Abstract
Cardiac fibrosis is known to alter cardiac conduction and promote reentry. Recent evidence indicates that fibrosis characterized by increased interstitial collagen accumulation and increased myofibroblast proliferation also promotes enhanced automaticity and early afterdepolarizations (EADs) causing triggered activity. Fibrosis then becomes an effective therapeutic target for the management of lethal cardiac arrhythmias. While oxidative stress with hydrogen peroxide (H2O2) is shown to readily promote EADs and triggered activity in isolated rat and rabbit ventricular myocytes however, this same stress fails to cause EADs in well-coupled, non-fibrotic hearts due to source-to-sink mismatches arising from cell-to-cell coupling. The triggered activity in the aged fibrotic hearts causes focal ventricular tachycardia (VT) that degenerates within seconds to ventricular fibrillation (VF) after the emergence of spatially discordant action potential duration alternans leading to wavebreak, reentry and VF. Computer simulations in 2D tissue incorporating variable degrees of fibrosis showed that intermediate (but not mild or very severe) fibrosis promoted EADs and TA. Human studies have shown that myocardial fibrosis was an independent predictor for arrhythmias including sustained VT and VF. A variety of drug classes including, torsemide, a loop diuretic, that inhibits the enzyme involved in the myocardial extracellular generation of collagen type I molecules and the inhibitors of the renin-angiotensin-aldosterone system (RAAS), the mineralocorticoid receptors and endothelin receptors reduce cardiac fibrosis with reduction of myocardial stiffness and improved ventricular function. It is hoped that in the near future effective antifibrotic drug regimen would be developed to reduce the risk of fibrosis related VT and VF.
Keywords: Early afterdepolarization, triggered activity, ventricular tachycardia, ventricular fibrillation, fibrosis, myofibroblast, optical mapping, discordant alternans, oxidative stress
Introduction
Increased cardiac fibrosis is shown to be associated with cardiac conduction block and reentry in isolated-perfused animal and diseased human cardiac tissues [1-3] as well as in isolated Langendorff-perfused explanted human hearts with dilated cardiac myopathy [4, 5]. While alterations of cardiac conduction [6, 7] and the resulting reentrant wavefront of excitation[8] are uniformly accepted arrhythmic consequences of increased cardiac fibrosis, recent experimental findings in isolated whole heart studies, indicate that fibrosis may also importantly modulate the formation of cardiac after-potentials notably early afterdepolarizations (EADs) that lead to triggered activity causing atrial fibrillation (AF) [9] and ventricular fibrillation (VF) [10-12]. These findings extend previous cardiac monolayer studies that showed myofibroblast coupling to cardiomyocytes through gap junction formation imparts enhanced automaticity to cardiomyocytes when coupled to a finite (critical) number of myofibro-blasts [13]. Taken together these findings indicate that increased cardiac fibrosis promotes arrhythmias not only by the mechanism of reentry but also by the mechanism of triggered activity and enhanced automaticity potentially making cardiac fibrosis a highly effective antiarrhythmic target.
In this brief review we delineate step-by-step how the interaction of aged fibrotic ventricles with oxidative stress leads to the emergence of early afterdepolarizations (EADs), triggered activity and VF. We emphasize the importance of fibrosis as non-fibrotic hearts when stressed similarly or at even higher stress levels do no manifest any arrhythmic events. Specifically, we describe the dynamic scenario starting from cellular EADs that evolves to rapid focal ventricular tachycardia (VT) caused by triggered activity which then degenerates to VF. In the second part we point out briefly on recent experimental [14, 15] and the potential of drug-induced prevention and/or reduction of ventricular fibrosis as an antiarrhythmic strategy in humans [16-20].
