Abstract
Background
Implantable device therapy of atrial fibrillation (AF) is limited by pain from high-energy shocks. We developed a low-energy multi-stage defibrillation therapy and tested it in a canine model of AF.
Methods and Results
AF was induced by burst pacing during vagus nerve stimulation. Our novel defibrillation therapy consisted of three stages: ST1 (1-4 low energy biphasic shocks), ST2 (6-10 ultra-low energy monophasic shocks), and ST3 (anti-tachycardia pacing). Firstly, ST1 testing compared single or multiple monophasic (MP) and biphasic (BP) shocks. Secondly, several multi-stage therapies were tested: ST1 versus ST1+ST3 versus ST1+ST2+ST3. Thirdly, three shock vectors were compared: superior vena cava to distal coronary sinus (SVC>CSd), proximal coronary sinus to left atrial appendage (CSp>LAA) and right atrial appendage to left atrial appendage (RAA>LAA). The atrial defibrillation threshold (DFT) of 1BP shock was less than 1MP shock (0.55 ± 0.1 versus 1.38 ± 0.31 J; p =0.003). 2-3 BP shocks terminated AF with lower peak voltage than 1BP or 1MP shock and with lower atrial DFT than 4 BP shocks. Compared to ST1 therapy alone, ST1+ST3 lowered the atrial DFT moderately (0.51 ± 0.46 versus 0.95 ± 0.32 J; p = 0.036) while a three-stage therapy, ST1+ST2+ST3, dramatically lowered the atrial DFT (0.19 ± 0.12 J versus 0.95 ± 0.32 J for ST1 alone, p=0.0012). Finally, the three-stage therapy ST1+ST2+ST3 was equally effective for all studied vectors.
Conclusions
Three-stage electrotherapy significantly reduces the AF defibrillation threshold and opens the door to low energy atrial defibrillation at or below the pain threshold.
Keywords: atrial fibrillation, defibrillation, cardioversion, vagal stimulation
Introduction
Atrial fibrillation (AF) is the most common tachyarrhythmia worldwide. The number of Americans afflicted by AF will increase to over 12 million by 2050,1 placing a significant additional burden on the healthcare system. Moreover, patients with AF suffer serious consequences including increased rates of thromboembolic stroke, congestive heart failure, cognitive dysfunction, and mortality.2-4
Catheter ablation has become increasingly common for the treatment for AF but its success rate varies widely and it is beset by a significant rate of major and minor complications. While success rates vary depending upon operator and patient selection, arrhythmia-free survival rates after a single catheter ablation procedure are less than 29% at 5 years.5 Complication rates will likely improve over time, yet risks such as stroke, pulmonary vein stenosis, phrenic nerve injury, and the rare but often fatal atrioesophageal fistula have made this a less than ideal treatment modality.6-9
For symptomatic patients, cardioversion of AF to sinus rhythm using high-voltage external shocks, with or without antiarrhythmic drugs remains a mainstay of therapy. External cardioversion is painful and can lead to brady- and tachy- arrhythmias, necessitating anesthesia and careful peri-procedural patient monitoring. Hospitalization, anesthesia, personnel and ancillary procedures costs associated with AF is estimated to cost Medicare more than $15.7 billion annually.10
These considerations have prompted efforts to design an implantable device that converts AF to sinus rhythm safely. Such a system would enable rapid cardioversion soon after the onset of AF, ameliorating symptoms and importantly, reducing the likelihood of atrial remodeling.11 Moreover, since internal atrial cardioversion requires significantly less energy than external cardioversion,12 it may be possible to deliver this therapy below the pain threshold, generally considered to be between 0.1-1 J.13, 14 Several internal atrial cardioverters have been developed in the past15, 16 but received limited acceptance primarily due to the discomfort associated with shocks.17-20
Experimental and clinical studies have demonstrated that vagus nerve stimulation promotes sustained AF by decreasing the atrial effective refractory period (AERP) and AERP rate adaption in an anatomically heterogeneous manner, thus creating the substrate for maintenance of sustained AF.21, 22 This acute model readily produces sustained AF and has been used extensively to study mechanisms of and therapies for atrial fibrillation.23 The AF generated by this model was shown to be of significantly longer duration and higher dominant frequency compared to that induced by chronic rapid pacing, making it a reasonable model to study the electrotherapy of AF.24
Previously, we compared multiple monophasic (MP) shocks to a single shock for defibrillation of atrial fibrillation and flutter in an ex vivo rabbit model25 and in a rabbit chronic infarction model of ventricular tachycardia.26 In both models, multiple monophasic shocks significantly lowered the defibrillation threshold. The goal of this study was to develop a low-energy therapy for defibrillation of AF in vivo.
Methods
Surgical Procedures
Mongrel dogs (n=16) weighing 20-25 kg were intubated and anesthetized with propofol (7.5 mg/kg intravenously) and 2-3% inhaled isoflurane in oxygen (Model 2000; Hallowell EMC, Pittsfield, MA). The right femoral artery was cannulated for continuous blood pressure monitoring and periodic measurements of arterial blood gas and electrolytes. After median sternotomy, the pericardium was opened to expose the heart. Bilateral carotid cut downs were performed to isolate the Vagus nerves. Cuff electrodes (A0004-6, Evergreen Medical Technologies, LLC, St. Paul, MN) were placed around each nerve for subsequent stimulation.
Electrode Placement
Bipolar electrodes were sutured to the right atrium for pacing, and left atrium and ventricular apex for sensing. Atrial and ventricular epicardial electrograms (EG) and surface electrocardiogram (ECG) were recorded continuously.
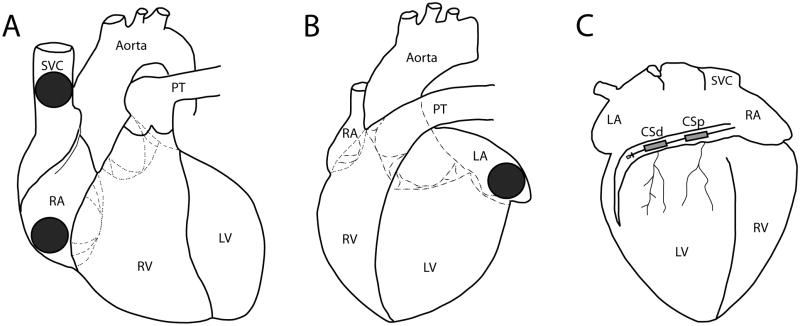
Custom defibrillation disc electrodes of 1.27 cm or 2.54 cm diameter were sutured to the right atrial appendage (RAA), left atrial appendage (LAA) and superior vena cava (SVC). A custom made 4 French lead with two shock coils, each 2.54 cm in length separated by an intercoil distance of 1.27 cm was placed into the coronary sinus (CS; Evergreen Medical Technologies, St. Paul, MN); the proximal and distal coils are referred to as CSp and CSd, respectively. A schematic of the defibrillation electrodes is shown in Figure 1.
Figure 1.
