Abstract
Aim: Whole-body vibration exercise (WBV) acutely decreases brachial-ankle pulse wave velocity (baPWV), an index of systemic arterial stiffness. However, the effect of WBV on segmental PWV and aortic hemodynamics is unknown. We examined the acute effects of WBV on arterial function. Methods: Fifteen young men performed ten 1-min sets of static squat with WBV (40 Hz, 1 mm, 5.37 G) and without WBV (no-WBV). Brachial and aortic blood pressure (BP), heart rate (HR), augmentation index (AIx), baPWV, carotid-femoral PWV (cfPWV), and femoral-ankle (faPWV), were recorded before and 5, 15, and 30 min after both trials. Results: Brachial and aortic SBP (P < 0.01), and HR (P < 0.01) were increased only at 5 min after both exercise trials. AIx was elevated through the recovery after no-WBV while decreased at 15 and 30 min after WBV exercise. FaPWV was decreased (P < 0.01) at 5 min after both trials, but returned to baseline at 15 min after no-WBV exercise and was maintained decreased at 15 and 30 min after WBV exercise. There were no significant changes in brachial and aortic diastolic BP, cfPWV and baPWV after both trials. Conclusions: Our findings indicate that regardless of WBV, static squat causes a small transient increase in hemodynamic responses during early recovery. WBV counteracts the increase in AIx induced by static squat and reduces wave reflection magnitude through a local effect on arterial stiffness.
Keywords: Aortic hemodynamics, pulse wave velocity, wave reflection, static exercise
Introduction
Whole-body vibration (WBV) is a relatively new exercise training mode that has shown to improve muscle strength and mass [1, 2], bone mineral density [3], and glycemic control [4]. Although exercise with WBV training requires less time per session to improve health parameters than resistance exercise training [4], only a few studies have evaluated the acute cardiovascular responses to WBV. Recent studies have reported a decrease in brachial-ankle pulse wave velocity (baPWV) [5], an index of systemic arterial stiffness. Additionally, mild increases [6] or no changes [5, 7] in heart rate (HR) and brachial blood pressure (BP) have been reported after a session of static squat with WBV at a vibration frequency of 26 Hz. These data suggest that WBV appears to exert low levels of cardiovascular stress and possible reduction in systemic PWV. However, the specific effect of static squat and WBV on the main components of baPWV (aortic PWV and leg PWV) [8] needs to be examined before recommending them to individuals with high cardiovascular risk.
Static squat acutely increases aortic SBP by ∼8 mmHg and pressure wave reflection (augmentation index, AIx) by ∼18.7% suggesting an increase in left ventricular afterload in young healthy adults [9]. Although static squat is the most common exercise used in WBV training, the aortic hemodynamic and regional PWV responses after an acute bout of static squat with and without WBV are unknown. Some studies have shown a decrease in leg PWV after acute single-leg static [10] and dynamic resistance exercise. Despite no change in aortic PWV, vasodilation of peripheral arteries may decrease leg PWV and the magnitude of the reflected wave and, thus, the aortic AIx after exercise [11-14]. Thus, it is possible that acute WBV would have a predominant effect on leg PWV and AIx as previously shown by an increase in leg muscle blood flow during and immediately after static squat with WBV [7, 15].
The purpose of this study was to determine the effects of a single bout of static exercise with and without WBV on aortic hemodynamics, AIx, and arterial stiffness. We hypothesized that WBV would reduce aortic hemodynamic, AIx, and PWV responses compared to static squat without WBV.
Methods
Subjects
Fifteen apparently healthy men (21 ± 4 years) were recruited from the local university population. Eligibility criteria were that participants should be normotensive (BP <140/90 mm Hg), without overt chronic diseases as evaluated by health history. Participants were nonsmokers and either sedentary or moderately physically active (≤ 1.5 hours/week). Exclusion criteria were prescribed medications or supplements known to affect cardiovascular parameters and contraindications for WBV including recent fractures or wounds and gallbladder or kidney stones. This study was approved by the Institutional Review Board of The Florida State University and all subjects gave informed consent.
