Abstract
Background: Left ventricular (LV) hypertrophy is an independent predictor of increased cardiovascular morbidity and mortality. It remains unclear whether components of the metabolic syndrome are associated with LV hypertrophy. Methods and Results: Accordingly, we analyzed echocardiograms in 192 consecutive ambulatory patients referred for echocardiography from October to December 2004. Patients were excluded if they had atrial fibrillation, significant valvular heart disease or failed to cooperate for echocardiogram. Of these, 126 (66%) patients met Adult Treatment Panel (ATP) III diagnostic criteria for the metabolic syndrome. 29% had any 3 metabolic syndrome components, 18% had any 4 metabolic syndrome components and 17% had all 5 metabolic syndrome components. In analyses of variance adjusted for age and sex, LV mass and LV mass adjusted to its allometric relation to height2.7 (LV mass/height2.7) were higher in patients with metabolic syndrome compared to those without metabolic syndrome (237 g [228-239 95%CI] vs. 224 g [206-239 95%CI] p=0.005 and 62 g/m2.7 [59-65 95%CI] vs. 56 g/m2.7 [52-60 95%CI] p=0.014, respectively). The prevalence of LV hypertrophy using prognostically-validated gender-specific partition values for LV mass/height2.7 was significantly higher in metabolic syndrome patients than in those without metabolic syndrome (81 v. 58%, p<0.001). There was a step-wise increase in LV mass/height2.7 in those with no metabolic syndrome components to those with increasing number of metabolic syndrome components (Figure, p<0.001). In this study of high-risk patients, the significant independent predictors of LV hypertrophy were only high blood pressure (OR=3.2, p=0.008) and increased waist circumference (OR=2.8, p=0.006) with no interaction between blood pressure and waist circumference. Conclusion: Metabolic syndrome is associated with higher LV mass and prevalence of LV hypertrophy. Increasing number of metabolic syndrome components is associated with step-wise increases in LV mass. Identification of LV hypertrophy in metabolic syndrome patients may provide an additional prognostic tool to further risk-stratify these patients.
Keywords: Metabolic syndrome, hypertension, left ventricle, hypertrophy, abdominal obesity
Introduction
The metabolic syndrome is constellation of interrelated risk factors of metabolic origin that are associated with heightened risk for cardiovascular disease cardiovascular mortality and diabetes [1-4]. The Third Report of the National Cholesterol Education Program Expert Panel of Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults provided a definition of the metabolic syndrome [5]. Studies have shown higher prevalence the metabolic syndrome in African Americans and Hispanics and suggest that these ethnic groups may be at higher cardiac risk [3-4].
Left ventricular (LV) hypertrophy has been shown to be a strong independent predictor of increased cardiovascular morbidity and mortality [1-2, 6-8] and has been used a surrogate to assess cardiovascular risk in epidemiologic studies. Population-based studies have indicated an association between the metabolic syndrome and abnormal LV geometry [9-14]. However, it remains unclear whether metabolic syndrome is associated with LV mass independently of its components in high-risk patients. Therefore, we conducted a cross-sectional study determine the relation of the metabolic syndrome and its components to abnormal LV geometry in an ambulatory clinical cohort of Hispanic and non-Hispanic blacks adults.
Methods
Study population
We reviewed results of clinical, laboratory and echocardiographic examinations of all ambulatory patients who were referred for echocardiography evaluation from October to December 2004. Patients with coronary heart disease, atrial fibrillation, significant (>2+) valvular heart disease or failed to cooperate for the echocardiographic procedure were excluded from the analyses. The protocol was reviewed and approved by the Institutional Review Board of the Bronx-Lebanon Hospital Center.
We abstracted data on the following variables: age, race, height, weight, blood pressure (BP), fasting lipid profile (serum total cholesterol, high density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides and fasting glucose. In addition, information on diagnosis and treatment for hypertension, type 2 diabetes mellitus (DM) and dyslipidemia was also collected. Hypertension was defined based on the Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure recommendations or taking antihypertensive medications [15]. DM was defined based on the American Diabetes Association recommendations or taking insulin or oral hypoglycemic agents [16]. Body mass index (BMI) was calculated as weight in kg divided by height (m2).
