Abstract
Aortic valve stenosis (AS) is the commonest form of valvular heart disease in the Western world. Its prevalence increases exponentially with age and it is present in 2-7% of all patients over 65 years of age. In view of the considerable cardiovascular morbidity and mortality associated not only with AS, but even its earlier stage, aortic sclerosis, many investigations have been directed towards better understanding of its pathogenesis, with the ultimate objective of developing strategies to retard its progression. Although risk factors and downstream mediators appear similar for AS and atherosclerosis (older age, male sex, hypertension, smoking, hypercholesterolemia, and diabetes, as many as 50% of patients with AS do not have clinically significant atherosclerosis. On the basis both of recent experimental evidence and clinical trials, it appears that atherogenesis is not pivotal to the pathogenesis of AS. On the other hand, there is increasing evidence of active involvement of aortic valve fibroblasts with resultant increased production of reactive oxygen species, active pro-inflammatory and pro-fibrotic processes culminating in calcification. We also discuss the evidence of involvement of the nitric oxide system in the pathogenesis of AS. The renin-angiotensin system has also emerged as a major player in the pathogenesis of AS. Histologically, there is increased ACE expression and elevated angiotensin II levels in stenotic valves, while we have just demonstrated amelioration of AS with the use of ACE inhibitors in an animal model. We further discuss intervention studies aimed at retarding AS progression, including recent failures of statins to retard progression of AS in large randomized clinical studies. Finally, we discuss the special case of bicuspid aortic valve, including its genetics and unique associated features.
Keywords: Aortic valve stenosis, bicuspid aortic valve, nitric oxide, oxidative stress, inflammation, fibrosis, calcification, intervention studies
Introduction
Aortic stenosis (AS) may be defined as narrowing of the aortic valve, due primarily to a combination of progressive fibrosis and calcification of the matrix, with consequent increase in valve stiffness, progressive reductions in valve area and concomitant increases in left ventricular afterload and work. The earliest stages of AS have been designated aortic valve sclerosis (ASc), implying disordered valve morphology (including potential calcification as well as fibrosis) in the absence of marked obstruction to left ventricular outflow.
AS is currently the most common form of valvular heart disease in the Western world [1], in large part because the most frequently occurring form of AS develops predominantly in individuals of advancing age. For example, in the Helsinki Ageing Study [2], the proportion of individuals with detectable valve calcification increased from approximately 40% to 75% between ages of 65 and 85 years, while approximately 3% of subjects over 75 years of age had severe AS.
Given that the only proven therapy for severe AS is aortic valve replacement, and that there is currently no definitive evidence that any treatment can retard progression of the disease, the development of severe symptomatic AS in elderly individuals presents an increasing medical and health economic dilemma. On the one hand, severe AS rarely remains asymptomatic for any great length of time: patients classically develop variable components of exertional dyspnoea, angina (irrespective of the presence or absence of epicardial coronary artery disease) and atrial or ventricular arrhythmias, resulting in poor quality of life, increased rates of hospitalization and increased mortality rates [3]. Although the precise natural history of severe AS in the elderly is uncertain, a number of studies suggested that mortality is high in the somewhat selected subgroup of individuals who do not undergo aortic valve replacement [4-7]. Additionally, both ASc and AS may be associated with cardiovascular problems which are apparently remote from the valve itself. For example, there is considerable evidence that ASc is an independent marker of increased risk of cardiovascular events [8, 9], while severe AS is associated with increased risk of haemorrhage, especially into the gastrointestinal tract, primarily because of the development of acquired von Willebrand's factor deficiency [10, 11].
It should also be stated at the outset that AS is a substantially heterogeneous disease, but with only two “common causes”. These are AS developing in a previously normal trileaflet valve, and AS associated with congenitally bicuspid aortic valves (BAV). These and other, rarer, causes of AS are summarized in Table 1.
Table 1.
Causes of AS. Wide frequency range generally reflects the age group(s) assessed by individual studies as well as population subgroups studied [79, 115-117].
| Etiology | Approximate frequency (%) | Associated features |
|---|---|---|
| Aging/calcific | 50 - 70 | Increased risk of coronary events. |
| Bicuspid aortic valve | 6 - 40 | Dilatation or dissection of the aorta, involving the aortic root, ascending aorta, or aortic arch. |
| Rheumatic | 2 - 11 | Mitral valve almost always affected as well |
| Unicuspid aortic valve | <1 | Dilatation or dissection of the aorta, involving the aortic root, ascending aorta, or aortic arch. |
| Post-endocarditis | <1 | Extra-cardiac embolic phenomena. |
Given that AS frequently develops in otherwise normal valves in aged individuals, this has been regarded as degenerative AS, implying a relationship to the normal process of “wear and tear” within the valve [12]. The purpose of this review is to demonstrate that AS is not primarily a “degenerative” process, but rather the result of a progressive inflammatory process within the valve matrix. Furthermore, we will present evidence that the development and progression of AS, rather than occurring with the implied inevitability of a purely degenerative disease, should theoretically be amenable to pharmaco-therapy.
Aortic valve anatomy and physiology: what are the homeostatic determinants?
The normal aortic valve consists of several layers of fibroblast-rich tissue, containing both collagen and elastin fibres, covered by a monolayer of endothelial cells [13]. There are also minute intravalvular blood vessels [14], consistent with a substantial oxygen demand by the other matrix cells.
The normal aortic valve interstitial cells are probably mainly fibroblasts, but some smooth muscle cells have been identified [15], raising the issue of potential variability in tone of the valve, an area which has been pursued in a number of experimental studies. For example, Pompilio et al [16] showed that intact porcine aortic valve contracted in response to phenylephrine, and also exhibited (endothelium-dependent) relaxation with acetylcholine. Furthermore, normal aortic valve interstitial cells (presumably including smooth muscle cells) have been shown to contract in response to 5-hydroxytryptamine, endothelin-1 and norepinephrine [17]. An important, but incompletely resolved issue relates to the prevalence and function of myofibroblasts, which exhibit some contractile properties, within normal aortic valves. The role of the myofibroblasts will be discussed more extensively in the context of valve pathology.
The physiological role of the aortic valve endothelium has attracted considerable attention over the past 15 years, although its role is still incompletely understood. A number of studies suggest that valve endothelium behaves in a qualitatively similar manner to vascular endothelium, for example releasing nitric oxide (NO) in response to stimuli such as acetylcholine [16], or 5-hydroxytryptamine [18].
