Abstract
The most widespread form of cardiovascular disease (CVD) in the western world today is atherosclerosis (AS), notably of the major arteries. Despite decades of laboratory, animal, and clinical investigation providing multiple leads and intervention strategies that have resulted in a progressive decline in CVD-related mortality rates, the etiology of AS is incomplete; hence, the need for management and prevention remains imperative. AS is initiated by endothelial cell (EC) injury whereas its progression is promoted by EC interaction with cells that are recruited to the injured endothelium, and additionally, bioactive cytokines and chemokines elaborated by the intercellular interplay. A primary research interest of our laboratory has been the mechanisms that underlie the “French paradox” - an epidemiological association of the co-existence of risk factors with low CVD incidence/mortality rates postulated to attribute to low-to-moderate consumption of red wine and grape-derived polyphenol resveratrol. This review summarizes effects and targets of resveratrol in cultured human aortic and pulmonary aortic endothelial cells (HAEC and HPAEC) and animal tissues, with focus on the resveratrol target protein RTP, N-ribosyldihydro-nicotinamide:quinone oxidoreductase (NQO2).
Keywords: Resveratrol, cardioprotection, RTPs (resveratrol target proteins), human endothelial cells, wistar rat tissues
Cardioprotection by resveratrol - weight of evidence
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in the United States and it is most frequently manifested as atherosclerosis (AS) of the major arteries. In spite of intense research on AS using a combination of laboratory, animal, and clinical studies, understanding of its etiology is still incomplete; hence development of cost-effective and readily compliant prevention and management strategies remains imperative.
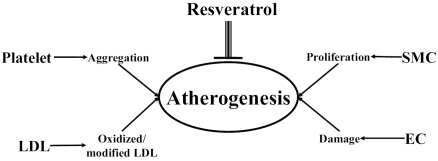
The “response-to-injury” hypothesis by Ross in the 1970's posited that AS is initiated by recurrent episodes of injury to the endothelium by a myriad of risk factors; notably, cigarette smoking, hypercholesterolemia, and high blood pressure [1]. Damage to the endothelium induces multiple changes in the vessel architecture and blood components, as exemplified by an increase in hemodynamic flow, recruitment and attachment of leukocytes and platelets, migration of smooth muscle cells to the intima, and deposition of extracellular matrix, which collectively contribute to thrombus formation and eventual occlusion of the vessel (Figure 1).
Figure 1.
Scheme depicting cardioprotection by resveratrol as attributed to its ability to act on the same multiple cellular targets impinging on atherogenesis - manifestation of multiple cellular dysfunctions including LDL oxidation, platelet aggregation, and proliferation of smooth muscle cells (SMC) and injury of endothelial cells (EC).
A progressive decline in age-adjusted CVD-related mortality over the past few decades has been reported in the United States, due to advances in anti-CVD strategies and also to the discovery of dietary agents with cardioprotective activities. Since 1998, research activities in this laboratory have focused on cardioprotection by grape-derived polyphenol, resveratrol, in large part to address the French paradox - the epide-miological phenomenon linking co-existence of CVD risk factors with low incidence/mortality rates which purportedly may attribute to low-to-moderate consumption of red wine and alcoholic beverages [2, 3]. Our studies have shown that resveratrol: i) inhibits oxidation of low density lipoprotein (LDL) isolated from normoten-sive individuals [4-6], ii) suppresses platelet aggregation [7-9], ii) reduces in ADP-induced platelet aggregation rate in rabbits [7, 10], iii) inhibits human and rabbit smooth muscle cell (SMC) proliferation in vitro, in response to stimulation by PDGF, endothelin, angitension II, and serum-mitogens [11-13], iv) reduces EC proliferation [14-16], v) attenuates intimal thickening following endothelium denudation in rabbits [17, 18] and reduces the mean area of atherosclerotic plaques [19], and vi) dose-dependently increases the protein expression of tumor suppressor gene p53, heat shock protein HSP27, quinone reductase 1 and 2, and induces nitric oxide synthase and its altered sub-cellular distribution [20]. Together, these results support cardioprotection by resveratrol acting by a plethora of activities impinging on events key to AS and CVD and reinforcing the notion that age-adjusted CVD deaths may be independently modulated by diet-based strategies including use of resveratrol.