The metabolic syndrome was defined based on modified National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria [5] and the methodology used elsewhere [5, 17] was applied. The NCEP ATP III [5] guidelines define the metabolic syndrome as having three or more of the following abnormalities: waist circumference (WC) in females > 88 cm and WC>102 cm in men ,fasting serum glucose ≥ 110 mg/dL, fasting serum triglycerides ≥ 150 mg/dL, HDL cholesterol < 50mg/dl, and blood pressure ≥ 130/85 mm/Hg. Patients treated for hypertension, DM and dyslipidemia were regarded as having high blood pressure, hyperglyceridemia, elevated triglycerides and low HDL cholesterol respectively when MBS parameters were attributed to the study patients. WC measurements were not obtained in all study patients. Previous studies have shown [18-21] a significant correlation between BMI and waist circumference. In our study, we used the previously described methodology for supplementing BMI as a surrogate of WC [22]. As a reference population to determine the cut-off values of BMI corresponding to ATP III defined WC cut-off values, we used data from National Health and Nutrition Examination Survey (NHANES) 2001-2002 participants. From the NHANES 2001-2002 dataset, we extracted the subset of n=2376 participants with following characteristics: demographically Non-Hispanic black 43 % and Hispanic 57%, with mean age of 45.96 (95% CI 45.2-46.68) years and BMI of 28.73 (95% CI 28.5 -29.0) kg/m2. In this NHANES 2001-2002 subset population, a waist circumference of 88 cm in women corresponded to a BMI of 26.08 kg/m2 (r=0.86, p< 0.0001) and waist circumference of 102 cm in men corresponded to a BMI of 29.64 kg/m2 (r=0.80, p< 0.0001).
Echocardiographic protocol
The parasternal acoustic window was used to record 35 consecutive beats of 2-dimensional and M-mode recordings of the LV internal diameter and wall thicknesses at or just below the tips of the mitral valve leaflets in long and short-axis views at baseline and immediately port-exercise. Correct orientation of planes for imaging and Doppler recordings was verified using standard procedures [16, 23]. All echocardiographic measurements were made using a computerized review station by an experienced reader (JNB) blinded to patient demographics.
LV internal dimension and interventricular septal and posterior wall thicknesses were measured at end-diastole and end-systole according to American Society of Echocardiography recommendations on up to 3 cycles [23]. When optimal orientation of the LV could not be obtained by M-mode, correctly oriented 2-D linear dimensions were made by the leading-edge convention according to ASE recommendation [23]. A close correlation between these two methods has been previously reported [24]. End-diastolic ASE LV dimensions were used to calculate LV mass by a formula that yields values closely related (r=0.90, p<0.001) to necropsy LV weight [25] and which showed excellent reproducibility (intraclass correlation coefficient = 0.93, p < 0.001) between two separate echocardiograms in 183 hypertensive patients [26] LV hypertrophy was defined using ex-specific partition values in men and women for LV mass adjusted to its allometric relation to height (height2.7) [10, 27]. Values of LV mass/height 2.7 more than 49.2 g/m 2.7 in men or 46.7 g/m 2.7 in women identified LV hypertrophy [28]. Relative wall thickness, a measure of LV concentricity, was calculated as 2 x end-diastolic posterior wall thickness/LV internal diameter [23]. Normal geometry was present when LV mass index and relative wall thickness were normal, normal LV mass index and increased relative wall thickness was classified as concentric remodeling, increased LV mass index but normal relative wall thickness identified eccentric LV hypertrophy and increases in both variables identified concentric LV hypertrophy [29].
Statistical analysis
Statistical analysis was performed with the JMP statistical software, version 4.0 and SAS statistical software version 8.2 (SAS Institute Inc). Results are reported as the means with 95% confidence intervals or percentages. We assessed the normality of the distribution of all variables using the normal quintile plot method. For comparison of continuous variables between the two groups, we used the unpaired two sided tests t-test. The analysis of covariance model was used to adjust for age, sex and BMI as confounding variables. Least square means are reported for adjusted continuous variables. Cochran-Armitage trend test was used to analyze trend in groups with ordinal variables. Dichotomous variables were compared by chi-square analysis using the Pearson test. Logistic regression analysis models were used in model for ascertaining effects of various independent variables on LV geometry, LV mass and to calculate odds ratios. Parameter estimates was used to evaluate interation between variables. Two-tailed p-values < 0.05 were indicated statistical significance.
