Abstract
Cardiovascular disease accounts for 1 of every 2.9 deaths in the United States, thus the burden of the disease remains high. Given the high mortality and escalating healthcare cost for the disease, it is of urgent need to treat cardiovascular disease effectively. Heme oxygenase-1 (HO-1) catalyzes the oxidation of heme to generate carbon monoxide, biliverdin, and iron. These reaction products of HO-1 have potent anti-inflammatory and anti-oxidative functions. Although HO-1 is expressed at low levels in most tissues under normal basal conditions, it is highly inducible in response to various pathophysiological stresses. Numerous studies have indicated that HO-1 induction is an adaptive defense mechanism to protect cells and tissues against injury in many disease settings. This review highlights the role of HO-1 in inflammation and several cardiovascular diseases—atherosclerosis, myocardial infarction, graft survival after heart transplantation, and abdominal aortic aneurysm. Given that inflammation and oxidative stress are associated with development of cardiovascular disease and that HO-1 has anti-inflammatory and anti-oxidative properties, HO-1 is emerging as a great potential therapeutic target for treating cardiovascular disease.
Keywords: Heme oxygenase-1 (HO-1), inflammation, cardiovascular disease
Introduction
According to the 2011 update on heart disease and stroke statistics from American Heart Association, the 2007 overall death rate from cardiovascular disease was 251.2 per 100,000. Cardiovascular disease accounts for 33.6% of all 2,243,712 deaths in 2007, or 1 of every 2.9 deaths in the United States [1]. It is clear that the burden of cardiovascular disease remains high. Given the high mortality and the escalating healthcare cost, it is of urgent need to treat cardiovascular disease effectively. Numerous studies have shown that the development of cardiovascular disease is associated with inflammation and oxidative stress. Therefore, targeting inflammation and oxidative stress may be of great potential for treating cardiovascular disease.
Heme oxygenase (HO), the rate-limiting enzyme in heme degradation, catalyzes the oxidation of heme to generate several biologically active molecules—carbon monoxide (CO), biliverdin, and ferrous ion [2]. The endogenously produced CO can serve as a second messenger affecting several cellular functions, including inflammation, proliferation, and apoptosis [3, 4]. Biliverdin is subsequently reduced to bilirubin, both of which have antioxidant properties. Ferrous iron induces ferritin expression, which is important for iron sequestration. There are three isoforms in the HO family-HO-1, HO-2, and HO-3. They are products of different genes and are different in their regulation. HO-1 is normally expressed at low levels in most tissues/organs except for spleen; however, it is highly inducible in response to a variety of stimuli to protect cells against oxidative and inflammatory injury [4]. HO-2 is constitutively expressed in most tissues. HO-3 has similar protein structure to HO-2 but with lower enzymatic activity and is less well characterized. Through its induction in response to various pathophysiological stresses and the anti-inflammatory and anti-oxidative functions, numerous studies have indicated the therapeutic potential of HO-1 in the prevention and treatment of cardiovascular disease.
The first genetic deficiency of HO-1 in human was reported in a young boy in 1999 [5] and a second case was reported recently in a young girl [6]. Both patients died in a young age, 6 and 15 years old, respectively [6, 7]. Both cases showed severe inflammatory phenotypes including elevated expression of inflammatory markers such as C-reactive protein, ferritin, and von Willebrand factor. They also had coagulopathy, nephritis, chronic inflammation, and increased susceptibility to atherosclerosis. The human HO-1 deficiency reveals a critical immunomodulatory role of HO-1 and highlights the essential function of HO-1 in human health and disease. This article reviews the roles of HO-1 in inflammation and cardiovascular disease such as atherosclerosis, myocardial infarction, graft survival after heart transplantation, and abdominal aortic aneurysm.
