Abstract
Objective: The goals of this study were to determine: 1) if the CHADS2 score correlates with left atrial (LA) or left atrial appendage (LAA) thrombus on pre-cardioversion transesophageal echocardiography (TEE) in nonvalvular atrial fibrillation (NVAF); and 2) what, if any, components of the CHADS2 score are most important in predicting LA/LAA thrombus. Background: It is unknown if CHADS2 score, a marker of thromboembolic risk in NVAF, accurately predicts LA/LAA thrombus on pre-cardioversion TEE. Methods: We retrospectively studied patients undergoing precardioversion TEE for NVAF at a tertiary hospital. TEE reports were reviewed for presence of LA/LAA thrombus. Using medical records and an ICD-9 coding database, a CHADS2 score was derived, and the association between CHADS2 and thrombus was evaluated with Mantel-Haenszel Chi-Square. The relation between the singular components of CHADS2 and thrombus were analyzed using Pearson's Chi-Square. Results: In 643 consecutive patients undergoing pre-cardioversion TEE, LA/LAA thrombus was identified in 46 (7.2 %). A strong association was present between CHADS2score and LA/LAA thrombus (p = 0.0005). No thrombi were identified in patients with CHADS2 = 0. Among 46 patients with thrombus, all (100%) had CHF. Of the singular components, CHF was the only factor independently associated with thrombus (p < 0.0001). Conclusions: In non-valvular atrial fibrillation, CHADS2 is strongly associated with LA thrombus on TEE. Our findings suggest pre-cardioversion TEE may be unnecessary if the CHADS2 score = 0. Of the components of the CHADS2 score, CHF was the only independently associated risk factor which correlated with LA/LAA thrombus.
Keywords: Atrial fibrillation, left atrial appendage thrombus, transesophageal echocardiography, CHADS2
Introduction
Atrial Fibrillation (AF) currently affects 2.2 million individuals in the United States alone [1] and its prevalence is projected to increase over the next several decades as the population ages [2]. AF represents a major risk factor for thromboembolic events, accounting for approximately 15% of all ischemic strokes [3]. Several risk stratification schemes have been developed and validated to predict thromboembolic risk in non-valvular atrial fibrillation (NVAF) [3-6]. The CHADS2 index is one such scoring algorithm that assesses risk of systemic throm-boembolism based on clinical factors and guides the selection of AF patients at sufficiently high risk of stroke to justify systemic anti-coagulation for stroke prevention [4].
Based on the risk of systemic thromboembolism at the time of cardioversion for atrial fibrillation, current guidelines recommend either several weeks of anticoagulation or precardioversion transesophageal echocardiography (TEE) to exclude intra-cardiac thrombus prior to restoration of sinus rhythm [1]. Given the increasing prevalence of atrial fibrillation and expanding pressure to reduce healthcare expenditures [1], avoidance of precardioversion anticoagulation or TEE may be warranted if low-risk individuals could be reliably identified. Although low CHADS2 scores have been demonstrated in several studies to be associated with a sufficiently low risk of stroke that anticoagulation over the long-term is not recommended [1,4,6], it is unknown if CHADS2 score can identify individuals at low risk for intra-cardiac thrombus on precardioversion TEE. Accordingly, we sought to delineate an association between CHADS2 score and the presence of left atrial thrombus on TEE in patients about to undergo cardioversion.
Methods
Study population
The clinical reports for all TEEs performed between January 1, 2006 and January 31, 2009 at William Beaumont Hospital, Royal Oak, a large tertiary hospital, were reviewed for the study indication. The study was approved by the William Beaumont Hospital Human Investigational Committee. If the clinical indication for TEE was to evaluate for the presence of LA thrombus prior to direct current cardioversion (DCCV), the following data was collected from the TEE report: 1) Age of patient at the time of TEE; 2) Presence of intra-cardiac thrombus; and 3) Left ventricular systolic function. An ICD-9 coding database was used to derive a CHADS2 score [4] ranging from 0-6. This was derived by assigning 1 point for congestive heart failure (CHF), hypertension, age >/= 75, and diabetes mellitus, and 2 points for stroke or transient ischemic attack. The CHADS2 score was confirmed by reviewing the hospital electronic medical records for verification of their ICD-9 coding diagnoses.
Transesophageal echocardiogram
TEE was performed with a standard TEE probe after anesthetizing the posterior pharynx and providing conscious sedation. Multiple views were obtained. All patients gave written consent prior to the TEE. A thrombus was defined as an echo-dense mass with a uniform texture different to that of the LA/LAA endocardial wall [7,8]. Left ventricular systolic function was classified as greater than or equal to an ejection fraction of 50% or an ejection fraction less than 50%.