Results
A total of 290 patients were evaluated during the specified time period. Of these, 192 patients fulfilled the inclusion criteria. The study population (n=192) consisted of non-Hispanic blacks 55 % (n=106) and Hispanic 45% (n=86). Females were 58% (n=111) and male 42 % (n=81). The overall prevalence of the metabolic syndrome in the study participants was 66 % (n=126). The clinical characteristics of the patients with and without the metabolic syndrome are summarized in Table 1. Metabolic syndrome was significantly more prevalent among Non-Hispanic blacks than Hispanic 61% vs. 39 % respectively (p = 0.02) and among female than male 63% vs. 37 % (p 0.03) in the population. The prevalence of previously diagnosed and treated hypertension, DM type 2, dislipidemia, and coronary artery disease were 68%, 43%, 34%, and 12% respectively. The study population did not differ by age, diastolic BP, total cholesterol and LDL levels among those with and without the metabolic syndrome, but by definition those with metabolic syndrome had significantly higher BMI, systolic BP, fasting glucose and triglyceride levels and lower HDL levels (Table 1).
Table 1.
Clinical features of patients with and without the metabolic syndrome
| Variable | MBS (N = 126) | No MBS (N = 66) | P |
|---|---|---|---|
| Age, years | 61 (95% CI: 58-64) | 60 (95% CI: 59-64) | 0.50 |
| Female, N (%) | 80 (63%) | 31 (47%) | 0.028 |
| Hispanic Ethnicity, N (%) | 49 (39%) | 37 (56%) | 0.023 |
| Body Mass Index, kg/m2 | 32 (95% CI: 30-33) | 25 (95% CI: 23-27) | <0.001 |
| Systolic BP, mm Hg | 131 (95% CI: 131-138) | 128 (95% CI: 123-133) | 0.04 |
| Diastolic BP, mm Hg | 76 (95% CI: 73-78) | 73(95%CI: 70-76) | 0..14 |
| Fasting Glucose, mg/dL | 149 (95% CI: 135-164) | 113 (95% CI: 93-133) | 0.005 |
| Total Cholesterol, mg/dL | 172 (95% CI: 164-181) | 183(95%CI: 172-194) | 0.13 |
| LDL Cholesterol, mg/dL | 97 (95% CI: 91-104) | 99 (95% CI: 90-109) | 0.71 |
| HDL Cholesterol, mg/dL | 44 (95% CI: 41-47) | 61 (95% CI: 57-66) | <0.0001 |
| Triglycerides, mg/dL | 159 (95% CI: 145-173) | 101 (95% CI: 81-120) | <0.0001 |
| Diabetes Mellitus, N (%) | 70 (56%) | 13 (20%) | <0.0001 |
| Hypertension, N (%) | 99 (79%) | 31 (47%) | <0.0001 |
| Dyslipidemia, N (%) | 60 (48%) | 5 (8%) | <0.0001 |
| Coronary Artery Disease, N (%) | 17 (13%) | 6 (9%) | 0.36 |
Among individual components of the metabolic syndrome, most prevalent was high blood pressure (89%), followed by high fasting glucose (76%), high waist circumference (WC) (75%), hypertriglyceridemia (51%) and low HDL cholesterol (51%). In the study only 2 patients (1%) had no of metabolic syndrome components, 31 (16.1%) had any one metabolic syndrome component, 33 (17.2%) had any two metabolic syndrome components, 55 (28.6%) had any three metabolic syndrome components, 37 (19.3%) had any four metabolic syndrome components and 34 (17.7%) had all five metabolic syndrome components present.