A critical but unresolved issue is what is the physiological response of the valve endothelium to shear stress, a normal stimulus for NO release, but also potentially for activation of endothelial NAD(P)H oxidase [19, 20], which in turn would lead to release of superoxide anion (O2-) and “scavenging” of NO. Despite ongoing uncertainties, the differences between normal aortic valve and vascular endothelial cells regarding responses to shear stress were reviewed by Butcher et al [21], who demonstrated that the changes in gene expression in response to shear stress varied substantially.
Finally, little work has been done regarding other endothelial autocoids within the valve. Notably, little is known about the physiology of prostacyclin release from the valve, although it has been detected [22]. No studies have properly addressed the issue of tissue penetration of autocoids released from valvular endothelium, other than the obvious implications of studies measuring valve contraction. It is therefore uncertain to what extent endothelial NO and prostacyclin might modulate the function of valve interstitial cells, a physiologically important issue given evidence that in AS valve endothelium is dysfunctional or absent, as discussed below.
Cellular histopathology of AS
Features of stenotic aortic valve lesions
Histopathologic studies have demonstrated that development and progression of calcific AS are based on an active process that shares some similarities with atherosclerosis. It has been suggested that aortic valve lesions begin with disruption of valve endothelium predominantly on the aortic side due to high shear stress [23-25].
Inflammation and lipid deposition
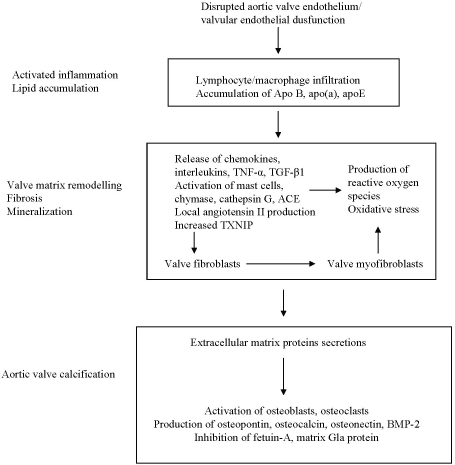
Aortic valve lesions typically present with areas of subendothelial thickening, which represents the early stage of aortic stenosis. Increased thickening of aortic valve leaflets is characterized by accumulation of inflammatory infiltrates of predominantly macrophages and T-lymphocytes, lipids, oxidized lipids, (summarized in Figure 1) [25-28] all of which potentially activate a host of pro-fibrotic and pro-inflammatory markers. Macrophages and T-lymphocytes have been detected and tend to be located near the surface of the lesion [25-28]. Immunohistochemical studies have found co-localization of apolipoproteins (apo) B, apo (a), apoE with lipid laden foam cells and macrophages [29] as well as oxidative modification of residential low density lipoproteins (LDLs) in early stenotic aortic valve lesions [30].
Figure 1.
Schema of postulated mechanisms underlying aortic valve lesion formation. Inflammatory infiltrations of T-lymphocytes and macrophages, along with lipid accumulation, is primarily responsible for early thickening of aortic valves. Interactions between chemical stimuli and disruption of valvular homeostasis: pro- and anti- fibrotic mechanisms. Later stages of aortic stenosis: - cytokine release and angiotensin II promote extracellular matrix proteins secretion at early stages of mineralization which in turns begin the processes of bone formation. This process occurs largely at the end stage of aortic stenosis where aortic valves mobility is significantly reduced due to a build up of bone-like calcific nodules.
Valvular matrix remodelling and fibrosis
The presence of macrophages and T-lymphocytes, along with oxidized LDL and apoli-poprotein accumulation activate several pro-fibrotic and pro-inflammatory cytokines which may modulate aortic valve remodelling and subsequent calcification. Transforming growth factor β1 (TGF-β1) [31] and interleukin-1β [32] have been found in valve matrix and are associated with increased local production of matrix metalloproteinases I and II (MMP-1 and MMP-2). All of these contribute to cell apoptosis, extracellular matrix formation, remodelling and consequently predispose to calcification. In addition to TGF-β1 and interleukin-1β, tumour necrosis factor-α (TNFα), another pro-inflammatory cytokine commonly responsible for immune regulation, inflammation and tissue remodelling, is co-localized with MMP-1 [33]. Furthermore, tenascin C, an extracellular matrix glyco-protein implicated in cell proliferation, migration, differentiation and apoptosis, which is involved in stimulation of bone formation and mineralization, is co-localized with MMP-2 in calcified aortic valve leaflets [34], and is associated with progression of AS [35].
Angiotensin II (Ang II), an important mediator of inflammation and fibrosis, could be formed by angiotensin converting enzyme (ACE) as well as the mast cell (MC)-derived neutral protease, chymase [36]. ACE has been identified in stenotic but not in normal aortic valves [37]. It has been shown that MC-derived chymase is also upregulated in stenotic valves, providing further evidence for local production of Ang II [38]. In addition, cathepsin G, another neutral protease also capable of generating Ang II, is present in increased concentrations throughout human stenotic aortic valves compared to normal valves [39]. These findings provide a potential basis for a role of angiotensin II in aortic valve remodelling along with other pro-fibrotic and pro-inflammatory mechanisms.
As summarized in Figure 1, current concepts of the pathogenesis of AS centre histologically on inflammation and lipid deposition, and biochemically on activation of cytokines and matrix metalloproteinases, together with generation of angiotensin II. These processes are postulated to induce injury of all valve components, leading to fibrosis and calcification.
Calcification
Calcification of aortic valve leaflets tends to occur more predominantly in the later stages of AS, and is located deeper in the lesion [25]. Active calcification is a major factor in reducing valvular mobility in severe AS. Early lesions of aortic valves show fine stippled mineralization, progressing to active bone formation resulting in gross calcification at later stages of the disease (Figure 1).
The process of calcification (and sometimes ossification) of aortic valve leaflets resembles that associated with atheroma formation. The presence of inflammation, fatty streak formation from lipid depositions, cytokine release, metalloproteinases, ACE, Ang II, all possibly contribute to the production of an extracellular matrix, and matrix vesicles that initiate mineralization.
Co-localization of macrophages and oxidized LDLs with osteopontin, a protein needed in bone formation, has been found and thought to be involved in extracellular matrix production in human stenotic aortic valves [40, 41]. Furthermore, Mohler et al [42] described an active process of calcification using immunohistochemistry in an extensive study of 347 human stenotic aortic valves. In addition to active osteoblasts and osteoclasts, this and other investigators [43] detected bone morphogenic proteins 2- and 4- (BMP-2, BMP-4). BMPs stimulate osteoblastic differentiation with subsequent calcification. In agreement with previous studies [25, 41], Mohler et al [42] also found that macrophages and lymphocytes accumulate in areas of calcification.
