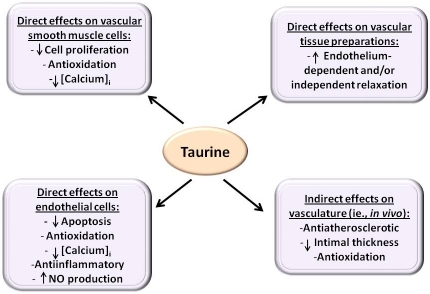
Abstract
Taurine is a sulfur-containing amino acid-like endogenous compound found in substantial amounts in mammalian tissues. It exerts a diverse array of biological effects, including cardiovascular regulation, antioxidation, modulation of ion transport, membrane stabilization, osmoregulation, modulation of neurotransmission, bile acid conjugation, hypolipidemia, antiplatelet activity and modulation of fetal development. This brief review summarizes the role of taurine in the vasculature and modulation of blood pressure, based on experimental and human studies. Oral supplementation of taurine induces antihypertensive effects in various animal models of hypertension. These effects of taurine have been shown to be both centrally and peripherally mediated. Consistent with this, taurine produces endothelium-dependent and independent relaxant effects in isolated vascular tissue preparations. Oral administration of taurine also ameliorates impairment of vascular reactivity, intimal thickening, arteriosclerosis, endothelial apoptosis, oxidative stress and inflammation, associated primarily with diabetes and, to a lesser extent with obesity, hypertension and nicotine-induced vascular adverse events. In rat aortic vascular smooth muscle cells (VSMCs), taurine acts as an antiproliferative and antioxidant agent. In endothelial cells, taurine inhibits apoptosis, inflammation, oxidative stress and cell death while increasing NO generation. Oral taurine in hypertensive human patients alleviates the symptoms of hypertension and also reverses arterial stiffness and brachial artery reactivity in type 1 diabetic patients. However, despite these favorable findings, there is a need to further establish certain aspects of the reported results and also consider addressing unresolved related issues. In addition, the molecular mechanism (s) involved in the vascular effects of taurine is largely unknown and requires further investigations. Elucidation of the mechanisms through which taurine affects the vasculature could facilitate the development of therapeutic and/or diet-based strategies to reduce the burdens of vascular diseases.
Keywords: Taurine, isolated vascular tissue preparations, VSMCs (vascular smooth muscle cells), endothelial cells, vasorelaxation, hypertension, diabetes, atherosclerosis, taurine deficiency
Introduction
Taurine (2-aminoethanesulfonic acid) is a naturally-occurring amino acid-like compound present in substantial amounts in many mammalian tissues [reviewed in 1-3]. It is mostly found in free form in the cytosol and plasma, and the concentrations in these compartments in humans are 50-200 μM and 5-30 mM, respectively. Further, taurine is not incorporated into protein structures. In humans, taurine is considered semi-essential since it can be synthesized endogenously.
Taurine was first isolated from the bile of ox (Latin Bos taurus) in 1827 by the Gerrnan scientists, Friedrick Triedemann and Leopold Gmelin [4]. Humans and animals can synthesize taurine from methionine and cysteine (or their precursors), primarily in the liver; it is then delivered to target tissues by the circulation (1). However, the biosynthetic capacity and turnover rate of taurine can vary significantly in different animal species (e.g., cats lack the ability for endogenous taurine biosynthesis while all others studied do not). Although humans can synthesize taurine, the majority of taurine stores derive from food sources of animal origin, especially eggs, meat and seafood. Taurine is readily absorbed from the gastrointestinal tract. Both ingested and endogenous taurine is transported into the interiors of cells across plasma membrane by a high-affinity active transport system designated taurine transporter (TAUT) [5]. In addition, at relatively high concentrations, taurine is also transported by diffusion [6]. The active taurine transport system is stereospecific and is inhibited by β-amino acids (eg., β-alanine), guanidino ethane sulfonate (GES) and γ-amino butyric acid. This transporter is proposed to help maintain the intracellular concentrations of taurine. While the distribution of taurine can differ markedly depending upon tissue/cell types, high levels of the compound are present in bile, intestine, heart, skeletal muscle, brain, nerve, liver, kidney, retina and leukocyts [1,2,7]. The excretion of taurine in mammals takes place mainly via the kidney and the rate of excretion is closely related to dietary intake of the nutrient.