The pathology of fibrosis
Fibrosis develops when the body's natural wound-healing process goes awry. Under normal (adaptive) conditions of wound healing, specialized cells known as fibroblasts become activated by transforming to myofibroblast. The myofibroblasts then undergo proliferation causing increased synthesis of collagen protein in the extracellular matrix composed predominantly of type I collagen and to a lesser extent type III collagen (normal wound healing process). What is initially an adaptive process, perhaps meant to enhance tensile strength, can progress to maladaptive (pathologic) conditions when the “healing” process persists with the development of diffuse and heterogeneous myocardial fibrosis characterized by a matrix made of increased collagen deposits and myofibroblast numbers [18, 21-23]. While resident cardiac fibroblasts may be activated and transformed to myofibroblasts there is also the potential of fibroblasts originating from endothelial cells, suggesting an endothelial-mesenchymal transition (EndMT). For example, it has been shown that the transforming growth factor-beta 1 (TGF-β1) induces endothelial cells to undergo EndMT, whereas bone morphogenic protein 7 (BMP-7) preserves the endothelial phenotype. The demonstration of the systemic administration of recombinant human BMP-7 (rhBMP-7) to significantly inhibit EndMT and the progression of cardiac fibrosis in mouse models of pressure overload may provide novel therapeutic approaches to control the progression of cardiac fibrosis [24].
Whole heart animal model of fibrosis and oxidative VF
In 1956, Harmann [25] made his groundbreaking observations on the role of reactive oxygen species (ROS) in the aging process. Thereafter, the concept of ROS became widely accepted in theories of aging [26]. While atrial and ventricular fibrosis may increase with aging, however, fibrosis per se does not promote cardiac arrhythmias [27-30]. Instead, fibrosis as we will see below, provides a substrate that when coupled to a mild form of stress that is of no arrhythmic consequence in non-fibrotic hearts, causes cardiac arrhythmias in the fibrotic heart. In this respect fibrosis becomes a significant risk factor for increased vulnerability to cardiac arrhythmias. We provide experimental evidence in isolated-perfused whole heart preparations that fibrosis acts as the “first hit,” a necessary and a required substrate, and that increased oxidative stress acts as a “second hit,” both hits being required to promote spontaneous VF. We call this the “double hit hypothesis” of spontaneous VF. We now describe the dynamic evolution as to how oxidative stress (hydrogen peroxide, H2O2) in aging-related fibrotic ventricles promotes spontaneous VF.
Oxidative stress
Hydrogen peroxide (H2O2) is shown to readily promote EADs and triggered activity in isolated rat and rabbit ventricular myocytes by increasing the late sodium current (INA-L) [31-33] However, this same stress fails to cause EADs in well-coupled, non-fibrotic cardiac tissue due to source-to-sink mismatches arising from cell-to-cell coupling. That is, a small current which is sufficient to reverse repolarization and cause an EAD in an isolated cardiac myocyte will be diluted into adjacent repolarizing myocytes (unless they are also simultaneously primed for an EAD), thereby suppressing the EAD. We investigated this issue by examining the effects of H2O2 on arrhythmias in Langendorff-perfused rat and rabbit hearts. Consistent with the predicted suppressive effects of well-coupled tissue on EADs, we found that oxidative stress with H2O2 failed to induce any ventricular arrhythmias in young adult rat or rabbit hearts with no fibrosis. In sharp contrast however, fibrotic aged rat and fibrotic middle-aged rabbit hearts, when exposed to similar levels of oxidative stress with 0.1 mM H2O2 readily induced VT/VF in these two species [10, 11]. Importantly it needs to be emphasized that in adult non-fibrotic hearts raising H2O2concentration up to 2 mM still failed to induce arrhythmias in adult rat and adult (3-5 months old) rabbit hearts indicating that it is not the greater susceptibility of aged hearts to oxidative stress relative to adult hearts that was responsible for the differential arrhythmic response in the two age groups of both species. These findings are consistent with the hypothesis that EADs are suppressed by cell -to-cell coupling in tissues, since H2O2 concentrations which consistently induce EADs and triggered activity in isolated rat and rabbit ventricular myocytes uniformly failed to induce EAD-related arrhythmias in non-fibrotic adult rat and rabbit intact hearts studied [10, 11].
Fibrosis
Histological analysis using Masson's trichrome collagen showed marked increase in fibrosis in the aged (24-26 months) rat ventricles compared to adult ventricles averaging 45±26% of the ventricles. The distribution of fibrosis however, was highly heterogeneous, varying between 10% in the RV to 90% in the left ventricular (LV) endocardium and at intermediate level at the base of the LV epicardium. Middle-aged (3-5 years old) rabbit hearts however, had less extensive fibrosis averaging from 15% in the RV and mid LV wall to 25-35% in the anterior and posterior LV near the base of the heart. In contrast, fibrosis was minimal in adult rat hearts, averaging 4±2.5% of the ventricles, and 3±1% in adult rabbit ventricles (P<0.001 compared to aged rat and middle-aged rabbit ventricles)[10].