Study design
Measurements were performed during the morning in a quiet temperature-controlled room (23° C) and at the same time of the day (± 1 hour) to minimize potential diurnal variations. Subjects were tested in the postprandial (≥3 hours) condition on two separate visits at least 72 hours apart. Participants abstained from caffeine and alcohol for 12 hours and avoided intense exercise 48 hours prior to testing. After electrocardiogram (ECG), BP, and arterial tonometry instrumentation, participants rested supine for at least 20 min before a 5 min baseline period. Thereafter, subjects performed static squat with (WBV) or without WBV (no-WBV) in a randomized order. Immediately after exercise, subjects reassumed the supine position for 30 min of recovery. Post-exercise measurements were collected at 5 min, 15 min, and 30 min.
Exercise trials
During the no-WBV and WBV trials, subjects stood on the vibration platform (Powerplate, Badhoevedorp, The Netherlands) with both knees flexed at 120° (full extension 180°) and slightly grasped the handlebar with the right hand. The vibration frequency and peak-by-peak amplitude for the WBV trial were 40 Hz and 1 mm, respectively, which corresponds to an acceleration of ∼5.37 G. The protocol consisted of ten 1-min sets of static squat separated by 1-min inter-set rest periods in the seated position. The rationale for the number of bouts and durations was based on a previous study that has shown a decrease in baPWV after acute WBV [5].
Pulse wave velocity
PWV and brachial BP were measured using an automatic device (VP-2000, Omron Healthcare Inc., Vernon Hills. IL, USA). BP cuffs were wrapped around both arms (brachial artery) and ankles (posterior tibial artery) while two tonometers were placed over the right carotid and femoral arteries to obtain PWV measurements from three arterial segments: baPWV, carotid-femoral PWV (cfPWV), and femoral-ankle PWV (faPWV). The cfPWV and faPWV are considered measurements of aortic and leg arterial stiffness, respectively [16]. BaPWV is an index of systemic arterial stiffness, which mainly includes aortic PWV and leg PWV [8, 17]. ECG electrodes were placed on the forearms, while a heart sound microphone was placed on the left parasternal border of the second intercostal space. Transit time was automatically determined from the time delay between the feet of the pulse waves related to the R-wave of a simultaneously recorded ECG. We got the time delay between brachial and tibial arteries (Tba), carotid and femoral arteries (Tcf), and femoral and tibial arteries (Tfa). The distance from the carotid to femoral artery (Dcf) was measured with a nonelastic tape measure. The distances from the suprasternal notch to brachial arteries (Dhb), from the suprasternal notch to femoral arteries (Dhf), and from femoral to tibial arteries (Dfa) were calculated automatically according to the following formulas: Dhb= 0.220 x height (cm) - 2.07, Dhf= 0.564 x height (cm) - 18.4, and Dfa= 0.249 x height (cm) + 30.7 [8,17]. PWV was calculated for each arterial segment using the following formulas: aortic PWV= Dcf/ Tcf, faPWV= Dfa/Tfa, and baPWV= (Dhf + Dfa -Dhb)/Tba [17]. Two measurements were collected at each time point and averaged. HR was determined from the ECG.
Pulse wave analysis
Pulse waveforms were obtained from a 10 sec epoch using a high-fidelity tonometer (SPT-301B; Millar Instruments, Houston, TX, USA) placed on the left radial artery. Radial pressure waveforms were calibrated against brachial SBP and diastolic BP (DBP) because of comparable hemodynamic properties in the upper limb arteries [18]. Aortic pressure waveform was derived using a generalized transfer function (SphygmoCor, AtCor Medical, Sydney, Australia), which has been validated at rest [19] and during exercise [20]. The first (P1) and second (P2) systolic peaks of the aortic pressure wave are the result of a forward traveling wave produced by the stroke volume and a reflected wave returning to the ascending aorta from the peripheral arteries. The augmentation pressure (AP) is defined as the difference between P2 and P1 of the central aortic pressure wave and it can increase or decrease depending on the magnitude of P2 and P1 [11]. The AIx was defined as the AP expressed as a percentage of the aortic pulse pressure (SBP-DBP). AIx was normalized to a HR of 75 beats/min (AIx@75) because it is influenced by HR [23]. AIx is an index of wave reflection intensity, which is affected by peripheral and aortic PWV [21]. The average of 2 measurements of brachial BP and high-quality (operator index ≥ 80%) aortic hemodynamics was used in the analysis.