CHADS2 score
The ICD-9 diagnosis coding database was accessed in order to obtain the correct comorbid conditions and to calculate a CHADS2 score [4,9].These ICD-9 diagnoses were then confirmed by reviewing the hospital electronic database. Congestive heart failure (CHF) was defined as clinical heart failure (Stage C or D) according to the 2009 Focused Update: ACC/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults [10]. Hypertension included any history of hypertension or currently treated hypertension at the time of TEE. Age of 75 years or greater at the time of TEE was considered a risk factor. Diabetes mellitus included any history of diabetes or currently treated diabetes at the time of TEE. TIA and CVA are clinically evident focal neurologic events with or without imaging confirmation.
Statistical analysis
The association between CHADS2 and thrombus was evaluated with Mantel-Haenszel Chi-Square. The relation between the singular components of CHADS2 and thrombus were analyzed using Pearson's Chi-Square. A p-value < 0.05 was considered statistically significant.
Results
Two thousand five hundred and sixty one TEEs were performed at William Beaumont Hospital, Royal Oak, between January 1, 2006 and January 30, 2009. Of these, 643 were precardioversion TEEs for atrial fibrillation/flutter. All 643 were included in the analysis. Table 1 lists the patient baseline characteristics. The mean age was 66.9±13.7 years, and 66% were male. The majority of patients (56%) fell into the intermediate risk group of a CHADS2 score of 1 (26%) or 2 (30%). Hypertension was the most common risk factor, and was present in 64% of the study population.
Table 1.
Baseline characteristics
| CHADS2 Score | |||||||
|---|---|---|---|---|---|---|---|
| Clinical Variable | 0 (n =102) (5.9%) | 1 (n =168) (26.1%) | 2 (n =195) (30.3%) | 3 (n =128) (19.9%) | 4 (n =36) (5.6%) | 5 or 6 (n =14) (2.1%) | Total (n =643) |
| Male | 73 (71.6%) | 119 (70.8%) | 121 (62.1%) | 82 (64.1%) | 22 (61.1%) | 10 (71.4%) | 427 (66.4%) |
| Age (years) | 54.4±11.9 | 62.1±11.7 | 70.3±12.3 | 73.7±10.7 | 77.6±10.2 | 79.1±9.1 | 66.9±13.7 |
| Age ≥ 75 years | 0 (0.0%) | 16 (9.5%) | 75 (38.5%) | 74 (57.8%) | 29 (80.6%) | 12 (87.5%) | 206 (32%) |
| Hypertension | 0 (0.0%) | 88 (52.4%) | 155 (79.5%) | 122 (95.3%) | 34 (94.4%) | 14 (100%) | 413 (64%) |
| Diabetes Mellitus | 0 (0.0%) | 11 (6.5%) | 55 (28.2%) | 67 (52.3%) | 29 (80.1%) | 4 (28.6%) | 166 (26%) |
| Heart Failure | 0 (0.0%) | 47 (28%) | 96 (49.2%) | 111 (86.7%) | 32 (88.9%) | 12(85.7%) | 298 (46%) |
| CHFor LVEF < 50% | 0 (0.0%) | 53 (31.5%) | 103 (52.8%) | 115 (89.8%) | 32 (88.9%) | 13 (92.9%) | 316 (49.1%) |
| LVEF < 50% | 0 (0.0%) | 38 (22.6%) | 56 (28.7%) | 62 (48.4%) | 16 (44.4%) | 9 (64.3%) | 181 (28%) |
| Previous CVA/TIA | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 3 (2.3%) | 10 (27.8%) | 14 (100%) | 28 (4.4%) |
CHF = congestive heart failure; CVA/TIA = cerebral vascular accident or transient ischemic attack; LVEF = left ventricular ejection fraction.