Inflammation and atherosclerosis
Coronary heart disease (CHD) remains a major health issue in the United States and developing countries. The CHD has a prevalence of 7.0 % in adults 20 years of age and it causes 1 of every 6 deaths with the mortality of 406,351 in the United States in 2007 [1]. CHD is a result of manifestations of atherosclerosis. Atherosclerotic lesions in coronary arteries can lead to blockage of blood flow and subsequent ischemia of the heart, which then results in myocardial infarction. The culprit for the initiation of atherosclerosis is thought to be circulating low-density lipoprotein (LDL). LDL particles can accumulate in the intima, become oxidized, and cause endothelial dysfunction. Monocytes and T cells are then attracted to these sites, adhere, and then migrate into the artery wall, forming the earliest atherosclerotic lesion—fatty streak [8]. As such, fatty streak is a pure inflammatory lesion with only monocyte-derived macrophages and T lymphocytes [9]. Further activation of immune cells in the early lesion propagates and amplifies the inflammatory response, attracting more immune cells and inducing medial smooth muscle cell (SMC) migration and proliferation into the lesion, leading to formation of advanced, complicated atherosclerotic lesions. In addition, the inflammatory mediators can cause apoptosis and necrosis of cells within the lesion, resulting in formation of necrotic core and a potential for plaque rupture [8, 10]. It is clear from all the evidences that inflammation plays a key role in the pathogenesis of atherosclerosis, including initiation and progression, and thus atherosclerosis is recognized as a chronic inflammatory disease [8, 10-12]. Strategies targeting inflammation may be of therapeutic benefits in the prevention and treatment of atherosclerosis and cardiovascular disease.
HO-1 in inflammation and atherosclerosis
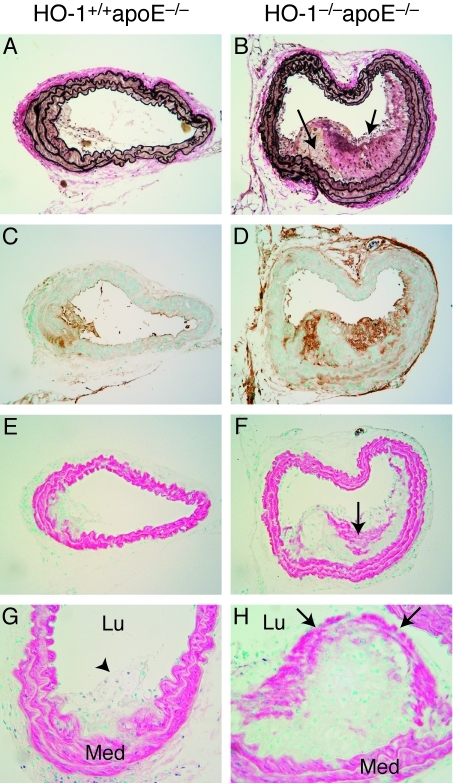
The role of HO-1 in inflammation was revealed in a report showing that upregulation of HO-1 results in suppression of immune effector functions [13]. Furthermore, HO-1 induction by cobalt protoporphyrin decreases the lymphoproliferative alloresponse and differentiation of cytotoxic T cells [13]. Given the anti-inflammatory function of HO-1, it may have a role in atherosclerosis. Interestingly, HO-1 expression is detected in atherosclerotic lesions, supporting a role for HO-1 in atherogenesis [14]. Indeed, HO-1 overexpression by pharmacological inducers or viral gene transfer inhibits atherogenesis in hypercholesterolemic animal models [15-17]. On the other hand, genetic ablation of both apoE and HO-1 in mice accelerates the development of atherosclerosis and exacerbates lesion formation (Figure 1), demonstrating unequivocally an essential role of HO-1 in protecting against atherogenesis [18]. The role of HO-1 in inflammatory cells and atherosclerosis is further emphasized in a study showing that HO-1 expression in macrophages increases antioxidant protection and decreases inflammatory components of atherosclerotic lesions [19]. Decreased and absent HO-1 expression in macrophages correlates with increased proinflammatory cytokines expression, such as monocyte chemotactic protein 1 and IL-6, scavenger receptor A expression, and foam cell formation [19]. It is particularly of interest that the anti-atherogenic effects of a number of mediators, including statins are mediated through HO-1 induction [20, 21]. Additionally, treatment of animals with Ginkgo biloba extract, known for its anti-atherogenic and vascular protective effects, enhances HO-1 expression in circulating monocytes and reduces leukocyte adherence to injured arteries [22]. Collectively, these studies establish that HO-1 is critical in anti-inflammation and protecting against vascular diseases that result from the manifestations of inflammation, such as atherosclerosis.
Figure 1.
