Abstract
The human liver is enriched in natural killer cells (NK cells) which are potent effectors of the innate immune system. We have determined that liver NK cells freshly isolated from surgical specimens from patients with hepatic malignancy have less cytolytic activity than autologous blood NK cells. This difference was due to a higher proportion of CD16− NK cells in the liver and reduced cytotoxicity by CD16+ liver NK cells compared with their blood counterparts. CD16+ liver NK cells had similar expression of activating NK receptors and had similar intracellular granzyme B and perforin content compared with CD16+ blood NK cells. CD16+ liver NK cells contained a reduced fraction of cells with inhibitory killer immunoglobulin-like receptors specific for self-MHC class I (self-KIR) and an increased fraction of self-KIRnegNKG2Apos and self-KIRnegNKG2Aneg cells. Using single-cell analysis of intracellular interferon-gamma (IFN-γ) production and cytotoxicity assays, we determined that CD16+ liver NK cells expressing self-KIR were more responsive to target cells than those cells that did not express self-KIR molecules. CD16+ liver NK cells gained cytolytic function when stimulated with IL-2 or cultured with LPS or PolyI:C-activated autologous liver Kupffer cells. Thus, the human liver contains NK cell subsets which have reduced effector function, but under appropriate inflammatory conditions become potent killers.
Keywords: Human, Liver, Natural killer cells, KIR, NKG2A
INTRODUCTION
The liver is continuously confronted with a large antigenic load that includes toxins, harmless dietary proteins, and antigenic elements from commensal intestinal organisms. The liver is also a common site of chronic viral and parasitic infection and is the most common location for distant metastatic disease in cancer. Therefore, the hepatic immune system must differentiate harmless foreign antigens from invasive threats in order to deliver appropriate immune responses. While the local immune mechanisms that are required to direct immunogenic or tolerogenic responses to these varied antigenic challenges are largely unknown, the overall balance in hepatic immunity tends to favor tolerance. For example, human leukocyte antigen (HLA) matching between donor and recipient does not affect the outcome of liver transplantation whereas HLA-disparate heart, lung, and kidney transplants are frequently rejected (1). Furthermore, compared with other solid organ transplants, T cell mediated rejection of liver allografts is uncommon (2). Additionally, the liver is believed to play a central role in oral tolerance, a phenomenon in which systemic immune responses to specific antigens are suppressed by prior administration of the antigen via the oral route or portal vein (3).
To cope with potentially toxic insults without launching harmful systemic immune responses, the liver may rely on its innate immune system. The lymphoid system of the liver is uniquely weighted with innate cells. While peripheral blood lymphocytes are dominated by T and B cells that possess clonotypic antigen-specific receptors and are capable of mediating adaptive responses, the lymphoid system of the liver is comprised chiefly of innate immune cells such as NK cells, and NKT cells, and γδ T cells which use invariant receptors and are capable of detecting and responding rapidly to pathogens and malignant cells (4).
NK cells are bone marrow derived lymphocytes that are able to recognize and kill transformed and virus-infected cells without the need for prior sensitization. NK cells are a predominant lymphocyte population in the murine and human liver. Whereas NK cells account for less than 20% of all circulating lymphocytes, they may comprise up to 50% of the total lymphocyte pool in the human liver (4). In humans, two main functional subsets of NK cells exist based on their surface density of CD56 and CD16 (5). CD56dimCD16pos NK cells (referred to hereafter as CD16+ NK cells) comprise upwards of 90% of NK cells in the periphery, contain an abundance of lytic granules, and have high cytotoxic potential. CD56brightCD16neg NK cells (referred to hereafter as CD16− NK cells) constitute a minor population of circulating NK cells and have decreased cytotoxic ability, but are capable of producing copious amounts of cytokines (6).
The distribution of human NK cell subsets varies within solid organs possibly reflecting a tissue-specific division of labor. Although rare in the blood, CD16− NK cells comprise approximately 75% of NK cells in lymph nodes and 50% of NK cells in the spleen (7,8). NK cells in lymphoid tissues can be found in close proximity to dendritic cells (DCs) (8,9). Through production of IFN-γ, NK cells may act to polarize adaptive T cell responses (10,11) and can limit EBV triggered B cell transformation (12). In the uterus and placenta, a unique subset of NK cells with the CD16− phenotype predominates (13,14). Although these NK cells do contain an abundance of cytolytic granules, they do not lyse the HLA-A and HLA-B negative extravillous trophoblasts that invade the decidua during pregnancy and when freshly isolated, demonstrate weak cytolytic function (13). In the placenta, these tissue resident NK cells appear to be specialized for regulating specific developmental processes at the fetal-maternal interface (15).
The effector functions of NK cells are regulated in part by their interaction with MHC class I (MHC-I) molecules. Ligation of NK cell inhibitory receptors by their specific MHC-I ligands on target cells results in inhibition of NK cell function. Therefore, targets with normal MHC-I expression are more resistant to killing than those lacking MHC-I, and target cells lacking MHC-I are sensitive to NK cytotoxicity. Through a variety of inhibitory and activating receptors and their ligands on target cells, NK cells identify cells that have lost or altered MHC-I expression, as occurs in viral infection and malignant transformation. Recently, the “missing-self” paradigm (16) has been modified to accommodate the observation that a substantial subset of NK cells are lacking all MHC class I specific receptors or inhibitory receptors for “self” MHC-I. NK cells require “licensing” or “education” by an inhibitory receptor that can interact with a self-MHC-I ligand to elicit strong responses to targets without self-MHC-I molecules, while NK cells without such receptors are hyporesponsive (17–23). The functional NK cell repertoire is therefore controlled by the inhibitory receptors that recognize self-MHC-I ligands. In humans, inhibitory NK receptors that recognize MHC-I include the polymorphic killer immunoglobulin-like receptors (KIRs) and the lectin-like CD94-NKG2A heterodimer (24). The inhibitory KIRs consist of KIR2DL1 which recognizes the group of HLA-C molecules with a Lys80 residue (HLA-C2 group), KIR2DL2/2DL3 which recognize the group of HLA-C with an Asn80 residue (HLA-C1 group), and KIR3DL1 which recognize Bw4-containing HLA-B alleles (HLA-Bw4 group). The CD94-NKG2A heterodimer recognizes a complex of the non-classical MHC-I molecule HLA-E bound to peptides from the leader sequences of other MHC-I molecules. Using fresh surgical specimens from patients with malignancy involving the liver, we show that the human liver NK cell repertoire as based on inhibitory receptor status is substantially different from its circulating counterpart. We have characterized the phenotype and function of NK cells in paired autologous liver and blood samples. We have found that human liver NK CD16+ NK cells display a decreased functional response towards MHC-I deficient target cells and that this is caused by a decrease in the proportion of NK cells with a licensed phenotype. Our findings emphasize the multitude of ways in which immune responses can be regulated in an organ-specific manner.