Prevalence of LA/LAA thrombus
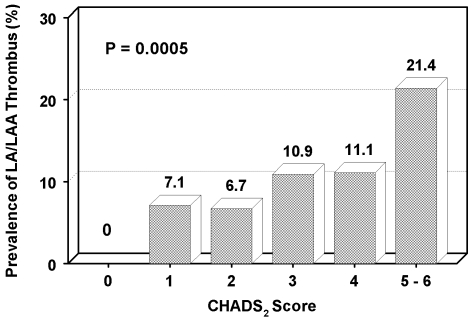
Left atrial thrombus was identified in 46 patients (7.2%) (Table 2). A strong association was present between CHADS2 score and presence of LA/LAA thrombus (p=0.0005) (Figure 1). No thrombi were identified in patients with CHADS2 = 0. In contrast, three out of fourteen patients (21%) with a CHADS2 = 5-6 had evidence of LA/LAA thrombus pre-cardioversion TEE. A CHADS2 score = 1 was associated with a 7.1% risk of thrombus. Of the singular components of CHADS2, CHF was the only factor independently associated with thrombus (p < 0.0001), whereas no association was present for hypertension (p = 0.43), age (p = 0.42), diabetes (p = 0.18), or stroke/TIA (p = 0.13) (Table 3).
Table 2.
Prevalence of thrombus by CHADS2 score
| CHADS2 Score | N | LA/LAA Thrombus |
|---|---|---|
| 0 | 102 | 0 (0%) |
| 1 | 168 | 12 (7.1%) |
| 2 | 195 | 13 (6.7%) |
| 3 | 128 | 14 (10.9%) |
| 4 | 36 | 4 (11.1%) |
| 5-6 | 14 | 3 (21.4%) |
| Total | 643 | 46 (7.2%) |
LA/LAA = left atrial/left atrial appendage
Figure 1.
Prevalence of LA/LAA thrombus on pre-cardioversion TEE by CHADS2 Score. *LA/LAA = left atrial/left atrial appendage; TEE = transesophageal echocardiography.
Table 3.
Prevalence of CHADS2 variables in patients with and without thrombus
| No Thrombus N = 596 (%) | Thrombus N = 46 (%) | P value | Odds Ratio | 90% Confidence Interval | |
|---|---|---|---|---|---|
| CHADS2 Score | 1.7 ± 1.2 | 2.4 ± 1.2 | 0.0005 | 1.528 | 1.203 – 1.940 |
| CHF | 270 (45.2) | 46 (100) | <0.0001 | * | |
| Hypertension | 381 (63.8) | 32 (69.6) | 0.43 | 1.296 | 0.751 – 2.236 |
| Age >75 | 189 (31.6) | 17 (36.9) | 0.42 | 1.266 | 0.750 – 2.135 |
| Diabetes | 158 (26.5) | 8 (17.4) | 0.18 | 0.585 | 0.303 – 1.129 |
| Stroke or TIA | 24 (4.0) | 4 (8.7) | 0.13 | 2.274 | 0.900 – 5.742 |
No odds ratio as all thrombus patients had CHF.
CHF = congestive heart failure; TIA = transient ischemic attack.
Discussion
Atrial fibrillation affects more than 10 percent of people over the age of 80 years, and the lifetime risk of developing AF is approximately 25% [11,12]. Atrial fibrillation is an independent risk factor for stroke, but the risk is highly variable depending on the associated co-morbidities and use of anticoagulation [13-15].Today, the most widely used clinical model to predict stroke is the CHADS2 score, developed by Gage et al [4]. Derived from the AFI and SPAF prediction models, and originally validated using data from a registry of hospitalized Medicare beneficiaries with AF, the CHADS2 score is derived by assigning one point for each of the following: CHF, hypertension, age >/= 75 years, diabetes mellitus, and two points for a history of TIA/CVA. Patients having a CHADS2 score of 0 are assigned as being at low risk for ischemic stroke with a 1.9% annual stroke rate. Patients with a score of 1-2 are assigned to moderate risk with a 2.8% and 4.0% annual stroke rate respectively. Finally, patients with a CHADS2 score of 3 -6 are at high risk, with an annual stroke ranging from 5.9 - 18.2% per 100 patient years [1,3,4,16]. According to the most recent ACC/AHA practice guidelines for the management of patients with AF, patients with a stroke risk of 2% per year or less do not benefit substantially from oral anti-coagulation, which would require treatment of 100 or more patients for 1 year to prevent a single stroke. A daily aspirin is the recommended therapy for this low risk group. For high risk AF patients with stroke rates of 6% per year or greater, the comparable number needed to treat is 25 or fewer, strongly favoring anticoagulation. Intermediate risk, those with a CHADS2 score of 1-2, and an estimated annual stroke rate of 3%-5% respectively, remains divided, and the risk of hemorrhage must be weighed. Clinical trials suggest that the absolute risk of major hemorrhages in patients with AF on warfarin is about 2% per year, with approximately one quarter of these attributed to intracranial hemorrhages [17,18]. Use of aspirin reduces the risk of stroke by 22% and warfarin reduces the risk of stroke by 62% [4]. The overall average CHADS2 score in our study population was 1.8, falling within the intermediate risk group.