Anthropometrics
Height was measured using a stadiometer to the nearest 0.5 cm and body weight was measured using a seca scale (Sunbeam Products Inc., Boca Raton, FL, USA) to the nearest 0.1 kg.
Statistical analysis
Data are presented as mean ± SEM. Student t-tests were used to determine possible baseline differences between trials. Differences in mean values for each variable between trials were compared by analysis of variance with repeated measures [trial (no-WBV vs. WBV) by time (baseline and 5, 15, and 30 min post-exercise)], followed by Fisher's LSD test. Appropriate post hoc analysis was used where significant main effect and interaction occurred. P < 0.05 was considered to be significant. SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
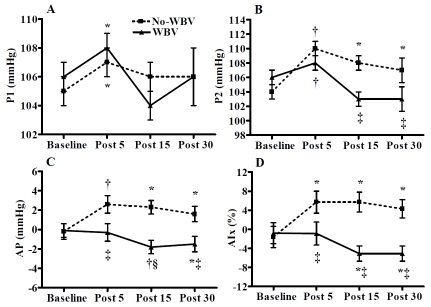
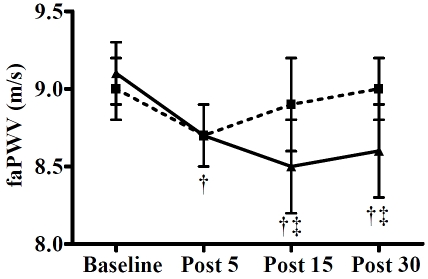
Weight and height were 92.7 ± 3.7 kg and 1.80 ± 0.02 m, respectively. Peripheral and central hemodynamics are shown in Table 1 and Table 2. Brachial and aortic SBP (P < 0.01), P1 (P < 0.05, Figure 1), P2 (P < 0.01, Figure 1), and HR (P < 0.01) were increased at 5 min after both exercise trials. All these parameters returned to baseline values at 15 min post-exercise, except P2, which remained elevated (P < 0.05) at 15 and 30 min after the no-WBV trial. The AP and AIx increased (P < 0.05) at 5, 15, and 30 min after the no-WBV trial, while they decreased (P < 0.05) at 15 and 30 min after the WBV trial (Figure 1). Changes in AIx and AIx@75 were similar. FaPWV decreased (P < 0.01) at 5 min after both trials, but it was maintained lower than baseline values (P < 0.01) at 15 and 30 min only after the WBV trial (Figure 2). A time-by-trial interaction (P < 0.05) was detected for AP, AIx, and AIx@75 at 5, 15, and 30 min post-exercise and for P2 and faPWV at 15 and 30 min post-exercise. There were no significant changes in brachial and aortic DBP, cfPWV, and baPWV after either trial.
Table 1.
Peripheral and central hemodynamics before and after static squat without (no-WBV) and with whole-body vibration (WBV) trials in young men (n=15)
| Baseline | Post 5 min | Post 15 min | Post 30 min | |
|---|---|---|---|---|
| Brachial SBP (mm Hg) | ||||
| No-WBV | 127 ± 1 | 131 ± 2† | 129 ± 1 | 128 ± 2 |
| WBV | 128 ± 1 | 130 ± 2† | 126 ± 1 | 126 ± 2 |
| Brachial DBP (mm Hg) | ||||
| No-WBV | 70 ± 2 | 71 ± 2 | 71 ± 1 | 71 ± 2 |
| WBV | 71 ± 2 | 73 ± 2 | 69 ± 1 | 67 ± 2 |
| Brachial MAP (mm Hg) | ||||
| No-WBV | 86 ± 1 | 89 ± 2 | 89 ± 2 | 88 ± 2 |
| WBV | 88 ± 1 | 89 ± 2 | 85 ± 2‡ | 84 ± 2*‡ |
| Aortic SBP (mm Hg) | ||||
| No-WBV | 106 ± 1 | 110 ± 1† | 109 ± 1 | 108 ± 2 |
| WBV | 107 ± 1 | 110 ± 1† | 105 ± 1‡ | 105 ± 2 |
| Aortic DBP (mmHg) | ||||
| No-WBV | 71 ± 2 | 72 ± 2 | 73 ± 2 | 72 ± 2 |
| WBV | 72 ± 2 | 74 ± 3 | 70 ± 1 | 68 ± 2‡ |
| Aortic MAP (mmHg) | ||||
| No-WBV | 87 ± 1 | 89 ± 2 | 89 ± 1 | 88 ± 2 |
| WBV | 88 ± 1 | 89 ± 2 | 85 ± 1 | 84 ± 1‡ |
| HR (beats/min)) | ||||
| No-WBV | 64 ± 3 | 69 ± 4† | 66 ± 3 | 63 ± 3 |
| WBV | 64 ± 3 | 70 ± 4† | 67 ± 4 | 64 ± 3 |
Values are mean ± SEM. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; Significant difference from baseline at
P < 0.05
P<0.01
Significant difference from No-WBV at
P < 0.05
Table 2.