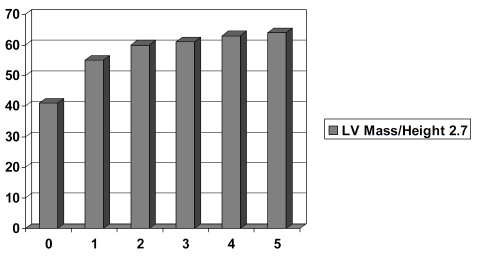
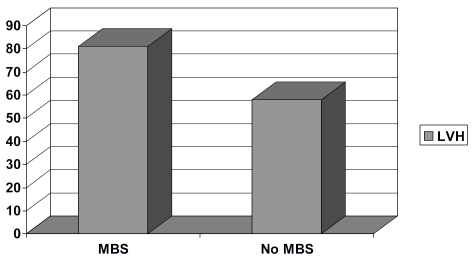
In ANCOVA adjusting for age, sex, and WC, patients with metabolic syndrome had significantly higher mean LV mass and LV mass/height2.7 (Table 2). There were step-wise increases in LV mass/height2.7 from no metabolic syndrome to one metabolic syndrome component to all five metabolic syndrome components in the study population (Figure 1). Furthermore, the prevalence of LV hypertrophy was statistically significantly higher in metabolic syndrome patients than in those without metabolic syndrome (Figure 2). Prevalence of the study participants with concentric LV geometry, defined as relative wall thickness (RWT) > 0.42, was 54% (n=103).
Table 2.
Left ventricular geometry in patients with and without the metabolic syndrome
| Variable | MBS (N=126) | No MBS (N=66) | P |
|---|---|---|---|
| LV Mass, g | 238 (95% CI: 227-250) | 220 (95% CI: 204-236) | 0.001 |
| LV Mass/Body Surface Area, g/m2 | 129 (95% CI: 123-136) | 126 (95% CI: 117-136) | 0.147 |
| LV Mass/Height2.7, g/m2.7 | 62 (95% CI: 59-65) | 56 (52-60) | <0.0001 |
| Relative Wall Thickness | 0.43 (95% CI:0.42-0.44) | 0.40 (0.40-0.44 | 0.048 |
Figure 1.
LV mass/height 2.7 (g/m2.7, y-axis) in relation to number of MBS components (x-axis).
Figure 2.
Prevalence of LV hypertrophy (y-axis) in patients with and without MBS.
Logistic regression models were used for concentric LV geometry and LV hypertrophy adjusted for height2.7 as response variables and individual metabolic syndrome components among model effects. In logistic regression, only high blood pressure (OR=3.2, p=0.008) and increased WC (OR=2.8, p=0.006) were significant predictors of LV hypertrophy (Table 3). When similar analysis was done for concentric LV geometry, none of MBS parameters predicted concentric LV geometry (data not shown).
Table 3.
Risk of left ventricular hypertrophy with components of the metabolic syndrome
| Component | Unadjusted OR (95% CI), p | Adjusted OR (95% CI), p |
|---|---|---|
| High Blood Pressure | 4.1 (1.91-90.3), 0.0003 | 3.2 (1.34-7.49), 0.008 |
| High Waist Circumference | 2.9 (1.42-5.86), 0.004 | 2.8 (1.35-5.93), 0.006 |
| Hyperglycemia | 1.9 (0.93-3.86), 0.08 | 1.8 (0.84-3.62), 0.01 |
| Hypertriglyceridemia | 1.1 (0.51-2.41), 0.8 | 1.1 (0.49-2.43), 0.8 |
| Low HDL Cholesterol | 1.0 (0.46-2.17), 0.9 | 1.1 (0.49-2.44), 0.58 |
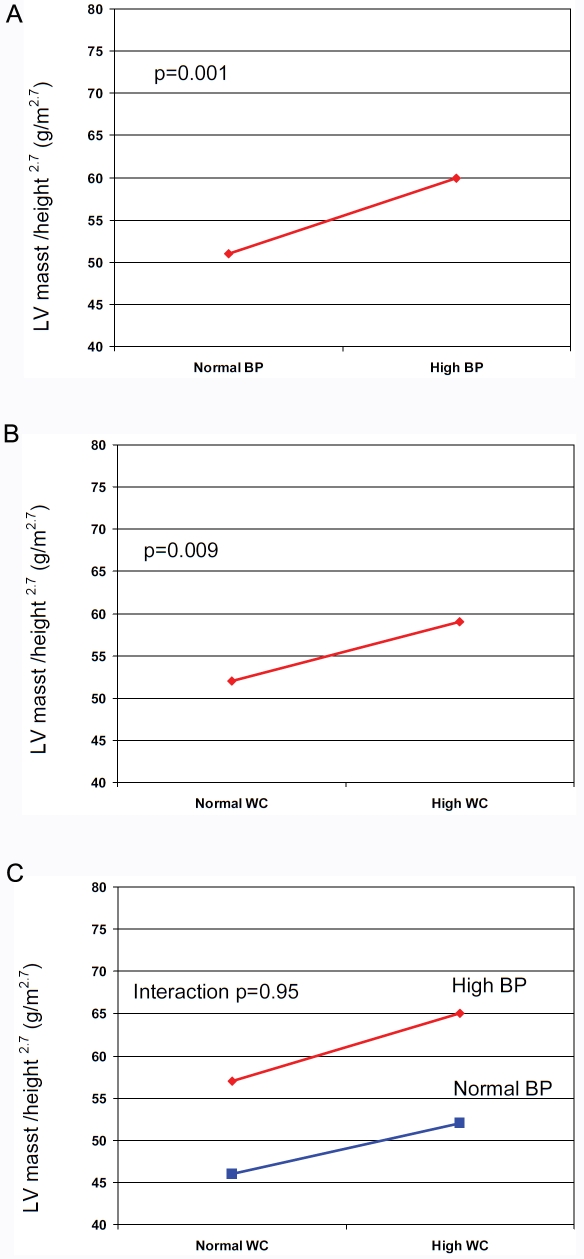