Taurine has been shown to exert a diversity of biological effects, enabling it to influence the functions of multiple organ systems. More commonly reported effects include cardiovascular regulation, antioxidation, modulation of ion transport, membrane stabilization, osmoregulation, modulation of neurotransmission, bile acid conjugation, hypolipidemia, antiplatelet activity and fetal development [1,2,8]. Most of these effects of taurine have been suggested to be a reflection of its modulatory role in membrane structure and function. In this regard, intracellular taurine, by virtue of its chemical nature, interacts electrostatically with polar groups of phospholipids, with possible effects on membrane permeability and fluidity [1,2,8]. This in turn influences the susceptibility of the structures and functions of a range of membrane-bound proteins (eg., receptors, transport proteins, ion channels, G-proteins and effector enzymes) to covalent modification and modulation [1,2,8].
Due to the important role taurine plays in biological and physiological functions, deficiency of the nutrient has been associated with various pathological conditions [9,10]. Indeed, prolonged low dietary intake of taurine has been observed to be linked to a number of disorders including retinal degeneration, retardation of growth and development, cardiovascular dysfunctions, CNS abnormalities, immune impairment and hepatic disorders. Most of these conditions have been shown to be effectively prevented and/or reversed by taurine supplementation. In clinical trials, taurine and some of its analogs have been utilized with varying degrees of success in the treatment of a wide variety of related conditions, such as congestive heart failure, hypertension, hypercholesterolemia, epilepsy/seizures, retinal disorders, Alzheimer's disease, hepatic problems, alcoholism, fatigue, cancer and cystic fibrosis [11-14].
The present article briefly reviews the role of taurine in the vasculature by examining the evidence from in vitro, animal and/or human studies in regard to its tissue content, transporter, and biological/physiological effects, aspects that have received relatively little attention.
Vascular taurine content and transporter
There is limited information in the literature on the distribution and content of taurine in vascular tissue. Korang et al. [15] studied these aspects of taurine in rat aorta and vena cava. The investigators found that the basal levels of taurine in these tissues were 2,129 ± 195 and 6,249 ± 310 nmol/g, respectively. Injection of taurine into rats as 0.8 g/kg ip elevated its contents in the aorta and vena cava by 3 and 2 folds, respectively, after 30 min. Simultaneous measurement of plasma taurine demonstrated that changes in plasma concentrations do not necessarily predict the changes in tissue contents; the relevance of this observation to other tissue types remains to be established. We have also shown a nearly similar basal level of taurine in the rat aorta [16]. On the other hand, treatment of rats with 3% β-alanine in drinking water for 3 weeks caused a significant reduction of taurine content in the aorta by about 40%, as reported for the kidney and heart [16-19]. It should be noted that compared to other major organs, the basal levels of taurine determined in these blood vessels are generally low. For example, Korang et al. [15] found that the corresponding value in the rat heart was 31,362 ± 1,886 nmol/g. The relevance of variations in tissue levels of taurine to physiological functions remain to be established. While there is insufficient information regarding the pathophysiological significance of taurine contents in the vasculature, one recent report [20] has described a decrease in taurine uptake and levels in aortic wall from spontaneously hypertensive rats (SHR), but without evaluating the contribution of the individual components of the aortic wall to the impaired taurine uptake.
From the above reports, the cellular source(s) of taurine within the vascular tissue is not clear. This issue can be resolved by determining taurine contents in individual components of blood vessels, including vascular smooth muscle and endothelial cells.
Regarding localization of taurine in vascular endothelium, porcine pulmonary arterial endothelial cells have been shown to accumulate [3H]taurine in hypertonic medium, a well-described phenomenon in response to regulatory volume changes [21,22]. Furthermore, immunohistological studies utilizing [3H]-taurine demonstrated intense staining in vascular endothelial cells in testis and in cultured cells. On the other hand, to our knowledge, the localization of taurine in vascular smooth muscle cells, per se, is not known.
Studies in different nonvascular cells have shown that taurine is actively transported by a taurine transporter (TAUT) protein [23,24]. This protein has been cloned and its gene expression has been found to be altered by several factors. In these nonvascular cells, taurine transport is coupled to the transport of sodium and chloride ions [23,24]. The presence of a similar active transport mechanism for taurine was verified in blood vessel by determining the expression of TAUT and the kinetic parameters of the uptake process in rat aortic smooth muscle cells [26]. Accordingly, it was demonstrated that mRNA and protein of TAUT were expressed significantly in these vascular cells and this was accompanied by marked taurine uptake. Iimmunohistochemistry experiments also revealed the expression of TAUT protein in the intact rat aorta [26,27]. Taken together, the results of these experiments provided evidence for the expression of functional TAUT in vascular smooth muscle cells (VSMCs). Similar experiments need to be performed on endothelial cells. However, the accumulation of [3H]-taurine and immuno-staining of endothelial cells, as described above, are suggestive of the existence of a TAUT system in these cells too.