Identification of RTPs (resveratrol target proteins) in bovine pulmonary aortic endothelial cells (BPAEC) and tissues from different age Wistar rats
To determine the mechanism of cardioprotection by resveratrol we advanced the hypothesis that resveratrol acts through interaction with distinct resveratrol target proteins, denoted RTPs. By using an affinity purification strategy in which column immobilized resveratrol serves as the RTP-retention bait, in combination with Matrix Assisted Laser Desorption/Ionization Time-of-Flight mass spectrometry and cloning, we identified quinone reductase NQO2 as a specific RTP with a high affinity for resveratrol (KD≤50 nM) [20-23].
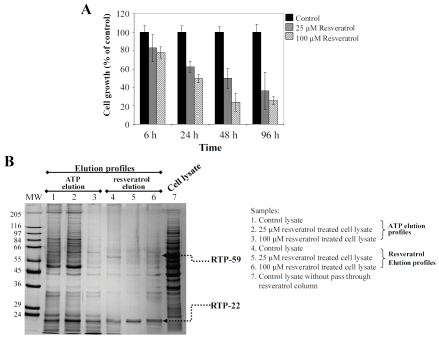
Since proliferation of BPAEC was significantly suppressed by resveratrol in a time- and dose-dependent manner [14, 15] (Figure 2A), we tested the presence of RTPs in cultured BPAEC using the resveratrol-directed affinity chromatography approach. Passage 4-6 BPAEC (obtained from Dr. Olson in this department) was grown to 70% confluence, and treated for 48 h with 25 and 100 μM resveratrol. Cells were harvested after 48 h treatment and lysates were prepared using our previously published procedures. Extracts containing 1 mg protein from control and treated cells were individually applied to mini-columns each containing 0.2 ml of resveratrol-coupled agarose resin. Each column was sequentially eluted with NaCl, ATP and resveratrol [15, 20, 21]. The ATP and resveratrol eluates were concentrated and resolved on 10% SDS-PAGE, followed by silver staining. Figure 2B depicts the stained protein patterns. Treatment with resveratrol resulted in prominent suppression (RTP-59) and slight induction (RTP-22) of proteins. Since both proteins appeared in the fraction eluted with resveratrol, it is evident that they bound avidly with high affinity to the resveratrol column. Interestingly, several silver stained proteins in the ATP-eluated fraction from the 25 μM resveratrol-treated cell extract displayed a profile distinct from a similarly eluted fraction of the control extract. RTP-22 was shown to be NQO2 with a high affinity for resveratrol [20, 21, 24].
Figure 2.
Panel A. Time- and dose-dependent inhibition of proliferation of cultured bovine pulmonary endothelial cells, BPAEC. Panel B. Fractionation of cytosolic cell extracts prepared from cultured BPAEC, with and without treatment for 48 h with 25 and 100 mM resveratrol, using resveratrol-immobilized affinity column. Lane marked MW, molecular weight marker; lanes 1-3 represent proteins eluted from affinity column using 1 mM ATP; lanes 4-6 represent proteins eluted from affinity column using 2 mM resveratrol; lane 7, unfractionated cytosolic extract from control cells. Lanes 1 and 4, eluted fractions from control cells; lanes 2 and 5, eluted fractions from cells treated with 25 mM resveratrol; lanes 3 and 6, eluted fractions from cells treated with 100 mM resveratrol. ATP and resveratrol eluted fractions were concentrated using a defined methanol:chloroform:water mixture [21, 47], separated by SDS-PAGE, and visualized by silver staining.