Increased atherosclerotic lesion formation in HO-1-/-apoE-/- mice. Representative histological analysis of HO-1+/+apoE-/- and HO-1-/-apoE-/- vessel sections after 8 weeks on Western diet. A and B, Verhoeff's staining for elastin (black) was performed on sections from the proximal brachiocephalic arteries from HO-1+/+apoE-/- (A) and HO-1-/-apoE-/- (B) mice. C and D, MOMA-2 staining for macrophages (brown) in proximal brachiocephalic arteries (x100) from HO-1+/+apoE-/- (C) and HO-1-/-apoE-/- (D) mice. E through H, SM a-actin-stained (red) vessels from HO-1+/+apoE-/- (E and G) and HO-1-/-apoE-/- (F and H) mice. In B, arrows indicate foam cells. In F, arrow indicates smooth muscle cells in the lesion. In G, arrowhead marks a small noncomplex lesion. In H, arrows mark the fibrous cap. Lu, lumen; Med, medial smooth muscle layers. Original magnification xlOO (A through F), and x200 (G and H). Reproduced from Yet etal. [18] with permission.
HO-1 in myocardial infarction
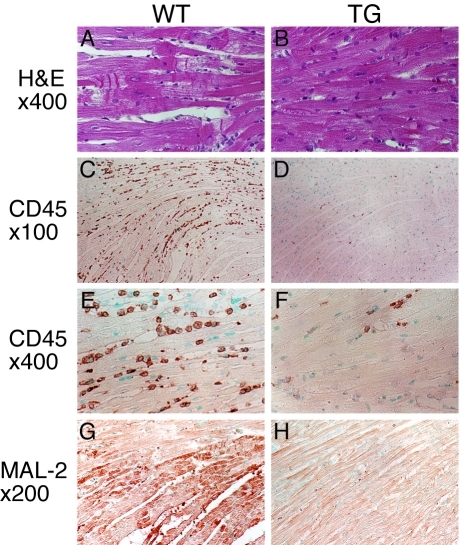
A role for HO-1 in cardiac homeostasis was first implicated in a study showing that HO-1 expression in the heart is increased in response to hyperthermia [23]. A follow-up study showed that ischemia/reperfusion substantially enhances HO-1 expression in the porcine heart, suggesting a potential role of HO-1 in the defense against pathophysiological stress [24]. In a genetic loss-of-function approach using HO-1 null (HO-1-/-) mice, we demonstrate that in contrast to wild type mice, hypoxia induces severe right ventricular dilatation and infarction in HO-1 -/- mice [25]. In addition, an absence of HO-1 exacerbates ischemia/reperfusion-induced myocardial damage [26]. Gain-of-function experiments using cardiac-specific HO-1 transgenic mice reveal that HO-1 overexpression reduces myocardial infarct size and inflammatory cell infiltration following ischemia/reperfusion injury (Figure 2), further demonstrating the protective role of HO-1 against myocardial infarction [27]. Studies using these cardiac-specific HO-1 transgenic mice in a heart failure model show that HO-1 overexpression significantly improved postinfarction survival and alleviated postinfarction pathological left ventricular remodeling [28]. In addition, HO-1 overexpression promotes neovascularization and ameliorates apoptosis in the heart failure model [28]. In light of the cardioprotective function of HO-1 and to avoid unwanted side effects of constitutively overexpressing HO-1, Tang et al. designed a hypoxia-regulated HO-1 gene therapy system, which can sense myocardial ischemia and turn on exogenous HO-1 expression [29, 30]. This vigilant plasmid-mediated HO-1 gene transfer improves contractile and diastolic performance after myocardial infarction [29, 30], indicating the therapeutic potential of HO-1.
Figure 2.
Reduced cardiomyocyte injury, inflammatory cell infiltration, and oxidative damage in transgenic mouse hearts after ischemia and reperfusion. Representative histological analysis of wild-type (WT, n = 4) and TG.H transgenic (TG, n = 4) heart sections after 1 h ischemia and 24 h reperfusion. A, Hematoxylin and eosin (H&E) stained left ventricles from the infarcted area of WT mice. B, H&E stained left ventricles from similar ischemic areas of TG mice. CD45 stained left ventricles from WT (C and E) and TG (D and F) mice. CD45 positive cells stained brown. MAL-2 immu-nostaining (brown) of the left ventricles from WT (G) and TG (H) mice. Original magnification: x400 (A and B), x100 (C and D), x400 (E and F), x200 (G and H). Reproduced from Yet et al. [27] with permission.