In vivo vascular effects of taurine in animals
Taurine has been investigated for its in vivo effects in relation to the vasculature either by using it as a supplement or by causing taurine deficiency with depleting agents. Thus, the role of taurine in the vasculature in animals is discussed by considering these experimental approaches separately.
Taurine supplementation studies
The in vivo effects of taurine supplementation on the vasculature in animals have been investigated primarily in hypertensive rat models [reviewed in 10]. These animal models include SHR, stroke-prone SHR (SHRSP, a variant of SHR), deoxycorticosterone-acetate NaCl rats (DOCA-salt rats), Dahl salt-sensitive rats (Dahl-S rats), renovascular hypertensive rats (RHR, Goldblatt hypertension), fructose-induced hypertensive rats (FHR) and ethanol-induced hypertensive rats (EHR). The major characteristics of these animal models in relation to hypertension are summarized below.
SHR: Genetic hypertension related to age and exhibiting various lesions of essential hypertension; multifactorial mechanisms involving abnormalities in hypothalamic-autonomic axis or hypothalamus-pituitary-adrenocortical system; variants of SHR are available including SHRSP;
DOCA-salt rat: DOCA-induced hypertension with salt diet; associated with increased sympathetic activity or turnover of norepinephrine (NE) and epinephrine (E);
Dahl-S rat: Hypertension developed in a specific stain on high-salt diet; associated with decreased renal kallikrein gene expression;
RHR: Renin-dependent hypertension induced by renal artery-clipping; usually associated with left ventricular hypertrophy and calcium overload;
FHR: Fructose-induced hypertension; associated with hyperinsulinemia and insulin resistance;
EHR: Ethanol-induced hypertension; associated with formation of acetaldehyde, sodium retention, reduced urinary output and abnormal intra-cellular cation levels;
The major effects of taurine in these and other animals with respect to the vasculature are briefly reviewed.
In SHR and SHRSP, 3% oral taurine supplementation with drinking water for 10 weeks has been shown to lower resting blood pressure, with significant reduction occurring starting from 4 weeks of age [28,29]. The supplement was found to be more effective in decreasing blood pressure in the SHRSP, animals that displayed greater blood pressure elevation to start with. Similar antihypertensive effect of taurine was also reported in SHR supplemented with 1% in drinking water for a longer period of time; that is, 16 weeks [30]. However, taurine did not reduce blood pressure to control levels in both the SHR and SHRSP groups, suggesting that it acts to stabilize the disorder only to a certain extent. On the other hand, taurine produced no significant effects on blood pressure in non-hypertensive control Wistar Kyoto (WKY) rats. Nonetheless, in WKY rats subjected to stress, 1.5% oral taurine supplementation for 8 weeks resulted in a reduction in mean blood pressure and total peripheral resistance, although more marked effects were observed in stressed SHR [31]. Attenuation of both hemodynamic and stress-induced catecholamine/sympathetic activity changes has been suggested to be involved in these effects of taurine. Furthermore, in SHR and SHRSP treated with high-salt diet, the administration of 1.5% taurine in drinking water for 21 weeks induced a pronounced decrease in mean blood pressure in association with cardio- and reno-protection [32]. Other studies have shown that oral taurine treatment directly modulates the activity of local renin-angiotensin system (RAS) in the brains of SHR [33]. In this regard, 3% taurine in drinking water inhibited the development of hypertension induced by renin or angiotensin II injection into the preoptic area in the SHR. Overall, these results provide evidence that taurine produces beneficial antihypertensive effects in SHR, SHRSP and in stressed WKY rats made hypertensive under different conditions that impair blood pressure regulation,. Such an effect suggests the ability of taurine to counter diverse cardiovascular conditions and maintain homeostasis.