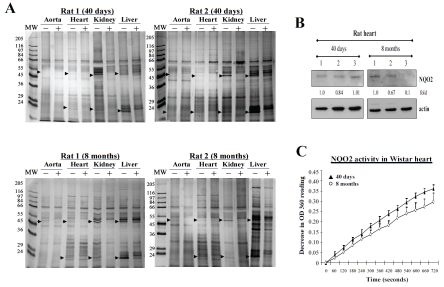
To further explore the versatility of resveratrol affinity column approach, its ability for fractionating extracts prepared from animal tissues was investigated. Studies were performed using several tissues, respectively, aorta, heart, kidney and liver, isolated from different age male Wistar rats (40 days and 8 months, from Charles River Laboratories). Homogenates were prepared from tissues and fractionated on resveratrol affinity column, as described. Figure 3A shows results of silver stained bands in the resveratrol-eluted fraction (with and without prior competition with excess resveratrol). Several interesting results are evident in these experiments: i) RTP-22 was below detection in the aorta in both age rats, ii) RTP-22 was most prominent in the liver and kidney among 4 tissues tested and appeared to decrease in 8-month rats, compared to 40-day rats, iii) RTP-22 was detected in the heart extract fractionated on resveratrol affinity column; the intensity of silver-stained RTP-22 appeared to be slightly less in 8-month-old rats. Evidence of decreased expression of RTP-22 as NQO2 in 8-month rat heart compared to 40-day rat heart was obtained by immunoblot analysis (Figure 3B) and enzyme activity assays (Figure 3C). The observed data show that rat tissues are appropriate for testing presence of RTPs and may be suitable in feeding experiments using resveratrol, red wine extracts and other dietary agents with potential cardioactive properties. It is also noteworthy that results using animal tissues further confirm the utility and sensitivity of this biospecific affinity platform as a quick, cost-effective, and reproducible method for profiling protein patterns exhibiting differential binding properties to resveratrol.
Figure 3.
Panel A. Presence of RTPs (identified by arrows) in extracts of tissues, respectively, aorta, heart, kidney, and liver dissected from 40 day and 8-month-old male Wistar rats, which were fractionated on resveratrol affinity column using procedures detailed in Figure 2 legend. For each tissue extract tested, lanes marked (-) and (+) represent extracts without and with prior incubation with 1 mM resveratrol prior to binding and fractionation on resveratrol affinity column. RTPs binding with high affinity to the affinity column can be effectively competed by prior incubation with 1 mM resveratrol. Panel B. Immunoblot blot analysis demonstrating NQO2 as an identified RTP in extracts prepared from three individual rat hearts from 40 day and 8 month old animals. Panel C. Assay of NQO2 activity using heart extracts prepared from 40 day and 8-month-old male Wistar rats.
Uptake of [3H]resveratrol in cultured human aortic endothelial cells
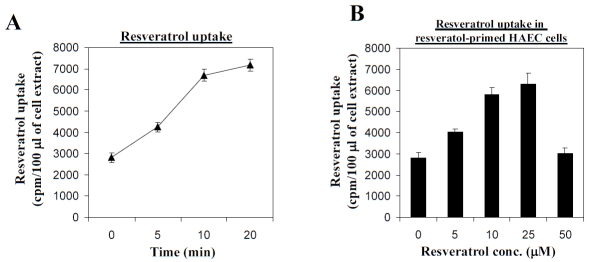
Presence of RTPs in BPAEC and animal tissues suggests that resveratrol is readily taken up in responsive cell types. This possibility was tested using cultured HAEC obtained from commercial sources (Lonza Walkerrsville Inc., Walkersville, MD, USA). Subconfluent cells maintained in Clonetics EGM-2 BulletKit containing endothelial cell basal medium-2 and growth supplements as recommended by the manufacturer were treated for 2 days with 0, 5, 10, 25, and 50 μM resveratrol. Control and treated cells were incubated with 5 nM [3H] resveratrol (specific activity, 15 Ci/mmol), from Moravek Biochemicals (Brea, CA, USA)) for 0, 5, 10, and 20 min in serum-free medium at 37°C, 95% humidity and 5% CO2. At the end of the labeling period, cells were harvested, washed three times with ice-cold phosphate buffered saline to remove unincorporated resveratrol. Attached cells were lysed with 1 ml 0.1 N NaOH. The amount of cell-associated radioactivity was determined by mixing the pooled cell lysates containing 6-16 mg soluble proteins with 4 ml of scintillation cocktail, Liquiscint (National Diagnostics, Atlanta, GA, USA), and counting using a liquid scintillation counter (Beckman