From EADs to VF
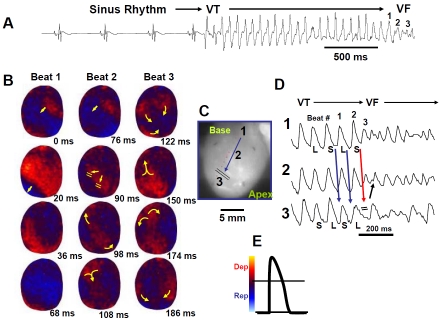
The mechanism of oxidative stress-mediated spontaneous VF (i.e., VF not induced by electrical stimulation) was studied systematically using optical activation map and single cell recordings with glass microelectrode in aged rats. For this purpose we used simultaneous voltage-and calcium (V-Ca) sensitive dyes to map activation pattern and define the underlying intracellular calcium dynamics (Cai2+). In response to 0.1 mM H2O2, 66 of 70 of the aged rat hearts (94%) developed spontaneous VF after a mean perfusion time of 17.6±7.1 min (Figure 1). LV epicardial activation map showed that the VF was preceded by a transient period of focal VT (mean CL of 70±18 ms) which arose suddenly from regular sinus rhythm with a mean CL of 380±162 ms. Within 2 sec, the VT degenerated to sustained VF (CL of 55±16 ms) (Figure 1), requiring electrical shock for termination. However, spontaneous VT/VF reoccurred repeatedly after 2-3 min of cardioversion. The focal VT preferentially originated from the base of the LV anterior epicardium where the degree of fibrosis was intermediate (30-40%). This unique feature of spontaneous VF onset allowed us to capture the onset of the focal unstable VT during optical and glass microelectrode action potential recordings. Similar findings were observed in the middle-aged rabbit hearts when LV epicardial fibrosis averaged 35±16% of the LV. As in the case of aged rats, the VF in the middle aged rabbits was also preceded by a transient period of VT (mean CL of 130±20 ms) which arose suddenly from regular sinus rhythm with a mean CL of 460±80 ms.
Figure 1.
Spontaneous initiation of VT/VF in an aged rat heart exposed to 0.1 mM H2O2. Panel A is an ECG showing the last 5 sinus beats before the sudden onset of VT leading to VF. Panel B are voltage snap shots of the last beat of the VT (beats #1) and of the first two beats of the VF (beats #2 & #3). In each snap shot activation time in ms is shown at the bottom right with time zero (arbitrary) coinciding with the onset of beat #1. The red color in the snap shots represents depolarization and the blue repolarization as shown in panel E. The yellow arrows in the snap shots represent the direction of the wavefront propagation with double horizontal lines denoting the site of conduction block. The VT originates from a focal site at the LV base and propagates as single wavefront towards the apex and undergoes functional conduction block at site 3. The two lateral edges of the front however, continue to propagate laterally (snap shot 98 ms) forming f igure-8 reentry (snap shot 108). During the second reentrant wavefront another wavefront emerges from the apical site of the LV (snap shot 122 ms) disrupting the activation pattern and signaling the onset of VF. Panel D, shows 3 optical action potentials (labeled 1, 2 and 3) recorded from sites identified on the heart silhouette (panel C). The two downward pointing blue arrows indicate the direction of propagation from site 1 to site 3 with the red downward pointing arrow showing block at site 3 followed by retrograde activation (upward pointing arrow). Notice the emergence of spatially discordant action potential duration (APD) alternans preceding conduction block at site 3 when the front with short APD (S) at site 1 encroaches a site (site 3) with long APD (L). S indicates short and L long APD. (From reference number 10, Morita et al)