Wave reflection and arterial stiffness before and after static squat without (no-WBV) and with whole-body vibration (WBV) trials in young men (n= 15)
| Baseline | Post 5 min | Post 15 min | Post 30 min | |
|---|---|---|---|---|
| AIx@75 (%) | ||||
| No-WBV | -6.9 ± 1.8 | 3.1 ± 2.8† | 2.6 ± 2.8† | -1.1 ± 2.5* |
| WBV | -6.1 ± 1.7 | -3.3 ± 3.0*‡ | -9.1 ± 2.3*§ | -9.6 ± 2.1*§ |
| cfPWV (m/s) | ||||
| No-WBV | 8.7 ± 0.3 | 9.1 ± 0.4 | 8.9 ± 0.3 | 8.8 ± 0.2 |
| WBV | 8.8 ± 0.3 | 9.1 ± 0.4 | 8.9 ± 0.3 | 8.5 ± 0.3 |
| baPWV (m/s) | ||||
| No-WBV | 12.0 ± 0.4 | 12.0 ± 0.3 | 12.0 ± 0.3 | 12.2 ± 0.4 |
| WBV | 12.0 ± 0.4 | 11.9 ± 0.3 | 11.8 ± 0.3 | 11.8 ± 0.3 |
Note: Values are mean ± SEM. AIx @ 75, augmentation index adjusted at HR 75; PWV, pulse wave velocity; cf, carotid-femoral; ba, brachial-ankle. Significant difference from baseline at
P < 0.05
P<0.01.
Significant difference from No-WBV at
P < 0.05
P<0.01.
Figure 1.
Aortic hemodynamic responses before (baseline) and 5, 15, and 30 min following a bout of static squat with whole-body vibration (WBV) and without WBV (no-WBV) trials in young men (n= 15). P1, first systolic peak pressure (A); P2, second systolic peak pressure (B); AP, augmentation pressure (C); and AIx, augmentation index (D). Values are mean ± SEM. *P < 0.05 and † P<0.01 vs. baseline; ‡P < 0.05 and §P<0.01 vs. No-WBV.
Figure 2.
Femoral-ankle pulse wave velocity (faPWV) before (baseline) and 5, 15, and 30 min following a bout of static squat with whole-body vibration (WBV) and without WBV (no-WBV) trials in young men (n= 15). Values are mean ± SEM. †P<0.01 vs. baseline; ‡P<0.05vs. No-WBV.
Discussion
The main findings of the present study are that hemodynamic parameters that influence the incident wave were transiently increased whereas faPWV was decreased at 5 min after both trials. In addition, wave reflection was increased and faPWV was not affected 15 to 30 min after no-WBV exercise while both parameters were reduced after WBV exercise.