MATERIALS AND METHODS
Cell isolation
Blood and matched liver samples were collected from 46 patients undergoing elective hepatic resection at Memorial Sloan-Kettering Cancer Center (MSKCC). The indications for operation included metastatic disease (n=34), primary cancer (n=10), and benign disease (n=2). Detailed clinicopathologic data are shown in Supplementary Table 1. Informed consent was obtained according to an IRB approved protocol. Blood was drawn intraoperatively and peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation over Ficoll-Paque Plus (GE Healthcare). Liver tissue was procured greater than 5cm away from any visible tumor and processed immediately after resection. Notably, we have not found any difference in expression of the activation markers HLA-DR and CD69 on NK cells in the peritumoral (within 2 cm from the tumor) versus distant liver (not shown). To remove contaminating circulating blood cells, hepatic vessels were flushed with a solution of HBSS containing type IV collagenase (1 mg/ml, Sigma), DNase (50 ng/ml, Roche Diagnostics), and 2%, endotoxin-free human AB+ serum (Invitrogen). The liver specimen was then morselized and digested in the collagenase solution at 37°C for 30 minutes. The tissue slurry was quenched with cold HBSS containing DNase (25 ng/ml) and passed through a 100 µm filter. The resulting cell suspension was centrifuged twice at 250g for 10 minutes to remove fat and debris and then hepatocytes were removed with a low speed spin (30g for 1 minute). The resulting non-parenchymal cells were layered over a Ficoll-Paque density gradient and centrifuged at 1000g for 20 minutes at which time the interface layer containing liver mononuclear cells (LMNCs) was harvested.
HLA genotyping
HLA typing was performed preoperatively on select patients using a combination of intermediate resolution sequence-specific typing for HLA-A, HLA-B, and HLA-Cw loci (Dynal Systems), followed by high resolution typing by sequence-specific oligo probe analysis (Orchid Diagnostics) as previously described (25). HLA-KIR ligand groups C1, C2, and Bw4 were assigned to each donor and shown in Supplementary Table 1 (26).
Flow cytometry
Immunophenotypic analysis of cells was performed with seven-color flow cytometry on a FACS Aria cytometer (BD Biosciences) using monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein-Cy5.5 (PerCP-Cy5.5), phycoerythrin-Cy7 (PE-Cy7), allophycocyanin (APC), allophycocyanin-Cy7 (APC-Cy7), and Alexa Fluor700® (AF700). Antibodies used for cell analysis included FITC-CD16 (3G8), FITC-CD62L (Dreg56), FITC-CD94 (HP-3D9), PE-NKp30 (P30-15), PE-NKp44 (P44-81), PE-NKp46 (9E2), PE-TRAIL (RIK-2), PE-NKG2D (1D11), PerCP-Cy5.5-CD3 (SK7), PE-Cy7-CD16 (3G8), APC-CD161 (DX12), APCy-Cy7-HLA-DR (L243), APC-Cy7-CD69 (FN50), AF700-CD56 (B159) (all from BD Biosciences), PE-CD56 (AF12-7H3), APC-KIR2DL1/2DS1 (11PB6), APC-KIR2DL2/2DL3 (DX27), APC-KIR3DL1 (DX9) (all from Miltenyi Biotec), PE-NKG2A (Z199, Beckman-Coulter), and PE-NKG2C (134591, R&D Systems). Intracellular perforin and granzyme content was detected using FITC-perforin (dG9, eBioscience) and PE-granzyme B (eBioGrB, eBioscience) following cell permeabilization (cytofix/cytoperm, BD Biosciences). Immunoglobulin isotype controls were used where appropriate. Flow cytometry data were analyzed with FlowJo software (TreeStar).
Fluorescence-activated cell sorting (FACS)
LMNCs or PBMCs were stained with the following antibodies for cell separation: FITC-CD16, PE-CD56, PE-Cy7-CD11b (ICR-F44, BD Biosciences), APC-KIR2DL1/2DL2, APC-KIR2DL2/2DL3, APC-KIR3DL1, APC-CD3 (SK7, BD Biosciences), APC-Cy7-CD3 (SK7, BD Biosciences), and APC-Cy7-CD14 (MΨP9, BD Biosciences). Cell sorting was performed using a MoFlo (DakoCytomation) or BD FACSAria (BD Biosciences) cell sorter and post sort purities were routinely >98%. NK cells were selected from the lymphocyte gate as CD56posCD3neg cells and in some cases as CD56dimCD16posCD3neg cells. Liver Kupffer cells (KC) were sorted as CD14posCD11bhi cells contained within the monocyte gate.
Cytotoxicity Assay
Standard cytotoxicity assays were performed using freshly isolated NK cells and 51Cr-labeled MHC class I negative-target cells. The K562 cell line was obtained from the American Type Culture Collection and the LCL721.221 cell line was provided by P. Parham (Stanford University, Palo Alto, CA). Cell lines were maintained in complete RPMI containing 10% heat inactivated fetal calf serum, 2 mM L-glutamine, 0.1% 2-mercaptoethanol, 100 U/ml penicillin, and 100 µg/ml streptomycin. Two million target cells were labeled with 100 µCi of Na51CrO4 (PerkinElmer Life and Analytical Sciences) at 37° Celsius for 90 minutes and then washed with media. Lysis assays were performed in triplicate for 4 hours at the indicated effector to target (E:T) ratio. In some experiments, NK cells were treated with 100 ng/ml concanamycin-A (CMA, ICN Biomedical) or a dimethylsulfoxide (DMSO, Fischer Scientific) vehicle for 2 hours at 37° Celsius before the addition of targets. Chromium release was measured with a TopCount NXT microplate scintillation and luminescence counter (PerkinElmer) and percent specific lysis was calculated as (cpm experimental − cpm spontaneous release) × 100/(cpm maximum release − cpm spontaneous release). In other experiments, freshly isolated NK cells were cultured overnight in media alone, in media containing 200 IU/ml recombinant human IL-2 (Proleukin, Novartis), or with autologous liver KC at an NK:KC ratio of 5:1. Before co-culture, KC were pulsed for 1 hour with LPS (1µg/ml from E. coli 055:B5, Sigma) or polyI:C (50ng/ml, Invivogen) and then washed. Following the culture period, NK cells were recounted for use in cytotoxicity assays.