Mechanism of focal VT
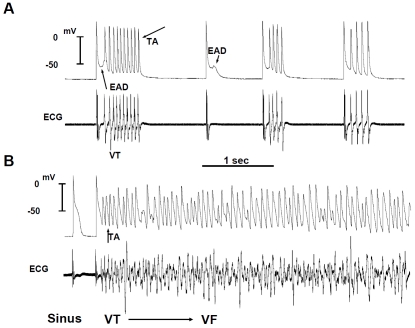
To gain insight into the cellular mechanisms of focal VT, after the cardioversion of the VF with an electrical shock, we continuously recorded with a roving glass microelectrode single cell action potentials from the epicardial surface of the LV base, where the focal VT frequently originated from to capture the onset of focal VT. With this approach, we show that the focal VT was initiated by an EAD-mediated triggered activity that arose from a mean take-off potential of -51±16 mV (Figure 2). The mean CL of the TA was 66±10 ms and was not significantly different from the mean CL of the VT (70±18 ms). The EAD preceded the QRS complex of a simultaneously recorded ECG by a mean of 8±4 ms and occurred during an isoelectric interval on the ECG indicating absence of electrical activity elsewhere in the heart during the EAD formation (Figure 2). The EADs arose when Cai2+ remained high relative to the diastolic resting level reflecting a slowed rate of decline of Cai2+. Maintained elevation of Cai2+ could activate the sodium-calcium exchanger (NCX) current providing a net inward depolarizing current [12] which along with the increased INA-L caused by H2O2 reduces the repolarization reserve and facilitates the emergence of reactivated L-type calcium (I-Ca-L) current causing EADs and triggered activity. These findings indicate that oxidative stress promotes EADs in areas of the LV where the level of fibrosis is intermediate by reducing ventricular repolarization reserve leading to EADs, triggered activity and focal VT.
Figure 2.
Simultaneous microelectrode and ECG recordings at the onset of VT/VF in an aged rat heart exposed to 0.1 mM H2O2. Panel A, onset of early afterdepolarization (EAD)-mediated triggered activity (TA) causing ventricular tachycardia (VT) 5 min after H2O2 exposure. Note the smooth emergence of EAD (upward pointing arrow) during the isoelec-tric interval on the ECG followed by a run of 10 TA (downward pointing arrow) causing non-sustained VT on the ECG. The onset of the EAD precedes the QRS complex of the VT by 8 ms indicating absence of electrical activity elsewhere in the heart. Two additional short runs of VT with 4 beats each are also shown that follow a single subthreshold EAD (downward small arrow) with no TA. Panel B shows the degeneration of the TA to VF 15 min after H2O2 exposure. (From reference number 10, Morita et al)
Why fibrosis is important? The source-to sink mismatch
Our findings highlight the importance of cell-to-cell coupling on the ability of EADs to form and cause arrhythmias in tissue. While isolated ventricular myocytes readily develop EADs and triggered activity in response to oxidative stress, normal non-fibrotic tissue or hearts however can not generate EADs or triggered when exposed to similar or more intense stressful conditions. This discrepancy supports the supposition that cell-to-cell coupling is a potent mechanism suppressing EAD formation in tissue, by creating a source-to-sink mismatch that prevents local EAD currents generated by a small group of myocytes from reversing repolarization when they are electrotonically coupled to a large group of adjacent normally repolarizing myocytes.