There is also evidence of angiogenesis, which is essential for longitudinal bone growth in stenotic valves. T-lymphocytes aggregates tend to co-localize with sites of neoangiogenesis within ossified valves [42]. This and the presence of heat shock protein 60 (hsp60) [44], commonly expressed by cells under stress conditions, together indicate a highly active, and chronic immunomediated process from stress to inflammation to calcification.
It has also been shown that the calcification process of aortic valves may also be regulated by receptor activator of nuclear factor κB, its ligand (RANK, and RANKL), and the soluble receptor osteoprotegerin (OPG) [45]. The study detected increased concentrations of RANKL in calcified compared to control valves. Furthermore, there was a significant reduction of OPG positive cells in aortic stenotic valves compared to controls. It has been shown that in mice deficient for OPG, vascular calcification was associated with increased expression of RANKL [46]. Additionally, in Kaden et al [45] shown that long -term cell culture of stenotic aortic valves in the presence of RANKL induced significant increase in matrix calcium deposition and the formation of cell nodules compared to controls. Thus, the RANKL/OPG pathway may also be involved in the calcific process of aortic valve stenosis.
A recent study [47] also revealed an association between presence of aortic stenosis and low serum levels of the anti-calcific protein fetuin-A. Furthermore, there was evidence that fetuin-A was deposited in calcific aortic valves. Nevertheless, the relative importance of these findings to the overall pathogenesis of aortic stenosis remains uncertain.
Matrix Gla-protein (MGP), another anti-calcific protein implicated in development of AS, is synthesised in different tissues and undergoes vitamin K dependent γ-carboxylation (reviewed by [48]. The γ-carboxylated form of MGP is involved in inhibition of tissue calcification possibly by preventing differentiation of vascular smooth muscle cells into chondrocyte-like cells or by blocking BMP function [48]. Patients with aortic valve calcification were found to have lower concentrations of uncarboxylated MGP compared with normal controls [49]; additionally both renal dysfunction and oral warfarin therapy were predictive of low MGP levels. Interestingly, another study found elevated levels of both uncarboxylated and carboxylated de-phosporylated forms of MGP in patients with severe AS [50]. The exact biological significance of these findings is still unclear.
Oxidative stress
A number of studies have documented increased intravalvular content of a variety of pro-oxidants in models of AS and in clinical samples [23, 51-54]. Importantly, this evidence of increased oxidative stress was not specifically associated with extent of local atherogenesis in any study.
Thioredoxin, thioredoxin reductase, NADPH oxidase and thioredoxin binding protein (TXNIP) are components of a ubiquitous system, which regulates intracellular redox stress [55]. TXNIP in particular is a fundamental mediator of increased redox stress as it binds to, and inactivates thioredoxin [56]. Increased expression of TXNIP is associated with activation of apoptosis signalling pathways [57], and is suppressed by endothelial NO release [58]. We have recently demonstrated in a rabbit model of mild AS with histological features similar to that of human disease, increased intravalvular concentration of TXNIP compared to control animals [53]. Furthermore, co-treatment with ramipril retarded the development of AS in this model, as measured by reduction both in transvalvular velocity and valve echogenicity on echocardiography [59]. This retardation further correlated with reduction in valvular calcium and macrophage infiltration and a reduction in TXNIP accumulation within the valve matrix. This finding further underscores the importance of ACE - Ang II system in pathogenesis of AS.
Evidence of the role of nitric oxide in the pathogenesis of aortic stenosis
AS, even in its early phases is associated with the pathogenesis of acute coronary syndromes (ACS) [8, 60, 61], which are paralleled by platelet hyper-aggregability [62]. Stenotic aortic valves constitute a pro-aggregatory milieu, potentially contributing to thromboembolism [24, 63]. It has been shown that patients with AS exhibit increased platelet reactivity [64], and thrombus formation has been documented on severely stenosed valves [65]. AS has recently been linked to the phenomenon of nitric oxide (NO) resistance, even at its earliest stages [66, 67].
NO is a physiological modulator of both vasomotor tone and platelet aggregation. These effects of NO are predominantly mediated by cyclic guanosine-3,'5'-monophosphate (cGMP), via activation of soluble guanylate cyclase (sGC). However, in patients with ischemic heart disease, platelets and coronary/peripheral arteries respond poorly to the anti-aggregatory and vasodilator effects of NO donors (e.g. nitroglycerin) [68]. This “NO resistance” represents a multi-faceted disorder at sites of abnormal NO-driven physiology, and as such may contribute to the increased risk of ischemic events. NO resistance results from a combination of “scavenging” of NO by superoxide radical (O2−) and of inactivation of sGC. The haem moiety of sGC is a “receptor” for NO and a mediator of NO -dependent activation. However, reactive oxygen species, and O2-in particular, diminish sGC sensitivity to NO because of the oxidation of the enzyme-bound haem and its subsequent loss (haem-deficient sGC).
We have investigated [66] whether AS, either with or without concomitant coronary artery disease, is associated with impaired NO responsiveness. Two major abnormalities of platelet function were documented in patients with AS. First, platelets manifested increased aggregability in response to ADP. Second, the anti-aggregating effects of NO donor SNP were significantly reduced, thus representing NO resistance at the platelet level. However, presence of the former abnormality did not account for the occurrence of the latter.
The severity of NO resistance was independent of presence/absence of hemodynamically significant coronary artery disease.These results indicate therefore that AS represents an additional “marker” of platelet NO resistance. Interestingly, there was no correlation between AVS severity and the extent of platelet NO resistance. This suggests that platelet abnormalities and impairment of NO-related mechanisms may appear early in the clinical course of AVS.
Indeed, in our recent cross-sectional population study evaluating biochemical and physiological correlates of the presence of aortic valve sclerosis (ASc), a precursor to AS, in aging individuals [67] we have documented that ASc was also associated with impaired platelet responsiveness to NO. In that study, the extent of ASc manifestation was strongly associated with the extent of platelet resistance to NO. This finding is of potential relevance to the association between ASc and thrombotic events [8]. Furthermore, tissue resistance to NO per se might also contribute to the calcification process [69].
Asymmetric dimethylarginine (ADMA) is an endogenous competitive inhibitor of NO synthase (eNOS) and a marker and mediator of endothelial dysfunction [70-72]. We have also demonstrated that plasma concentrations of ADMA are elevated in patients with AS, compared with controls [73]. Thus, this finding provides further support to the suggestion that NO generation is impaired in AS.
A critical question which arises is whether the pathogenesis of AS is fundamentally related to dysfunction of the valve endothelium. NO resistance in AS may be paralleled by impairment of aortic valve endothelial function. Previous studies [63, 74, 75] have demonstrated that even early aortic valve calcification is associated with some decrease in endothelial function and even loss of valvular endothelium. Narrowing of the aortic valve orifice in AS, together with deformation of leaflets and increasingly rough surface of the valve, contributes to local turbulence in blood flow which creates shear stress, affecting both valve endothelium and passing platelets [76]. Furthermore, loss of aortic valve endothelium may predispose towards calcification of the aortic valve leaflets [69, 77, 78]. Potentially, aortic valve endothelium plays a critical role in maintaining normal aortic valve function.