Intracellular IFN-γ detection
Detection of intracellular IFN-γ was performed as previously described with minor modifications (26). After the percentage of NK cells in LMNCs and PBMCs was determined by flow cytometry, 1 million LMNCs or PBMCs were cultured in 96-well round bottom plates in complete media with K562 cells at an NK cell to target cell ratio of 1:1. Brefeldin-A (10 µg/ml, BD Biosciences) was added after 1 hour and the cells were cultured for an additional 12 hours. For detection of intracellular IFN-γ following CD16 receptor cross-linking, similar experiments were performed using anti-CD16 coated plates prepared by labeling 96-well enzyme immunoassay high-binding plates (Fisher Scientific) with purified anti-CD16 antibody (3G8, BD Biosciences) in PBS overnight at 4°C. After culture, the cells were stained with antibodies to CD3, CD56, CD16, NKG2A, and inhibitory self-KIR receptors before fixation and permeabilization and staining with a FITC-conjugated anti-IFN-γ Ab (Intracellular cytokine detection kit, BD Biosciences).
Statistics
Data were analyzed for statistical significance using paired Student’s t-test tests (Prism statistical software, GraphPad). P values <0.05 were considered statistically significant.
RESULTS
Human liver contains a lower percentage of CD16+ NK cells
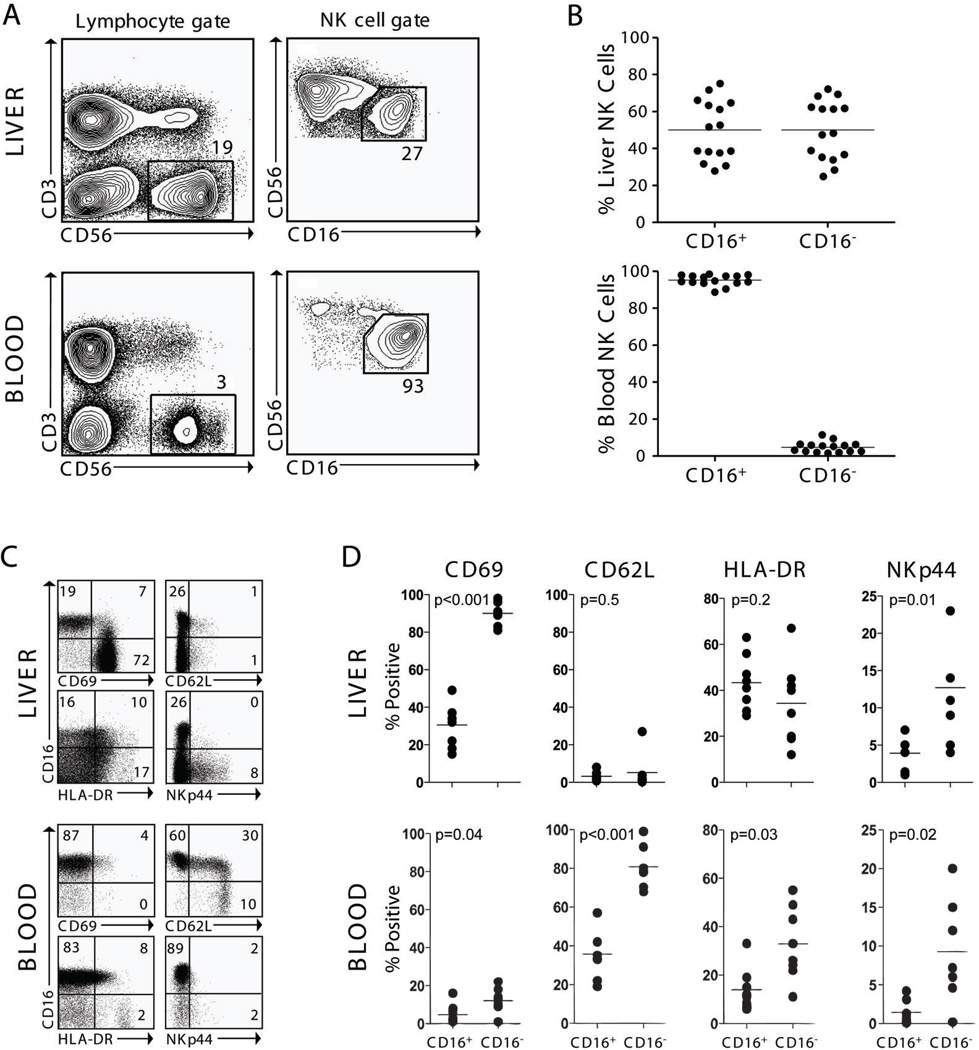
Using flow cytometry, we analyzed LMNCs and autologous PBMCs from 15 patients to determine the composition of NK cell subsets in the liver (Fig. 1A–B). There was considerable variability in NK cell subset composition within the liver. Whereas CD16+ NK cells accounted for a mean (±SEM) of 95±1% (range 89% to 98%) of NK cells in the blood, CD16+ NK cells represented 50±4% (range 27% to 75%) of all liver NK cells (Fig. 1B). Furthermore, liver NK cells displayed an activated phenotype when compared with blood NK cells as they had higher expression of CD69, HLA-DR, and NKp44 and lower CD62L (Fig. 1C–D).
Figure 1. Human liver contains a variable percentage of CD16+ NK cells.
A) Contour plots from one representative patient demonstrate the gating strategy used for analysis by flow cytometry and cell sorting of liver and blood mononuclear cell preparations. Numbers represent the percentage of cells within the indicated gate. B) Scatter plots for 15 patients show the percentages of CD16+ and CD16− NK cells in liver and blood mononuclear cell preparations. C) Representative dotplots from 1 of 8 patients are gated on CD56+CD3− cells to demonstrate the phenotype of liver compared with blood NK cells. Numbers represent the percentage of cells in their respective quadrants. D) Summary plots for activation marker expression on CD16+ and CD16− NK cells in matched blood and liver samples from 8 patients.
