Abstract
Objective: Diabetes mellitus (DM) leads to accelerated progression of arteriosclerosis with an increased risk of coronary events in comparison to non-diabetic patients with coronary artery disease (CAD). The precise and early detection of DM-induced vascular alterations is crucial to identify patients with high risk for cardiovascular complications. Thus, we aimed at simultaneously characterizing functional, physicomechanical, and structural vascular alterations in diabetic patients using a non-invasive approach. Research Design and Methods: In CAD patients with and without type 2 diabetes mellitus (n=50), we non-invasively measured flow-mediated dilation (FMD) of the brachial artery as a marker for endothelial function, fractional diameter changes (FDC) as a marker for physicomechanical properties, intima-media thickness (IMT) as a marker for structural properties, and forearm blood flow (FBF) as a marker for microvascular function. Results: DM was associated with reduced FMD (2.5±0.2 vs 4.8±0.4%; p≤0.001) indicating impaired macrovascular endothelial function. In parallel, reduced FDC (0.024±0.002 vs 0.034±0.004; p≤0.05) and increased IMT (0.38±0.01 vs 0.31±0.01mm; p≤0.001) indicated increased stiffness and enhanced structural alterations. Furthermore, reduced forearm blood flow during reactive hyperemia (10.7±1.0 vs. 15.3±1.4mL/min*100mL; p≤0.05) was found indicating microvascular dysfunction. Plasma glucose and HbA1c correlated with FMD (glucose: r=-0.32; HbA1c: r=-0.45), IMT (glucose: r=0.54; HbA1c: r=0.48) and FBF (glucose: r=-0.30) suggesting diabetes-specific effects on vascular properties. Conclusion: In patients with CAD, DM leads to functional and structural vascular alterations of the peripheral vasculature which are determined by the control of the disease underlining the relevance of a strict control of the DM to prevent accelerated atherosclerosis.
Keywords: Diabetes mellitus, coronary artery disease, endothelial function, intima media thickness, microcirculation
Introduction
The number of patients with diabetes mellitus (DM) is increasing due to population growth, aging, urbanization, and increasing prevalence of obesity and physical inactivity [1]. It was estimated that there were 171 million people suffering from diabetes mellitus in 2000 and the number is expected to nearly double to 366 million people in North America and Europe by 2030 [1]. The expected rise in the number of diabetic patients will lead to an enormous increase in the socioeconomic burden. Beside the severe microvascular complications, which become clinically evident as diabetic nephropathy, retinopathy or neuropathy [2,3], macrovascular complications including coronary artery disease (CAD) are frequent among diabetic patients. DM leads to premature and accelerated atherosclerosis with an increased risk of cardiovascular events [4,5]. Moreover, myocardial ischemia due to coronary atherosclerosis commonly occurs without symptoms in patients with diabetes [6]. As a result, multivessel atherosclerosis is often present before ischemic symptoms occur and before treatment is instituted. A delayed recognition of various forms of arteriosclerotic complications undoubtedly worsens the prognosis for survival for many diabetic patients [7].
The early and effective detection of macrovascular and microvascular alterations is of highest interest for the primary and secondary prevention of cardiovascular disease and monitoring of optimal medical treatment. Recently, we have introduced a novel non-invasive methodology simultaneously monitoring functional, physi-comechanical, and structural properties of the brachial artery using high resolution ultrasound in a “one stop shop” fashion [8]. In a single session, the flow-mediated dilation (FMD), the fractional diameter change (FDC), and the intima-media thickness (IMT) of the brachial artery can be determined in the same vascular segment simultaneously.
The aforementioned non-invasive methods may help in the early detection of vascular functional and structural alterations and may identify patients with high risk for cardiovascular complications. We aimed to assess diabetes-associated additive vascular functional, physicomechanical and structural alterations in the conduit arteries as well as functional alterations in the microvascular bed in patients with CAD using the “one-stop shop” approach complemented with venous occlusion plethysmography, a standard read-out of microvascular function.