Many studies have demonstrated the promising role of adult bone marrow-derived stem cells in regenerating damaged myocardium [31-33]. However, the efficacy of cell therapy is limited by poor viability of graft cells, which might be due in part to ischemia and inflammatory response in the ischemic heart [34]. Interestingly, a hypoxia-regulated HO-1 vector modification of mesenchymal stem cells (MSCs) improves the survival of engrafted MSCs by protecting cells from apoptosis after implantation [35]. The ischemic hearts treated with HO-1-modified MSCs have reduced proinflammatory cytokine production, less inflammatory cell infiltration, and more importantly, have a better cardiac function [35]. In another gene transfer study, injection of adenoviral-HO-1-transduced MSCs into rat hearts 1 h after myocardial infarction reduces infarct size and significantly improves cardiac performance of HO-1-MSCs-treated hearts [36]. Furthermore, HO-1-MSCs-treated hearts have decreased proinflammatory cyto-kine but increased anti-inflammatory cytokine IL -10 expression [36]. Interestingly, HO-1-MSC increases TIMP2/3 expression and decreases MMP2/9 expression and thus normalizes the ratio of MMPs/TIMPs in transplanted hearts, suggesting modulating MMPs/TIMPs system might be another mechanism contributing to cardioprotective effect of HO-1 [37]. In a rat infarction model, transplantation of HO-1-overexpressing-MSCs results in increased resistance to cell apoptosis and death and increased capillary density [38]. Taken together, combining stem cell therapy with HO-1 overexpression might provide a new therapeutic strategy for heart disease.
HO-1 in heart transplantation
Heart transplantation is the most effective therapy for end-stage heart failure. However, patients need lifelong immunosuppression remedy to prevent acute and chronic rejection by immune system for allograft survival and function. Current immunosuppressive drug treatments are effective for acute but not chronic rejection and are associated with significant side effects including renal toxicity, dyslipidemia, and diabetes [39]. Since HO-1 and its byproducts mediate many cytoprotective effects such as anti-inflammation, antioxidant, and anti-apoptosis, HO-1 may play a role in promoting graft survival after transplantation.
Induction of HO-1 has been shown to improve allograft/isograft survival for liver [40], thyroid [41], and kidney [42]. In a mouse cardiac allograft model, induction of HO-1 protects grafts against development of transplant arteriosclerosis, characteristic of chronic rejection [43]. In a mouse-to-rat cardiac xenograft model, the combination therapy of cobra venom factor (CVF) and cyclosporin A (CyA) in recipients effectively prolongs long-term graft survival [44]. Interestingly, this phenomenon is accompanied with HO -1 induction in endothelial cells, SMCs, and cardiac myocytes [45]. The importance of HO-1 in xenograft survival is further demonstrated in a genetic loss-of-function approach by using HO-1 -/- mice. When HO-1+/+ or HO-1-/- hearts are transplanted into rats treated with CVF plus CyA, HO-1+/+ hearts survived long-term (more than 60 days) in rats whereas all HO-1-/- hearts are rejected -3-4 days after transplant [45]. The rejected HO-1-/- hearts exhibit severe thrombosis in major coronary arteries, myocardial infarction, and activated host leukocyte infiltration [45]. On the other hand, hemin treatment induces HO-1 expression and increases the survival time of discordant cardiac xenograft by decreasing interstitial edema, lymphocyte infiltration, myocardial cell apoptosis, and thrombus formation in small vessels, and deposition of xenoantibodies IgM in the intima of vessels in transplanted heart [46]. These results indicate that HO-1 protects xenografts from injury and thus preventing rejection. To explore the therapeutic potential of HO-1 overexpression by gene transfer, recombinant adeno-associated virus (rAAV) was used to increase HO-1 expression in heart grafts in a rat heart transplantation model [47]. rAAV-mediated HO-1 expression not only increases long-term graft survival but also reduces graft atherosclerosis. Furthermore, rAAV/ HO-1 treatment results in fewer infiltrating T cells and macrophages and lower expressions of macrophage migration inhibitory factor, TNF-α, and TGF-β1 [47].