Human liver NK cells are poorly cytolytic
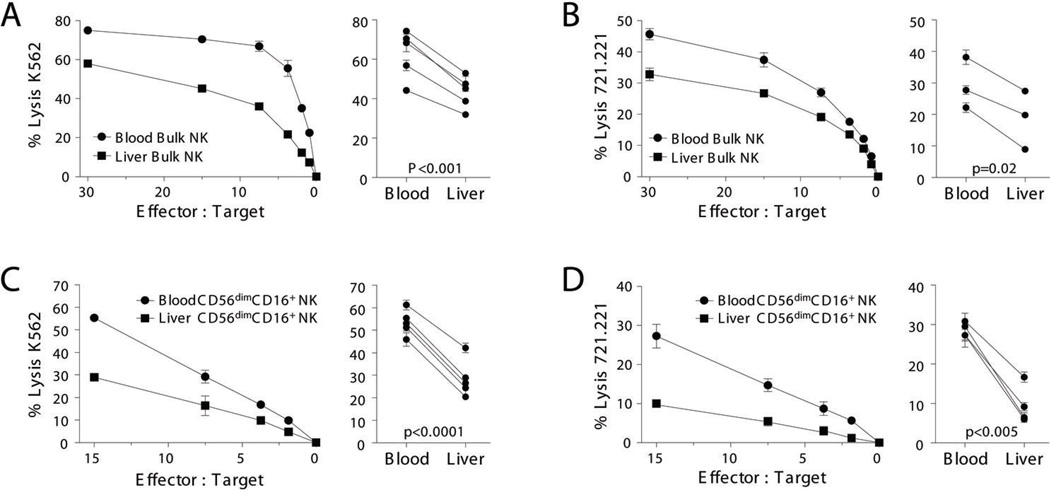
Because of their activated phenotype, we hypothesized that resident liver NK cells would have greater cytolytic function than circulating NK cells. On the contrary, we found that freshly isolated blood NK cells were consistently more potent than autologous liver NK cells in their ability to kill K562 and MHC-I deficient LCL721.221 targets (Fig. 2A–B). To determine whether subset composition was merely responsible for the difference in lysis between blood and liver bulk NK cells, we compared the lytic function of blood and liver CD16+ NK cells. Strikingly, CD16+ liver NK cells demonstrated markedly less killing (Fig. 2C–D). The low number of CD16− NK cells within our limited number of paired blood and liver samples that were freshly isolated did not allow for further analysis. However, in agreement with previous reports (27), CD16− blood NK cells were less cytolytic than CD16+ blood NK cells (not shown) and in 2 patients where adequate numbers could be isolated to compare sorted liver and blood CD16− NK cells, lysis of class I negative targets was similar (liver:30.9±0.6% vs. blood:32.9±1.3% at E:T 15:1 for K562; and liver:12.8±1.6% vs. blood:10.6±0.2% at E:T 15:1 for LCL721.221). Therefore, human liver is enriched in NK cells with decreased cytotoxic function because of both a higher percentage of CD16− NK cells and overall decreased cytolytic activity of liver CD16+ NK cells.
Figure 2. Human liver NK cells have weaker cytolytic function than blood NK cells.
Bulk NK cells were sorted from matched blood and liver mononuclear cell preparations and tested for their ability to lyse chromium labeled K562 (A, 5 patients) and 721.221 target cells (B, 3 patients). Similarly, CD16+ NK cells were sorted from matched blood and liver mononuclear cell preparations and their lytic ability was tested in chromium release assays using K562 (C, 5 patients) and 721.221 (D, 4 patients) as targets. One representative patient is shown in the left panel and in the right panel, data from blood and liver samples of individual patients are summarized by percent specific lysis at the highest effector to target ratio.
CD16+ liver NK cells express activating receptors and contain intracellular perforin and granzyme B
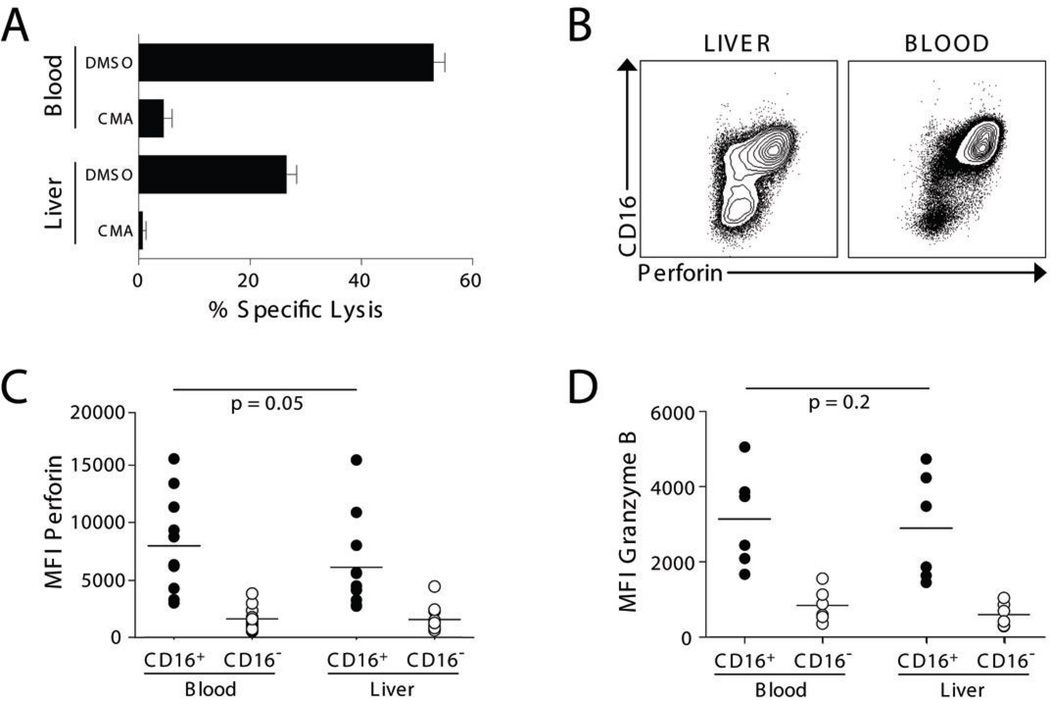
We next sought to determine the reason for decreased killing by CD16+ liver NK cells. It has become clear that activating receptors contribute substantially to NK cell specificity and may provide the critical threshold of signals needed to override the counterbalancing effects of inhibitory receptors in order to mount a productive response (28). We did not find a difference in the expression of activating receptors between blood and liver CD16+ NK cells that could account for their disparate cytotoxicity (Supplementary Table 2). Using concanamycin-A to inhibit the granule exocytosis pathway (29), we found that both liver and blood CD16+ NK cells killed K562 targets predominantly through granule release (Fig. 3A). Both liver and blood CD16+ NK cells contained intracellular perforin and granzyme B, although a trend towards decreased perforin content was observed in liver NK cells (Fig. 3B–D). Therefore, CD16+ liver NK cells are armed for killing but have overall decreased cytotoxic activity.