Anatomic Positions of Defibrillation Electrodes. Schematic of a canine heart with locations of electrodes from which defibrillation therapies were delivered depicted. A: right anterior oblique (RAO) view showing superior vena cava and right atrial appendage disc electrodes. B: left anterior oblique (LAO) view showing left atrial appendage disc electrode. C: posteroanterior (PA) view showing distal and proximal coronary sinus coils. SVC, superior vena cava; RA, right atrium; PT, pulmonary trunk; RV, right ventricle; LV, left ventricle; CSp, proximal coronary sinus; CSd, distal coronary sinus.
Determination of atrial and ventricular Shock Excitation Threshold
After the placement of electrodes, atrial and ventricular shock excitation thresholds (SET) were measured for each defibrillation electrode pair tested. Atrial SET was defined as the minimum voltage at which a monophasic 10 ms shock captured the atrium. Similarly, the ventricular SET was defined as the minimum voltage at which a 10 ms monophasic shock captured the ventricle. Shocks were delivered during the diastolic interval while the animal was in sinus rhythm. Atrial and ventricular capture was determined by evaluating the surface ECG and the bipolar atrial and ventricular sensing electrodes. In general, the atrial SET was lower in voltage than the ventricular SET. In the rare instances where there was no difference between the atrial and ventricular SETs for a given vector, leads were repositioned and the atrial and ventricular SETs were re-measured.
Determination of Atrial Effective Refractory Period
Atrial Effective Refractory Period (AERP) was determined by programmed stimulation delivered from the bipolar right atrial pacing electrode. A pacing train (S1) was delivered at 300 ms, followed by single premature extrastimulus (S2). S1-S2 coupling interval was serially decremented by 10 ms until the S2 stimulus failed to capture the atrium, and this value of S2 was determined to be the AERP. AERP was determined prior to and during vagal stimulation to assess the effectiveness of vagal stimulation on the atrium.
Vagus nerve stimulation and Induction of AF
Bilateral vagus nerve stimulation was performed with 10-20 V pulses of 1 ms duration starting at 4 Hz via a stimulator (SD9; Grass, West Warwick, RI). Frequency of vagal stimulation was increased in 1 Hz steps until AF lasted 10 minutes or longer. AERP was measured immediately before and 30 s after vagus nerve stimulation. After 30 seconds of stimulation, AF was induced via right atrial burst pacing at 10-20 Hz at four times the atrial capture threshold via a stimulus isolator (A365R; World Precision Instrument, Sarasota, FL). AF was defined as a rapid, irregular atrial rhythm with varying atrial electrogram morphology, while atrial flutter (AFl) was defined as a rapid but regular atrial rhythm with consistent atrial electrogram morphology. If AFl was induced, anti-tachycardia pacing (ATP) or multiple-shock therapy was applied to restore sinus rhythm. ATP consisted of 6-10 bipolar pacing stimuli, each 5 ms in duration, delivered at an amplitude of four times the atrial pacing capture threshold, and at a cycle length equal to 88% of the AF cycle length, with no decrement. Vagus nerve stimulation was then resumed with a higher frequency, and AERP was re-measured. After every successful termination of AF or AFl, stimulation was withheld for five minutes to allow for recovery of the nerve.
Individual Stages of Multi-Stage Electrotherapies
Stage 1
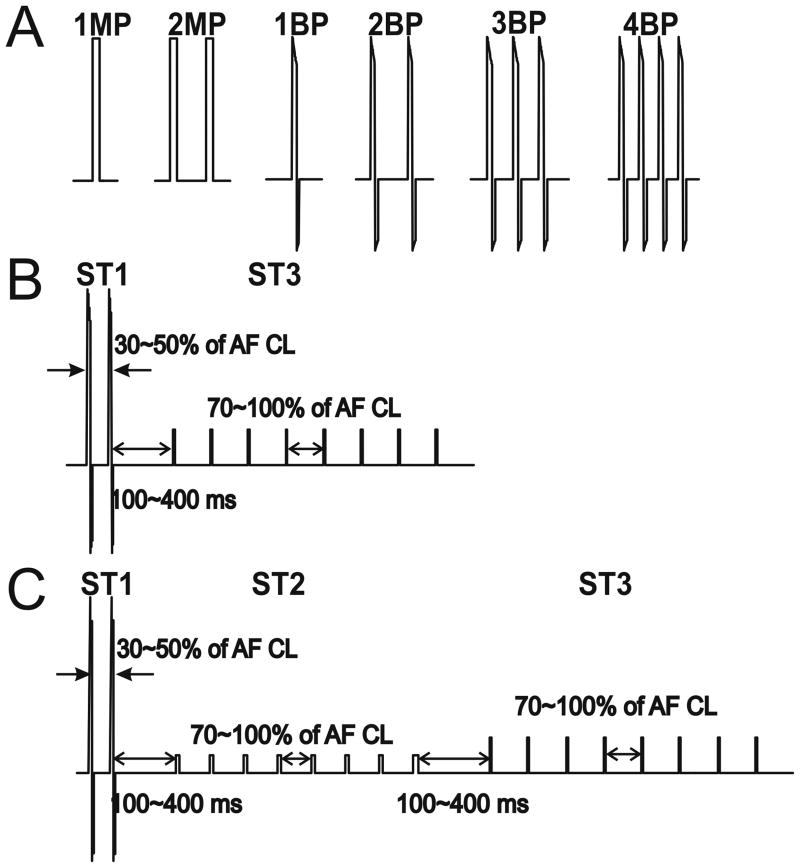
Initially, we tested the abilities of single or multiple, monophasic or biphasic shocks to terminate AF. These waveforms are referred to as Stage 1 (ST1) of the multi-stage electrotherapy and are depicted in Figure 2A. Single monophasic (1MP, 10 ms) or biphasic (1BP, 6/4 ms) shocks, and multiple monophasic (2MP) or biphasic shocks (2BP, 3BP, 4BP) were delivered on the R-wave and triggered via the ventricular electrogram or surface ECG. The multiple shocks were applied within 100 ms, which was shorter than the ventricular ERP, to avoid induction of ventricular fibrillation.
Figure 2.
Waveforms and Stages of Low Energy Defibrillation Therapy. A: Individual waveforms tested in Stage 1 (ST1) of the three-stage therapy. B: ST1+ST3 therapy. ST1 consists of 2 biphasic shocks delivered within 30-50% of the AF cycle length. ST3 consists of 8 bipolar atrial pacing stimuli delivered at 70-100% of the AF cycle length. C: ST1+ST2+ST3 therapy. ST1 consists of 2 biphasic shocks. ST2 consists of 8 lower voltage monophasic shocks, and ST3 consists of 8 atrial pacing stimuli. Each pulse in ST2 and ST3 is delivered at 70-100% of the AF cycle length. In both B and C, a delay of 100-400 ms separates each stage of therapy. MP, monophasic shock; BP, biphasic shock; AF, atrial fibrillation; CL, cycle length. ST1, stage 1; ST2, stage 2; ST3, stage 3.
Stage 2
Stage 2 (ST2) of the multi-stage electrotherapy consisted of 6-10 MP shocks of 10ms duration applied across the same vector as in ST1. To avoid initiation of ventricular tachyarrhythmias, all ST2 shocks were delivered above the atrial shock excitation threshold (SET) but no higher than 60% of the ventricular SET, thus capturing atrial but not ventricular tissue. ST2 shocks were delivered at a rate 70-100% that of the AF CL. For example, if the AF CL was 120 ms, ST2 MP shocks were delivered at a rate of every 84-120 ms.