In the present study, there were increases in SBP, HR, and P1 at 5 min after squat with and without WBV. We showed an increase in both brachial and aortic SBP (∼4 mm Hg) during the early recovery of an acute bout of static squat exercise that was not affected by WBV. The magnitude of change in aortic SBP after our two trials was smaller than those reported during the early recovery of resistance exercise (9-12 mm Hg) [11, 22]. It is known that SBP and AIx are determined by HR, pulse wave reflection, and aortic PWV [16, 23]. Although an inverse relationship exists between HR and AIx [23], a low HR (∼66-76 bpm) does not influence the increase in AIx after static handgrip [24] and squat exercise [9]. Since there was no increase in aortic PWV, the increases in aortic SBP and AIx after no-WBV squat were likely influenced by an increase in the magnitude of the reflected wave (P2), which has been attributed to peripheral vasoconstriction [25]. Although the pressures of the first (P1) and second systolic (P2) peaks increased 5 min after both trials, a significant increase in AP occurred only after no-WBV exercise resulting in an increased AIx. Conversely, the similar increase in P2 and P1 did not change the AP and, hence, the AIx after WBV. The increases in hemodynamic factors that influenced the incidence wave returned to baseline levels 15 min after static squat with and without WBV.
P2, AP, AIx and AIx@75 remained elevated 15 and 30 min after no-WBV squat exercise indicating an increased wave reflection from peripheral sites to the aorta. After isometric exercise, the acute increase in sympathetic activity evokes an increase in AIx [24], which leads to an increase in left ventricle afterload [9]. It is likely that the factors that influenced P2 and the AIx at 5 min post-exercise persisted for the remaining of the recovery only after the no-WBV trial. Conversely, WBV caused a decrease in AP and AIx after an acute bout of static squat. In agreement with our findings, previous studies have observed reductions in AIx 15 and 30 min after maximal cycling exercise [13, 14] and low-intensity resistance exercise [11]. However, the decrease in AIx in the previous studies was likely influenced by the increased HR due to the inverse relationship between the two parameters [23]. In the present study, HR was recovered and P2 was lower after the WBV trial compared with the no-WBV trial. It is most likely that HR did not influence the decrease in wave reflection after WBV exercise. Thus, the decrease in wave reflection magnitude was due to vasodilation of peripheral arteries [14]. Our findings indicate that WBV counteracts the acute increase in AIx induced by isometric exercise [9, 24] and may prevent the increase in AIx noted after resistance exercise training [26].
Central artery stiffness is increased via structural changes in the arterial media such as increased collagen and fragmentation of elastin fibers [27]. In peripheral arteries, stiffness is influenced by acute changes in vascular tone regulated by sympathetic activity, endothelial and contraction-related factors. We previously noted that leg PWV and SBP can be increased by intense vascular sympathetic activation during muscle ischemia after no-WBV exercise [28]. The concurrent increases in PWV and SBP after a bout of exercise suggests that acute changes in PWV may be pressure-dependent [22]. In the present study, we observed a decrease in leg PWV concomitant with a small increase in SBP immediately after both exercise trials, which is consistent with previous data [29, 30]. Since BP returned to resting levels 15 min after both trials, it is likely that the decrease in leg PWV may be due to changes in local arterial function independent of BP [10, 29, 31]. Our data support that the decrease in leg PWV influenced the decrease in AIx after WBV exercise. Previous studies have shown that the reduction in leg PWV after acute resistance [12], static [10], and endurance exercise [29, 31, 32] has been attributed to contraction-related vasodilatory factors. However, the reduction in leg PWV after no-WBV did not remain after 5 min post-exercise, suggesting the effect of other vasodilatory factors. An increased vasodilation in the inactive vibrated limb has been attributed to increased nitric oxide production [33]. Thus, the prolonged decrease in leg PWV after WBV exercise could be attributed mainly to the local effect of vibration on the leg arteries.
In contrast to our findings, Otsuki et al. [5] showed a decrease in baPWV 20 to 40 min after a session of WBV squat. Since the exercise protocols in both studies were comparable in number and duration of sets, the discrepancy in post-exercise baPWV could be due to the greater vibration frequency used in the present (40 Hz) than in the previous study (26 Hz). Vibration transmissibility to the trunk is completely abolished with frequencies greater than 30 Hz [34]. Thus, reduced vibration transmissibility to the trunk and no effect on the aorta may explain the no apparent effect of WBV on cfPWV and baPWV in the present study. However, the effect of antihypertensive treatment on peripheral arteries appears to be more related to the reduction in wave reflection magnitude rather than cfPWV [35]. Thus, the vascular effects of WBV may have relevant implications for individuals with conditions characterized by elevated AIx such as hypertension and stroke [35, 36].