CHADS2 and prevalence of thrombus
Increasing CHADS2 score has been validated to correlate with an increased risk of stroke. The mechanism for this increased stroke risk in AF has been attributed to the fact that there is an increased propensity to develop LA/LAA thrombus in AF secondary to stasis, and this thrombus serves as the source for the thromboembolic cerebral event. The finding of LAA thrombus on TEE has been shown to be independently associated with high thromboembolic risk in patients with AF [19], however, the relationship between the clinical increasing CHADS2 risk index and propensity of finding LA/LAA thrombus on TEE has not been well validated. In our study, there is a strong and statistically significant correlation between CHADS2 and LA/LAA thrombus on pre-cardioversion TEE (p<0.0005). These findings are similar to the study by Puwanant et al which demonstrated increasing prevalence of a thrombogenic milieu (as defined by thrombus or sludge on TEE) with ascending CHADS2 scores [20], and Rader et al who also reported increasing prevalence of LA/LAA thrombus with increasing CHADS2 score [21].
CHADS2 = 0 population
In our study, 102 patients had a CHADS2 score of zero. In this subset, there were no LA/LAA thrombi identified, consistent with their overall low stroke risk. Our findings support the current guidelines that state that anticoagulation is unnecessary in this subset, and aspirin can suffice. This finding is similar to that published by Puwanant et al. who reported no LA/LAA thrombi identified in patients with a CHADS2 =0 on TEE prior to pulmonary vein isolation, albeit the majority of these patients were anticoagulated at the time of TEE [20]. In a similar study by McCready et al, there were no LA/LAA thrombi identified on TEE prior to AF ablation in low risk patients (identified by echocardiography and a clinical risk score) [22]. The current ACC/AHA guidelines are ambiguous in regard to the need for TEE prior to electrical cardioversion in this recognized low stroke risk group. Although the safety profile for TEE has been well documented: major complication rates of <0.02%, they are not without some inherent risk to the patient, can be uncomfortable and inconvenient to the patient, and add a cost burden to the patient and society [23]. Our findings suggest that a TEE prior to DCCV in this low risk group may be unnecessary. A patient may be able to undergo DCCV followed by the recommended four weeks of anticoagulation, and avoid the unnecessary risk, cost, and inconvenience of an invasive procedure without sacrificing safety. These findings however, are not simiiar to Rader et al who found evidence of LAA thrombus on TEE in 2/75 patients with a CHADS2=0. [21] Clearly, more studies are necessary to help clarify.
CHADS2 = 1 or 2 population
A CHADS2 score of 1 or 2 is considered intermediate risk for stroke with an estimated annual stroke rate of 2.8% and 4.0% respectively. In our study, 168 patients (26.1%) had a CHADS2 score of 1, and 195 patients (30.3%) had a CHADS2 score of 2. A total of 12 left atrial thrombi were found in the CHADS2 =1 group correlating to a prevalence of 7.1%. Thirteen LA/LAA thrombi were discovered in the CHADS2=2 group for a prevalence of 6.7%. Opinions regarding the use of anticoagulation in this intermediate risk group are divided. Some experts call for routine anticoagulation in patients in this category without a high bleeding risk. Others advocate for selective anticoagulation on an individual basis with a strong emphasis on bleeding risk and the patient's individual preference [1]. The 2006 ACC/AHA/ESC practice guidelines recommend either ASA or warfarin in individuals with a CHADS2=1, and warfarin for patients with more than 1 moderate risk factor (age >/=75 years, HTN, heart failure, LVEF </=35%, DM). The relatively high prevalence of LA/LAA thrombus on pre-cardioversion TEE in patients in this intermediate group may argue for more liberal use of warfarin in the CHADS2=1 or 2 subgroups, especially for those patients at a relatively low risk for bleeding.
Congestive heart failure
In the original study by Gage et al, recent CHF exacerbation (within 100 days), rather than any history of CHF was used to determine CHF as a risk factor when calculating a CHADS2 score. Because of the retrospective nature of this study, we used any history of CHF as a risk factor. Using this model, CHF was present in over 49% of the study population. Of the 46 patients who had LA/LAA thrombus on pre-cardioversion TEE, all (100%) had CHF as a risk factor, making it the only statistically significant risk factor (p <0.0001). Both clinical heart failure and decreased left ventricular systolic function have been identified as significant risk factors for stroke in the setting of atrial fibrillation [1,2,20-22,24-26]. Our data is in agreement with previous published reports.