Materials and methods
Study populations
We studied 50 patients with CAD with or without type 2 diabetes mellitus (65 ±1 years). All subjects were screened by clinical history, physical examination, ECG at rest, and routine chemical analysis. Diabetes mellitus was defined by symptoms of hyperglycemia and casual plasma glucose ≥200 mg/dL, a fasting glucose concentration ≥126 mg/dL or medical antidiabetic treatment. Diabetic patients were treated by dietetic control, oral hypoglycemic agents, or insulin therapy. CAD was diagnosed by coronary angiography. Patients with chronic heart failure, chronic renal failure, a malignant disease, an inflammatory disease, vasculitis, or Raynaud's syndrome were excluded. The study was approved by the local ethics committee and written informed consent was obtained from all study subjects prior to enrolment.
Vascular studies
All investigations were performed in the morning between 8:00 and 11:00 a.m. in an air-conditioned room at a temperature of 23±2°C in supine position. Cigarettes, beverages containing caffeine, and alcohol were prohibited for at least 12 hours prior to the investigation. Each subject rested in a supine position quietly for at least 10 minutes before the first scan.
Flow-mediated dilation
Flow-mediated brachial artery dilation was measured as previously described [9,10]. Briefly, the diameter of the brachial artery (BA) was measured from images assessed with a 15 MHz linear array transducer (Vivid i, GE Healthcare) using an automated software analysis (Brachial Analyzer, Medical Imaging Applications, Iowa City, IA, USA). Following measurement of the baseline diameter of the brachial artery, a blood pressure tourniquet located at the proximal forearm was inflated to a pressure of 200 mmHg for 5 minutes. Brachial artery dilation following reactive hyperemia was recorded for 60-90 seconds after release of the cuff. Endothelium-independent dilation of the brachial artery was measured after sublingual nitroglycerin (NTG 400 μg). The time interval between end of hyperemia and NTG test was 20 min to the re-establish baseline conditions. Both FMD and endothelium-independent vasodilation following nitroglycerin were expressed as the percent increase compared to the diameter of the resting scans.
Fractional diameter change
Images for the measurement of FDC of the brachial artery were assessed during the same setting with the FMD as previously described [8]. In order to evaluate FDC, a scan was made in a longitudinal section with regards to a clear differentiation of the intima-media complex of the anterior and posterior wall. FDC was determined during one heart cycle and calculated as the difference between maximum systolic diameter and the minimum diastolic diameter in relation to the minimum diastolic diameter using an automated analysis system (Brachial Analyzer, Medical Imaging Applications, Iowa City, IA, USA).
Intima-media thickness
Images for the measurement of the brachial artery IMT were assessed during the same setting with the FMD as previously described [8]. In order to evaluate IMT, a scan was made in a longitudinal section with regards to a clear differentiation of the intima-media complex of the posterior wall. PC-based measurement of IMT was performed according to the method of Wendelhag et al. [11] using an automated analysis system (Artery Measurement System, AMS, Wallenberg Laboratory for Cardiovascular Research, Goteborg, Sweden).
Venous occlusion plethysmography
Forearm blood flow (FBF) was measured by mercury-in-rubber strain gauge plethysmography (Periquant 833, Gutman, Eurasburg, Germany) according to standard techniques as previously described [12]. FBF was measured at rest and during reactive hyperemia and expressed as mL•min-1•100 mL-1 of tissue.
Statistical analysis
Data are expressed as means ± SEM. Comparisons between groups were analyzed for normal distribution using Shapiro-Wilk test and differences between groups by the unpaired Student t-test. Chi square test was used for comparison of non-continuous data. Univariate correlations were calculated using Pearson's coefficient (r). P-values ≤ 0.05 were accepted as statistically significant. Data processing was performed with the software modules of SPSS® (Statistical package for analysis in social sciences, Predictive analysis software release 18, SPSS Inc., Chicago, USA).