LS 6500, Jersey City, NJ, USA). Figure 4A showed [3H] resveratrol bound to HAEC extracts when labeling was immediately terminated by harvesting (within 1 min) and processing (within 2 min), suggesting that there was an initial, rapid association of [3H] resveratrol with cellular components. By following the amount of radioactivity in cell extracts after 5, 10 or 20 min of labeling, it can be seen that uptake of [3H]resveratrol increased progressively in both control and resveratrol-pretreated HAEC (Figure 4A). Uptake of resveratrol may be further modulated by resveratrol since in HAEC cells pretreated with increasing doses (5-50 μM) of unlabeled resveratrol for 2 days, the uptake of [3H]resveratrol into treated HAEC increased in a dose-dependent manner up to 25 μM, more substantially than the control, (Figure 4B), suggesting that pretreatment may affect the cell membrane properties in ways that stimulated the uptake of resveratrol. It is also noteworthy that in control HAEC, the uptake of radioactive resveratrol within the first 10 minutes was more rapid and robust, and is followed by a steady, maintenance level of labeled resveratrol from 10-20 min (Figure 4A). These results are consistent with the existence of a membrane-associated transport mechanism for resveratrol.
Figure 4.
The uptake of [3H]resveratrol by human aortic endothelial cells (HAEC). PanelA. Time-course of resveratrol uptake by HAEC. Control cells were incubated with [3H]-labeled resveratrol for the indicated time durations. The uptake of resveratrol was measured as described in the text. Panel B. Effects of pretreatment with resveratrol on uptake of resveratrol in HAEC. Cells were pretreated with 0, 5, 10, 25 or 50 μM resveratrol for 2 days, washed extensively to remove resveratrol, and then incubated with [3H]-labeled resveratrol for 10 minutes. The uptake of resveratrol was measured as described in the text. Data points are expressed as mean ± SD from 3-6 determinations.
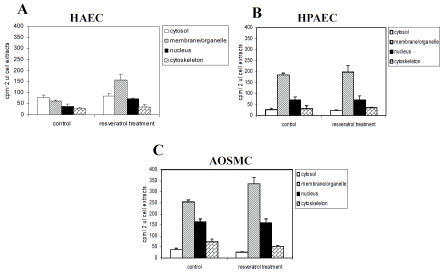
Subcellular distribution of resveratrol in HAEC, HPAEC and AOSMC
Determination of the intracellular disposition of resveratrol is of interest since it might provide information on the identity and nature of cellular targets with which resveratrol binds specifically. We analyzed the intracellular localization of resveratrol in HAEC, HPAEC and AOSMC. Cells were incubated with [3H]resveratrol for 10 min, harvested and lysed. Fractionation of the lysate from control and treated cells was performed using the Subcellular Proteome extraction kit, into four cellular compartments, respectively, F1 (cytosol), F2 (membrane/organelle), F3 (nucleus), and F4 (cytoskeleton). Protein content of cell lysates and cellular fractions was determined by coomassie protein assay kit (Pierce, Rockford, IL, USA) with BSA as standard.
The amount of radioactive resveratrol in each compartment was determined. In parallel experiments, we also tested whether the subcellular distribution of this polyphenol may be altered by prior exposure of cells for 2 days to 25 μM resveratrol. Figure 5 shows that i) distribution of radioactive resveratrol in untreated HAEC was different from partitioning of resveratrol in HPAEC, ii) the subcellular distribution of resveratrol followed the pattern of membrane/organelle > nucleus > cytoskeleton = cytosol in control and treated HPAEC and AOSMC, and in resveratrol-treated HAEC, iii) subcellular profile of radioactive resveratrol was similar in untreated and treated HPAEC and AOSMC; in contrast, treatment by resveratrol markedly changed the cellular distribution of labeled resveratrol in HAEC, iv) treatment by resveratrol significantly increased (30-100%) the amount of radioactivity in the organelle/membrane fraction in AOSMC and HAEC. These results suggest that except for untreated HAEC, once resveratrol gains entry into cells, it is rapidly and preferentially delivered to the organelle/membrane fraction.