A study with 18 heart transplant patients shows an inverse correlation between HO-1 expression and the degree of cardiomyocyte apoptosis in biopsies from patients [48], suggesting HO-1 protects the graft. Supporting these findings, endomyocardial biopsies of transplanted hearts have higher HO-1 expression in the first two months [49], suggesting induction of HO-1 may protect transplanted hearts from injury [49]. In humans, a (GT)n dinucleotide repeat in the HO-1 gene promoter shows a length polymorphism that modulates the level of gene transcription [50]. Short (<25 GT) repeats are associated with an increased HO-1 upregulation in response to inflammatory stimuli than are longer repeats [51]. This ability to upregulate HO-1 serves as an endogenous protective mechanism in many human cardiovascular diseases [52]. Thus, one would predict that heart transplants with a short (GT)n HO-1 gene promoter polymorphism have a better outcome. However, a study with 152 heart allograft recipients with at least one-year survival reveals that HO-1 gene promoter polymorphism does not show an association with the development of cardiac allograft vasculopathy in heart transplants [53]. Another study also indicates no influence of HO-1 gene promoter polymorphism on the development of heart failure, graft survival, acute rejection, or transplant atherosclerosis [54]. Interestingly, Ohmann et al. [55] recently found that HO-1 polymorphisms with higher expression of HO-1 correlate with a reduced risk of late post-transplantation infection in pediatric heart. Although it is clear that HO-1 protects heart graft in animal models, the role of HO-1 in human heart transplants is somewhat controversial and thus warrants further study.
HO-1 in abdominal aortic aneurysm
Abdominal aortic aneurysm (AAA) is a relatively common and often fatal condition that primarily affects older patients [56, 57]. It is a leading cause of sudden death in men older than 55 years [58]. AAA is a localized dilatation of the abdominal aorta exceeding the normal diameter (∼2 cm) by more than 50% [59], which is characterized by chronic aortic wall inflammation, loss of medial SMCs, and connective tissue degradation and remodeling. AAA is associated with old age, male gender, cigarette smoking, atherosclerosis, hypertension, and a genetic disposition [60, 61]. Screening studies in Europe show that -5% of men 65 years of age have AAAs of 3 cm or more [62]. In the United States, the prevalence of AAAs 2.9 to 4.9 cm in diameter ranges from 1.3% in men 45 to 54 years of age to 12.5% in men 75 to 84 years of age [1]. With an aging population, the incidence and prevalence of AAA is certain to go up. Although the pathogenesis of AAA is not completely elucidated yet, it is well accepted that inflammation and oxidative stress are key factors inducing AAA formation. Inflammation-mediated proteolysis and disorganized extracellular remodeling within the aortic wall are critical pathophysiological events leading to progressive aortic enlargement and ultimate rupture [63]. In experimental animal models of AAA, genetic and pharmacological inhibition of reactive oxygen species (ROS) production and MMPs suppress aneurysm formation [64-66]. Thus, reducing ROS generation and inflammation and inhibiting MMP activity might be useful therapeutic strategies.
Interestingly, patients with AAA are less frequently carriers of short (<25 GT) repeat in the HO-1 gene promoter than healthy subjects. This suggests that short alleles, and thus, increased upregulation of HO-1, may be a protective anti-inflammatory factor against the development of AAA [67]. In animal models, flow loading significantly increases HO-1 expression and attenuates AAA formation [64]. Concomitant with increased HO-1 expression, ROS production is significantly reduced in flow-loaded AAAs, suggesting that flow loading and HO-1 induction may attenuate AAA enlargement via wall shear or strain-related reductions in oxidative stress [64]. Further supporting the notion that HO-1 may affect development and progression of AAA, HO-1 is shown to reduce levels of MMPs and inflammation. For example, HO-1 has been suggested to reduce MMP9 levels in human carotid enarterectomy tissues with intraplaque hemorrhage [68] and in human atheromatous plaque [69]. The effects of HO-1 on MMPs may influence the stability of AAAs by attenuating elastin degradation and thereby preventing AAA rupture. The medial SMC apoptotic death contributes to the reduction of cellularity and subsequent impairment for the repair and maintenance of the arterial extracellular matrix in AAAs [70]. HO-1 also has anti-apoptotic activity [71], thus, HO-1 may participate in decreasing SMC apoptosis. Taken together, with the anti-oxidative, anti-inflammatory, and anti-apoptotic activities of HO-1, HO-1 has a great potential to prevent/attenuate the development/ progression of AAA. The role of HO-1 in the pathogenesis of AAA certainly warrants further investigation.