Figure 3. CD16+ liver NK cells contain cytolytic granules and express perforin and granzymes.
(A) Sort-purified blood and liver CD16+ NK cells were assayed for their ability to lyse K562 target cells in the presence or absence of CMA, an inhibitor of perforin release, or a dimethyl sulfoxide (DMSO) vehicle control. One of 3 representative experiments with similar results is shown. (B) Representative contour plots of perforin expression in liver and blood NK cells. (C–D) The mean fluorescence index (MFI) of intracellular perforin (11 patients) and granzyme B (6 patients) in blood and liver CD16+ NK cells and CD16− NK cells is shown.
A lower proportion of liver NK cells possesses inhibitory KIR receptors for self-MHC-I
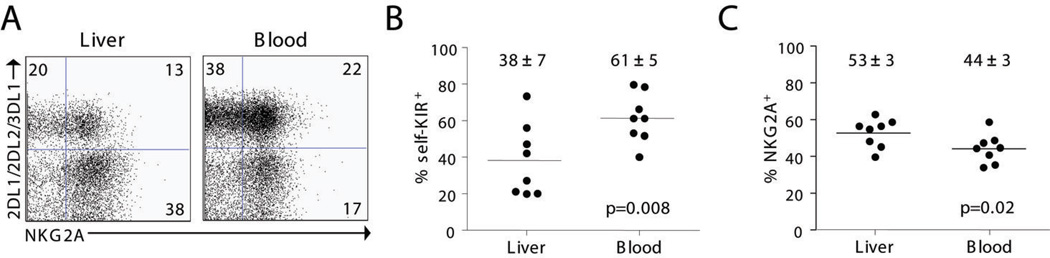
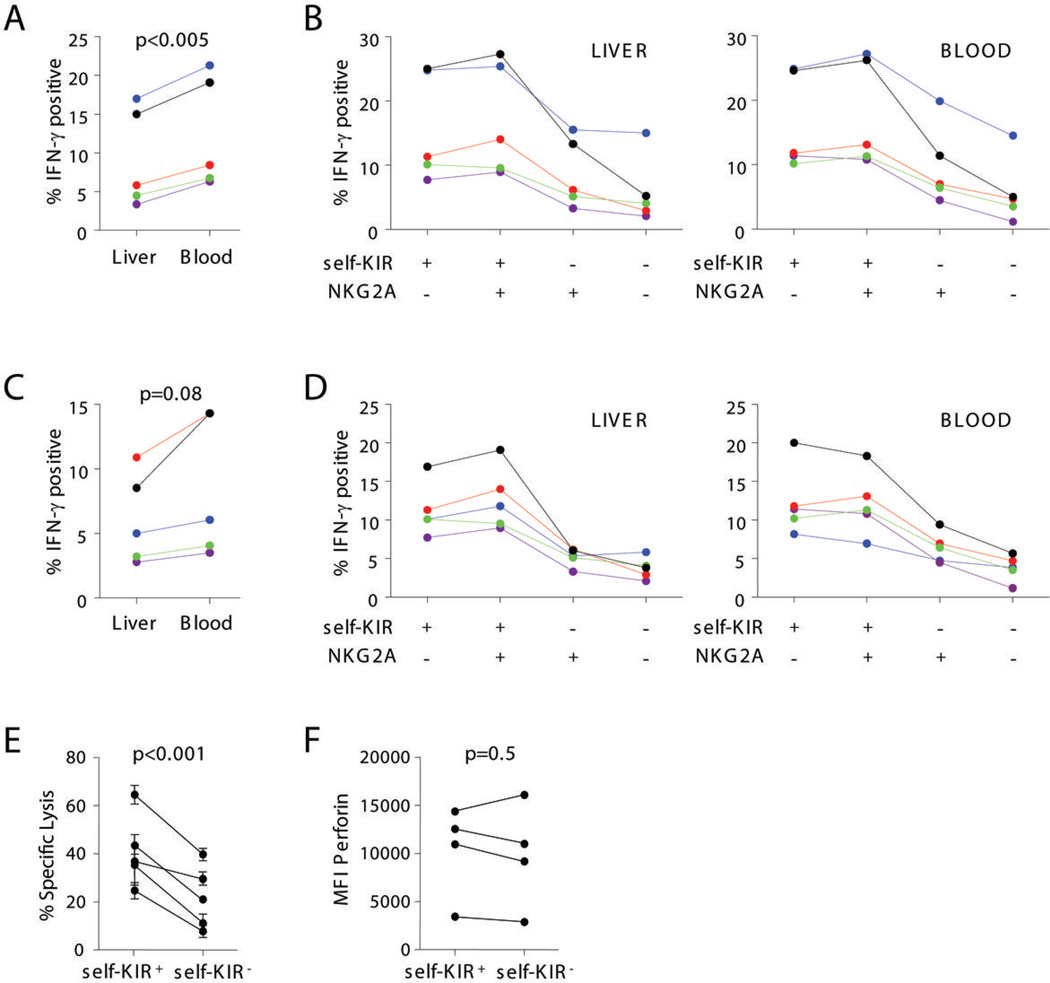
NK cells that possess inhibitory receptors with specificity for self-MHC class I antigens are endowed with functional competence (i.e. licensed) and are highly responsive to tumor targets (17–23). Therefore, we investigated if the expression of these inhibitory receptors was different on liver and blood NK cells. HLA-KIR ligand groups C1, C2, and Bw4 were assigned preoperatively to each patient by HLA genotyping and KIRs specific to self-MHC-I were identified (self-KIR). NK cell expression of self-KIR and NKG2A receptors was determined for 8 patients (Fig. 4 and Table I).
Figure 4. Liver NK cells express less inhibitory receptors for self-MHC-I than blood NK cells.
Inhibitory KIRs with affinity for self-MHC-I (self-KIR) were determined preoperatively as described in the Materials and Methods. A) Dotplots from one representative patient show the distribution of self-KIR and NKG2A on CD16+ liver and blood NK cells. In this C1/C2 Bw4/Bw6 patient self-KIR expressing NK cells are represented by KIR2DL1+, KIR2DL2+, or KIR3DL1+ NK cells. The percent self-KIR+ (B) or NKG2A+ CD56dimCD16+ NK cells (C) is shown for 8 patients.
Table 1.