Study limitations
The limitations of this study include those inherent to any retrospective study. Several potentially important details were unable to be reliably obtained based upon chart review including duration of atrial fibrillation, type of atrial fibrillation (paroxysmal, persistent, new onset vs. recurrent). Presence of systemic anticoagulation at time of pre-cardioversion TEE was not reliably provided. TEEs were performed by various echo-cardiographers, however all are highly experienced in the usage of TEE. The study was a single center study. TEE is known to have a sensitivity of >95% for detecting thrombus, but TEE may miss thrombi < 2mm, [23] and this could possibly lead to underestimating the prevalence of thrombus.
Conclusions
In non valvular atrial fibrillation, CHADS2 is strongly associated with LA thrombus on pre-cardioversion TEE. Our findings support using PCTEE if the CHADS2 score is ≥ 1 and suggest that it may be unnecessary before DCCV if the CHADS2 score = 0. Of the components of the CHADS2 score, CHF was the only independent risk factor associated with the finding of thrombus.
Glossary
Abbreviations
- (LA)
Left Atrial
- (LAA)
Left Atrial Appendage
- (NVAF)
Non-valvular Atrial Fibrillation
- (AF)
Atrial Fibrillation
- (TEE)
Transesophageal Echocardiography
- (CVA)
Cerebral Vascular Accident
- (TIA)
Transient Ischemic Attack
- (CHF)
Congestive Heart Failure
- (AFI)
Atrial Fibrillation Investigators
- (SPAF)
Stroke Prevention and Atrial Fibrillation
- (DCCV)
Direct Current Cardioversion
- (LVEF)
Left Ventricular Ejection Fraction
- (ACC)
American College of Cardiology
- (AHA)
American Heart Association
- (ESC)
European Society of Cardiology
Declaration of conflicts of interest
None
References
- 1.ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation. J Am Coll Cardiol. 2006;48:e149–e246. [Google Scholar]
- 2.Antithrombotic Therapy in Atrial Fibrillation: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:429S–456S. doi: 10.1378/chest.126.3_suppl.429S. [DOI] [PubMed] [Google Scholar]
- 3.Fang MC, Go AS, Chang Y, Borowski L, Pomernacki N, Singer D. Comparison of Risk Stratification Schemes to Predict Thromboembolism in People With Nonvalvular Atrial Fibrillation. J Am Coll Cardiol. 2008;51:810–815. doi: 10.1016/j.jacc.2007.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 5.Atrial Fibrillation Investigators. Risk Factors for Stroke and Efficacy of Antithrombotic Therapy in Atrial Fibrillation. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 6.Anderson D, Halperin J, Hart R, McAnulty J, McBride R, Pearce L, Sherman D. Patients with Nonvalvular Atrial Fibrillation at Low Risk of Stroke During Treatment with Aspirin: Stroke Prevention in Atrial Fibrillation III Study. JAMA. 1998;279:1273–1277. [PubMed] [Google Scholar]
- 7.Seward JB, Khanderia BK, Oh JK, Freeman WK, Tajik AJ. Critical appraisal of transesophageal echocardiography: limitations, pitfalls and complications. J Am Soc Echo. 1992;5:288–305. doi: 10.1016/s0894-7317(14)80352-0. [DOI] [PubMed] [Google Scholar]
- 8.Aschenberg W, Schluter M, Kremer P, Schroder E, Siglow V, Bliefeld W. Transesophageal two-dimensional echocardiography for the detection of left atrial appendage thrombus. J Am Coll Cardio. 1986;7:163–6. doi: 10.1016/s0735-1097(86)80275-3. [DOI] [PubMed] [Google Scholar]
- 9.Rietbrock S, Heeley E, Plumb J, Van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or TIA (CHADS2) risk stratification scheme. Am Heart J. 2008;156:57–64. doi: 10.1016/j.ahj.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 10. ACC/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults. 2009 Focused Update.