Stage 3
Stage 3 (ST3) of the multi-stage electrotherapy consisted of ATP, consisting of 6-10 bipolar pacing stimuli, each 5 ms in duration, delivered at an amplitude of four times the atrial pacing capture threshold, and at a cycle length equal 88% of the AF cycle length, with no decrement.
Multi-Stage Electrotherapies
The following combinations of stages were tested: (i) ST1, (ii) ST1+ST3, and (iii) ST1+ST2+ST3. Results from testing ST1 therapies determined that 2BP shocks was the optimal ST1 therapy in terms of peak voltage and total energy required, so 2BP shocks was used as the ST1 waveform in all subsequent mult-stage testing. The delay between each stage was 100-400 ms. Pilot studies showed that ST2 alone or ST3 alone did not terminate AF (data not shown), therefore these stages could not be tested individually. Instead, they were incorporated in the multi-stage electrotherapy, in all cases, after ST1 therapy had been applied. Due to limitations on the number of combinations that could be formally evaluated, ST1+ST2 alone was not tested formally. Rather, the incremental advantage of ST1+ST2+ST3 was compared to ST1+ST3 alone.
Electrotherapy Protocol
This study was carried out in two parts. First, the atrial DFTs of single versus multiple monophasic or biphasic shocks were determined according to a randomized protocol. A random number generator was used to generate the specific therapy to be tested after each induction of sustained AF. From this first part we concluded that 2 biphasic shocks represented the optimal compromise between peak shock voltage and total energy. In the second part, we compared the atrial DFTs of ST1, ST1+ST3, and ST1+ST2+ST3 electrotherapies, again according to a randomized protocol.
The atrial DFTs of each waveform tested for ST1 depicted in Figure 2A were determined using a step-up protocol with voltage increments. After induction of AF, electrotherapies were delivered according to a randomized protocol. The initial peak voltage of the ST1 waveform was 10V. If the individual therapy failed to terminate AF within 10 s, the peak voltage of the ST1 waveform was increased in 5 V increments to a maximum of 100 V. Following successful termination and a wait period to allow vagus nerve recovery, AF was re-induced and the next ST1 electrotherapy was tested, according to a randomized protocol.
The atrial DFTs of multi-stage therapies depicted in Figure 2B and Figure 2C were measured according to an identical step-up protocol with voltage increments, in which the peak voltage of the ST1 waveform was increased until AF was terminated. All multi-stage electrotherapies used two biphasic shocks (2BP) shocks as the ST1 waveform. The voltage of the ST2 component of multi-stage therapy, set at 60% of the ventricular SET, and the voltage of the ST3 component, set at four times the atrial pacing capture threshold during sinus rhythm, respectively, did not vary.
Determination of Atrial Defibrillation Thresholds
Atrial DFT voltage was defined as the peak leading edge voltage of the ST1 shock that terminated AF using the step-up protocol. Atrial DFT total energy, was calculated as the sum of the ST1 and ST2 and electrotherapies delivered, i.e., the square of the total voltage integrated over time, divided by the shock impedance. ST3 energy was negligible and therefore not included in the calculation.
Finally, we determined the atrial DFTs of three defibrillation vectors: SVC to CSd, LAA to CSp, and RAA to LAA. For each vector, three combinations of therapy (described above) were tested. All therapies were delivered by a custom built LabVIEW software program (National Instruments, Austin, TX) and amplified by a computer-controlled, regulated power supply (BOP 100-4M; Kepco, Flushing, NY). The impedance of each defibrillation vector was measured using a current probe (A622; Tektronix, Inc., Beaverton, OR).
Statistical Analysis
Student's t-test was used to compare the average vagus nerve stimulation frequencies that induced AF versus AFl, the dominant frequency of AF versus AFl, atrial ERP before versus after vagus nerve stimulation and atrial/ventricular shock excitation thresholds. Atrial and ventricular shock excitation thresholds, shock vector impedances were compared using one-way analysis of variance (ANOVA). The protocol randomized treatment sequence to prevent confounding effects of treatments with respect to time. Atrial DFT for each waveform and defibrillation vector tested were analyzed with a linear mixed random effects models with animal ID as a random effect and the period between treatments as a fixed effect; animal ID*Vector and animal ID*Therapy were also analyzed as random effects to test for interactions. Estimates were calculated with the MIXED procedure in SAS (Version 9.2, SAS Institute Inc., Cary, NC). Results are reported as mean ± standard deviation. A p value of ≤ 0.05 was considered significant.
Results
Atrial Fibrillation Model
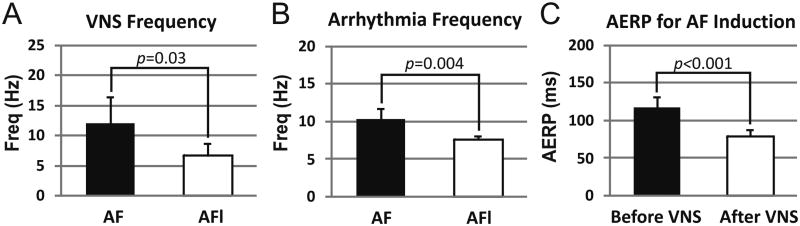
Sustained AF was induced in twelve of sixteen dogs. The average number of AF episodes induced per dog was 25 ± 8. The average duration of an experiment was 4 ± 1.5 hours. Characteristics of the AF generated are shown in Figure 3. AERP decreased significantly during vagal stimulation (79.6 ± 8.7 ms vs. 117.1 ± 14.7 without stimulation, p < 0.001). In general, durations of AF increased with the frequency of vagus nerve stimulation, and the stimulation frequency that sustained AF was significantly shorter than that which sustained atrial flutter (AFL; 6.7 ± 2.1 Hz vs. 12.0 ± 4.4 Hz, p = 0.03). As expected, average dominant frequency of sustained AF was significantly higher than that of AFl (7.7 ± 0.4 Hz vs. 10.4 ± 1.4 Hz, p = 0.004).
Figure 3.
Characteristics of Vagus Nerve Stimulation and Induced Arrhythmias. A: Mean frequency of vagal nerve stimulation (VNS) during induction of atrial fibrillation (AF) and atrial flutter (AFl). B: Mean dominant frequency of AF and AFl induced by burst pacing during VNS. C: Effect of VNS on atrial effective refractory period (ERP). All values are reported as mean ± standard deviation.