Inhibitory Receptor Expression on Liver NK cells
| self-KIR+NKG2A− | self-KIR+NKG2A+ | self-KIR−NKG2A+ | self-KIR−NKG2A− | |
|---|---|---|---|---|
| Bulk NK cells | ||||
| Liver | 10.4 ± 2.1 | 9.2 ± 1.9 | 52.3 ± 2.4 | 30.8 ± 4.0 |
| Blood | 29.4 ± 5.7 | 20.2 ± 2.6 | 26.3 ± 3.3 | 20.2 ± 2.4 |
| p value | 0.009 | 0.002 | <0.0001 | 0.016 |
| CD16− NK cells | ||||
| Liver | 2.9 ± 0.6 | 3.6 ± 0.6 | 63.6 ± 3.2 | 29.7 ± 3.1 |
| Blood | 3.7 ± 1.1 | 4.9 ± 1.3 | 64.1 ± 5.0 | 24.5 ± 2.8 |
| p value | 0.28 | 0.14 | 0.87 | 0.09 |
| CD16+ NK cells | ||||
| Liver | 22.8 ± 4.2 | 15.5 ± 3.0 | 37.2 ± 4.8 | 23.8 ± 3.1 |
| Blood | 39.7 ± 4.4 | 21.7 ± 2.8 | 22.5 ± 3.0 | 15.8 ± 2.2 |
| p value | 0.002 | 0.02 | 0.001 | 0.007 |
Matched liver and blood samples are shown for 8 individual patients.
The phenotype distribution of CD16+ NK cells was markedly different between liver and blood. CD16+ NK cells in the liver contained a statistically significant decreased percentage of both self-KIRposNKG2Aneg and self-KIRposNKG2Apos NK cells, and a higher percentage of self-KIRnegNKG2Apos and self-KIRnegNKG2Aneg NK cells (Table I and Fig. 4 B and C). In contrast, the KIR and NKG2A phenotype of CD16− NK cells in blood and liver were similar (Table I). These data demonstrate that the liver when compared with blood contains a decreased number of licensed NK cells that express inhibitory receptors for self-MHC class I. These results would suggest that this difference in NK cell phenotype should be associated with decreased effector function against MHC class I deficient target cells.
The relative distribution of NK cell subpopulations in the liver and blood accounts for the differences in effector function in CD16+ NK cells
We investigated how the inhibitory receptor subset phenotype of liver CD16+ NK cells contributed to their functional capacity. We observed that liver CD16+ NK cells had modestly decreased intracellular IFN-γ responses to K562 target cells (Fig. 5A–B) and CD16 crosslinking than their matched blood CD16+ NK cells (Fig. 5C–D). We next compared the four subsets of NK cells defined by self-KIR and NKG2A and their IFN-γ production following stimulation with K562 (Fig. 5B) and CD16 cross-linking (Fig. 5D). There were no significant differences between the responses for each subset of NK cells in blood and liver. For example, the two self-KIRpossubsets (i.e. self-KIRposNKG2Aneg and self-KIRposNKG2Apos) had higher responses than the two self-KIRneg subsets in both blood and liver and displayed a similar hierarchy of responsiveness (Fig. 5B and D). Cytolytic effector function of the self-KIR expressing liver CD16+ NK cells was compared with liver CD16+ NK cells that did not possess self-KIR using standard chromium-release assays. Consistent with the NK cell licensing hypothesis, self-KIRpos liver NK cells had higher cytolytic activity than self-KIRneg cells (Fig. 5E). We have not found the degree of CD107a membrane expression on liver NK cells to correlate reliably with classical chromium-based lysis assays (data not shown). Similar to a previously published comparison between licensed and unlicensed blood NK cells (19), we did not find a difference in perforin expression between self-KIRpos and self-KIRneg subsets of liver CD16+ NK cells that could account for the observed difference in killing (Fig. 5F). Taken together, the decreased frequency of self-KIR expressing CD16+ NK cells in the liver compared with the blood accounts for the overall decreased cytolytic potential of liver CD16+ NK cells.
Figure 5. Self-KIR and NKG2A endow similar function to blood and liver CD16+ NK cells.
Matched liver and blood mononuclear cells were cultured with K562 cells at an NK:K562 ratio of 1:1 (A–B) or on anti-CD16 coated plates (C–D) in the presence of Brefeldin-A. The percentage of IFN-γ+ CD16+ NK cells is shown for 5 individual patients in A and C. In each blood and liver, the percentage of self-KIR and/or NKG2A expressing CD16+ NK cells that are IFN-γ+ is shown for 5 patients in B and D. Each color-coded set of points represents a single patient. (E) Liver CD16+ NK cells from 5 individual patients were sorted into self-KIR+ and self-KIR− subsets and tested for their ability to lyse K562 targets. (F) Intracellular perforin expression in self-KIR+ and self-KIR− CD16+ liver NK cells was compared in 4 patients.
The cytolytic restriction on liver CD16+ NK cells is removed by IL-2 or an activated liver APC
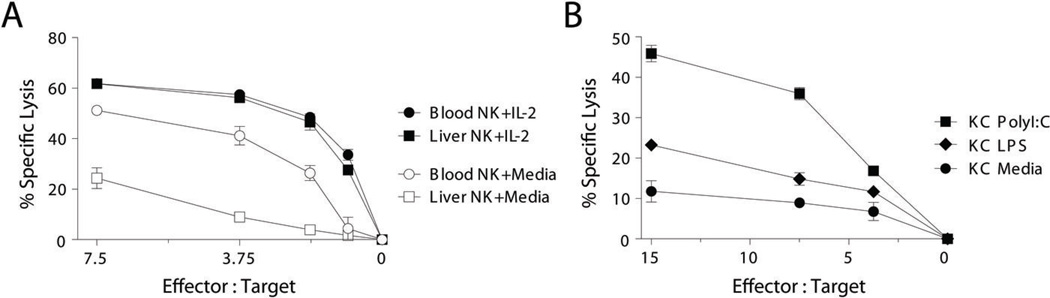
IL-2 potentiates the cytotoxic capability of NK cells by upregulating cytolytic machinery and increasing intracellular perforin (30). Moreover, human blood NK cells gain an equal level of cytotoxicity when cultured in IL-15 regardless or the expression of inhibitory receptors for MHC class I (20). To determine whether liver CD16+ NK cells were anergic or whether their cytolytic restriction could be overcome, they were cultured overnight in IL-2. NK cells became robust cytolytic effectors after activation with IL-2 with comparable potency to IL-2 cultured blood CD16+ NK cells (Fig. 6A). Similar results were obtained using IL-15 (not shown). Although a predominance of NK cells with weak resting cytolytic function may be advantageous to prevent hyperactivity in the potentially volatile, endotoxin-rich environment of the liver, their cytolytic function could be beneficial during an infectious or metastatic insult. Therefore, we asked whether an activated resident liver immune cell could provide the necessary signals to boost their killing potential. When freshly isolated liver CD16+ NK cells were cultured overnight with autologous Kupffer cells activated either by LPS or poly I:C, they became more potent killers of K562 targets (Fig. 6B). Hence, although the liver contains an abundance of CD16+ NK cells with cytolytic potential that is limited to some degree by their inhibitory receptor expression, they are capable of mounting effective lytic responses in the appropriate inflammatory settings.