Since high blood pressure and elevated WC significantly predicted LV hypertrophy, we assessed the effects of these metabolic syndrome components on LV hypertrophy (Figure 3). As seen in Figure 3A, adjusted LV mass was significantly higher among patients with the metabolic syndrome and high blood compared to whose with normal blood pressure (61 vs. 51 g/m 2.7 respectively, p=0.001). Similarly, in patients with the metabolic syndrome and increased WC, adjusted LV mass was significantly higher than in those with normal WC (60 vs. 53 g/m 2.7 respectively, p=0.009) (Figure 3B). When blood pressure and WC were used as combined variable, the parameter estimate for the analysis was negative for interaction (p=0.95). Hence, the effects of high blood pressure and increased WC on LV hypertrophy are additive (Figure 3C). Similar analysis was performed for the RWT as independent variable did not reveal differences between blood pressure and WC groups (data not shown).
Figure 3.
Impact of high blood blood pressure only (3A), waist circumference only (3B) and both high blood pressure and waist circumference on adjusted LV mass in patients with the metabolic syndrome.
Discussion
Our study indicates that in an inner city, ambulatory clinical cohort of Non-Hispanic blacks and Hispanic patients, metabolic syndrome is highly prevalent and is associated with higher LV mass and prevalence of LV hypertrophy. In our study population with high burden of cardiovascular risk factors, high blood pressure and increased WC, were significant predictors of LV hypertrophy and those effects were additive.
It is well-established that the prevalence of the metabolic syndrome differs among various population groups [3-4, 17, 22, 30] and it is increasing within US population [31]. When methodology similar to our study was used by Ford et al. [31] to evaluate prevalence of the metabolic syndrome in representative US population, NHANES 1999-2000, age-adjusted prevalence of metabolic syndrome was 25.1 % with a slightly higher prevalence in men (27.9%) than in women (22.6%). Among blacks, women had a 50% higher age-adjusted prevalence of the metabolic syndrome than did men (36.7 vs 24.5%) [24]. In the American Indian participants in the Strong Heart Study (SHS), diagnosis of the metabolic syndrome was made in 43% of the study population with significantly higher prevalence in women than in men (51% vs 30%) [12]. Similarly, the San Antonio Heart Study of 1656 Mexican-American subjects showed that the metabolic syndrome was 13% higher in Mexican-American women than in men (32.8 vs 29%) [4]. Our study extends these observations from population-based studies that indicate a higher prevalence of metabolic syndrome in women than in men. The higher prevalence of the metabolic syndrome in our cohort (66%) is due to that fact that this is a clinical cohort of ambulatory patients referred for an echocardiogram.