In isolated porcine and canine aortic valves, it was shown that the aortic valve endothelium releases NO and prostacyclin, which both exert local anti-thrombotic effects and reduce leaflet tone [16, 18, 63]. The main “luminal” implication of these studies was that the stenotic aortic valve constitutes a pro-aggregatory milieu: - the clinical consequences of a loss of valve anti-aggregatory function must be considered together with platelet hyperaggregability and platelet resistance to NO occurring in AVS [66]. However, it is equally possible that disordered valve endothelial function contributes to development of valve matrix pathology. In our recent studies [69] with porcine aortic valve interstitial cell cultures, calcific nodule formation and associated redox stress were inhibited by NO donors. This suggests that NO has a direct protective effect extending to the inhibition of the calcification processes in aortic valve cells.
Bicuspid aortic valve (BAV): a special case?
BAV represents the most common congenital cardiac abnormality, with estimates of its prevalence ranging from 0.5 to 2% of the general population [79]. Structurally, BAV is heterogeneous, depending on the pattern of valve leaflet fusion, and its association with extravalvular disease, as summarized in Table 2. However, the most common pattern, accounting for about 75% of BAV cases, is fusion of the right and left (R-L: Type I) valve leaflets, while Type II BAV (fusion of the right and non-coronary cusps) accounts for virtually all other cases.
Table 2.
Subtypes of BAV by anatomical structure and associated features [118].
| Subtype of BAV | Valvular anatomical features | Associated features |
|---|---|---|
| Type I (70-75%) | Fusion of the right and left coronary cusp resulting in anterior – posterior commissural orientation | More common in males. Aortic root dilatation and higher prevalence of aortic coarctation. |
| Type II (25-30%) | Fusion of the right and non-coronary cusp result in right and left commissural orientation. | More common in females. Higher association with progression to aortic stenosis and regurgitation |
| Type III (∼1%) | Fusion of the non-and left coronary cusp. |
A fundamental and unique issue with BAV is the association with dilatation of the aortic root and arch, which is a particularly prominent feature of Type I BAV, and which is associated with risk of aortic dissection and rupture. On the other hand, progression of AS appears to be more rapid in Type II [79], where most patients require eventual aortic valve replacement.
From a pathophysiological point of view, BAV is unique and intriguing because it presents as a basis for a rapidly progressive form of AS and/or aortopathy, and because of the possible insight it provides regarding the pathogenesis of AS.
As regards the rapid progression, in a retrospective study of 156 adult patients with BAV, Thanassoulis et al [80] have demonstrated a mean increase in diameter of ascending aorta of 0.37mm/year and of aortic sinus of Valsava of 0.17mm/year. Tzemos et al [81] documented increase in aortic valve gradient of 0.7 mmHg per annum in a prospective cohort of 642 subjects with BAV.
As regards available data on the pathogenesis of BAV, despite a very large number of recent investigations, both basic and clinical, no coherent picture has emerged. Rather, it needs to be emphasised that there is increasing evidence of heterogeneity of pathogenesis, notably between Type I and Type II BAV, with increasing evidence of redox stress and inflammatory activation for Type I, and of predominant endothelial dysfunction for Type II [82]. Furthermore, there appears to be a substantial genetic component to BAV pathogenesis.
As regards the issue of inheritance of BAV, although all series show a predominance of male patients, the overall thrust of genetic investigations is to suggest autosomal dominant inheritance in most cases. A number of studies reveal multiple cases within kindred [83-85]. The most extensively documented mutations underlying BAV occur in the NOTCH1 gene [86], which would also predispose towards valve calcification, given that NOTCH signalling has been linked to expression of osteogenic genes osteopontin and osteocalcin [87]. However the relevant signal transduction pathways for AS development in BAV remain uncertain [88] and there is also evidence of multiple mutations associated with BAV [85].
The issue of association of BAV with endothelial dysfunction and possibly with impaired eNOS signalling has attracted considerable attention since it was observed that mice lacking the eNOS gene frequently also had BAV (but not other congenital cardiovascular abnormalities) [89]. However, Fernandez et al [82] have more recently clarified this observation: the abnormality is actually Type II BAV (right - non-coronary cusp fusion). Interestingly, these investigators documented that Type I BAV is present in inbred Syrian hamsters, without any known association with eNOS deficiency
While no other endothelial function studies have specified BAV subtype, there is substantial additional evidence that BAV may indeed be associated with eNOS deficiency and/or endothelial dysfunction. Aicher et al [90] quantitated eNOS protein expression in the aortic wall (but not the valve) at the time of surgical valve replacement and observed lower levels of eNOS in BAV versus tricuspid (“normal”) valves in the presence of AS. Furthermore, eNOS expression was negatively correlated with aortic diameter. A further study has examined brachial flow-mediated dilatation (FMD): - in a group of men with BAV, there was an association between aortic dilatation and impaired FMD [91], consistent with findings of Archer el al [90]. A number of other studies have evaluated the association between AS and NO deficiency, but not always in the context of BAV: these findings will be discussed later.
The concept of inflammatory change and oxidative stress is a relevant component of the pathogenesis of BAV, and is in a sense linked to that of NO deficiency. For example, Tzemos et al [91] observed that aortic dilatation in BAV was associated not only with impaired FMD, but also with increased plasma levels of matrix metalloproteinase 2 (MMP-2), which might have contributed to the dilatation process. Furthermore, Phillippi et al [92] demonstrated that susceptibility to oxidative stress was increased, and responsive expression of metallothionein decreased in BAV aortae. While these findings implicate oxidative stress and inflammatory activation, there is also evidence that endothelial progenitor cell mobilization in response to these changes may be abnormal [93], contributing to perturbation of aortic and valvular endothelial function.
Intervention studies to retard the progression of AS
Statins
Statins were the first commercially available drugs studied in aortic stenosis given their established benefit in primary and secondary prevention of cardiovascular diseases, as well as evidence of association of lipid infiltration of aortic valve in AS [25, 29, 30]. Whilst there are many “dyslipidemic” animal models of AS (see animal model section), only one model so far has shown benefit of statin therapy [94].
There were a number of very encouraging human retrospective studies of statins in AS, suggesting significant reduction in the rate of progression of AS with statin use [95-99]. Yet, only one small prospective open-label non-randomized observational study of rosuvastatin in patients with moderate AS showed slowing of haemodynamic progression of AS [100]. Three large prospective double-blind randomized placebo control trials of statins in AS failed to show any retardation of AS progression [101-103].