Shock Excitation Threshold
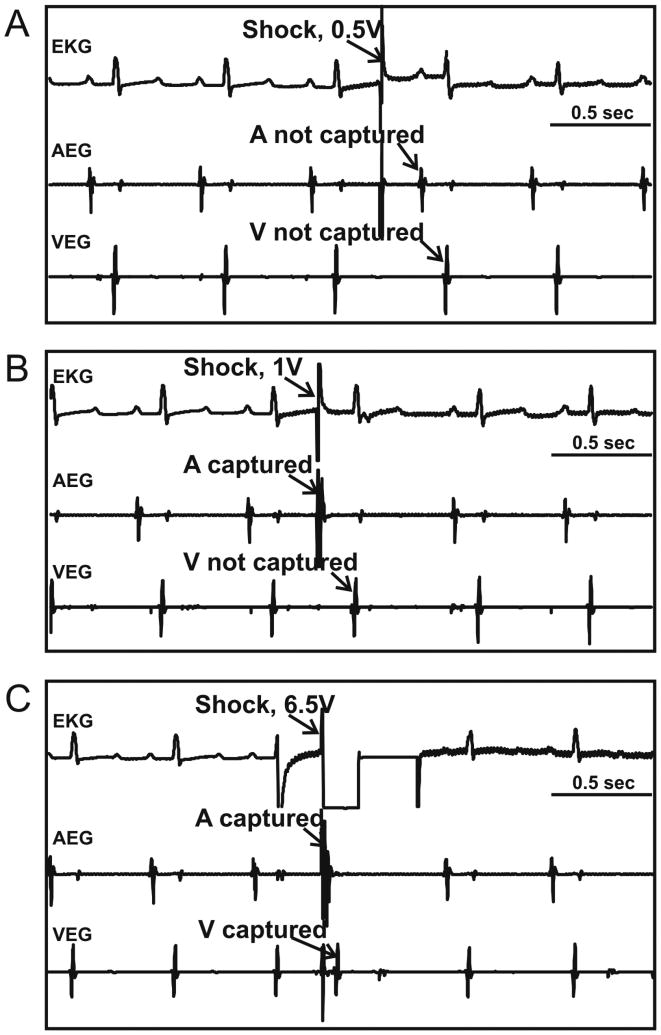
Atrial and ventricular SETs were measured for each defibrillation vector and for both polarities during sinus rhythm before induction of AF. A representative example of SET measurement is shown in Figure 4. In this case, a 0.5 V, 10 ms shock failed to capture atrial or ventricular tissue (Figure 4A), while a 1 V shock captured the atria but not the ventricles (Figure 4B). Increasing the shock amplitude to 6.5 V captured both atria and ventricles (Figure 4C).
Figure 4.
Determination of Atrial and Ventricular Shock Excitation Thresholds. An example of the surface electrocardiogram (EKG), atrial (AEG) and ventricular (VEG) electrograms, respectively, recorded during the measurement of atrial and ventricular shock excitation thresholds (SET) for shock vector RAA>LAA is shown. A: 0.5 V shock captured neither atrial nor ventricular tissue. B: 1 V shock captured atrial but not ventricular tissue. C: 6.5 V shock captured atrial and ventricular tissue. A, atrium; V, ventricle.
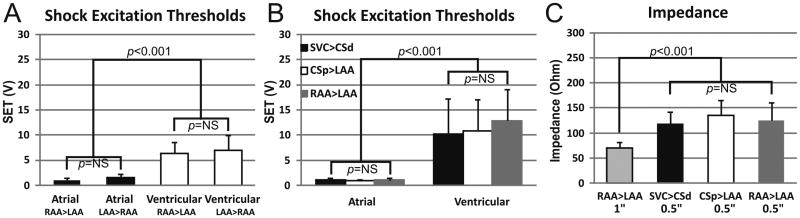
Changing shock polarity did not significantly alter atrial or ventricular SET (Figure 5A). The atrial SET was significantly less than the ventricular SET for each vector tested (Figure 5B). This finding enabled the application of ST2 shocks above the atrial SET and below the ventricular SET delivered outside the ventricular refractory window without inducing ventricular tachycardia or fibrillation (VT or VF, respectively).
Figure 5.
Shock Excitation Thresholds are Independent of Polarity and Electrode Position Mean atrial and ventricular shock excitation threshold (SET) is shown for different electrode sizes and individual vectors tested. A: Reversing polarity of the shock vector did not reduce SET significantly. Atrial (black bars) and ventricular (white bars) SETs from two vectors using 1.0 in diameter disc electrodes placed on the left and right atrial appendages (LAA and RAA, respectively) is depicted. For each vector, notation is anode > cathode. B: Atrial and ventricular SETs for the three vectors tested using 0.5 in diameter disc electrodes and a 4F CS lead with two 1 in coils. C: Impedances for each vector tested. Note that the RAA>LAA impedance using 0.5 inch electrodes (0.5″) was larger than for 1.0 inch electrodes (1″), resulting in increased ventricular SETs for the 0.5 inch electrodes.
Electrotherapy Applications and AF Terminations
An average of 287 electrotherapies per animal were applied using the voltage-regulated step-up protocol. An average of 22 AF episodes were terminated per animal by the electrotherapies applied.
Testing MP versus BP and single versus multiple shocks
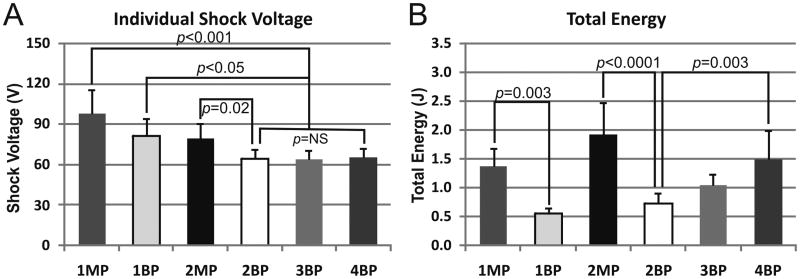
Atrial DFTs for ST1 shock waveforms are summarized in Figure 6, with peak shock voltage in the upper panel and total energy in the lower panel. Total energy was calculated as the sum of the energies of individual shocks. In all cases, AFl was easily terminated by a single BP shock at 0.0003 ± 0.0001 J or anti-tachycardia pacing (ATP) with successful rate of 100% for each therapy. For termination of AF, the atrial DFT of 1BP shock was significantly lower than that of 1MP shock (0.55 ± 0.1 J vs. 1.38 ± 0.31 J, p =0.003). Similarly, the atrial DFT of 2BP shocks was significantly lower than that of 2MP shocks (0.72 ± 0.19 J vs. 1.92 ± 0.56 J, p < 0.0001). Interestingly, the atrial DFT of 4BP shocks was significantly higher than that of 2BP (1.50 ± 0.50 vs. 0.72 ± 0.02 J, p = 0.003), since the voltages required to terminate AF were similar for 2, 3 and 4 BP shocks. Additionally, though the efficacy of 1 BP shock was lower compared 2 BP shocks, the peak voltage was significantly higher. Therefore, we deemed 2 BP shocks to be the optimal waveform and chose it as the first stage (ST1) of subsequent multiple-stage therapy.
Figure 6.
Peak Voltage and Total Energy of Stage 1 therapies. For each therapy tested in Stage 1 (ST1), peak voltage (A) and total energy (B) is shown. MP, monophasic shock; BP, biphasic shock; the number preceding the shock type describes the number of shocks delivered, e.g., 1MP denotes a single MP shock while 4BP denotes four sequential BP shocks.