Figure 6. Liver CD16+ NK cell cytolytic function is increased by IL-2 or activated Kupffer cells.
CD16+ NK cells were purified from the blood or liver of the same patient and cultured overnight in either media or IL-2 (200 IU/ml). The following day the lytic capacity of the cultured NK cells was assayed using chromium-labeled K562 targets. This data is representative of experiments from 4 individual patients. (B) CD16+ liver NK cells were cultured overnight with autologous sort-purified KCs that were first pulsed with PolyI:C, LPS, or media. The following day their lytic ability was tested using chromium-labeled K562 targets. This data are representative of experiments from 3 individual patients.
DISCUSSION
The threshold for activation of innate immune responses varies among different organs. These activation profiles are controlled by different organ-specific regulatory mechanisms that are required to sustain immune homeostasis yet can result in immunopathology if disrupted (31). For example, the teeming microbial mass in the colon does not induce overt inflammation in the intestinal mucosa, suggesting the existence of specialized regulatory mechanisms that curtail initiation of inflammatory and bactericidal programs against the luminal microflora. Recent studies have suggested that intestinal epithelial cells may have evolved polarized signaling mechanisms to maintain colonic homeostasis and regulate tolerance and immunity. While basolateral TLR9 stimulation mobilizes an inflammatory immune cascade, apical (luminal) TLR9 stimulation results in tolerogenic immune responses (32). Additionally, human intestinal macrophages do not express innate response receptors for LPS (CD14) and do not produce pro-inflammatory cytokines in response to a range of inflammatory stimuli (33). Similarly, human liver DCs promote CD4 T cell hyporesponsiveness and favor the generation of regulatory T cells (34) and human Kupffer cells respond to LPS paradoxically by secreting the anti-inflammatory cytokine IL-10 (35). In the lung, administration of TLR ligands induces high levels of the negative immunoregulator indolamine 2,3-dioxygenase (IDO) that can decrease Th2-driven experimental asthma (36).
The immune environment of the liver is unique because it is continually exposed to commensal-derived endotoxin that is present in the portal circulation and therefore lends itself to constitutive TLR4 ligation in the absence of an infectious threat. LPS is a powerful activator of both innate and adaptive immunity through the triggering of inflammatory cascades, secretion of pro-inflammatory cytokines, and maturation of specialized antigen-presenting cells. NK cells are present in abundant numbers in the liver and are responsible for hepatocyte death and liver inflammation during HBV infection (37). Furthermore, hepatocytes make up 60 to 80 percent of the cellular mass of the liver and the expression of MHC-I on human hepatocytes has been well documented to be low and even absent in vivo (38–40). This potentially MHC-I deficient environment lends itself to relief of the tonic inhibition on NK cells, however, NK cells do not appear to be hyperactive in the potentially hostile immune environment of the liver. Therefore, in order to prevent liver injury and compromise liver function, it is crucial that autoaggressive activation of NK cells be regulated.
It has been previously recognized that human liver NK cells are enriched in CD16− NK cells (41) and that this subset of NK cells, through cross-talk with activated resident liver macrophages, will produce IFN-γ (42). We have determined that the subset composition of NK cells in the human liver is variable but on average is represented by equal percentages of CD16+ and CD16− NK cells. Furthermore, we and others have found that NK cells in the human liver display an activated phenotype (43) and we show here that this phenotype is accounted for predominantly by the CD16− subset of liver NK cells. Similar to CD16− lymph node NK cells, this subset of human liver NK cells express high levels of HLA-DR, NKp44, CD69, and low levels of CD62L (8,44). Despite their activated phenotype, CD16− NK cells had poor cytotoxic capacity like their respective blood NK cell equivalents. However, CD16+ NK cells that are regarded as the cytotoxic subset of NK cells in the liver were also poorly cytotoxic, suggesting one potential regulatory means by which liver NK cell autoreactivity could be quelled.
The isolation of immune cells from solid organs is more difficult than isolating circulating lymphocytes and requires processing of the tissues. Using propidium iodide and annexin V staining, we initially established that the method of isolation did not influence the viability or killing ability of the cells (not shown). Furthermore, by subjecting whole blood to enzyme treatment and a mock isolation procedure identical to that used to isolate liver NK cells, we clarified that KIR and NKG2A expression were unaffected by the isolation process and importantly, did not find any difference in lysis of K562 cells (not shown).
To avoid inadvertent destruction of normal tissues, the potent effector functions of NK cells are controlled through their interactions with ubiquitously expressed MHC-I molecules. KIRs and NKG2A are inhibitory NK cell receptors that recognize MHC-I and play a major role in mediating the balance between self-tolerance and appropriately targeted effector responses. For example, these inhibitory NK cell receptors fulfill the missing-self hypothesis by inhibiting NK cell lysis and cytokine production when they are engaged by their cognate ligands on normal tissues. Conversely, NK cell effector functions are released when these ligands are missing from an infected or malignant target cell. It is now evident that these same inhibitory receptors are required for an NK cell to become functionally competent effectors. In other words, it has been shown that to be strongly reactive to missing-self targets, an NK cell is required to have at least one inhibitory receptor that can interact with a self-MHC-I ligand (17–23). This model for NK cell licensing provides an explanation for the decreased lytic responses of liver CD16+ NK cells based upon their repertoire of self-KIR deficient NK cells.