To explore the effects of cell-to-cell coupling on EAD suppression, we performed computer simulations in 2D cardiac tissue in which normal myocyte-to-myocyte coupling was disrupted by inserting fibroblasts into the tissue [10]. With too few fibroblasts, EADs and triggered activity were suppressed by cell-to-cell coupling, and with too many fibroblasts, while the EADs occurred but they were unable to propagate. With an intermediate myocyte-fibroblast ratio, however, EADs both formed and successfully propagated into the surrounding tissue. These findings agreed well with the experimental results in aged rat hearts, in which EADs and TA typically arose from regions with intermediate levels of fibrosis (30-40%), usually at the base of the LV epicardium. Two important factors tip the balance towards the emergence of EAD: 1) the process of synchronization of EADs.[34] and 2) increased cardiac fibrosis causing reduced gap junction coupling.[35] We recently described a synchronization mechanism for EADs based on the evidence that the irregularity of EADs is a form of dynamical chaos and that contiguous cells engaged in EAD formation actually synchronic over a finite length scale. However, when the tissue exceeds the critical size, electrotonic coupling can no longer globally synchronize EADs, resulting in regions of partial synchronization that shift in time and space. The regionally synchronized EADs then form premature ventricular complexes that propagate into recovered tissue without EADs causing premature ventricular depolarization and in the case of triggered activity focal VT. How EADs overcome electrotonic source-sink mismatches in tissue to trigger premature ventricular complexes remains incompletely understood. To study this question, we used a rabbit ventricular action potential model to simulate tissues in which a central area of contiguous myocytes susceptible to EADs was surrounded by unsusceptible tissue. In 1D tissue with normal longitudinal conduction velocity (0.55 m/s), the numbers of contiguous susceptible myocytes required for an EAD to trigger a propagating action potential was 70. In 2D tissue, the number of cells increases to 6940 and in 3D tissue to 696,910. The number of the cells decreases considerably when the gap junction conductance between the myocytes becomes reduced from 780 nS to 125 nS (6.25 fold) as might be expected to occur in fibrotic hearts. The decrease in the number of cells was proportionately more in 3D (93% reduction in the number of cells, i.e., 48,700 cells) than in 2D (84%, i.e., 1,100 cells) than in 1D (57%, i.e., 30 cells) [35]. These simulation studies have shown that the source-to-sink mismatch in well-coupled cardiac tissue provides a powerful mechanism to protect the heart from EAD-mediated arrhythmias. In contrast however when fibrosis develops and gap junctional couplings between myocytes decreases a considerable decrease in the number of myocytes needed to promote a PVD and VT occur presumably leading to increased vulnerability to VT/VF in intact fibrotic hearts.
Transition from focal VT to mixed reentrant and focal VF
The EAD-mediated focal VT degenerated within 3 sec of the onset to sustained VF (CL of 105±15 ms), requiring electrical shock for termination. The focal VT propagated as single wavefront from the base of the heart to the apex promoting spatially discordant action potential duration (APD) alternans (Figure 1) causing localized conduction block (wavebreak) midway between the base and the apex of the LV anterior epicardial surface. After the block, the wavefront continued to propagate laterally past the site of block forming a figure-8 type reentry, coinciding in time with the transition from the VT to VF on the ECG (Figure 1). This pattern of VT to VF transition occurred in about 70% of the VF episodes. In the remaining episodes the sites of the wavebreak could not be defined. Whether focal VTs seen in patients are also caused by EAD-mediated triggered activity remains to be elucidated [36,37-39].
Electrophysiological differences between young adult and aged rat hearts
Although disruption of normal cell-to-cell coupling by fibrosis provides a plausible explanation for the increased susceptibility of aged hearts to EADs and EAD-mediated arrhythmias, we cannot exclude the possibility that other aspects of aging-related remodeling (e.g. electrical, Cai+2 cycling, and gap junction remodeling) also make important contributions. Aging-related changes in electrophysiological and Ca cycling properties at the myocyte level may also be important in the genesis of EADs. In comparing young adult versus aged rat hearts, however, we found that H2O2 had no significant effect on action potential duration (APD) or the maximum APD restitution slope, both before and after exposure to H2O2. On the other hand, some differences in Cai2+cycling between young adult and aged rat hearts were noted. The mean rate constant of the Cai2+transient decline (fit to a single exponential) was significantly longer in aged compared to young adult ventricles (P<0.05), both before and after H2O2 exposure. Furthermore, there was greater regional heterogeneity in Cai2+handling in the aged than young adult rat hearts, with the slowest decline rate constant clustering at the base of the LV compared to mid and apical sites. Overall, our findings in the aged fibrotic hearts with oxidative VF support a critical role of partial cellular uncoupling in EAD formation at tissue level caused by increased interstitial tissue fibrosis.
Has the time come for clinical trials to manage cardiac arrhythmias with anti-fibrotic therapy?