Figure 5.
Subcellular distribution of resveratrol in HAEC, HPAEC and AOSMC. Panel A. Subcellular distribution of [3H] resveratrol in untreated and 25 μM resveratrol pre-treated HAEC. Panel B. Subcellular distribution of [3H]resveratrol in untreated and 25 μM resveratrol pre-treated HPAEC. Panel C. Subcellular distribution of [3H]resveratrol in untreated and 25 μM resveratrol pre-treated AOSMC. Cells were pretreated with 0 or 25 μM resveratrol for 2 days prior incubation with [3H]-labeled resveratrol. Cell extracts were analyzed by fractionation into the cytosol, membrane/ organelle, nucleus, and cytoskeleton as described in the text. The amount of radioactivity associated with each fraction was measured as described in the text. Data points are expressed as mean ± SD from 3-6 determinations.
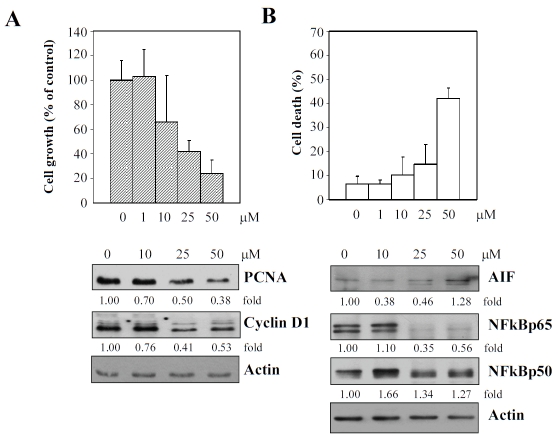
Resveratrol pronouncedly inhibits growth of human aortic endothelial cells (HAEC)
As mentioned previously, EC plays an essential role on AS and CVD; the “response-to-injury” hypothesis introduced by Ross places episodic insults to EC as pivotal events in the initiation of atherogenesis [25-28]. Accordingly, prevention of injury distinct from or concurrent with stimulation of repair to damaged endothelium might be intimately involved in mechanism of action of cardioprotective agents. Therefore, we tested the effects of resveratrol on proliferation of cultured HAEC. Subconfluent cells maintained in CloneticsÒEGMÒ-2 BulletKitÒ containing endothelial cell basal medium-2 and growth supplements as recommended by the manufacturer were treated with 0, 1, 10, 25, and 50 mM resveratrol. Control and treated cells were harvested at 48 h. Growth and viability was determined using a hemocytometer and by the trypan blue exclusion assay. Figure 6 shows that proliferation of HAEC (panel A) was inhibited by resveratrol in a concentration-dependent manner, and at the highest concentration of 50 μM resveratrol tested, also accompanied by substantial cell death (panel B). Next, immunoblot analysis was performed to examine changes in levels of several key proteins playing a critical role in the regulation of cell growth and death. These results are presented in the lower panel in Figure 6. Evidently, a concentration-dependent decrease in expression of PCNA and cyclin D1, NFkBp65 was observed. Notably, the 50 μM resveratrol-treated HAEC was also accompanied by an increase in AIF expression.
Figure 6.
Suppression of growth, as evidenced by immunoblot analysis of specific gene expression changes (panel A) and induction of cell death, based on determination of changes in expression of specific proteins shown (panel B) of HAEC by increasing concentrations of resveratrol. Results in (A) represent the mean of triplicate experiments± SD. Results in immunblot analysis were quantified using actin expression as the internal loading control.