Results
Patient characteristics
The clinical characteristics are presented in Table 1. The study groups were well matched for cardiovascular risk factors such as age, sex, blood pressure, or lipid levels. As expected, patients with DM had significantly increased body mass index (29.7±1.0 vs 26.6±0.7 kg/m2; p≤0.05), plasma glucose concentration (148±8 vs 105±8 mg/dL; p≤0.001) and HbA1c (7.0±0.2 vs 5.8±0.2%; p≤0.001) as compared to non-diabetic patients. The medication was comparable between the study groups except for clopidogrel which was present in a lower percentage of diabetic patients (20 vs 68%; p≤0.001).
Table 1.
Clinical characteristics of the study population
| Parameter | Unit | Diabetes mellitus | Control | p value |
|---|---|---|---|---|
| n | 25 | 25 | ||
| CAD | [n] | 25 | 25 | |
| Age | [years] | 65 ± 1.4 | 66 ±1.6 | n.s. |
| Male sex | [n] | 15 | 21 | n.s. |
| Body mass index | [kg/m2] | 29.7 ± 1.0 | 26.6 ±0.7 | p≤0.05 |
| Systolic blood pressure | [mmHg] | 151 ±5 | 142 ±3 | n.s. |
| Diastolic blood pressure | [mmHg] | 87 ±2 | 86 ±2 | n.s. |
| Mean arterial pressure | [mmHg] | 108 ±3 | 105 ±2 | n.s. |
| Triglycerides | [mg/dL] | 142 ± 12 | 133 ±9 | n.s. |
| Total cholesterol | [mg/dL] | 174 ±9 | 182 ±9 | n.s. |
| LDL cholesterol | [mg/dL] | 107 ±8 | 111 ±7 | n.s. |
| HDL cholesterol | [mg/dL] | 51 ±2 | 52 ±3 | n.s. |
| Plasma glucose | [mg/dL] | 148 ±8 | 105 ±8 | p≤0.001 |
| HbA1c | [%] | 7.0 ±0.2 | 5.8 ±0.2 | p≤0.001 |
| ASS | [%] | 88 | 100 | n.s. |
| Clopidogrel | [%] | 20 | 68 | p≤0.001 |
| Beta blocker | [%] | 80 | 84 | n.s. |
| ACE-I or ARB | [%] | 92 | 84 | n.s. |
| Statin | [%] | 84 | 96 | n.s. |
| Oral antidiabetics | [%] | 60 | 0 | p≤0.001 |
| Insulin | [%] | 44 | 0 | p≤0.001 |
CAD: coronary artery disease; LDL cholesterol: low density lipoprotein cholesterol; HDL cholesterol: high density lipoprotein cholesterol; ACE-I: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Endothelial function
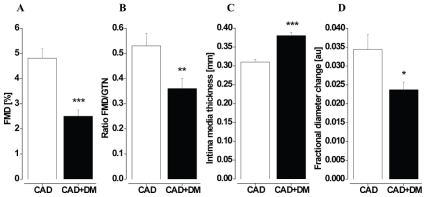
FMD of the BA was significantly reduced (2.5±0.2 vs 4.8±0.4%; p≤0.001; Figure 1A) in patients with DM as compared to control subjects. Endothelium-independent dilation of the BA was comparable between the study groups (8.4±0.7 vs 9.8±0.5%; p=n.s.). The ratio of FMD/GTN response was significantly lower in patients with DM (0.34±0.04 vs 0.53±0.05; p≤0.01; Figure 1B) thereby indicating endothelium specific vasodilator dysfunction of conduit arteries.
Figure 1.
Vascular function and structure in conduit arteries. Flow-mediated dilation (FMD; A), ratio FMD/GTN response (B); fractional diameter change (C) and intima-media thickness (D) of the brachial artery were significantly impaired in patients with coronary artery disease and type 2 diabetes mellitus (CAD+DM) in comparison to control patients with coronary artery disease (CAD). DM is presented as white bars, C as black bars. * p≤0.05; ** p≤0.01; *** p≤0.001.