In the DOCA-salt rat model, taurine supplementation in drinking water as 1%, 2% and 3 % was effective in preventing the development of hypertension in a dose-dependent fashion [34]. This effect of taurine has been suggested to be linked to reversal or normalization of sympathetic overactivity in these rats. This observation was supported by the correspondingly reduced turnover rate and/or levels of NE and/or E in the heart, hypothalamus, spleen, adrenal gland and plasma of these rats. However, similar to the blood pressure data, NE turnover was not affected by oral taurine supplementation in control rats. Moreover, augmented activity of the sympathetic nervous system and pressor responses to cold-stress and electrical stimulation in the hypothalamus of DOCA-salt rats were also attenuated by the administration of taurine [35,36]. In these animals, the antihypertensive effect of taurine was associated with enhanced natriuresis and activation of opioid receptors in the hypothalamus. Treatment with taurine increased the levels of taurine and endorphin-like immunoreactivity in the hypothalamus. Therefore, in the DOCA-salt rat model of hypertension, the hyportensive effect of taurine may at least involve normalization/reduction of augmented catecholamine metabolism/sympathetic activity and overactivity of endorphin-mediated mechanisms in the hypothalamus; this further indicates the multidimensional actions and homeostatic mechanisms associated with taurine.
Consistent with the above observations, hypotension was also demonstrated in the Dahl-S rat model of hypertension in response to taurine. Accordingly, oral administration of 3% taurine in drinking water inhibited the development of blood pressure in these rats, the effect of which was associated with greater urinary volume and increased excretion of kallikrein [37]. These results provide additional evidence for the effectiveness of taurine as an antihypertensive agent yet in another model of hypertension.
Dietary taurine was reported to induce hypotension in the RHR model in which renin-dependent mechanisms are believed to play a prominent role [38]. The simultaneous antagonism of renin with enalapril was found to be more effective in reducing blood pressure, normalizing calcium mobilization and reversing ventricular hypertrophy. Thus, taurine is also involved in ameliorating abnormal blood pressure in a rat model of hypertension linked to disorder of the renin-angiotensin system.
In fructose-fed insulin resistant (diabetic) rats, which are prone to develop hypertension, 1%-2% oral taurine in drinking water prevented a rise in blood pressure along with normalization of plasma insulin [39-41]. The taurine-induced effect was linked to increased urinary sodium and kallikrein secretion, which was abolished by a kinin B2 receptor antagonist. This observation suggests that, in this animal model, taurine may also stimulate the renal kallikrein-kinin system and act as a natriuretic agent to prevent enhancement of blood pressure. The concomitant reduction of plasma insulin by taurine may suggest the role of hyperinsulinemia in development of hypertension in this model. A more recent report showed that the prevention of the development of hypertension by taurine (2% in drinking water for 3 weeks) in the FHR was augmented by exercise [42]. The mechanism for this observation was suggested to involve anti-oxidation and the preservation of nitric oxide (NO) by taurine.
Further studies have been carried out on the role of taurine in the regulation of blood pressure in animal models of hypertension which seem to be “less conventional.” Hypertension induced in rats with the administration of 5% or 15% ethanol for 4 weeks (EHR model) was prevented with 1% oral taurine supplementation in drinking water [43]. This vasoprotective effect of taurine was associated with normalization of altered hemoglobin-bound acetaldehyde (HbAA) (by modulating acetaldehyde dehydrogenase activity) and cation transport/metabolism. In a hypertensive rat model developed by the administration of the NO synthase inhibitor, N-nitro -L-arginine methylester (L-NAME), 1% or 2% oral taurine in the drinking water also ameliorated elevation of blood pressure [44]. Along with this effect, taurine increased serum levels of NO, interfered with activity of the renin-angiotensin-aldosterone system, and minimized elevation of serum cytokine, endothelin, neuropeptide Y and thromboxane B2, among other actions. Taken together, these data indicate that, as shown in other rat models of hypertension, taurine can alleviate blood pressure elevation induced by different factors in these less popular models of hypertension as well. This finding provides further evidence for taurine's ability to act as an antihypertensive agent against various forms of hypertension and its overall role in cardiovascular modulation.
The literature further reveals that taurine exerts prenatal vascular effects when given to either hypertensive or healthy female rats. Accordingly, oral taurine supplementation of female SHRSP prior to or during pregnancy has been shown to cause a decrease in the development of hypertension in offspring that continued to feed on maternal milk of animals receiving taurine [28,45]. This observation indicates taurine's ability to reduce blood pressure in SHRSP, at least in part, as a result of prenatal vascular effects. This finding is consistent with taurine's antihypertensive effect reported for hypertensive rats. In contrast, other studies using healthy rats reported enhanced mean arterial pressure in male rats prenatally exposed to taurine (3% in drinking water from conception to weaning); however, the pressure increases in corresponding female offspring were seen only when the animals were subsequently maintained on high glucose diet [46]. These results alone indicate that prenatal taurine exposure influences blood pressure regulation of adult rats and this effect is gender specific. The reason(s) for differences between the results of the studies in the healthy rats and those in SHRSP are not clear but may be related to differences in the health status of the animals (i.e., hypertensive vs normotensive). However, despite this observation, given the generally accepted cardiovascular effects of taurine, the results of the prenatal exposure to the supplement in the healthy female rats was unexpected and further research is needed for clarification.