Development of a low energy multiple stage defibrillation therapy
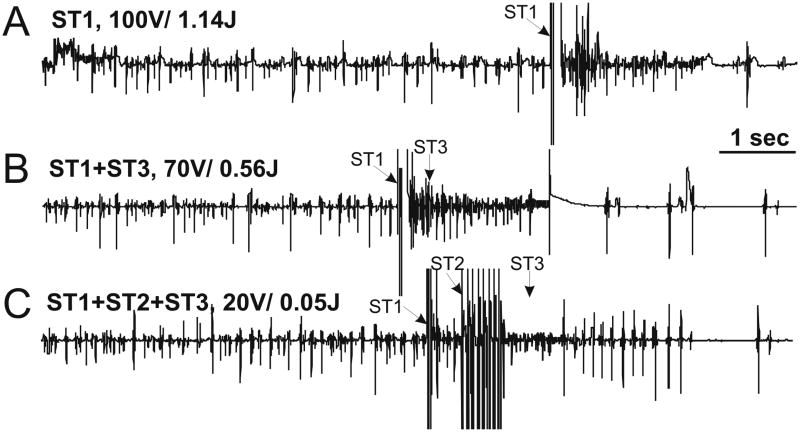
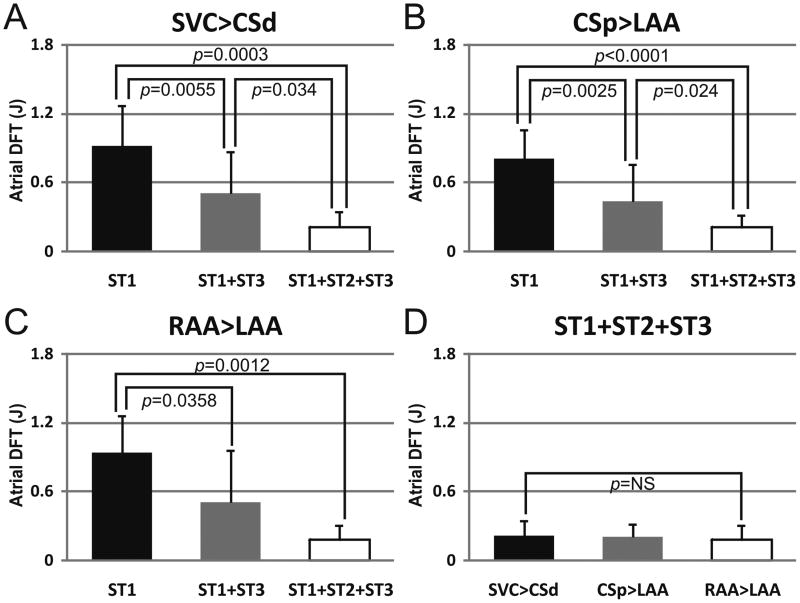
2 BP shocks (ST1) proved an optimal compromise with respect to peak voltage and total energy for cardioversion of AF in this model. Next, we asked whether additional stages, such as sub-ventricular excitation shocks (ST2) and ATP (ST3) further lowered the atrial DFT. Figure 7 shows a representative example of AF termination by one-stage, two-stage, and three-stage therapies and the corresponding atrial DFTs. The summarized results are shown in Figure 8. 2BP shocks followed by ATP (S1+S3) reduced the atrial DFT to 0.51 ± 0.46 J compared to 0.95 ± 0.32 J for ST1 alone (shock vector RAA>LAA, p=0.036). Significantly, atrial DFT was dramatically reduced further by the combination of 2 BP shocks followed by sub-ventricular SET shocks and then ATP (ST1+ST2+ST3). This “three-stage therapy” reduced the atrial DFT by nearly four-fold to 0.19 ± 0.12 J (vs 0.95 ± 0.32J for ST1 alone, p=0.001). The three-stage therapy was then tested across multiple vectors.
Figure 7.
Representative Atrial Electrograms of AF Terminations. For each panel, peak voltage in Stage 1 (ST1) and total energy is denoted above the corresponding atrial electrogram. The time that each individual therapy was delivered is indicated with an arrow. A: ST1 therapy of two 100 V biphasic shocks (total energy 1.14 J) terminated AF. B: Stage 1 plus Stage 3 (ST1+ST3) lowered the peak voltage of ST1 to 70 V and the atrial defibrillation threshold (DFT) to 0.56 J. C: Three-stage therapy (ST1+ST2+ST3) dramatically reduced the total energy to 0.05 J and the peak voltage of ST1 to 20 V.
Figure 8.
Atrial DFTs for each Shock Vector tested. Atrial DFTs of ST1, ST1+ST3 and ST1+ST2+ST3 are shown for each vector tested in total energy. A: Shock vector SVC>CSd. B: Shock vector CSp>LAA. C: Shock vector RAA>LAA. D: Atrial DFT of Three-Stage therapy (ST1+ST2+ST3) did not significantly differ with respect to shock vector tested. SVC, superior vena cava; CSd, distal coronary sinus; CSp, proximal coronary sinus; LAA, left atrial appendage; RAA, right atrial appendage.
The relationship of shock vector to atrial DFT
We applied the three-stage therapy across three vectors: LAA-RAA, RAA to CSd, and CSp-LAA. Surprisingly, we detected no significant difference in atrial DFT (Figure 8D).
Safety considerations
Two out of a total of 3444 AF termination attempts induced VF. One episode was caused by improperly triggered ST1 application (not delivered on the R-wave) due to unexpected noise in the ventricular electrogram. Adding an R-wave recognition algorithm in the trigger function and using the surface ECG as the trigger input when the signal-to-noise ratio of ventricular electrogram was poor overcame this problem. The second VF episode was induced by ST2 shocks when the applied shock amplitude was very close to the ventricular SET. This problem was avoided in subsequent trials by strictly limiting the amplitude of ST2 shocks to below 50% of the ventricular SET.
Discussion
The main findings of the present study are as follows:
Single and multiple biphasic shocks in ST1 were significantly more efficacious at terminating AF than the same number of monophasic shocks,
Increasing the number of biphasic shocks in ST1 above two did not decrease the atrial DFT in this model,
A three-stage therapy ST1+ST2+ST3 significantly reduced the atrial DFT compared to one-stage (ST1) and two-stage (ST1+ST3) therapies,
Three tested defibrillation vectors (RAA-LAA, RAA-CSd, LAA-CSp) were equally efficacious for all tested combinations of stages of therapy (ST1, ST1+ST3 and ST1+ST2+ST3).
A prevailing theory is that AF is induced and maintained by a single or a small number of stable, self-sustained mother rotors giving rise to exceedingly high frequency excitation, with resultant fibrillatory conduction in the atria.27 Rotors are organized around phase singularities which tend to anchor to anatomical or functional syncytial heterogeneities.28 Attempts to optimize electrotherapy of fibrillation should be based on directly targeting the phase singularities.27, 29, 30 We have, however, demonstrated that electrotherapy may fail to defibrillate despite successful termination of phase singularities that maintain the ongoing arrhythmia. This occurs because defibrillation simultaneously terminates existing phase singularities while inducing new phase singularities via the virtual electrode induced phase singularity mechanism.31 Biphasic shocks with appropriate energy ratios between the first and second phases can create homogeneous post-shock transmembrane polarization and phase distribution, which reduces the probability of inducing new phase singularities. 32 Therefore, an optimized biphasic shock therapy was proven safe and reliable in treating AF.15
The atrial DFT of a conventional single biphasic shock remains above the human pain threshold.17-20 This fact greatly limits implantable device therapy for cardioversion of AF. High voltage shocks also cause electroporation33 and impair efferent sympathetic neural function.34 The three-stage AF therapy developed and tested in the present study is below the thresholds of electroporation and nerve damage.33-35 Most importantly, the energies and voltages necessary to cardiovert AF using multi-stage therapy are well likely below the human pain threshold,36 making realistic the possibility of an implantable device for defibrillation of AF.