Physicomechanical properties
FDC of the BA was significantly lower in patients with DM as compared to control patients (0.024±0.002 vs 0.034±0.004 a.u.; p≤0.05; Figure 1C), indicating an increased arterial stiffness of the muscular conduit arteries.
Vascular structure: IMT of the BA was significantly increased (0.38±0.01 vs 0.31±0.01 mm; p≤0.001; Figure 1D) in patients with DM as compared to control patients. Increased IMT reflects accelerated structural alterations of the diabetic BA.
Microvascular function
Microvascular dysfunction in patients with diabetes mellitus was shown by reduced FBF at rest (2.2±0.2 vs 3.1±0.3 mL/min per 100mL tissue; p≤0.05) and during reactive hyperemia (10.7±1.0 vs. 15.3±1.4 mL/min per 100mL tissue; p≤0.05).
Correlation of vascular function and structure with plasma glucose and HbA1c
Plasma glucose concentration and HbA1c correlated with FMD (glucose: r=-0.321, p≤0.05; HbA1c: r=-0.447, p≤0.01), IMT (glucose: r=0.539, p≤0.001; HbA1c: r=0.479, p≤0.001), and FBF during reactive hyperemia (glucose: r=-0.304, p≤0.05; HbA1c: r=-0.285, p≤0.05). For FDC, the correlations did not reach statistical significance (glucose: r=-0.272, p=0.067; HbA1c: r=-0.239, p=0.110) (Table 2).
Table 2.
Correlations of vascular function and structure with plasma glucose concentration and HbA1c
| Plasma glucose | HbA1c | ||
|---|---|---|---|
| FMD | r | -0.321 | -0.447 |
| p value | 0.032 | 0.002 | |
| GTN | r | -0.135 | -0.252 |
| p value | 0.377 | 0.095 | |
| FDC | r | -0.272 | -0.239 |
| p value | 0.067 | 0.110 | |
| IMT | r | 0.539 | 0.479 |
| p value | <0.001 | 0.001 | |
| FBFRH | r | -0.304 | -0.285 |
| p value | 0.045 | 0.061 |
FMD: flow-mediated dilation; GTN: glyceroltrinitrate-induced dilation; IMT: intima-media thickness; FDC: fractional diameter change; FBFRH: forearm blood flow during reactive hyperaemia
Correlations of vascular function and structure
FMD correlated with FDC (r=0.429; p≤0.01) and with IMT (r=-0.532; p≤0.001) as simultaneously assessed by high resolution ultrasound of the BA. FMD also correlated with FBF during reactive hyperemia (r=0.307; p≤0.05) indicating a potential mutual interaction of macrovascular and microvascular function (Table 3).
Table 3.
Correlations of vascular function and structure
| FMD | IMT | FDC | FBFRH | ||
|---|---|---|---|---|---|
| FMD | r | 1 | -0.532 | 0.429 | 0.307 |
| p value | <0.001 | 0.003 | 0.038 | ||
| IMT | r | -0.532 | 1 | -0.523 | -0.140 |
| p value | <0.001 | <0.001 | 0.355 | ||
| FDC | r | 0.429 | -0.523 | 1 | 0.065 |
| p value | 0.003 | <0.000 | 0.671 | ||
| FBFRH | r | 0.307 | -0.140 | 0.065 | 1 |
| p value | 0.038 | 0.355 | 0.671 |
FMD: flow-mediated dilation; IMT: intima-media thickness; FDC: fractional diameter change; FBFRH: forearm blood flow during reactive hyperemia
Discussion
Herein, we demonstrate that in patients with CAD, diabetes mellitus leads to enhanced functional, physicomechanical and structural alterations on the macrovascular level as well as functional alterations on the microvascular level in the peripheral vasculature which are determined by the glucose control.