The regulation of NK cell responses by inhibitory receptor phenotypes applies to the resting state and the requirement for self-MHC recognition in establishing functional tolerance can be overcome by cytokine stimulation (17,20,23). Along these lines, we have found that CD16+ liver NK cells like blood CD16+ NK cells would become potent effectors by short culture in IL-2 or IL-15. Similarly, others have found that 4 days of culture in IL-2 will activate bulk human liver NK cells to become more potent cytolytic killers (45). Like human blood NK cells which can be activated by DCs (46–48) and macrophages (49), liver CD16− NK cells can be activated by autologous liver KCs (42). We have similarly found that the lytic capacity of liver CD16+ NK cells can be enhanced by activated liver DCs (not shown) or KCs. Taken together, this suggests that the lytic capacity of liver CD16+ NK cells is regulated by their inhibitory receptor phenotype in the resting state, but in an inflammatory state, they can become potent effectors through cross-talk with liver APCs. Whether the tumor-bearing liver has impact upon these results is difficult to determine as most hepatectomy specimens that we can obtain are from cancer patients. For example, it has been suggested that compared with normal donors, patients with hepatic malignancy have significantly reduced proportions of NK and NKT cells expressing KIR receptors and CD94 (50). However this study did not compare the proportions of CD16− NK cells and CD16+ NK cells in the diseased and normal livers as this ratio could potentially explain a difference in overall KIR and NKG2A expression. Furthermore, we have not found differences in CD94 expression between liver and blood NK cell subsets (Supplemental Table 2 and not shown).
In conclusion, the liver contains a repertoire of NK cells that are less cytolytic than circulating NK cells. One of the predominant contributing factors is that the subset of CD16+ NK cells expresses less inhibitory KIR molecules with specificity for self-MHC-I ligands and is less cytotoxic. We hypothesize that through some process of selection, the liver gains a repertoire of tolerant non-licensed CD16+ NK cells that may be beneficial in preventing liver injury and autoimmunity in its potentially hostile local immune environment.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Hebroon Obaid for technical support and Jan Hendrikx, Mark Kweens, and Patrick Anderson of the MSKCC Flow Cytometry Core Facility for assistance with cell sorting.
Footnotes
This work was supported by NIH grants DK068346 and AI70658 (RPD), CA23766 (ZZ and BD) and CA123938 (BMB), the William H Goodwin and Alice Godman Fund and Commonwealth Cancer Foundation for Research/Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center, and the Swim Across America Foundation.
REFERENCES
- 1.Opelz G, Wujciak T, Dohler B, Scherer S, Mytilineos J. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Rev Immunogenet. 1999;1:334–342. [PubMed] [Google Scholar]
- 2.Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, McMichael J, Fung JJ, Starzl TE. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243–249. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 4.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 5.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 7.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 8.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 9.Bajenoff M, Breart B, Huang AY, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immuno.l. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 11.Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol. 2006;36:2394–2400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 12.Strowig T, Brilot F, Arrey F, Bougras G, Thomas D, Muller WA, Munz C. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma. PLoS Pathog. 2008;4:e27. doi: 10.1371/journal.ppat.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci U S A. 2005;102:15563–15568. doi: 10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, Gruda R, Hurwitz A, Bdolah Y, Haimov-Kochman R, Yagel S, Mandelboim O. Endometrial NK cells are special immature cells that await pregnancy. J Immunol. 2008;181:1869–1876. doi: 10.4049/jimmunol.181.3.1869. [DOI] [PubMed] [Google Scholar]
- 15.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 16.Ljunggren HG, Kärre K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Cooley S, Xiao F, Pitt M, Gleason M, McCullar V, Bergemann TL, McQueen KL, Guethlein LA, Parham P, Miller JS. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Sunwoo JB, Yang L, Choi T, Song YJ, French AR, Vlahiotis A, Piccirillo JF, Cella M, Colonna M, Mohanakumar T, Hsu KC, Dupont B, Yokoyama WM. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsu KC, Dupont B. Natural killer cell receptors: regulating innate immune responses to hematologic malignancy. Semin Hematol. 2005;42:91–103. doi: 10.1053/j.seminhematol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, O'Reilly RJ, Horowitz MM, Dupont B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol. 2007;179:854–868. doi: 10.4049/jimmunol.179.2.854. [DOI] [PubMed] [Google Scholar]
- 27.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–3191. [PubMed] [Google Scholar]
- 28.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 30.Zhang J, Scordi I, Smyth MJ, Lichtenheld MG. Interleukin 2 receptor signaling regulates the perforin gene through signal transducer and activator of transcription (Stat)5 activation of two enhancers. J Exp Med. 1999;190:1297–1308. doi: 10.1084/jem.190.9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raz E. Organ-specific regulation of innate immunity. Nat Immunol. 2007;8:3–4. doi: 10.1038/ni0107-3. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 33.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, Gonen M, Young JW, DeMatteo RP. J Immunol. 2009;182:1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, Humphreys E, Afford S, Adams DH, Bertoletti A, Maini MK. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984;38:287. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Lau JY, Bird GL, Naoumov NV, Williams R. Hepatic HLA antigen display in chronic hepatitis B virus infection. Relation to hepatic expression of HBV genome/gene products and liver histology. Dig Dis Sci. 1993;38:888–895. doi: 10.1007/BF01295916. [DOI] [PubMed] [Google Scholar]
- 40.So SK, Snover DC, Ascher NL, Platt JL. Class I major histocompatibility complex antigens are induced on hepatocytes in rejecting human liver grafts. Transplant Proc. 1987;19:2464–2465. [PubMed] [Google Scholar]
- 41.Hata K, Zhang XR, Iwatsuki S, Van Thiel DH, Herberman RB, Whiteside TL. Isolation, phenotyping, and functional analysis of lymphocytes from human liver. Clin Immunol. Immunopathol. 1990;56:401–419. doi: 10.1016/0090-1229(90)90160-r. [DOI] [PubMed] [Google Scholar]
- 42.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu Z, Bozorgzadeh A, Crispe IN, Orloff MS. The activation state of human intrahepatic lymphocytes. Clin Exp Immunol. 2007;149:186–193. doi: 10.1111/j.1365-2249.2007.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizrahi S, Yefenof E, Gross M, Attal P, Ben Yaakov A, Goldman-Wohl D, Maly B, Stern N, Katz G, Gazit R, Sionov RV, Mandelboim O, Chaushu S. A phenotypic and functional characterization of NK cells in adenoids. J Leukoc Biol. 2007;82:1095–1105. doi: 10.1189/jlb.0407205. [DOI] [PubMed] [Google Scholar]
- 45.Ishiyama K, Ohdan H, Ohira M, Mitsuta H, Arihiro K, Asahara T. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 2006;43:362–372. doi: 10.1002/hep.21035. [DOI] [PubMed] [Google Scholar]
- 46.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nedvetzki S, Sowinski S, Eagle RA, Harris J, Vely F, Pende D, Trowsdale J, Vivier E, Gordon S, Davis DM. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 50.Norris S, Doherty DG, Curry M, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. Selective reduction of natural killer cells and T cells expressing inhibitory receptors for MHC class I in the livers of patients with hepatic malignancy. Cancer Immunol Immunother. 2003;52:53–58. doi: 10.1007/s00262-002-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.