Therapeutic opportunities
As described above, through its enzymatic products HO-1 mediates many cellular functions in protecting cells and tissues against inflammation and oxidative stress. Numerous studies have indicated the protective function of HO-1 in many cardiovascular diseases. In light of the socioeconomic burden of cardiovascular disease, the therapeutic potential of HO-1 is of particular interest. Thus, induction of HO-1 pharmacologically or by other means might be a promising therapeutic intervention for preventing and treating these inflammatory cardiovascular diseases.
Acknowledgments
This work was supported in part by National Health Research Institutes (Taiwan) grant CS-100-PP-04 (to S.-F.Y.) and National Science Council (Taiwan) grant 98-2320-B-400-004-MY3 (to S.-F.Y.). This research was conducted in part under the Graduate Program of Biotechnology in Medicine sponsored by the National Tsing Hua University and National Health Research Institutes.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, Roger VL, Turner MB. Heart Disease and Stroke Statistics 2011 Update: A Report From the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 3.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 4.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 5.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radhakrishnan N, Yadav SP, Sachdeva A, Pruthi PK, Sawhney S, Piplani T, Wada T, Yachie A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol. 2011;33:74–78. doi: 10.1097/MPH.0b013e3181fd2aae. [DOI] [PubMed] [Google Scholar]
- 7.Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 11.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 12.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 13.Woo J, Iyer S, Cornejo MC, Mori N, Gao L, Sipos I, Maines M, Buelow R. Stress protein-induced immunosuppression: inhibition of cellular immune effector functions following overexpression of haem oxygenase (HSP 32) Transpl Immunol. 1998;6:84–93. doi: 10.1016/s0966-3274(98)80022-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol. 1998;152:711–720. [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa K, Sugawara D, Wang X, Suzuki K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa K, Sugawara D, Goto J, Watanabe Y, Kawamura K, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104:1831–1836. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- 17.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 18.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 19.Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih DM, Agarwal A, Lusis AJ, Araujo JA. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res. 2007;100:1703–1711. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- 20.Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 21.Heeba G, Moselhy ME, Hassan M, Khalifa M, Gryglewski R, Malinski T. Anti-atherogenic effect of statins: role of nitric oxide, peroxynitrite and haem oxygenase-1. Br J Pharmacol. 2009;156:1256–1266. doi: 10.1111/j.1476-5381.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JS, Huang PH, Wang CH, Lin FY, Tsai HY, Wu TC, Lin SJ, Chen JW. Nrf-2 mediated heme oxygenase-1 expression, an antioxidantindependent mechanism, contributes to antiatherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis. 2011;214:301–309. doi: 10.1016/j.atherosclerosis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Ewing JF, Raju VS, Maines MD. Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3':5'-guanosine monophosphate. J Pharmacol Exp Ther. 1994;271:408–414. [PubMed] [Google Scholar]
- 24.Sharma HS, Maulik N, Gho BC, Das DK, Verdouw PD. Coordinated expression of heme oxygenase-1 and ubiquitin in the porcine heart subjected to ischemia and reperfusion. Mol Cell Biochem. 1996;157:111–116. doi: 10.1007/BF00227888. [DOI] [PubMed] [Google Scholar]
- 25.Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Wei J, Peng DH, Layne MD, Yet SF. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54:778–784. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- 27.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Protection from ischemic heart injury by a vigilant heme oxygenase-1 plasmid system. Hypertension. 2004;43:746–751. doi: 10.1161/01.HYP.0000120152.27263.87. [DOI] [PubMed] [Google Scholar]