Endothelial dysfunction in diabetes mellitus
We here show that in patients with CAD diabetes mellitus is associated with deterioration of endothelial function underlining the results from a previous study by Reyes-Soffer et al [13]. Remarkably, diabetes mellitus leads to a further aggravation of endothelial dysfunction in patients with CAD as a manifestation of atherosclerosis thereby indicating the pronounced effects of diabetes mellitus on vascular function. Hyperglycemia is considered as the most relevant cause of endothelial dysfunction and diabetic complications [14]. The CATHAY study has shown that even in non-diabetic individuals FMD was significantly associated with increasing levels of glycemia. Fasting glucose level was identified as an independent predictor of vascular function. Additionally, other conventional cardiovascular risk factors, including obesity, blood pressure, and an adverse lipid profile, were also related to levels of glycemia, further contributing to impaired vascular function [15]. Other factors promoting endothelial dysfunction in DM also include hyperinsulinemia, insulin resistance, and inflammation as recently reviewed elsewhere [14].
Complex vascular alterations in diabetes mellitus
Endothelial function is understood as an indicator of the integrity of the arterial wall reflecting both functional as well as structural and physicomechanical properties i.e. vessels may not be able respond to endothelial vasodilator mediators due to increased vascular stiffness or increased intima-media thickness. In addition to endothelial function, we measured physicomechanical and structural properties of the brachial artery in one examination at the same site. In parallel with endothelial dysfunction, we found increased arterial stiffness and increased IMT in the brachial artery thereby indicating extensive systemic physicomechanical alterations and structural alterations of the vascular wall in diabetes mellitus explaining part of the observed impaired FMD response. Typically, carotid-femoral pulse wave velocity (PWV) is considered as the gold-standard measurement of arterial stiffness [16]. However, local measurements of arterial stiffness are indicated for mechanistic analyses in pathophysiology, pharmacology, and therapeutics [16]. Henry et al. measured carotid, femoral, and brachial artery distensibility in a population-based cohort including study subjects with type 2 DM. They found an increased arterial stiffness in both elastic and muscular arteries in patients with DM which indicates early physicomechanical alterations in different arterial segments, even in peripheral arteries which are not typically affected by atherosclerosis [17]. In the presence of atherosclerotic disease, DM even leads to a further increase in arterial stiffness as detected in the carotid artery [18] as well as in peripheral arteries. In the above mentioned study by Henry et al., the increase in stiffness indices were explained by decreases in distension, increases in pulse pressure, an increase in carotid IMT, and, for the femoral artery, a decrease in diameter [17]. Typically, IMT is measured in the carotid artery and large studies have shown the predictive value of the carotid IMT for cardiovascular events [19]. It has been shown that diabetic patients without a history of myocardial infarction have a comparable IMT as non -diabetic patients with a history of myocardial infarction [20]. It may be assumed that DM leads to marked systemic structural alterations of the vascular wall affecting elastic as well as muscular arteries.
The presented approach of parallel measurement of FMD, FDC, and IMT in the same vascular segment may give further important insights into the mechanisms of vascular remodelling. We have shown that FDC and IMT correlated with FMD suggesting that physicomechanical and structural properties determine endothelial function or, more general, the vascular response to an increase blood flow. Soltesz et al. measured FMD of the brachial artery, IMT of the carotid artery and arterial stiffness in patients with systemic autoimmune disease who are at an increased risk of vascular dysfunction and increased cardiovascular mortality due to accelerated atherosclerosis. They showed a reduced FMD, an increased IMT and an increased PWV in patients with systemic autoimmune disease. In this patient group, FMD was inversely correlated with IMT and PWV [21]. Ravikumar and colleagues also measured FMD of the brachial artery, IMT of the carotid artery and arterial stiffness in patients with or without DM. They found a correlation of reduced FMD and augmentation index (AI) with the carotid IMT. FMD and AI correlated with plasma glucose concentration and HbA1c, respectively [22]. However, there is only a limited number of studies investigating endothelial function, physicomechanical, and structural properties in the same vascular bed [23]. Bjarnegard et al. showed in female patients with type 1 diabetes mellitus a reduced FMD and GTN response whereby IMT and arterial distensibility were comparable with non-diabetic control patients. HbA1c was an independent predictor of the reduced GTN response in DM [23]. In contrast to our results in patients with type 2 DM, in these patients with type 1 DM the impairment of endothelium-independent response has been seen as the most remarkable vascular alteration suggesting different pathways of vascular remodelling in type 1 and type 2 diabetes. Further longitudinal studies are needed to fully characterize the course of vascular remodelling in the peripheral vasculature in diabetes mellitus.