The link between ventricular fibrosis and ventricular arrhythmia risk suggests that targeting fibrosis may impart antiarrhythmic benefits. For example, a recent study found that in patients with hypertrophic cardiomyopathy, myocardial fibrosis as measured by the late gadolinium enhancement cardiovascular magnetic resonance (CMR) is an independent predictor of adverse outcome [40]. Interestingly these investigators found that the extent of myocardial fibrosis was an independent predictor for arrhythmias including sustained VT and VF [40]. In another study Lopez and associates found that patients receiving torsemide, a loop diuretic that inhibits the enzyme involved in the myocardial extracellular generation of collagen type I molecules (i.e., procollagen type I carboxy-terminal proteinase or PCP) reduced myocardial collagen volume fraction (CVF) as assessed in right septal endocardial biopsies from patients with chronic heart failure [41]. Interestingly changes in serum PCP activation were positively correlated with changes in CVF in these patients receiving torsemide [41]. These initial clinical studies suggest that ventricular fibrosis may indeed be decreased with antifibrotic therapy however its antiarrhythmic significance awaits confirmation in larger patient populations. The emergence of new cardiac imaging techniques of myocardial fibrosis with reasonable degree of accuracy [42] may help assess the efficacy of antifibrotic therapy and the associated cardiac arrhythmias. Antiarrhythmic therapy would thus be targeted to prevent disproportionate collagen accumulation and myofibroblast proliferation. Such measures are also bound not only to prevent arrhythmias but also help in the management of heart failure. Drugs that inhibit the renin-angiotensin-aldosterone system (RAAS) are known to suppress cardiac fibrosis indirectly via improved hemodynamics and directly through inhibition of myofibroblast activity and collagen synthesis. Experimental studies have shown that RAAS inhibitors (angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, such as eplerenone reduce cardiac fibrosis [14]. Similarly, block of miner-alocorticoid receptors or endothelin receptors reduce cardiac fibrosis along with reduction of myocardial stiffness and improved ventricular function [43].
Collectively, there are a wide range of possible antifibrotic treatments options that target the TGF-β, endothelin-1 (ET-1), connective tissue growth factor (CTGF), angiotensin II, and platelet -derived growth factor (PDGF) networks.[18] The experimental findings are encouraging and suggest that reduction of cardiac fibrosis is possible and may indeed reduce the risk of cardiac arrhythmias [14, 18, 24, 44-47]. For example, studies in rats have shown that pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias [48, 49]. Another animal study showed that the agent relaxin-1 reverses cardiac fibrosis and related cardiac dysfunction [50]. Relaxin is a potent antifibrotic peptide hormone that inhibits fibroblast activation (indicated by suppressed expression of α-smooth muscle actin) and collagen synthesis stimulated by angiotensin II or transforming growth factor-β [50].
It is hoped that these basic research findings will be translated to patients at risk of developing cardiac fibrosis-related related arrhythmias. To the extent that such a translation will be successful it is anticipated that a more rational and effective care of patients at risk of VT/VF may be developed.
References
- 1.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 2.Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986;58:356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- 3.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 4.Wu TJ, Ong JJ, Hwang C, Lee JJ, Fishbein MC, Czer L, Trento A, Blanche C, Kass RM, Mandel WJ, Karagueuzian HS, Chen PS. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J Am Coll Cardiol. 1998;32:187–196. doi: 10.1016/s0735-1097(98)00184-3. [DOI] [PubMed] [Google Scholar]
- 5.de Bakker JMT, Coronel R, Tasseron S, Wilde AAM, Opthof T, Janse MJ, van Capelle FJL, Becker AE, Jambroes G. Ventricular tachycardia in the infarcted, Langendorff-perfused human heart: Role of the arrangement of surviving cardiac fibers. J Am Coll Cardiol. 1990;15:1594–1607. doi: 10.1016/0735-1097(90)92832-m. [DOI] [PubMed] [Google Scholar]