- 30.Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J Cardiovasc Pharmacol Ther. 2005;10:251–263. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- 31.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 33.Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 2004;364:183–192. doi: 10.1016/S0140-6736(04)16632-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 35.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 36.Zeng B, Chen H, Zhu C, Ren X, Lin G, Cao F. Effects of combined mesenchymal stem cells and heme oxygenase-1 therapy on cardiac performance. Eur J Cardiothorac Surg. 2008;34:850–856. doi: 10.1016/j.ejcts.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 37.Shu T, Zeng B, Ren X, Li Y. HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue Cell. 2010;42:217–222. doi: 10.1016/j.tice.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Tsubokawa T, Yagi K, Nakanishi C, Zuka M, Nohara A, Ino H, Fujino N, Konno T, Kawashiri MA, Ishibashi-Ueda H, Nagaya N, Yamagishi M. Impact of anti-apoptotic and antioxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298:H1320–1329. doi: 10.1152/ajpheart.01330.2008. [DOI] [PubMed] [Google Scholar]
- 39.Vassalli G, Fleury S, Li J, Goy JJ, Kappenberger L, von Segesser LK. Gene transfer of cytoprotective and immunomodulatory molecules for prevention of cardiac allograft rejection. Eur J Cardiothorac Surg. 2003;24:794–806. doi: 10.1016/s1010-7940(03)00456-1. [DOI] [PubMed] [Google Scholar]
- 40.Ke B, Buelow R, Shen XD, Melinek J, Amersi F, Gao F, Ritter T, Volk HD, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase 1 gene transfer prevents CD95/Fas ligand-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Hum Gene Ther. 2002;13:1189–1199. doi: 10.1089/104303402320138970. [DOI] [PubMed] [Google Scholar]
- 41.Niimi M, Takashina M, Takami H, Ikeda Y, Shatari T, Hamano K, Esato K, Matsumoto K, Kameyama K, Kodaira S, Wood KJ. Overexpression of heme oxygenase-1 protects allogeneic thyroid grafts from rejection in naive mice. Surgery. 2000;128:910–917. doi: 10.1067/msy.2000.109968. [DOI] [PubMed] [Google Scholar]
- 42.Blydt-Hansen TD, Katori M, Lassman C, Ke B, Coito AJ, Iyer S, Buelow R, Ettenger R, Busuttil RW, Kupiec-Weglinski JW. Gene transferinduced local heme oxygenase-1 overexpression protects rat kidney transplants from ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:745–754. doi: 10.1097/01.asn.0000050760.87113.25. [DOI] [PubMed] [Google Scholar]
- 43.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 44.Bach FH, Ferran C, Hechenleitner P, Mark W, Koyamada N, Miyatake T, Winkler H, Badrichani A, Candinas D, Hancock WW. Accommodation of vascularized xenografts: expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- 45.Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 46.Zhen-Wei X, Jian-Le S, Qi Q, Wen-Wei Z, Xue-Hong Z, Zi-Li Z. Heme oxygenase-1 improves the survival of discordant cardiac xenograft through its anti-inflammatory and antiapoptotic effects. Pediatr Transplant. 2007;11:850–859. doi: 10.1111/j.1399-3046.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- 47.Tsui TY, Wu X, Lau CK, Ho DW, Xu T, Siu YT, Fan ST. Prevention of chronic deterioration of heart allograft by recombinant adenoassociated virus-mediated heme oxygenase-1 gene transfer. Circulation. 2003;107:2623–2629. doi: 10.1161/01.CIR.0000066911.03770.8D. [DOI] [PubMed] [Google Scholar]
- 48.Chok R, Senechal M, Dorent R, Mallat Z, Leprince P, Pavie A, Ghossoub JJ, Gandjbakhch I. Apoptosis and expression of heme oxygenase-1 in heart transplant recipients during acute rejection episode. Transplant Proc. 2002;34:2815–2818. doi: 10.1016/s0041-1345(02)03526-1. [DOI] [PubMed] [Google Scholar]
- 49.Souza AI, Felkin LE, McCormack AM, Holder A, Barton PJ, Banner NR, Rose ML. Sequential expression of three known protective genes in cardiac biopsies after transplantation. Transplantation. 2005;79:584–590. doi: 10.1097/01.tp.0000153154.37616.94. [DOI] [PubMed] [Google Scholar]
- 50.Kimpara T, Takeda A, Watanabe K, Itoyama Y, Ikawa S, Watanabe M, Arai H, Sasaki H, Higuchi S, Okita N, Takase S, Saito H, Takahashi K, Shibahara S. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum Genet. 1997;100:145–147. doi: 10.1007/s004390050480. [DOI] [PubMed] [Google Scholar]