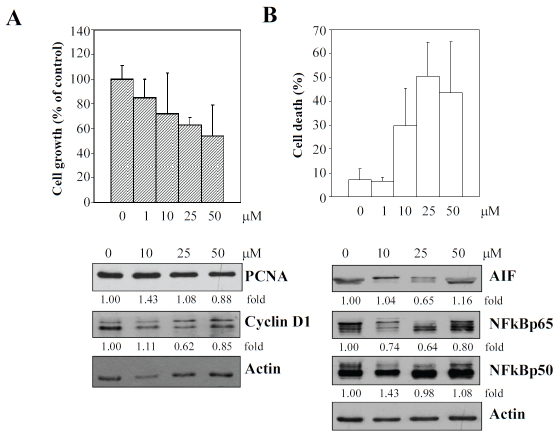
Resveratrol exerts a less prominent growth inhibitory effect on human pulmonary aortic endothelial cells (HPAEC)
Recent studies show that heterogeneity is intrinsically present among endothelial cells derived from anatomically distinct sites [29-33], and additionally, also from different species [34]. Endothelial cells from different vascular locations may also exhibit strikingly different responses when exposed to identical physical and biological challenges [35-46]. Furthermore, a remarkable focal distribution has been found in vascular diseases, suggesting that the function and regulatory response of endothelial cells to risk as well as protective factors may be contingent upon the source of the vasculature from which endothelial cells originate. Therefore, the effects of resveratrol on growth of cultured commercially available HPAEC was also performed, by exposing cells to an increasing concentration of the grape polyphenol, respectively, at 1, 10, 25, and 50 μM. At 48 h post treatment, cells were harvested for assessment of growth and viability, using the trypan blue exclusion assay. Figure 7 shows that proliferation of HPAEC was inhibited less significantly by resveratrol compared to HAEC; moreover, little effect of resveratrol on PCNA expression was observed. Of note, inhibition on cyclin D1 and NFkBp65 and stimulation on AIF in resveratrol-exposed HPAEC, was much more modest, compared to similarly treated HAEC (Figure 6).
Figure 7.
Suppression of growth, as evidenced by immunoblot analysis of specific gene expression changes (panel A) and induction of cell death, based on determination of changes in expression of specific proteins shown (panel B) of HPAEC by increasing concentrations of resveratrol. Results in (A) represent the mean of triplicate experiments± SD. Results in immunblot analysis were quantified using actin expression as the internal loading control.
Summary and conclusion
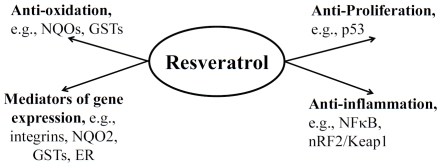
Red wine polyphenols have been proposed to confer protection against CVD. In this review, we have summarized data showing that biologically active red wine polyphenol, resveratrol acts by a plethora of activities and putative molecular targets (Figure 8), in support of its role as a deterrent targeting the endothelium and contributing to prevention of AS. Additional studies to determine the mechanism of cardioprotection by resveratrol may further advance the development of diet-based strategies to reduce the adverse health effects of risk factors on the endothelium and CVD.
Figure 8.
Proposed biological effects and molecular targets, both directly and indirectly of resveratrol contributing to its cardioprotective activities.
Acknowledgments
Studies on cardioprotection by resveratrol in our laboratory since 1998 had been supported by: California Grape Trade Commission, Phillip Morris USA Inc. and Phillip Morris International, and the Intramural Sponsored Research Program of New York Medical College to JMW.