ACE-inhibitors (ACEI)/Angiotensin receptor blockers (ARB)
Numerous studies have demonstrated increased ACE activity/expression and Ang II presence in stenotic valves [37-39], providing a rationale for investigation of benefits of ACEI/ARB therapy in AS. Treatment of cholesterol-fed rabbits with angiotensin receptor-1 blocker olmesartan was associated with decreased macrophage infiltration and reductions in osteopontin and ACE in aortic valves [104]. Unfortunately, no hemodynamic valvular measures of AS progression were performed. We have demonstrated hemodynamic retardation of AS, con-comitantly with reduction in calcification, macrophage infiltration, redox stress and improvement in endothelial function, with ACEI ramipril treatment in a rabbit model of AS [59].
As regards human studies, no prospective trials of either class of agents have been published to date. Two main retrospective evaluations of population studies have provided conflicting data: - Rosenhek et al [98], utilizing echocardo-graphic parameters, found no significant effect of ACE-I therapy on AS progression (albeit with a trend to slower progression), while O'Brien et al [105], in a study using CT-based calcification assessment, found lower rates of AS calcification, after correction for comorbidities.
Bisphosphonates
Bisphosphonates, usually used for treatment of osteoporosis in humans, inhibit bone resorption as they cause osteoclast apoptotosis [106, 107]. They also inhibit an enzyme in the cholesterol synthesis pathway, which causes abnormalities in the cytoskeleton in the osteoclast, thus reducing bone resorption [108]. Thus, bisphosphonates may directly reduce valvular calcification via their osteoblast action, as well as indirectly via inhibition of inflammation and resultant fibrosis. A study in a rat model of dialysis suggested that etidronate, now rarely used bisphosphanate, limited aortic calcification [109].
Two small retrospective human studies of bisphophonates demonstrated reduction in the AS progression rate [110, 111]. However the small size and observational nature of these studies make the results hypothesis generating at best.
Aldosterone blockade
Aldosterone has been implicated in animal studies in vascular inflammation and myocardial fibrosis, and these were ameliorated by aldosterone blockade [112]. Eplerenone, an aldoster-one-receptor antagonist, shown to improve outcomes in heart failure patients [113], was trialled in a randomized double-blind placebo-controlled study in patients with moderate-severe AS [114]. This small trial failed to show reduction in rate of progression of valve stenosis.
Conclusion
The major objective of this review is to present the case that pathogenesis of AS is an active process that involves a combination of inflammatory activation, increased oxidative stress, fibrosis and calcification, which should be amenable to therapeutic intervention.
AS is the commonest form of valvular heart disease and its prevalence is rising due to increasing longevity, especially in Western world. We present evidence that AS, previously thought to be “degenerative” disorder of aging, is a complex active process, involving valvular endothelium, fibroblasts and extracellular matrix. The process is characterized by inflammatory activation and lipid deposition within valve lesions. There is extensive valvular matrix remodelling and fibrosis with increased production of MMP-1 and -2, TGF-β1, interleukin-1β and TNFα. There is extensive evidence for increased production of Ang II, a major pro-inflammatory and pro-fibrotic mediator, within stenotic valves. This would lead to further fibrosis and calcification. Impaired activation of anti-calcific modulators, such as fetuin-A and MGP, is alos important in AS. There is concurrent increased in oxidative stress and evidence of impairment of the nitric oxide system as well as associated systemic endothelial dysfunction.
In BAV, representing the most common congenital cardiac abnormality, valvular inflammation is combined with aortopathy. BAV illustrates unique interplay of genetically driven inflammatory activation and, in some cases, deficiency of nitric oxide formation.
The most promising targets for pharmacological interventions have been thought to be lipid infiltration and atheroma formation. However, results of all randomized double-blind prospective studies of statins have been disappointing [101-103]. ACE-I/ARB therapy, on the other hand, shows promising results. Although post-hoc clinical data are limited and inconclusive, we have recently demonstrated in an animal model that ACE-I ramipril retards progression of mild AS [59]. In a separate study, olmesartan treatment was also associated with reductions in macrophage infiltration and ACE in aortic valves in a rabbit model [104].
Therefore AS represents the result of a prolonged inflammatory process leading to valve calcification and ossification, which represents a potential target for therapeutic interventions.
Acknowledgments
This work is supported in part by research grants from the National Health and Medical Research Council of Australia (NHMRC) and Cardiovascular Lipid Research Grants (Australia).
References
- 1.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 2.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E, Goldman L. Philadelphia: Saunders; 2003. Primary cardiology. [Google Scholar]
- 4.Ross J Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 5.Bakaeen FG, Chu D, Ratcliffe M, Gopaldas RR, Blaustein AS, Venkat R, Huh J, LeMaire SA, Coselli JS, Carabello BA. Severe aortic stenosis in a veteran population: treatment considerations and survival. Ann Thorac Surg. 2010;89:453–458. doi: 10.1016/j.athoracsur.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116–2122. doi: 10.1016/j.athoracsur.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 7.Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82:2111–2115. doi: 10.1016/j.athoracsur.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 8.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 9.Volzke H, Haring R, Lorbeer R, Wallaschofski H, Reffelmann T, Empen K, Rettig R, John U, Felix SB, Dorr M. Heart valve sclerosis predicts all-cause and cardiovascular mortality. Atherosclerosis. 2010;209:606–610. doi: 10.1016/j.atherosclerosis.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Veyradier A, Balian A, Wolf M, Giraud V, Montembault S, Obert B, Dagher I, Chaput JC, Meyer D, Naveau S. Abnormal von Wille-brand factor in bleeding angiodysplasias of the digestive tract. Gastroenterology. 2001;120:346–353. doi: 10.1053/gast.2001.21204. [DOI] [PubMed] [Google Scholar]
- 11.King RM, Pluth JR, Giuliani ER. The association of unexplained gastrointestinal bleeding with calcific aortic stenosis. Ann Thorac Surg. 1987;44:514–516. doi: 10.1016/s0003-4975(10)62112-1. [DOI] [PubMed] [Google Scholar]