We examined arterial function responses after a session of static squat with WBV at 40 Hz in young healthy men and the results may not be directly applied to different populations or WBV protocols. In addition, the vascular effects of exercise with WBV beyond 30 min of recovery were not assessed. Evaluation of lower WBV frequencies, greater exercise time, and longer recovery periods than those used in the present study is warranted in populations that would likely get more benefits from exercise with WBV such as the elderly and individuals with type 2 diabetes who have an intermediate increase in faPWV besides the preferential increase in central PWV [37].
In conclusion, a bout of static squat induced an acute increase in AIx due to increased magnitude of the reflected wave. WBV overcomes the increase in AIx during early recovery and decreases AIx during late recovery by decreasing pressure wave reflection and leg PWV.
Acknowledgments
The authors would like to express their gratitude to Edzard Zeinstra and Power Plate International for providing technical support and the vibrating platform.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Machado A, Garcia-Lopez D, Gonzalez-Gallego J, Garatachea N. Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand J Med Sci Sports. 2009 doi: 10.1111/j.1600-0838.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 2.Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sports Exerc. 2003;35:1033–1041. doi: 10.1249/01.MSS.0000069752.96438.B0. [DOI] [PubMed] [Google Scholar]
- 3.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 4.Baum K, Votteler T, Schiab J. Efficiency of vibration exercise for glycemic control in type 2 diabetes patients. Int J Med Sci. 2007;4:159–163. doi: 10.7150/ijms.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otsuki T, Takanami Y, Aoi W, Kawai Y, Ichikawa H, Yoshikawa T. Arterial stiffness acutely decreases after whole-body vibration in humans. Acta Physiol (Oxf) 2008;194:189–194. doi: 10.1111/j.1748-1716.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane DJ, Stannard SR, Sargeant AJ, Rittweger J. The rate of muscle temperature increase during acute whole-body vibration exercise. Eur J Appl Physiol. 2008;103:441–448. doi: 10.1007/s00421-008-0736-4. [DOI] [PubMed] [Google Scholar]
- 7.Kerschan-Schindl K, Grampp S, Henk C, Resch H, Preisinger E, Fialka-Moser V, Imhof H. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin Physiol. 2001;21:377–382. doi: 10.1046/j.1365-2281.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, Tanaka H. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19:401–406. doi: 10.1038/sj.jhh.1001838. [DOI] [PubMed] [Google Scholar]
- 9.Murakami T. Squatting: the hemodynamic change is induced by enhanced aortic wave reflection. Am J Hypertens. 2002;15:986–988. doi: 10.1016/s0895-7061(02)03085-6. [DOI] [PubMed] [Google Scholar]
- 10.Davies TS, Frenneaux MP, Campbell RI, White MJ. Human arterial responses to isometric exercise: the role of the muscle metaboreflex. Clin Sci (Lond) 2007;112:441–447. doi: 10.1042/CS20060276. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa A, Vicil F. Post-exercise aortic hemodynamic responses to low-intensity resistance exercise with and without vascular occlusion. Scand J Med Sci Sports. 2011;21:431–436. doi: 10.1111/j.1600-0838.2009.01061.x. [DOI] [PubMed] [Google Scholar]
- 12.Heffernan KS, Rossow L, Jae SY, Shokunbi HG, Gibson EM, Fernhall B. Effect of single-leg resistance exercise on regional arterial stiffness. Eur J Appl Physiol. 2006;98:185–190. doi: 10.1007/s00421-006-0259-9. [DOI] [PubMed] [Google Scholar]
- 13.Heffernan KS, Jae SY, Echols GH, Lepine NR, Fernhall B. Arterial stiffness and wave reflection following exercise in resistance-trained men. Med Sci Sports Exerc. 2007;39:842–848. doi: 10.1249/mss.0b013e318031b03c. [DOI] [PubMed] [Google Scholar]