As early as 1945, Gurvich tested two to four discharges of a single capacitor with different intervals ranging from 250 ms to 2 sec between each discharge for transthoracic defibrillation of ventricular fibrillation in dogs.37 He found that two to three multiple shocks with an interval of 1.33 to 2 sec were capable to terminate VF in dogs with voltage about 50% to 70% of the DFT of a single shock. In addition, he discovered that this multiple shock defibrillation technique could be improved by shortening the interval between pulses to a range of 333-250 ms.
We previously found that multiple monophasic shocks achieve lower defibrillation thresholds than biphasic shocks in an in vitro model of VT in chronically infarcted rabbit hearts.26 In contrast, this study shows that multiple biphasic shocks are superior for the defibrillation of AF, a finding that is consistent with the studies of traditional high energy, single shock defibrillation.38, 39 The apparent discrepancy in findings between these studies may be explained by different mechanisms of low-energy defibrillation in these different tachyarrhythmias. VT, in the rabbit model, was maintained by a single rotor with phase singularity attached to a chronic infarction scar. Successful multiple-shock low-voltage defibrillation was achieved by maintaining a small region refractory in the reentry circuit via the VEP effect induced by monophasic shocks until the wave front crashed into this refractory region and terminated. Accordingly, the second phase of biphasic shocks reverses the VEP effect of the first phase and is therefore disadvantageous.26 Hence, biphasic shocks have higher DFTs compared to monophasic shocks for termination of VT. In contrast, AF is sustained by multiple rotors with different frequencies, phases, and anatomical locations. Therefore, extinguishing all existing rotors is fundamental to the defibrillation of AF.25,40 This is better achieved by biphasic shocks, which create a more homogenous VEP pattern than monophasic shocks, and provides a mechanistic explanation of our findings in the present study.
Another important difference between the defibrillation of VT versus AF is the optimal number of shocks. In a canine infarct model of VT, we found that three monophasic shocks had the highest DFT, while ten monophasic shocks applied within two VT cycles had the lowest DFT compared to one, three, five, and seven monophasic shocks applied within one VT cycle.40 In the present study, we demonstrated that applying more than two biphasic shocks in ST1 did not lower the DFT. This difference suggests fundamental differences in the mechanisms of defibrillation of a relatively slow and more organized VT wave front versus a faster and more disorganized collection of wave fronts seen in AF; a longer train of shocks in a short interval could lead to defibrillation failure not by failing to terminate the existing disorganized wave fronts per se, but rather, by inducing new wave fronts.41 Moreover, from a neurological point of view, a smaller number of shocks is advantageous in developing a painless AF cardioversion strategy, as perceived pain increases with increasing number of shocks.42
Our three-stage therapy significantly lowers the energy for cardioversion of AF by application of three different stages of therapy, which we mechanistically relate to the: (1) unpinning of wave fronts that maintain AF, (2) prevent re-repinning of wave fronts to tissue heterogeneities such as scar, and (3) annihilation of remaining wave fronts. The unpinning stage uses multiple pulses aiming to unpin the reentry from the stabilizing resistive heterogeneity. The applied electric field creates stronger virtual electrode polarization at tissue heterogeneities, which causes excitation and then unpinning of the reentry.28, 43 However, this stage leaves behind a number of unpinned phase singularities which could then re-pin to heterogeneities and perpetuate AF. Thus, the anti-repinning stage likely prevents the meandering singularities from anchoring back to the tissue heterogeneities by applying an entrainment shock train.44 After the first two stages, the last stage adopts anti-tachycardia pacing to drive the remaining reentry circuits to the boundaries of the atria, i.e., tricuspid or mitral annulus, vena cavae, or pulmonary veins, thereby annihilating the arrhythmia to restore sinus rhythm25, 26, 45.
AF is rarely fatal, yet atrial defibrillation stimuli could lead to VF, as was found earlier46 and in our study. Accordingly, the safety of any atrial defibrillation therapy must be considered the highest priority. Our study revealed several techniques to improve safety. Synchronization of the first stage shocks to the R-wave to deliver it within the ventricular ERP is critical to avoiding potentially lethal post-shock ventricular arrhythmias. Similarly, the shock amplitude of the second stage has to be significantly below the ventricular shock excitation threshold. We found that delivering the second stage below 50% of the ventricular SET provided an adequate safety margin and prevented unintended ventricular excitation.
Prior studies of atrial defibrillation delivered shocks from a CS coil to an RA coil positioned along the lateral wall in order to minimize damage to the sinoatrial and atrioventricular nodes.47 Our studies indicated that a defibrillation vector involving the coronary sinus may enhance the probability of inducing VF due to the relative ease of exciting ventricular tissue from the canine CS. In contrast, the human coronary sinus is better insulated from the ventricular myocardium due to a thicker fatty and fibrotic tissue barrier that is more robust than that of canines (data not shown). Nevertheless, due to the larger size of human coronary sinus anatomy, the migration of such a lead after implantation could cause unintended ventricular capture during Stages 2 and 3 of the three-step therapy. Such migration may be prevented by cautious implantation to avoid placing the CS lead into sub-vessels from which the coil may easily migrate out of, and by designing leads that more stably maintain their position after implant.
Further reductions in atrial defibrillation energy may be achieved by thoughtful engineering. Larger impedances require higher voltage shocks to deliver adequate defibrillation energy, yet large voltage shocks are more likely to be painful. Previous studies showed that increasing electrode surface area by using multiple cathode or anode electrodes can significantly reduce the overall impedance.48 Importantly, such a reduction would also decrease the peak voltage necessary for defibrillation, which is directly linked to pain sensation. Another strategy is to deliver sequential shocks across multiple vectors using several different pairs of electrodes.48-50 This strategy could also be used to lower DFTs by eliminating the region of low field gradient created between two electrodes that are electrically tied together. In addition to multiple stage therapy, the engineering techniques discussed here may be crucial to keeping the atrial DFT under the pain threshold and should be explored in future studies.
This study demonstrates that multi-stage electrotherapy significantly reduces the atrial DFT compared to a single shock. It is important to point out that these findings are derived from an experimental, animal model of AF and do not replicate the spectrum of disease leading to atrial fibrillation in humans. Importantly, extrapolation of these results to human atrial fibrillation requires formal testing.