- 12.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 13.Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can J Cardiol. 1996;12:231–236. [PubMed] [Google Scholar]
- 14.Weind KL, Ellis CG, Boughner DR. The aortic valve blood supply. J Heart Valve Dis. 2000;9:1–7. discussion 7-8. [PubMed] [Google Scholar]
- 15.Cimini M, Rogers KA, Boughner DR. Aortic valve interstitial cells: an evaluation of cell viability and cell phenotype over time. J Heart Valve Dis. 2002;11:881–887. [PubMed] [Google Scholar]
- 16.Pompilio G, Rossoni G, Sala A, Polvani GL, Berti F, Dainese L, Porqueddu M, Biglioli P. Endothelial-dependent dynamic and anti-thrombotic properties of porcine aortic and pulmonary valves. Ann Thorac Surg. 1998;65:986–992. doi: 10.1016/s0003-4975(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 17.Filip D, Radu A, Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986;59:310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- 18.El-Hamamsy I, Balachandran K, Yacoub MH, Stevens LM, Sarathchandra P, Taylor PM, Yoganathan AP, Chester AH. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J Am Coll Cardiol. 2009;53:1448–1455. doi: 10.1016/j.jacc.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 19.Godbole AS, Lu X, Guo X, Kassab GS. NADPH oxidase has a directional response to shear stress. American Journal of Physiology -Heart and Circulatory Physiology. 2009;296:H152–H158. doi: 10.1152/ajpheart.01251.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. American Journal of Physiology - Heart and Circulatory Physiology. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 21.Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, Nerem RM. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arterioscler Thromb Vasc Biol. 2006;26:69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 22.Ku DD, Nelson JM, Caulfield JB, Winn MJ. Release of endothelium-derived relaxing factors from canine cardiac valves. J Cardiovasc Pharmacol. 1990;16:212–218. doi: 10.1097/00005344-199008000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Thomas SR, Keaney JF., Jr Beyond LDL oxidation: ROS in vascular signal transduction. Free Radic Biol Med. 2003;35:117–132. doi: 10.1016/s0891-5849(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 24.Mohler Iii ER. Mechanisms of aortic valve calcification. The American Journal of Cardiology. 2004;94:1396–1402. doi: 10.1016/j.amjcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 26.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23:1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 27.Wallby L, Janerot-Sjoberg B, Steffensen T, Broqvist M. T lymphocyte infiltration in nonrheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 2002;88:348–351. doi: 10.1136/heart.88.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren BA, Yong JL. Calcification of the aortic valve: its progression and grading. Pathology. 1997;29:360–368. doi: 10.1080/00313029700169315. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 30.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 31.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. discussion 465-456. [DOI] [PubMed] [Google Scholar]
- 32.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kilic R, Sarikoc A, Brueckmann M, Vahl C, Hagl S, Haase KK, Borggrefe M. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–211. doi: 10.1016/s0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 33.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kilic R, Sarikoc A, Pinol R, Hagl S, Lang S, Brueckmann M, Borggrefe M. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–87. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Jian B, Jones PL, Li Q, Mohler ER, 3rd, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-C in calcific aortic stenosis. Am J Pathol. 2001;159:321–327. doi: 10.1016/S0002-9440(10)61698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satta J, Melkko J, Pollanen R, Tuukkanen J, Paakko P, Ohtonen P, Mennander A, Soini Y. Progression of human aortic valve stenosis is associated with tenascin-C expression. J Am Coll Cardiol. 2002;39:96–101. doi: 10.1016/s0735-1097(01)01705-3. [DOI] [PubMed] [Google Scholar]
- 36.Nishimoto M, Takai S, Kim S, Jin D, Yuda A, Sakaguchi M, Yamada M, Sawada Y, Kondo K, Asada K, Iwao H, Sasaki S, Miyazaki M. Significance of chymase-dependent angiotensin II-forming pathway in the development of vascular proliferation. Circulation. 2001;104:1274–1279. doi: 10.1161/hc3601.094304. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien KD, Shavelle DM, Caulfield MT, McDonald TO, Olin-Lewis K, Otto CM, Probstfield JL. Association of angiotensinconverting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106:2224–2230. doi: 10.1161/01.cir.0000035655.45453.d2. [DOI] [PubMed] [Google Scholar]
- 38.Helske S, Lindstedt KA, Laine M, Mayranpaa M, Werkkala K, Lommi J, Turto H, Kupari M, Kovanen PT. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol. 2004;44:1859–1866. doi: 10.1016/j.jacc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 39.Helske S, Syvaranta S, Kupari M, Lappalainen J, Laine M, Lommi J, Turto H, Mayranpaa M, Werkkala K, Kovanen PT, Lindstedt KA. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J. 2006;27:1495–1504. doi: 10.1093/eurheartj/ehi706. [DOI] [PubMed] [Google Scholar]
- 40.Mohler ER, 3rd, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–552. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 42.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 43.Kaden JJ, Bickelhaupt S, Grobholz R, Vahl CF, Hagl S, Brueckmann M, Haase KK, Dempfle CE, Borggrefe M. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis. 2004;13:560–566. [PubMed] [Google Scholar]
- 44.Mazzone A, Epistolato MC, De Caterina R, Storti S, Vittorini S, Sbrana S, Gianetti J, Bevilacqua S, Glauber M, Biagini A, Tanganelli P. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J Am Coll Cardiol. 2004;43:1670–1676. doi: 10.1016/j.jacc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 45.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaden JJ, Reinohl JO, Blesch B, Brueckmann M, Haghi D, Borggrefe M, Schmitz F, Klomfass S, Pillich M, Ortlepp JR. Systemic and local levels of fetuin-A in calcific aortic valve stenosis. Int J Mol Med. 2007;20:193–197. [PubMed] [Google Scholar]
- 48.Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protethe calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603. [PubMed] [Google Scholar]
- 49.Koos R, Krueger T, Westenfeld R, Kuhl HP, Brandenburg V, Mahnken AH, Stanzel S, Vermeer C, Cranenburg EC, Floege J, Kelm M, Schurgers LJ. Relation of circulating Matrix Gla-Protein and anticoagulation status in patients with aortic valve calcification. Thromb Haemost. 2009;101:706–713. [PubMed] [Google Scholar]