- 11.Krahn A, Manfreda J, Tate RB, Mathewson F, Cuddy T. The Natural History of Atrial Fibrillation: Incidence, Risk Factors, and Prognosis in the Manitoba Follow-up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Wang TJ, Leip E, Larson MG, Levy D, Vasan RS, D'agostino R, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime Risk for Development of Atrial Fibrillation: The Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 13.Wolf PA, Dawber TR, Thomas HE, Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham Study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 14.Cairns JA, Connolly SJ, Nonrheumatic atrial fibrillation Risk of stroke and role of antithrombotic therapy. Circulation. 1991;84:469–481. doi: 10.1161/01.cir.84.2.469. [DOI] [PubMed] [Google Scholar]
- 15.Kleemann T, Becker T, Strauss M, Schneider S, Karlheinz S. Prevalence and Clinical Impact of Left Atrial Thrombus and Dense Spontaneous Echo Contrast in Patients with Atrial Fibrillation and Low CHADS2 Score. Euro J of Echo. 2009;10:383–388. doi: 10.1093/ejechocard/jen256. [DOI] [PubMed] [Google Scholar]
- 16.Rockson SG, Albers GW. Comparing the Guidelines: Anticoagulation Therapy to Optimize Stroke Prevention in Patients With Atrial Fibrillation. J Am Coll Cardiol. 2004;43:929–935. doi: 10.1016/j.jacc.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Weir NU. An Update on Cardioembolic Stroke. Postgrad Med J. 2008;84:133–142. doi: 10.1136/pgmj.2007.066563. [DOI] [PubMed] [Google Scholar]
- 18.Gage BF, VanWalraven C, Pearce L, Hart R, Koudstaal P, Boode B, Petersen P. Selecting Patients With Atrial Fibrillation for Anticoagulation Stroke Risk Stratification in Patients Taking Aspirin. Circulation. 2004;110:2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 19.Zabalgoitia M, Halperin JL, Pearce LA, Black-shear JL, Asinger RW, Hart RG. Transesophag-eal Echocardiographic Correlates of Clinical Risk of Thromboembolism in Nonvalvular Atrial Fibrillation. J Am Coll Cardiol. 1998;31:1622–1626. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 20.Puwanant S, Varr BC, Shrestha K, Hussain SK, Tang W, Gabriel RS, Wazni OM, Bhargava M, Saliba WI, Thomas JD, Lindsay BD, Klein AL. Role of the CHADS2 Score in the Evaluation of Thromboembolic Risk in Patients with Atrial Fibrillation Undergoing Transesophageal Echocardiography Before Pulmonary Vein Isolation. J Am Coll Cardiol. 2009;54:2032–2039. doi: 10.1016/j.jacc.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 21.Rader VJ, Khumri TM, Idupulapati M, Stoner CN, Magalski A, Main ML. Clinical Predictors of Left Atrial Thrombus and Spontaneous Echocardiographic Contrast in Patients with Atrial Fibrillation. J Am Soc Echo. 2007;20:1181–1185. doi: 10.1016/j.echo.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 22.McCready JW, Nunn L, Lambiase PD, Ahsan SY, Segal OR, Rowland E, Lowe MD, Chow AW. Incidence of Left Atrial Thrombus prior to Atrial Fibrillation Ablation: Is Pre-Procedural Transesophageal Echocardiography Mandatory? Europace. 2010;12:927–932. doi: 10.1093/europace/euq074. [DOI] [PubMed] [Google Scholar]
- 23.Klein AL, Murray RD, Grimm RA. Role of Transesophageal Echocardiography-Guided Cardio-version of Patients with Atrial Fibrillation. J Am Coll Cardiol. 2001;37:691–704. doi: 10.1016/s0735-1097(00)01178-5. [DOI] [PubMed] [Google Scholar]
- 24.Stroke Prevention in Atrial Fibrillation Investigators. Predictors of Thromboembolism in atrial Fibrillation: I. Clinical Features of Patients at Risk. Ann Intern Med. 1992;116:1–5. doi: 10.7326/0003-4819-116-1-1. [DOI] [PubMed] [Google Scholar]
- 25.Stroke Prevention in Atrial Fibrillation Investigators. Predictors of Thromboembolism in Atrial Fibrillation: II. Echocardiographic Features of Patients at Risk. Ann Intern Med. 1992;116:6–12. doi: 10.7326/0003-4819-116-1-6. [DOI] [PubMed] [Google Scholar]
- 26.Khan MN, Usmani A, Noor S, Elayi S, Ching CK, Biase LD, Patel D, Burkhardt JD, Cummings J, Schweikert R, Saliba W, Natale A. Low Incidence of Left Atrial or Left Atrial Appendage Thrombus in Patients with Paroxysmal Atrial Fibrillation and Normal EF Who Present for Pulmonary Vein Antrum Isolation Procedure. J Cardiovascular Electrophysiology. 2008;19:356–8. doi: 10.1111/j.1540-8167.2007.01070.x. [DOI] [PubMed] [Google Scholar]