- 14.Munir S, Jiang B, Guilcher A, Brett S, Redwood S, Marber M, Chowienczyk P. Exercise reduces arterial pressure augmentation through vasodilation of muscular arteries in humans. Am J Physiol Heart Circ Physiol. 2008;294:H1645–1650. doi: 10.1152/ajpheart.01171.2007. [DOI] [PubMed] [Google Scholar]
- 15.Lythgo N, Eser P, de Groot P, Galea M. Whole-body vibration dosage alters leg blood flow. Clin Physiol Funct Imaging. 2009;29:53–59. doi: 10.1111/j.1475-097X.2008.00834.x. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 17.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 18.Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 19.Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 20.Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension. 2006;47:1203–1208. doi: 10.1161/01.HYP.0000223013.60612.72. [DOI] [PubMed] [Google Scholar]
- 21.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 22.Fahs CA, Heffernan KS, Fernhall B. Hemodynamic and vascular response to resistance exercise with L-arginine. Med Sci Sports Exerc. 2009;41:773–779. doi: 10.1249/MSS.0b013e3181909d9d. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueroa A, Hooshmand S, Figueroa M, Bada AM. Cardiovagal baroreflex and aortic hemodynamic responses to isometric exercise and post-exercise muscle ischemia in resistance trained men. Scand J Med Sci Sports. 2010;20:305–309. doi: 10.1111/j.1600-0838.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 25.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Cortez-Cooper MY, DeVan AE, Anton MM, Farrar RP, Beckwith KA, Todd JS, Tanaka H. Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am J Hypertens. 2005;18:930–934. doi: 10.1016/j.amjhyper.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Avolio A, Jones D, Tafazzoli-Shadpour M. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension. 1998;32:170–175. doi: 10.1161/01.hyp.32.1.170. [DOI] [PubMed] [Google Scholar]
- 28.Figueroa A, Gil R, Sanchez-Gonzalez MA. Whole-body vibration attenuates the increase in leg arterial stiffness and aortic systolic blood pressure during post-exercise muscle ischemia. Eur J Appl Physiol. 2010 doi: 10.1007/s00421-010-1746-6. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara J, Maeda S, Otsuki T, Tanabe T, Ajisaka R, Matsuda M. Effects of nitric oxide synthase inhibitor on decrease in peripheral arterial stiffness with acute low-intensity aerobic exercise. Am J Physiol Heart Circ Physiol. 2004;287:H2666–2669. doi: 10.1152/ajpheart.00077.2004. [DOI] [PubMed] [Google Scholar]
- 30.Campbell R, Fisher JP, Sharman JE, McDonnell BJ, Frenneaux MP. Contribution of nitric oxide to the blood pressure and arterial responses to exercise in humans. J Hum Hypertens. 2010 doi: 10.1038/jhh.2010.53. [DOI] [PubMed] [Google Scholar]
- 31.Kingwell BA, Berry KL, Cameron JD, Jennings GL, Dart AM. Arterial compliance increases after moderate-intensity cycling. Am J Physiol. 1997;273:H2186–2191. doi: 10.1152/ajpheart.1997.273.5.H2186. [DOI] [PubMed] [Google Scholar]
- 32.Sugawara J, Otsuki T, Tanabe T, Maeda S, Kuno S, Ajisaka R, Matsuda M. The effects of low-intensity single-leg exercise on regional arterial stiffness. Jpn J Physiol. 2003;53:239–241. doi: 10.2170/jjphysiol.53.239. [DOI] [PubMed] [Google Scholar]
- 33.Maloney-Hinds C, Petrofsky JS, Zimmerman G, Hessinger DA. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther. 2009;11:39–43. doi: 10.1089/dia.2008.0011. [DOI] [PubMed] [Google Scholar]
- 34.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine (Phila Pa 1976) 2003;28:2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 36.Tuttolomondo A, Di Sciacca R, Di Raimondo D, Serio A, D'Aguanno G, Pinto A, Licata G. Arterial stiffness indexes in acute ischemic stroke: relationship with stroke subtype. Atherosclerosis. 2010;211:187–194. doi: 10.1016/j.atherosclerosis.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Kimoto E, Shoji T, Shinohara K, Inaba M, Okuno Y, Miki T, Koyama H, Emoto M, Nishizawa Y. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes. 2003;52:448–452. doi: 10.2337/diabetes.52.2.448. [DOI] [PubMed] [Google Scholar]