- 51.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Ullrich R, Exner M, Schillinger M, Zuckermann A, Raith M, Dunkler D, Horvat R, Grimm M, Wagner O. Microsatellite polymorphism in the heme oxygenase-1 gene promoter and cardiac allograft vasculopathy. J Heart Lung Transplant. 2005;24:1600–1605. doi: 10.1016/j.healun.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Holweg CT, Balk AH, Uitterlinden AG, Niesters HG, Maat LP, Weimar W, Baan CC. Functional eme oxygenase-1 promoter polymorphism in relation to heart failure and cardiac transplantation. J Heart Lung Transplant. 2005;24:493–497. doi: 10.1016/j.healun.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Ohmann EL, Brooks MM, Webber SA, Girnita DM, Ferrell RE, Burckart GJ, Chinnock R, Canter C, Addonizio L, Bernstein D, Kirklin JK, Naftel DC, Zeevi A. Association of genetic polymorphisms and risk of late posttransplantation infection in pediatric heart recipients. J Heart Lung Transplant. 2010;29:1342–1351. doi: 10.1016/j.healun.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Bickerstaff LK, Hollier LH, Van Peenen HJ, Melton LJ, 3rd, Pairolero PC, Cherry KJ. Abdominal aortic aneurysms: the changing natural history. J Vasc Surg. 1984;1:6–12. [PubMed] [Google Scholar]
- 57.Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson RW. Detection and management of small aortic aneurysms. N Engl J Med. 2002;346:1484–1486. doi: 10.1056/NEJM200205093461910. [DOI] [PubMed] [Google Scholar]
- 59.Pennell RC, Hollier LH, Lie JT, Bernatz PE, Joyce JW, Pairolero PC, Cherry KJ, Hallett JW. Inflammatory abdominal aortic aneurysms: a thirty-year review. J Vasc Surg. 1985;2:859–869. [PubMed] [Google Scholar]
- 60.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 61.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994-2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 62.Scott RA, Ashton HA, Kay DN. Abdominal aortic aneurysm in 4237 screened patients: prevalence, development and management over 6 years. Br J Surg. 1991;78:1122–1125. doi: 10.1002/bjs.1800780929. [DOI] [PubMed] [Google Scholar]
- 63.Shah PK. Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation. 1997;96:2115–2117. doi: 10.1161/01.cir.96.7.2115. [DOI] [PubMed] [Google Scholar]
- 64.Nakahashi TK, Hoshina K, Tsao PS, Sho E, Sho M, Karwowski JK, Yeh C, Yang RB, Topper JN, Dalman RL. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2002;22:2017–2022. doi: 10.1161/01.atv.0000042082.38014.ea. [DOI] [PubMed] [Google Scholar]
- 65.Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, Pure E, FitzGerald GA. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II -induced abdominal aortic aneurysm formation. Circulation. 2008;117:1302–1309. doi: 10.1161/CIRCULATIONAHA.107.731398. [DOI] [PubMed] [Google Scholar]
- 66.Wu G, Chen T, Shahsafaei A, Hu W, Bronson RT, Shi GP, Halperin JA, Aktas H, Qin X. Complement regulator CD59 protects against angiotensin II-induced abdominal aortic aneurysms in mice. Circulation. 2010;121:1338–1346. doi: 10.1161/CIRCULATIONAHA.108.844589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schillinger M, Exner M, Mlekusch W, Domanovits H, Huber K, Mannhalter C, Wagner O, Minar E. Heme oxygenase-1 gene promoter polymorphism is associated with abdominal aortic aneurysm. Thromb Res. 2002;106:131–136. doi: 10.1016/s0049-3848(02)00100-7. [DOI] [PubMed] [Google Scholar]
- 68.Choudhary S, Higgins CL, Chen IY, Reardon M, Lawrie G, Vick GW, 3rd, Karmonik C, Via DP, Morrisett JD. Quantitation and localization of matrix metalloproteinases and their inhibitors in human carotid endarterectomy tissues. Arterioscler Thromb Vasc Biol. 2006;26:2351–2358. doi: 10.1161/01.ATV.0000239461.87113.0b. [DOI] [PubMed] [Google Scholar]
- 69.Cheng C, Noordeloos AM, Jeney V, Soares MP, Moll F, Pasterkamp G, Serruys PW, Duckers HJ. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009;119:3017–3027. doi: 10.1161/CIRCULATIONAHA.108.808618. [DOI] [PubMed] [Google Scholar]
- 70.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 71.Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]