed 18 Oct 2011). [Google Scholar]
- 8.US Preventive Services Screening for prostate cancer: U.S. Preventive services Task Force recommendation statement (DRAFT). 2011 http://www.uspreventiveservicestaskforce.org/draftrec3.htm (accessed 18 Oct 2011).
- 9.US Preventive Services Screening for Prostate Cancer. 2008. http://www.ahrq.gov/clinic/uspstf/uspsprca.htm (accessed 2 Jun 2008). [Google Scholar]
- 10.Dartmouth Atlas Projeect Preference sensitive care. A Dartmouth Atlas Project Topic Brief. 2011. http://www.dartmouthatlas.org/downloads/reports/preference_sensitive.pdf [PubMed] [Google Scholar]
- 11.O'Donnell J. Help me in my confusion: should we think more about mammography and colonoscopy as “preference sensitive care”? J Cancer Educ 2010;25:471–2 [DOI] [PubMed] [Google Scholar]
- 12.Gyrd-Hansen D, Sogaard J. Analysing public preferences for cancer screening programs. Health Econ 2001;10:617–34 [DOI] [PubMed] [Google Scholar]
- 13.Marshall DA, Johnson FR, Phillips KA, et al. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health 2007;10:415–30 [DOI] [PubMed] [Google Scholar]
- 14.Marshall DA, McGregor E, Currie G. Measuring preferences for colorectal cancer (CRC) screening – What are the implications for moving forward? The Patient - Patient Centred Outcomes Research 2010;3:79–89 [DOI] [PubMed] [Google Scholar]
- 15.Salkeld G, Solomon M, Short L, et al. Evidence-based consumer choice: a case study in colorectal cancer screening. Aust N Z J Public Health 2003;27:449–55 [DOI] [PubMed] [Google Scholar]
- 16.Howard K, Salkeld G. Does attribute Framing in discrete choice experiments influence Willingness to Pay? Results from a discrete choice experiment in screening for colorectal cancer. Value Health 2009;121:354–3 [DOI] [PubMed] [Google Scholar]
- 17.Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics 2008;26:661–77 [DOI] [PubMed] [Google Scholar]
- 18.Bridges JF, Kinter E, Kidane L, et al. Things are looking up since we started listening to patients: recent trends in the application of conjoint analysis in health 1970-2007. The Patient 2008;1:273–82 [DOI] [PubMed] [Google Scholar]
- 19.Marshall DA, Bridges JF, Hauber AB, et al. Conjoint Analysis Applications in Health - How are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. The Patient 2010;3:249–56 [DOI] [PubMed] [Google Scholar]
- 20.Bridges JF, Hauber AB, Marshall DA, et al. Conjoint analysis Applications in health-a Checklist: a report of the ISPOR good research practices for conjoint analysis Task Force. Value Health 2011;14:5. [DOI] [PubMed] [Google Scholar]
- 21.Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009;339:b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber J, Zwerina K. The importance of utility balance in efficient choice design. J Market Res 1996;XXXIII:307–17 [Google Scholar]
- 23.Sandor Z, Wedel M. Profile construction in experimental designs for mixed logit models. Marketing Science 2002;21:455–75 [Google Scholar]
- 24.Hensher DA, Rose JM, Greene WH. Applied choice analysis. A Primer. 1st edn Cambridge: Cambridge University Press, 2005 [Google Scholar]
- 25.Louviere J, Hensher DA, Swait JD, et al. Stated Choice Methods - Analysis. Cambridge University Press, 2000 [Google Scholar]
- 26.Hensher DA, Greene WH. The mixed logit model: the state of practice. Transportation 2003;30:133–76 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.