Interaction of macrocirculation and microcirculation
We found a reduced forearm blood flow at rest and during reactive hyperemia suggesting concomitant microvascular dysfunction in patients with type 2 DM. From clinical studies, it is well known that DM is characterized by its microvascular complications such as diabetic nephropathy, retinopathy or neuropathy. In addition, DM is also associated with homeostatic alterations which are suggested to produce a procoagulant state [24,25]. In previous studies, reduced microvascular response to endothelium-dependent and endothelial-independent stimuli in resistance arteries and capillaries has been presented by different methods in patients with diabetes mellitus [26,27,28]. Again, forearm blood flow during reactive hyperemia correlated with FMD of the brachial artery indicating an interaction between macrovascular and microvascular function as shown before [12,29]. It may be argued that the reduced forearm blood flow in diabetic patients led to a reduced stimulus of flow during reactive hyperemia which might contribute to the impaired FMD.
Conclusion
In patients with CAD, DM leads to functional and structural vascular alterations which are determined by the glycemic control underlining the relevance of a strict control of the DM to prevent accelerated atherosclerosis. The approach of a single non-invasive one-stop-shop examination allows a frequent and non-invasive monitoring of vascular status in diabetics.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Marshall SM, Flyvbjerg A. Prevention and early detection of vascular complications of diabetes. BMJ. 2006;333(7566):475–480. doi: 10.1136/bmj.38922.650521.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 4.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D'Agostino RB, Sr, Wilson PW, Savage PJ. Trends in cardiovascular complications of diabetes. JAMA. 2004;292(20):2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 6.Wingard DL, Barrett-Connor EL, Scheidt-Nave C, McPhillips JB. Prevalence of cardiovascular and renal complications in older adults with normal or impaired glucose tolerance or NIDDM. A population-based study. Diabetes Care. 1993;16(7):1022–1025. doi: 10.2337/diacare.16.7.1022. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 8.Balzer J, Boos M, Rassaf T, Heiss C, Preik M, Matern S, Schoebel F, Kelm M, Lauer T. “One Stop-Shop” ultrasound diagnosis of functional, structural and physicomechanical properties of the brachial artery. Vasa. 2007;36:100–106. doi: 10.1024/0301-1526.36.2.100. [DOI] [PubMed] [Google Scholar]
- 9.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 10.Heiss C, Lauer T, Dejam A, Kleinbongard P, Hamada S, Rassaf T, Matern S, Feelisch M, Kelm M. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol. 2006;47:573–579. doi: 10.1016/j.jacc.2005.06.089. [DOI] [PubMed] [Google Scholar]
- 11.Wendelhag I, Liang Q, Gustavsson W, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke. 1997;28:2195–2200. doi: 10.1161/01.str.28.11.2195. [DOI] [PubMed] [Google Scholar]
- 12.Lauer T, Heiß C, Preik M, Balzer J, Hafner D, Strauer BE, Kelm M. Reduction of peripheral flow reserve impairs endothelial function in conduit arteries of patients with essential hypertension. J Hypertens. 2004;23:563–569. doi: 10.1097/01.hjh.0000160213.40855.b7. [DOI] [PubMed] [Google Scholar]