- 6.Spach MS, Heidlage F, Dolber PC, Barr RC. Mechanism of origin of conduction disturbances in aging human atrial bundles: Experimental and model study. Heart Rhythm. 2007;4:175–185. doi: 10.1016/j.hrthm.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 8.Spach MS, Dolber PC, Heidlage JF. Interaction of inhomogeneities of repolarization with anisotropic propagation in dog atria. A mechanism for both preventing and initiating reentry. Circ Res. 1989;65:1612–1631. doi: 10.1161/01.res.65.6.1612. [DOI] [PubMed] [Google Scholar]
- 9.Ono N, Hayashi H, Kawase A, Lin SF, Li H, Weiss JN, Chen PS, Karagueuzian H. Spontaneous atrial fibrillation initiated by triggered activity near the pulmonary veins in aged rats subjected to glycolytic inhibition. Am J Physiol Heart Circ Physiol. 2007;292:639–648. doi: 10.1152/ajpheart.00445.2006. [DOI] [PubMed] [Google Scholar]
- 10.Morita N, Sovari AA, Xie Y, Fishbein MC, Mandel WJ, Garfinkel A, Lin SF, Chen PS, Xie LH, Chen F, Qu Z, Weiss JN, Karagueuzian HS. Increased Susceptibility of Aged Hearts to Ventricular Fibrillation During Oxidative Stress. Am J Physiol Heart Circ Physiol. 2009;297:H1594–H1605. doi: 10.1152/ajpheart.00579.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita N, Lee JH, Xie Y, Sovari A, Qu Z, Weiss JN, Karagueuzian HS. Suppression of reentrant and multifocal ventricular fibrillation by the late sodium current blocker ranolazine. J Am Coll Cardiol. 2011;57:366–375. doi: 10.1016/j.jacc.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita N, Lee JH, Bapat A, Fishbein MC, Mandel WJ, Chen PS, Weiss JN, Karagueuzian HS. Glycolytic Inhibition Causes Spontaneous Ventricular Fibrillation in Aged Hearts. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 14.Stein M, Boulaksil M, Jansen JA, Herold E, Noorman M, Joles JA, van Veen TA, Houtman MJ, Engelen MA, Hauer RN, de Bakker JM, van Rijen HV. Reduction of fibrosis-related arrhythmias by chronic renin-angiotensinaldosterone system inhibitors in an aged mouse model. Am J Physiol Heart Circ Physiol. 2010;299:H310–321. doi: 10.1152/ajpheart.01137.2009. [DOI] [PubMed] [Google Scholar]
- 15.Mulder P, Devaux B, Richard V, Henry JP, Wimart MC, Thibout E, Mace B, Thuillez C. Early versus delayed angiotensin-converting enzyme inhibition in experimental chronic heart failure. Effects on survival, hemodynamics, and cardiovascular remodeling. Circulation. 1997;95:1314–1319. doi: 10.1161/01.cir.95.5.1314. [DOI] [PubMed] [Google Scholar]
- 16.Lin CS, Pan CH. Regulatory mechanisms of atrial fibrotic remodeling in atrial fibrillation. Cell Mol. Life Sci. 2008;65:1489–1508. doi: 10.1007/s00018-008-7408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Mello WC. Beneficial effect of eplerenone on cardiac remodelling and electrical properties of the failing heart. J Renin Angiotensin Aldosterone Syst. 2006;7:40–46. doi: 10.3317/jraas.2006.005. [DOI] [PubMed] [Google Scholar]
- 18.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich JR, Biliczki P, Hohnloser SH, Nattel S. Atrial-selective approaches for the treatment of atrial fibrillation. J Am Coll Cardiol. 2008;51:787–792. doi: 10.1016/j.jacc.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 20.Madrid AH, Escobar C, Rebollo JM, Marin I, Bernal E, Nannini S, Limon L, Peng J, Moro C. Angiotensin receptor blocker as adjunctive therapy for rhythm control in atrial fibrillation: results of the irbesartan-amiodarone trial. Card Electrophysiol Rev. 2003;7:243–246. doi: 10.1023/B:CEPR.0000012391.95928.d2. [DOI] [PubMed] [Google Scholar]
- 21.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am. J Physiol Heart Circ. Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 22.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 23.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 25.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 26.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 27.Boyle AJ, Shih H, Hwang J, Ye J, Lee B, Zhang Y, Kwon D, Jun K, Zheng D, Sievers R, Angeli F, Yeghiazarians Y, Lee R. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011 doi: 10.1016/j.exger.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eghbali M, Eghbali M, Robinson TF, Seifter S, Blumenfeld OO. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res. 1989;23:723–729. doi: 10.1093/cvr/23.8.723. [DOI] [PubMed] [Google Scholar]