As expected from a clinical cohort, prevalence of diagnosed and treated hypertension, type II DM and dyslipidemia were high: 68%, 43% and 34 %, respectively. These proportions are consistent with findings of the Atherosclerosis Risk in Communities (ARIC) study of a representative sample selected from black residents in Jackson Mississippi (mean age=59 years), where 54 % of women and 42 % of men had hypertension, 24 % of women and 21% of men had type II DM and 38 % of women and 39% of men had dyslipidemia [9].
Several population-based studies have shown the association of ATP III-defined metabolic syndrome with abnormal cardiac structure and function. In the American Indian SHS participants, mean LV mass was higher in participants with metabolic syndrome than in those without metabolic syndrome by 17 g (about 11%) [10]. Similarly, among black participants of ARIC study, age-adjusted mean LV mass/height increased progressively with increasing components of the metabolic syndrome in both men and women from 125 and 131 g/m in those with no metabolic syndrome component to 169 and 170 g/m in those with three metabolic syndrome components in men and women, respectively [9]. Furthermore, in a clinical cohort of hypertensive non-diabetic patients [12], LV mass was higher in patients with metabolic syndrome (220 g) than in patients without metabolic syndrome (178 g). Our results extend the results of these studies by showing that LV mass was higher in an ambulatory clinical cohort of Hispanic and non-Hispanic black adults by 18 g (about 8%). Furthermore, after adjusting for covariates, LV mass adjusted to its allometric relation to height (height2.7) was higher in patients with MBS than in patients without MBS (62 g/m 2.7 vs. 56 g/m 2.7).
The Hypertension Genetic Epidemiology Network (HyperGEN) Study indicated that LV mass/height2.7 increased with the number of metabolic risk factors, after adjusting for covariates [32]. In the ARIC study [9] significant trend was observed in increasing LV mass/height with increasing metabolic syndrome components. Our study extend these results and indicate that there was a step-wise increase in adjusted LV mass and number of metabolic syndrome components and prevalence of LV hypertrophy was significantly higher in patients with the metabolic syndrome compared to those without the metabolic syndrome. Furthermore, high blood pressure and increased waist circumference were the strongest predictors of LV hypertophy; there was no interaction between blood pressure and WC in predicting LV hypertrophy indicating that these effects were additive. These suggest that optimal control of hypertension and obesity in patients with the metabolic syndrome could be efficacious in regressing LV hypertrophy and perhaps, reduce cardiovascular morbidity and mortality associated with LV hypertrophy.
Potential limitations include that the cross-sectional design of this study does not permit establishing of any causal relationships. This study was performed in an ambulatory clinical cohort of Hispanic and non-Hispanic black patients and thus, may not be directly applicable to the general population and to other ethnicities. However, several population-based studies of varying ethnicity have documented evidence of association between metabolic syndrome and abnormal LV geometry.
Conclusion
In a clinical ambulatory cohort of Hispanic and non-Hispanic black adults with high burden of cardiovascular risk factors, the metabolic syndrome is associated with higher LV mass and prevalence of LV hypertrophy. Increasing number of metabolic syndrome components is associated with step-wise increases in LV mass. High blood pressure and increased WC separately and additively contribute to LV hypertrophy suggesting that optimal blood pressure control and weight loss may be sufficient to regress LV hypertrophy. Further studies are needed to determine the prognostic impact of changes in metabolic syndrome components on cardiovascular morbidity and mortality.