- 50.Ueland T, Gullestad L, Dahl CP, Aukrust P, Aakhus S, Solberg OG, Vermeer C, Schurgers LJ. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med. 2010;268:483–492. doi: 10.1111/j.1365-2796.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- 51.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J, Jr, Pomerantzeff PM, Laurindo FR. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 53.Ngo DT, Stafford I, Kelly DJ, Sverdlov AL, Wuttke RD, Weedon H, Nightingale AK, Rosenkranz AC, Smith MD, Chirkov YY, Kennedy JA, Horowitz JD. Vitamin D(2) supplementation induces the development of aortic stenosis in rabbits: interactions with endothelial function and thioredoxin-interacting protein. Eur J Pharmacol. 2008;590:290–296. doi: 10.1016/j.ejphar.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 54.Ngo DT, Stafford I, Sverdlov AL, Qi W, Wuttke RD, Zhang Y, Kelly DJ, Weedon H, Smith MD, Kennedy JA, Horowitz JD. Ramipril retards development of aortic valve stenosis in a rabbit model: mechanistic considerations. Br J Pharmacol. 2011;162:722–732. doi: 10.1111/j.1476-5381.2010.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World CJ, Yamawaki H, Berk BC. Thioredoxin in the cardiovascular system. J Mol Med. 2006;84:997–1003. doi: 10.1007/s00109-006-0109-6. [DOI] [PubMed] [Google Scholar]
- 56.Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 57.Xiang G, Seki T, Schuster MD, Witkowski P, Boyle AJ, See F, Martens TP, Kocher A, Sondermeijer H, Krum H, Itescu S. Catalytic degradation of vitamin D up-regulated protein 1 mRNA enhances cardiomyocyte survival and prevents left ventricular remodeling after myocardial ischemia. J Biol Chem. 2005;280:39394–39402. doi: 10.1074/jbc.M502966200. [DOI] [PubMed] [Google Scholar]
- 58.Schulze PC, Liu H, Choe E, Yoshioka J, Shalev A, Bloch KD, Lee RT. Nitric oxidedependent suppression of thioredoxininteracting protein expression enhances thioredoxin activity. Arterioscler Thromb Vasc Biol. 2006;26:2666–2672. doi: 10.1161/01.ATV.0000248914.21018.f1. [DOI] [PubMed] [Google Scholar]
- 59.Ngo DT, Stafford I, Sverdlov AL, Qi W, Wuttke RD, Zhang Y, Kelly DJ, Weedon H, Smith MD, Kennedy JA, Horowitz JD. Ramipril retards development of aortic valve stenosis in a rabbit model: mechanistic considerations. Br J Pharmacol. 2011;162:722–732. doi: 10.1111/j.1476-5381.2010.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aronow WS, Ahn C, Shirani J, Kronzon I. Comparison of frequency of new coronary events in older persons with mild, moderate, and severe valvular aortic stenosis with those without aortic stenosis. Am J Cardiol. 1998;81:647–649. doi: 10.1016/s0002-9149(97)00966-1. [DOI] [PubMed] [Google Scholar]
- 61.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 62.Theroux P, Fuster V. Acute coronary syndromes: unstable angina and non-Q-wave myocardial infarction. Circulation. 1998;97:1195–1206. doi: 10.1161/01.cir.97.12.1195. [DOI] [PubMed] [Google Scholar]
- 63.Chirkov YY, Mishra K, Chandy S, Holmes AS, Kanna R, Horowitz JD. Loss of antiaggregatory effects of aortic valve tissue in patients with aortic stenosis. J Heart Valve Dis. 2006;15:28–33. [PubMed] [Google Scholar]
- 64.Riddle JM, Stein PD, Magilligan DJ, Jr, McElroy HH. Evaluation of platelet reactivity in patients with valvular heart disease. J Am Coll Cardiol. 1983;1:1381–1384. doi: 10.1016/s0735-1097(83)80039-4. [DOI] [PubMed] [Google Scholar]
- 65.Stein PD, Sabbah HN, Pitha JV. Continuing disease process of calcific aortic stenosis. Role of microthrombi and turbulent flow. Am J Cardiol. 1977;39:159–163. doi: 10.1016/s0002-9149(77)80185-9. [DOI] [PubMed] [Google Scholar]
- 66.Chirkov YY, Holmes AS, Willoughby SR, Stewart S, Horowitz JD. Association of aortic stenosis with platelet hyperaggregability and impaired responsiveness to nitric oxide. Am J Cardiol. 2002;90:551–554. doi: 10.1016/s0002-9149(02)02536-5. [DOI] [PubMed] [Google Scholar]
- 67.Ngo DT, Sverdlov AL, Willoughby SR, Nightingale AK, Chirkov YY, McNeil JJ, Horowitz JD. Determinants of occurrence of aortic sclerosis in an aging population. JACC Cardiovasc Imaging. 2009;2:919–927. doi: 10.1016/j.jcmg.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 68.Chirkov YY, Horowitz JD. Impaired tissue responsiveness to organic nitrates and nitric oxide: a new therapeutic frontier? Pharmacol Ther. 2007;116:287–305. doi: 10.1016/j.pharmthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Kennedy JA, Hua X, Mishra K, Murphy GA, Rosenkranz AC, Horowitz JD. Inhibition of calcifying nodule formation in cultured porcine aortic valve cells by nitric oxide donors. Eur J Pharmacol. 2009;602:28–35. doi: 10.1016/j.ejphar.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Sydow K, Schwedhelm E, Arakawa N, Bode-Boger SM, Tsikas D, Hornig B, Frolich JC, Boger RH. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res. 2003;57:244–252. doi: 10.1016/s0008-6363(02)00617-x. [DOI] [PubMed] [Google Scholar]
- 71.Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–833. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- 72.Boger RH, Bode-Boger SM, Brandes RP, Phivthong-ngam L, Bohme M, Nafe R, Mugge A, Frolich JC. Dietary L-arginine reduces the progression of atherosclerosis in cholesterolfed rabbits: comparison with lovastatin. Circulation. 1997;96:1282–1290. doi: 10.1161/01.cir.96.4.1282. [DOI] [PubMed] [Google Scholar]
- 73.Ngo DT, Heresztyn T, Mishra K, Marwick TH, Horowitz JD. Aortic stenosis is associated with elevated plasma levels of asymmetric dimethylarginine (ADMA) Nitric Oxide. 2007;16:197–201. doi: 10.1016/j.niox.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Brener SJ, Duffy CI, Thomas JD, Stewart WJ. Progression of aortic stenosis in 394 patients: relation to changes in myocardial and mitral valve dysfunction. J Am Coll Cardiol. 1995;25:305–310. doi: 10.1016/0735-1097(94)00406-g. [DOI] [PubMed] [Google Scholar]
- 75.Wu HD, Maurer MS, Friedman RA, Marboe CC, Ruiz-Vazquez EM, Ramakrishnan R, Schwartz A, Tilson MD, Stewart AS, Winchester R. The lymphocytic infiltration in calcific aortic stenosis predominantly consists of clonally expanded T cells. J Immunol. 2007;178:5329–5339. doi: 10.4049/jimmunol.178.8.5329. [DOI] [PubMed] [Google Scholar]
- 76.Joris I, Zand T, Majno G. Hydrodynamic injury of the endothelium in acute aortic stenosis. Am J Pathol. 1982;106:394–408. [PMC free article] [PubMed] [Google Scholar]
- 77.Dahm M, Dohmen G, Groh E, Krummenauer F, Hafner G, Mayer E, Hake U, Oelert H. Decalcification of the aortic valve does not prevent early recalcification. J Heart Valve Dis. 2000;9:21–26. [PubMed] [Google Scholar]