Conclusions
In the present study, we found that multiple biphasic shocks were significantly more efficacious for cardioversion of AF than monophasic shocks, and that two sequential biphasic shocks was optimal in terms of atrial DFT and peak voltage. We also showed that a novel three-stage therapy we achieved the lowest atrial DFT. Importantly, the atrial DFT of three-stage therapy is at or below the pain threshold (0.19 ± 0.12 J). Finally, our study demonstrated that defibrillation vector did not significantly alter atrial DFT. Based on this study, we conclude that three-stage therapy may allow pain-free cardioversion of atrial fibrillation.
Acknowledgments
Funding Sources: This work was supported by NIH grant R01HL-067322 and unrestricted educational grant from Cardialen, Inc.
Abbreviations
- AERP
atrial effective refractory period
- AF
atrial fibrillation
- AFl
atrial flutter
- BP
biphasic
- SET
shock excitation threshold
- MP
monophasic
Footnotes
Conflict of Interest Disclosures: Dr. Efimov is a chairman of the scientific advisory board, a member of the board of directors, and owns stock in CardiaLen, Inc.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Petersen RC, Cha SS, Bailey KR, Gersh BJ, Casaclang-Verzosa G, Abhayaratna WP, Seward JB, Iwasaka T, Tsang TS. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: Data from a community-based cohort. Eur Heart J. 2007;28:1962–1967. doi: 10.1093/eurheartj/ehm012. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D'Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: The framingham heart study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 4.Seiler J, Stevenson WG. Atrial fibrillation in congestive heart failure. Cardiol Rev. 18:38–50. doi: 10.1097/CRD.0b013e3181c21cff. [DOI] [PubMed] [Google Scholar]
- 5.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 6.Gaita F, Caponi D, Pianelli M, Scaglione M, Toso E, Cesarani F, Boffano C, Gandini G, Valentini MC, De Ponti R, Halimi F, Leclercq JF. Radiofrequency catheter ablation of atrial fibrillation: A cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation. 122:1667–1673. doi: 10.1161/CIRCULATIONAHA.110.937953. [DOI] [PubMed] [Google Scholar]
- 7.Di Biase L, Fahmy TS, Wazni OM, Bai R, Patel D, Lakkireddy D, Cummings JE, Schweikert RA, Burkhardt JD, Elayi CS, Kanj M, Popova L, Prasad S, Martin DO, Prieto L, Saliba W, Tchou P, Arruda M, Natale A. Pulmonary vein total occlusion following catheter ablation for atrial fibrillation: Clinical implications after long-term follow-up. J Am Coll Cardiol. 2006;48:2493–2499. doi: 10.1016/j.jacc.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Bai R, Patel D, Di Biase L, Fahmy TS, Kozeluhova M, Prasad S, Schweikert R, Cummings J, Saliba W, Andrews-Williams M, Themistoclakis S, Bonso A, Rossillo A, Raviele A, Schmitt C, Karch M, Uriarte JA, Tchou P, Arruda M, Natale A. Phrenic nerve injury after catheter ablation: Should we worry about this complication? J Cardiovasc Electrophysiol. 2006;17:944–948. doi: 10.1111/j.1540-8167.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 9.Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso F, Torracca L, Benussi S, Alfieri O, Hong R, Lau W, Hirata K, Shikuma N, Hall B, Morady F. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 10.Lee WC, Lamas GA, Balu S, Spalding J, Wang Q, Pashos CL. Direct treatment cost of atrial fibrillation in the elderly american population: A medicare perspective. J Med Econ. 2008;11:281–298. doi: 10.3111/13696990802063425. [DOI] [PubMed] [Google Scholar]
- 11.Weigner MJ, Caulfield TA, Danias PG, Silverman DI, Manning WJ. Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med. 1997;126:615–620. doi: 10.7326/0003-4819-126-8-199704150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt C, Alt E, Plewan A, Ammer R, Leibig M, Karch M, Schomig A. Low energy intracardiac cardioversion after failed conventional external cardioversion of atrial fibrillation. J Am Coll Cardiol. 1996;28:994–999. doi: 10.1016/s0735-1097(96)00274-4. [DOI] [PubMed] [Google Scholar]
- 13.Ladwig KH, Marten-Mittag B, Lehmann G, Gundel H, Simon H, Alt E. Absence of an impact of emotional distress on the perception of intracardiac shock discharges. Int J Behav Med. 2003;10:56–65. doi: 10.1207/s15327558ijbm1001_05. [DOI] [PubMed] [Google Scholar]
- 14.Lok NS, Lau CP, Tse HF, Ayers GM. Clinical shock tolerability and effect of different right atrial electrode locations on efficacy of low energy human transvenous atrial defibrillation using an implantable lead system. J Am Coll Cardiol. 1997;30:1324–1330. doi: 10.1016/s0735-1097(97)00298-2. [DOI] [PubMed] [Google Scholar]
- 15.Wellens HJ, Lau CP, Luderitz B, Akhtar M, Waldo AL, Camm AJ, Timmermans C, Tse HF, Jung W, Jordaens L, Ayers G. Atrioverter: An implantable device for the treatment of atrial fibrillation. Circulation. 1998;98:1651–1656. doi: 10.1161/01.cir.98.16.1651. [DOI] [PubMed] [Google Scholar]
- 16.Jung W, Luderitz B. Implantation of an arrhythmia management system for ventricular and supraventricular tachyarrhythmias. Lancet. 1997;349:853–854. doi: 10.1016/S0140-6736(05)61758-8. [DOI] [PubMed] [Google Scholar]
- 17.Steinhaus DM, Cardinal DS, Mongeon L, Musley SK, Foley L, Corrigan S. Internal defibrillation: Pain perception of low energy shocks. Pacing Clin Electrophysiol. 2002;25:1090–1093. doi: 10.1046/j.1460-9592.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- 18.Haffajee CI, Chaudhry GM, Casavant D, Pacetti PE. Efficacy and tolerability of automatic nighttime atrial fibrillation shocks in patients with permanent internal atrial defibrillators. Am J Cardiol. 2002;89:875–878. doi: 10.1016/s0002-9149(02)02207-5. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell AR, Spurrell PA, Gerritse BE, Sulke N. Improving the acceptability of the atrial defibrillator for the treatment of persistent atrial fibrillation: The atrial defibrillator sedation assessment study (adsas) Int J Cardiol. 2004;96:141–145. doi: 10.1016/j.ijcard.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 20.Spurrell P, Mitchell A, Kamalvand K, Sulke N. Quality of life after use of the patient activated atrial defibrillator. Int J Clin Pract. 2003;57:30–34. [PubMed] [Google Scholar]