- 13.Reyes-Soffer G, Holleran S, Di Tullio MR, Homma S, Boden-Albala B, Ramakrishnan R, Elkind MS, Sacco RL, Ginsberg HN. Endothelial function in individuals with coronary artery disease with and without type 2 diabetes mellitus. Metabolism. 2010 doi: 10.1016/j.metabol.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120(13):1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas GN, Chook P, Qiao M, Huang XS, Leong HC, Celermajer DS, Woo KS. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study. Arterioscler Thromb Vasc Biol. 2004;24(4):739–743. doi: 10.1161/01.ATV.0000118015.26978.07. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 17.Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, Kamp O, Westerhof N, Bouter LM, Stehouwer CD. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation. 2003;107(16):2089–2095. doi: 10.1161/01.CIR.0000065222.34933.FC. [DOI] [PubMed] [Google Scholar]
- 18.Martens FM, van der GY, Dijk JM, Olijhoek JK, Visseren FL. Carotid arterial stiffness is marginally higher in the metabolic syndrome and markedly higher in type 2 diabetes mellitus in patients with manifestations of arterial disease. Atherosclerosis. 2008;197(2):646–653. doi: 10.1016/j.atherosclerosis.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocar-dial infarction: the Rotterdam Study. Circulation. 1997;96(5):1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 20.Lee CD, Folsom AR, Pankow JS, Brancati FL, Atherosclerosis Risk in Communities (ARIC) Study Investigators Cardivascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation. 2004;109:855–860. doi: 10.1161/01.CIR.0000116389.61864.DE. [DOI] [PubMed] [Google Scholar]
- 21.Soltesz P, Der H, Kerekes G, Szodoray P, Szucs G, Danko K, Shoenfeld Y, Szegedi G, Szekanecz Z. A comparative study of arterial stiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima-media in patients with systemic autoimmune diseases. Clin Rheumatol. 2009;28(6):655–662. doi: 10.1007/s10067-009-1118-y. [DOI] [PubMed] [Google Scholar]
- 22.Ravikumar R, Deepa R, Shanthirani C, Mohan V. Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow mediated dilatation in diabetic and nondiabetic subjects (The Chennai Urban Population Study [CUPS-9]) Am J Cardiol. 2002;90:702–707. doi: 10.1016/s0002-9149(02)02593-6. [DOI] [PubMed] [Google Scholar]
- 23.Bjarnegard N, Arnqvist HJ, Lindstrom T, Jonasson L, Jonsson A, Lanne T. Long-term hyperglycaemia impairs vascular smooth muscle cell function in women with type 1 diabetes mellitus. Diab Vasc Dis Res. 2009;6(1):25–31. doi: 10.3132/dvdr.2009.005. [DOI] [PubMed] [Google Scholar]
- 24.Jax TW, Peters AJ, Plehn G, Schoebel FC. Relevance of hemostatic risk factors on coronary morphology in patients with diabetes mellitus type 2. Cardiovasc Diabetol. 2009;8:24. doi: 10.1186/1475-2840-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jax TW, Peters AJ, Plehn G, Schoebel FC. Hemostatic risk factors in patients with coronary artery disease and type 2 diabetes - a two year follow-up of 243 patients. Cardiovasc Diabetol. 2009;8:48. doi: 10.1186/1475-2840-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27(3):567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 27.Schmiedel O, Schroeter ML, Harvey JN. Microalbuminuria in Type 2 diabetes indicates impaired microvascular vasomotion and perfusion. Am J Physiol Heart Circ Physiol. 2007;293(6):H3424–H3431. doi: 10.1152/ajpheart.00558.2007. [DOI] [PubMed] [Google Scholar]
- 28.Fredriksson I, Larsson M, Nystrom FH, Lanne T, Ostgren CJ, Stromberg T. Reduced arterio-venous shunting capacity after local heating and redistribution of baseline skin blood flow in type 2 diabetes assessed with velocity-resolved quantitative laser Doppler flowmetry. Diabetes. 2010;59(7):1578–1584. doi: 10.2337/db10-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauer T, Heiss C, Balzer J, Keymel S, Kelm M, Preik M, Rassaf T. Resting microvascular resistance and conduit artery tone: relevance to endothelium-dependent flow-mediated dilation. Eur J Cardiovasc Prev Rehabil. 2008;15:677–682. doi: 10.1097/HJR.0b013e32830eb6d8. [DOI] [PubMed] [Google Scholar]