- 29.de Souza RR. Aging of myocardial collagen. Biogerontology. 2002;3:325–335. doi: 10.1023/a:1021312027486. [DOI] [PubMed] [Google Scholar]
- 30.Hacker TA, McKiernan SH, Douglas PS, Wanagat J, Aiken JM. Age-related changes in cardiac structure and function in Fischer 344 x Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2006;290:H304–H311. doi: 10.1152/ajpheart.00290.2005. [DOI] [PubMed] [Google Scholar]
- 31.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. Journal of Physiology. 1997;500(Pt 3):631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 33.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative Stress-Induced Afterdepolarizations and Calmodulin Kinase II Signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato D, Xie LH, Sovari AA, Tran DX, Morita N, Xie F, Karagueuzian H, Garfinkel A, Weiss JN, Qu Z. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc. Natl. Acad Sci U.S.A. 2009;106:2983–2988. doi: 10.1073/pnas.0809148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99:1408–1415. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pogwizd SM. Focal mechanisms underlying ventricular tachycardia during prolonged ischemic cardiomyopathy. Circulation. 1994;90:1441–1458. doi: 10.1161/01.cir.90.3.1441. [DOI] [PubMed] [Google Scholar]
- 37.Ong JJC, Lee JJ, Fishbein MC, Karagueuzian HS, Chen PS. Initiation and maintenance of atrial fibrillation by focal and reentrant mechanisms: the role of complex 3-dimensional atrial anatomy. Circulation. 1996;95:I351–I351. [Google Scholar]
- 38.Zhou S, Chang CM, Wu TJ, Miyauchi Y, Okuyama Y, Hamabe A, Omichi C, Hayashi H, Brodsky LA, Mandel WJ, Ting CT, Fishbein MC, Karagueuzian HS, Chen PS. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2002;283:H1244–H1252. doi: 10.1152/ajpheart.01109.2001. [DOI] [PubMed] [Google Scholar]
- 39.Pogwizd SM, McKenzie JP, Cain ME. Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation. 1998;98:2404–2414. doi: 10.1161/01.cir.98.22.2404. [DOI] [PubMed] [Google Scholar]
- 40.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Lopez B, Gonzalez A, Beaumont J, Querejeta R, Larman M, Diez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:859–867. doi: 10.1016/j.jacc.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 42.Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, Quinones MA, Zoghbi WA, Entman ML, Roberts R, Marian AJ. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation. 2001;104:317–324. doi: 10.1161/hc2801.094031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, Van Wagoner DR. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. 3:369–379. doi: 10.1161/CIRCEP.109.924985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, Guerra PG, Ducharme A. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003;107:2926–2931. doi: 10.1161/01.CIR.0000072793.81076.D4. [DOI] [PubMed] [Google Scholar]
- 45.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of Angiotensinconverting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 46.Massare J, Berry JM, Luo X, Rob F, Johnstone JL, Shelton JM, Bassel-Duby R, Hill JA, Naseem RH. Diminished cardiac fibrosis in heart failure is associated with altered ventricular arrhythmia phenotype. J Cardiovasc Electrophysiol. 2010;21:1031–1037. doi: 10.1111/j.1540-8167.2010.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyralla K, Adamczak M, Benz K, Campean V, Gross ML, Hilgers KF, Ritz E, Amann K. High-dose enalapril treatment reverses myocardial fibrosis in experimental uremic cardiomyopathy. PLoS One. 2011;6:e15287. doi: 10.1371/journal.pone.0015287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen DT, Ding C, Wilson E, Marcus GM, Olgin JE. Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias. Heart Rhythm. 2010;7:1438–1445. doi: 10.1016/j.hrthm.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 49.Lee KW, Everett THt, Rahmutula D, Guerra JM, Wilson E, Ding C, Olgin JE. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–1712. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du XJ, Xu Q, Lekgabe E, Gao XM, Kiriazis H, Moore XL, Dart AM, Tregear GW, Bathgate RA, Samuel CS. Reversal of cardiac fibrosis and related dysfunction by relaxin. Ann N Y Acad Sci. 2009;1160:278–284. doi: 10.1111/j.1749-6632.2008.03780.x. [DOI] [PubMed] [Google Scholar]