References
- 1.Casale PN, Devereux RB, Milner M, Zullo G, Harshfield GA, Pickering TG, Laragh JH. Value of echocardiographic measurements of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med. 1986;105:173–178. doi: 10.7326/0003-4819-105-2-173. [DOI] [PubMed] [Google Scholar]
- 2.Cheal KL, Abbasi F, Lamendila C, McLaughlin T, Reaven GM, Ford ES. Relationship to insulin resistance of the adult treatment panel III diagnostic criteria for identification of the metabolic syndrome. Diabetes. 2004;53:1195–1200. doi: 10.2337/diabetes.53.5.1195. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. The metabolic syndrome and mortality form cardiovascular disease and all-causes: findings from the National Health and Nutrtion Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Meigs JB, Wilson PW, Nathan DM, D'Agostino RB, Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52:2160–2167. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 5.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 6.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echo-cardiographically-determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 8.Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease and ventricular dysfunction on survival among black adults. JAMA. 1995;273:1592–1597. [PubMed] [Google Scholar]
- 9.Burchfield CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, Taylor HA. Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2005;112:819–827. doi: 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- 10.Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick H, Lee ET, Best LG, de Simone G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong heart Study) Am J Cardiol. 2004;93:40–44. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Cuspidi C, Meani S, Fusi V, Severgnini B, Valerio C, Catinin E, Leonetti G, Magrini F, Zanchetti A. Metabolic syndorme and target organ damage in untreated essential hypertensives. J Hypertens. 2004;22:1991–1998. doi: 10.1097/00004872-200410000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Grandi AM, Maresca AM, Gidici E, Laurita E, Marchesi C, Solbiati F, Nicolini E, Guati L, Venco A. Metabolic syndorme and morphofunctional characteristics of the left ventricle in clinically hypertensive nondiabetic subjects. Am J Hypertens. 2006;19:199–205. doi: 10.1016/j.amjhyper.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Mule G, Nardi E, Cottone S, Cusimano P, Volpe V, Piazza G, Mongiovi R, Mezzatesta G, Andronico G, Cerasols G. Influence of the metabolic syndorme on hypertension-related target organ damage. J Intern Med. 2005;257:503–513. doi: 10.1111/j.1365-2796.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 14.Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, Siepi D, Vauso G, Mannarino E. Differnet impact of the metabolic syndrome on left ventricular structure and function in hyperetensive men and women. Hypertension. 2006;47:881–886. doi: 10.1161/01.HYP.0000216778.83626.39. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Rocella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 16.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebowitz H, Lernmark A, Natahn D, Palmer J, Rizza R, saudek C, Shaw J, Steffes M, stern M, Tuomlehto J, Zimmer P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Gile WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 18.Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Does waist circumference add to the predictive power of the the body mass index for coronary ris? Obes Res. 2001;9:685–695. doi: 10.1038/oby.2001.93. [DOI] [PubMed] [Google Scholar]
- 19.Janssen Katzmarzyk PT, Ross R. Body mass index, waist circumference and helth risk: evidence in support of current Institutes of Helath guidelines. Arch Intern Med. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Stampfer MJ, Giovannucii E, Spiegelman D, Colditz GA, Willet WC. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 22.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalenc and characteristics of the metabolic syndrome in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pelikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelins and Standards Committee and Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;158:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Devereux RB, de Simone G, Pickering TG, Schwartz JE, Roman MJ. Relation of left ventricular midwall function to cardiovascular risk factors and arterial structure and function. Hypertension. 1998;31:929–936. doi: 10.1161/01.hyp.31.4.929. [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sach I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 26.Palmieri V, Dahlof D, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB. Reliability of echoacrdiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J AM Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 27.de Simone G, Deveerux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 28.Wachtell K, Bella JN, Liebson PR, Gerdts E, Dahlof B, Aalto T, Roman MJ, Papademetriou V, Ibsen H, Rokkedal J, Devereux RB. Impact of different partition values on prevalences of left ventricular hypertensophy and concentric geometry in a large hypertensive population: the LIFE study. Hypertension. 2000;35:6–12. doi: 10.1161/01.hyp.35.1.6. [DOI] [PubMed] [Google Scholar]
- 29.Ganau A, Devereux Rb, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES. Prevalence of the metabolic syndrome in US populations. Endocrinol Metab Clin Noth Am. 2004;33:333–350. doi: 10.1016/j.ecl.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adulst. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 32.de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323–331. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]