References
- 1.Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review) Int J Mol Med. 2001;8:3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Das DK, Sato M, Ray PS, Maulik G, Engelman RM, Bertelli AA, Bertelli A. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res. 1999;25:115–120. [PubMed] [Google Scholar]
- 4.Zou JG, Huang YZ, Chen Q, Wei EH, Hsieh TC, Wu JM. Resveratrol inhibits copper ion-induced and azo compound-initiated oxidative modification of human low density lipoprotein. Biochem Mol Biol Int. 1999;47:1089–1096. doi: 10.1080/15216549900202213. [DOI] [PubMed] [Google Scholar]
- 5.Zou J, Huang Y, Chen Q, Wei E, Cao K, Wu JM. Effects of resveratrol on oxidative modification of human low density lipoprotein. Chin Med J (Engl) 2000;113:99–102. [PubMed] [Google Scholar]
- 6.Belguendouz L, Fremont L, Linard A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem Pharmacol. 1997;53:1347–1355. doi: 10.1016/s0006-2952(96)00820-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Zou J, Huang Y, Cao K, Xu Y, Wu JM. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin Med J (Engl) 2002;115:378–380. [PubMed] [Google Scholar]
- 8.Kirk RI, Deitch JA, Wu JM, Lerea KM. Resveratrol decreases early signaling events in washed platelets but has little effect on platalet in whole food. Blood Cells Mol Dis. 2000;26:144–150. doi: 10.1006/bcmd.2000.0289. [DOI] [PubMed] [Google Scholar]
- 9.Lin KH, Hsiao G, Shih CM, Chou DS, Sheu JR. Mechanisms of resveratrol-induced platelet apoptosis. Cardiovasc Res. 2009;83:575–585. doi: 10.1093/cvr/cvp139. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–79. [PubMed] [Google Scholar]
- 11.Zou J, Huang Y, Chen Q, Wang N, Cao K, Hsieh TC, Wu JM. Suppression of mitogenesis and regulation of cell cycle traverse by resveratrol in cultured smooth muscle cells. Int J Oncol. 1999;15:647–651. doi: 10.3892/ijo.15.4.647. [DOI] [PubMed] [Google Scholar]
- 12.Poussier B, Cordova AC, Becquemin JP, Sumpio BE. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J Vasc Surg. 2005;42:1190–1197. doi: 10.1016/j.jvs.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Brito PM, Devillard R, Negre-Salvayre A, Almeida LM, Dinis TC, Salvayre R, Auge N. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2009;205:126–134. doi: 10.1016/j.atherosclerosis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh TC, Juan G, Darzynkiewicz Z, Wu JM. Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21(WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2. Cancer Res. 1999;59:2596–2601. [PubMed] [Google Scholar]
- 15.Bruder JL, Hsieh T, Lerea KM, Olson SC, Wu JM. Induced cytoskeletal changes in bovine pulmonary artery endothelial cells by resveratrol and the accompanying modified responses to arterial shear stress. BMC Cell Biol. 2001;2:1. doi: 10.1186/1471-2121-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. Faseb J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 17.Zou J, Huang Y, Cao K, Yang G, Yin H, Len J, Hsieh TC, Wu JM. Effect of resveratrol on intimal hyperplasia after endothelial denudation in an experimental rabbit model. Life Sci. 2000;68:153–163. doi: 10.1016/s0024-3205(00)00925-5. [DOI] [PubMed] [Google Scholar]
- 18.Zou JG, Wang ZR, Huang YZ, Cao KJ, Wu JM. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int J Mol Med. 2003;11:317–320. [PubMed] [Google Scholar]
- 19.Wang Z, Zou J, Cao K, Hsieh TC, Huang Y, Wu JM. Dealcoholized red wine containing known amounts of resveratrol suppresses atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels. Int J Mol Med. 2005;16:533–540. [PubMed] [Google Scholar]
- 20.Wang Z, Chen Y, Labinskyy N, Hsieh TC, Ungvari Z, Wu JM. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem Biophys Res Commun. 2006;346:367–376. doi: 10.1016/j.bbrc.2006.05.156. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Hsieh TC, Zhang Z, Ma Y, Wu JM. Identification and purification of resveratrol targeting proteins using immobilized resveratrol affinity chromatography. Biochem Biophys Res Commun. 2004;323:743–749. doi: 10.1016/j.bbrc.2004.08.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh TC, Wang Z, Hamby CV, Wu JM. Inhibition of melanoma cell proliferation by resveratrol is correlated with upregulation of quinone reductase 2 and p53. Biochem Biophys Res Commun. 2005;334:223–230. doi: 10.1016/j.bbrc.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh TC, Wang Z, Deng H, Wu JM. Identification of glutathione sulfotransferase-pi (GSTP1) as a new resveratrol targeting protein (RTP) and studies of resveratrol-responsive protein changes by resveratrol affinity chromatography. Anticancer Res. 2008;28:29–36. [PMC free article] [PubMed] [Google Scholar]