- 78.Mirzaie M, Meyer T, Schorn B, Schwartz P, Baryalei M, Rastan A, Lotfi S, Dalichau H. Calcification tendency of various biological aortic valves in an experimental animal model. Cardiovasc Surg. 1999;7:735–741. doi: 10.1016/s0967-2109(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 79.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 80.Thanassoulis G, Yip JWL, Filion K, Jamorski M, Webb G, Siu SC, Therrien J. Retrospective study to identify predictors of the presence and rapid progression of aortic dilatation in patients with bicuspid aortic valves. Nat Clin Pract Cardiovasc Med. 2008;5:821–828. doi: 10.1038/ncpcardio1369. [DOI] [PubMed] [Google Scholar]
- 81.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, Webb GD, Siu SC. Outcomes in Adults With Bicuspid Aortic Valves. JAMA: The Journal of the American Medical Association. 2008;300:1317–1325. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 82.Fernandez B, Duran AC, Fernandez-Gallego T, Fernandez MC, Such M, Arque JM, Sans-Coma V. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol. 2009;54:2312–2318. doi: 10.1016/j.jacc.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 83.Clementi M, Notari L, Borghi A, Tenconi R. Familial congenital bicuspid aortic valve: a disorder of uncertain inheritance. Am J Med Genet. 1996;62:336–338. doi: 10.1002/(SICI)1096-8628(19960424)62:4<336::AID-AJMG2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 84.Glick BN, Roberts WC. Congenitally bicuspid aortic valve in multiple family members. Am J Cardiol. 1994;73:400–404. doi: 10.1016/0002-9149(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 85.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 86.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 87.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 88.Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102:1169–1181. doi: 10.1161/CIRCRESAHA.108.174318. [DOI] [PubMed] [Google Scholar]
- 89.Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation. 2000;101:2345–2348. doi: 10.1161/01.cir.101.20.2345. [DOI] [PubMed] [Google Scholar]
- 90.Aicher D, Urbich C, Zeiher A, Dimmeler S, Schafers HJ. Endothelial nitric oxide synthase in bicuspid aortic valve disease. Ann Thorac Surg. 2007;83:1290–1294. doi: 10.1016/j.athoracsur.2006.11.086. [DOI] [PubMed] [Google Scholar]
- 91.Tzemos N, Lyseggen E, Silversides C, Jamorski M, Tong JH, Harvey P, Floras J, Siu S. Endothelial function, carotid-femoral stiffness, and plasma matrix metalloproteinase-2 in men with bicuspid aortic valve and dilated aorta. J Am Coll Cardiol. 2010;55:660–668. doi: 10.1016/j.jacc.2009.08.080. [DOI] [PubMed] [Google Scholar]
- 92.Phillippi JA, Klyachko EA, Kenny JPt, Eskay MA, Gorman RC, Gleason TG. Basal and oxidative stress-induced expression of metallothionein is decreased in ascending aortic aneurysms of bicuspid aortic valve patients. Circulation. 2009;119:2498–2506. doi: 10.1161/CIRCULATIONAHA.108.770776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vaturi M, Perl L, Leshem-Lev D, Dadush O, Bental T, Shapira Y, Yedidya I, Greenberg G, Kornowski R, Sagie A, Battler A, Lev EI. Circulating Endothelial Progenitor Cells in Patients With Dysfunctional Versus Normally Functioning Congenitally Bicuspid Aortic Valves. Am J Cardiol. 2011;108:272–276. doi: 10.1016/j.amjcard.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 94.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemiainduced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–2665. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–2209. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- 96.Shavelle DM, Takasu J, Budoff MJ, Mao S, Zhao XQ, O'Brien KD. HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet. 2002;359:1125–1126. doi: 10.1016/S0140-6736(02)08161-8. [DOI] [PubMed] [Google Scholar]
- 97.Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–1730. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 98.Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 99.Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 100.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 102.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 103.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 104.Arishiro K, Hoshiga M, Negoro N, Jin D, Takai S, Miyazaki M, Ishihara T, Hanafusa T. Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J Am Coll Cardiol. 2007;49:1482–1489. doi: 10.1016/j.jacc.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 105.O'Brien KD, Probstfield JL, Caulfield MT, Nasir K, Takasu J, Shavelle DM, Wu AH, Zhao XQ, Budoff MJ. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med. 2005;165:858–862. doi: 10.1001/archinte.165.8.858. [DOI] [PubMed] [Google Scholar]
- 106.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem. 2005;280:7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 108.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tamura K, Suzuki Y, Matsushita M, Fujii H, Miyaura C, Aizawa S, Kogo H. Prevention of aortic calcification by etidronate in the renal failure rat model. Eur J Pharmacol. 2007;558:159–166. doi: 10.1016/j.ejphar.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 110.Innasimuthu AL, Katz WE. Effect of Bisphosphonates on the Progression of Degenerative Aortic Stenosis. Echocardiography. 2011;28:1–7. doi: 10.1111/j.1540-8175.2010.01256.x. [DOI] [PubMed] [Google Scholar]
- 111.Skolnick AH, Osranek M, Formica P, Kronzon I. Osteoporosis treatment and progression of aortic stenosis. Am J Cardiol. 2009;104:122–124. doi: 10.1016/j.amjcard.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 112.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–1810. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 113.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 114.Stewart RA, Kerr AJ, Cowan BR, Young AA, Occleshaw C, Richards AM, Edwards C, Whalley GA, Freidlander D, Williams M, Doughty RN, Zeng I, White HD. A randomized trial of the aldosterone-receptor antagonist eplerenone in asymptomatic moderate-severe aortic stenosis. Am Heart J. 2008;156:348–355. doi: 10.1016/j.ahj.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 115.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 116.Iung B, Baron G, Tornos P, Gohlke-Barwolf C, Butchart EG, Vahanian A. Valvular heart disease in the community: a European experience. Curr Probl Cardiol. 2007;32:609–661. doi: 10.1016/j.cpcardiol.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Novaro GM, Mishra M, Griffin BP. Incidence and echocardiographic features of congenital unicuspid aortic valve in an adult population. J Heart Valve Dis. 2003;12:674–678. [PubMed] [Google Scholar]
- 118.Fernandes SM, Sanders SP, Khairy P, Jenkins KJ, Gauvreau K, Lang P, Simonds H, Colan SD. Morphology of bicuspid aortic valve in children and adolescents. J Am Coll Cardiol. 2004;44:1648–1651. doi: 10.1016/j.jacc.2004.05.063. [DOI] [PubMed] [Google Scholar]