- 21.Alessi R, Nusynowitz M, Abildskov JA, Moe GK. Nonuniform distribution of vagal effects on the atrial refractory period. Am J Physiol. 1958;194:406–410. doi: 10.1152/ajplegacy.1958.194.2.406. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: Role of refractoriness heterogeneity. Am J Physiol. 1997;273:H805–816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 23.Fedorov VV, Sharifov OF, Beloshapko GG, Yushmanova AV, Rosenshtraukh LV. Effects of a new class iii antiarrhythmic drug nibentan in a canine model of vagally mediated atrial fibrillation. J Cardiovasc Pharmacol. 2000;36:77–89. doi: 10.1097/00005344-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Katsouras G, Sakabe M, Comtois P, Maguy A, Burstein B, Guerra PG, Talajic M, Nattel S. Differences in atrial fibrillation properties under vagal nerve stimulation versus atrial tachycardia remodeling. Heart Rhythm. 2009;6:1465–1472. doi: 10.1016/j.hrthm.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Ambrosi CM, Ripplinger CM, Efimov IR, Fedorov VV. Termination of sustained atrial flutter and fibrillation using low-voltage multiple-shock therapy. Heart Rhythm. 2011;8:101–108. doi: 10.1016/j.hrthm.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Ripplinger CM, Lou Q, Efimov IR. Multiple monophasic shocks improve electrotherapy of ventricular tachycardia in a rabbit model of chronic infarction. Heart Rhythm. 2009;6:1020–1027. doi: 10.1016/j.hrthm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalife J, Berenfeld O, Skanes A, Mandapati R. Mechanisms of atrial fibrillation: Mother rotors or multiple daughter wavelets, or both? J Cardiovasc Electrophysiol. 1998;9:S2–12. [PubMed] [Google Scholar]
- 28.Ripplinger CM, Krinsky VI, Nikolski VP, Efimov IR. Mechanisms of unpinning and termination of ventricular tachycardia. Am J Physiol Heart Circ Physiol. 2006;291:H184–192. doi: 10.1152/ajpheart.01300.2005. [DOI] [PubMed] [Google Scholar]
- 29.Efimov IR, Kroll MW, Tchou PJ. Cardiac bioelectric therapy. New York: Springer; 2009. [Google Scholar]
- 30.Efimov I, Ripplinger CM. Virtual electrode hypothesis of defibrillation. Heart Rhythm. 2006;3:1100–1102. doi: 10.1016/j.hrthm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Efimov IR, Cheng Y, Yamanouchi Y, Tchou PJ. Direct evidence of the role of virtual electrode-induced phase singularity in success and failure of defibrillation. J Cardiovasc Electrophysiol. 2000;11:861–868. doi: 10.1111/j.1540-8167.2000.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 32.Efimov IR, Cheng Y, Van Wagoner DR, Mazgalev T, Tchou PJ. Virtual electrode-induced phase singularity: A basic mechanism of defibrillation failure. Circ Res. 1998;82:918–925. doi: 10.1161/01.res.82.8.918. [DOI] [PubMed] [Google Scholar]
- 33.Al-Khadra A, Nikolski V, Efimov IR. The role of electroporation in defibrillation. Circ Res. 2000;87:797–804. doi: 10.1161/01.res.87.9.797. [DOI] [PubMed] [Google Scholar]
- 34.Ito M, Pride HP, Zipes DP. Defibrillating shocks delivered to the heart impair efferent sympathetic responsiveness. Circulation. 1993;88:2661–2673. doi: 10.1161/01.cir.88.6.2661. [DOI] [PubMed] [Google Scholar]
- 35.Fedorov VV, Kostecki G, Hemphill M, Efimov IR. Atria are more susceptible to electroporation than ventricles: Implications for atrial stunning, shock-induced arrhythmia and defibrillation failure. Heart Rhythm. 2008;5:593–604. doi: 10.1016/j.hrthm.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayanti V, Zviman MM, Nazarian S, Halperin HR, Berger RD. Novel electrode design for potentially painless internal defibrillation also allows for successful external defibrillation. J Cardiovasc Electrophysiol. 2007;18:1095–1100. doi: 10.1111/j.1540-8167.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 37.Gurvich NL. control of heart fibrillation by means of condensatory discharges of subthreshold power. Biull Eksp Biol Med. 1945;20:55–58. [PubMed] [Google Scholar]
- 38.Gurvich NL, Makarychev VA. defibrillation of the heart with biphasic electric impulsation. Kardiologiia. 1967;7:109–112. [PubMed] [Google Scholar]
- 39.Schuder JC, Gold JH, Stoeckle H, McDaniel WC, Cheung KN. Transthoracic ventricular defibrillation in the 100 kg calf with symmetrical one-cycle bidirectional rectangular wave stimuli. IEEE Trans Biomed Eng. 1983;30:415–422. doi: 10.1109/TBME.1980.326689. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Fedorov VV, Schuessler RB, Efimov IR. Low-voltage multiple pulse termination of ventricular tachycardia in 4-day infarct canine hearts. Circulation. 2009;120:S631. [Google Scholar]
- 41.Salama G, Kanai A, Efimov IR. Subthreshold stimulation of purkinje fibers interrupts ventricular tachycardia in intact hearts. Experimental study with voltage-sensitive dyes and imaging techniques. Circ Res. 1994;74:604–619. doi: 10.1161/01.res.74.4.604. [DOI] [PubMed] [Google Scholar]
- 42.Levy S. Internal defibrillation: Where we have been and where we should be going? J Interv Card Electrophysiol. 2005;13 1:61–66. doi: 10.1007/s10840-005-1824-6. [DOI] [PubMed] [Google Scholar]
- 43.Takagi S, Pumir A, Pazo D, Efimov I, Nikolski V, Krinsky V. Unpinning and removal of a rotating wave in cardiac muscle. Phys Rev Lett. 2004;93:058101. doi: 10.1103/PhysRevLett.93.058101. [DOI] [PubMed] [Google Scholar]
- 44.Trayanova N, Skouibine K, Aguel F. The role of cardiac tissue structure in defibrillation. Chaos. 1998;8:221–233. doi: 10.1063/1.166299. [DOI] [PubMed] [Google Scholar]
- 45.Kurian TK, Efimov IR. Mechanisms of fibrillation: Neurogenic or myogenic? Reentrant or focal? Multiple or single? Still puzzling after 160 years of inquiry. J Cardiovasc Electrophysiol. 2010;21:1274–1275. doi: 10.1111/j.1540-8167.2010.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray RA, Jalife J. Effects of atrial defibrillation shocks on the ventricles in isolated sheep hearts. Circulation. 1998;97:1613–1622. doi: 10.1161/01.cir.97.16.1613. [DOI] [PubMed] [Google Scholar]
- 47.Kusumoto FM. Internal atrial and ventricular defibrillation during electrophysiology procedures. J Interv Card Electrophysiol. 2005;13 1:71–78. doi: 10.1007/s10840-005-0753-8. [DOI] [PubMed] [Google Scholar]
- 48.Zheng X, Benser ME, Walcott GP, Girouard SD, Rollins DL, Smith WM, Ideker RE. Reduction of atrial defibrillation threshold with an interatrial septal electrode. Circulation. 2000;102:2659–2664. doi: 10.1161/01.cir.102.21.2659. [DOI] [PubMed] [Google Scholar]
- 49.Tsukerman BM, Bogdanov K, Kon MV, Kriukov VA, Vandiaev GK. defibrillation of the heart by a rotating current field. Kardiologiia. 1973;13:75–80. [PubMed] [Google Scholar]
- 50.Zheng X, Benser ME, Walcott GP, Smith WM, Ideker RE. Reduction of the internal atrial defibrillation threshold with balanced orthogonal sequential shocks. J Cardiovasc Electrophysiol. 2002;13:904–909. doi: 10.1046/j.1540-8167.2002.00904.x. [DOI] [PubMed] [Google Scholar]