- 24.Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, Zhang Z. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry. 2004;43:11417–11426. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 26.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 27.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts) N Engl J Med. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 28.Ross R, Glomset JA. The pathogenesis of atherosclerosis (second of two parts) N Engl J Med. 1976;295:420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- 29.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 30.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 31.Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res. 2006;98:159–162. doi: 10.1161/01.RES.0000204553.32549.a7. [DOI] [PubMed] [Google Scholar]
- 32.Aird WC. Endothelial cell heterogeneity and atherosclerosis. Curr Atheroscler Rep. 2006;8:69–75. doi: 10.1007/s11883-006-0067-z. [DOI] [PubMed] [Google Scholar]
- 33.Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- 34.Kjellstrom BT, Ortenwall P, Risberg B. Comparison of oxidative metabolism in vitro in endothelial cells from different species and vessels. J Cell Physiol. 1987;132:578–580. doi: 10.1002/jcp.1041320323. [DOI] [PubMed] [Google Scholar]
- 35.Wakefield TW, Hinshaw DB, Burger JM, Burkel WE, Stanley JC. Protamine-induced reductions of endothelial cell ATP. Surgery. 1989;106:378–385. [PubMed] [Google Scholar]
- 36.Welsby IJ, Newman MF, Phillips-Bute B, Messier RH, Kakkis ED, Stafford-Smith M. Hemodynamic changes after protamine administration: association with mortality after coronary artery bypass surgery. Anesthesiology. 2005;102:308–314. doi: 10.1097/00000542-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Levy JH, Tanaka KA. Management of surgical hemostasis: systemic agents. Vascular. 2008;16(Suppl 1):S14–21. [PubMed] [Google Scholar]
- 38.Farber HW, Center DM, Rounds S. Effect of ambient oxygen on cultured endothelial cells from different vascular beds. Am J Physiol. 1987;253:H878–883. doi: 10.1152/ajpheart.1987.253.4.H878. [DOI] [PubMed] [Google Scholar]
- 39.Friedman HM, Wolfe J, Kefalides NA, Macarak EJ. Susceptibility of endothelial cells derived from different blood vessels to common viruses. In Vitro Cell Dev Biol. 1986;22:397–401. doi: 10.1007/BF02623529. [DOI] [PubMed] [Google Scholar]
- 40.Johnson AR. Human pulmonary endothelial cells in culture. Activities of cells from arteries and cells from veins. J Clin Invest. 1980;65:841–850. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchan KW, Martin W. Modulation of barrier function of bovine aortic and pulmonary artery endothelial cells: dissociation from cytosolic calcium content. Br J Pharmacol. 1992;107:932–938. doi: 10.1111/j.1476-5381.1992.tb13388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiwara M, Jin E, Ghazizadeh M, Kawanami O. Differential expression of proteaseactivated receptors 1, 2, and 4 on human endothelial cells from different vascular sites. Pathobiology. 2004;71:52–58. doi: 10.1159/000072962. [DOI] [PubMed] [Google Scholar]
- 43.Manolopoulos VG, Samet MM, Lelkes PI. Regulation of the adenylyl cyclase signaling system in various types of cultured endothelial cells. J Cell Biochem. 1995;57:590–598. doi: 10.1002/jcb.240570403. [DOI] [PubMed] [Google Scholar]
- 44.Graven KK, Zimmerman LH, Dickson EW, Weinhouse GL, Farber HW. Endothelial cell hypoxia associated proteins are cell and stress specific. J Cell Physiol. 1993;157:544–554. doi: 10.1002/jcp.1041570314. [DOI] [PubMed] [Google Scholar]
- 45.Merker MP, Audi SH, Bongard RD, Lindemer BJ, Krenz GS. Influence of pulmonary arterial endothelial cells on quinone redox status: effect of hyperoxia-induced NAD(P)H:quinone oxidoreductase 1. Am J Physiol Lung Cell Mol Physiol. 2006;290:L607–619. doi: 10.1152/ajplung.00302.2005. [DOI] [PubMed] [Google Scholar]
- 46.Wu W, Platoshyn O, Firth AL, Yuan JX. Hypoxia divergently regulates production of reactive oxygen species in human pulmonary and coronary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L952–959. doi: 10.1152/ajplung.00203.2007. [DOI] [PubMed] [Google Scholar]
- 47.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]