Besides the effects observed with oral administration, taurine has also been shown to exert vascular effects with direct application into the central nervous system (CNS), in both hypertensive and normotensive rats. Accordingly, as seen with the use of oral taurine, the administration of taurine into the ventricles of the brain of SHR and RHR significantly alleviated hypertension [47,48]. Injection of taurine into the cerebrospinal fluid of normotensive rats also lowered arterial pressure, an effect replicated by intraventricular administration [49,50]. From experiments involving hypothalamic neurons that secrete vasopressin, it has also been shown that taurine has an inhibitory effect on the activity of these neurons, thereby reducing the release of vasopressin and its pressor and antidiuretc effects. The consequence of this is a decrease in blood pressure and promotion of diuresis. The centrally-mediated effect of taurine with regard to salt regulation is consistent with results of other studies [51] using oral taurine supplementation and taurine deficiency in rormotensive Wistar rats.
Central administration of taurine is also involved in modulation of baroreflex function. In this respect, the administration of taurine into the lateral ventricles of rats caused an acute decrease in blood pressure and heart rate while facilitating NE-induced reflex bradycardia [52]. Also, intraraphe administration of taurine caused greater lowering of mean arterial pressure and heart rate while enhancing the baroreflex response to E [53]. The hypotension and bradycardia induced by E were found to be greater with taurine. In another study, it was also shown that increased taurine release from the hypothalamus by hypervolumia or pressure, or its reduced release by hypotension in intact but not denervated aortas in rats suggested that the baroreflex pathway modulates taurine release from this brain area [50]. However, this observation is seemingly at variance with our investigation of lack of an effect of taurine deficiency on baroreflex function in normotensive rats [54]. A possible explanation for the difference is that the taurine level in our experimental model might not have been sufficiently decreased by the depleting agent (3% β-alanine in drinking water for 3 weeks) to cause detectable baroreflex impairment.
Other studies have provided evidence that taurine acts locally on the renin-angiotensin system in the brain to lower hypertension [50,55]. Angiotensin II is known to activate vasomotor neurons of the rostral ventrolateral medulla (RVLM) to increase blood pressure via sympathetic signaling, and the iontophoretic application of taurine resulted in inhibition of these cardiovascular neurons. To our knowledge, the effect of oral taurine treatment on theses neurons has not been studied.
Studies using taurine deficient animals
The role of endogenous taurine in the vasculature has also been studied using taurine deficient rats that were treated with the taurine-depleting amino acid, β-alanine. Our laboratory assessed the effect of taurine deficiency induced by 3% oral β-alanine (in drinking water for 3 weeks) on cardiovascular responses of healthy WKY rats to the vasoactive agents, phenylephrine (PE), angiotensin II and sodium nitroprusside [54]. It was found that taurine deficiency caused a selective reduction in pressor responses to angiotensin II without impairing baroreflex function and altering hemodynamic responses to either phenylephrine or sodium nitroprusside. Although the mechanism(s) for the impaired angiotensin II-mediated pressor response in the taurine deficient rat remains to be established, these observations signify the role of endogenous taurine as a modulator of the responses to this vasoactive peptide. In subsequent experiments, we also demonstrated that β-alanine-induced taurine deficiency (3% β-alanine in drinking water for 14 weeks) in uninephrectomized rats resulted in acceleration of the development of hypertension in response to high (8%) dietary NaCl, without alterations in baroreflex function [56]. These observations suggest that taurine deficiency modulates renal adaptation to a combination of uninephrectomy and dietary NaCl excess, suggesting the important role endogenous taurine plays as a homeostatic mechanism in the regulation of renal and cardiovascular functions. Collectively, the data concerning taurine deficiency are generally consistent with the reported functional role of the supplement in the vasculature.
The effect of taurine deficiency on arterial pressure was further studied in rats prenatally made deficient of taurine. Roysommuti et al. [57] reported that prenatal taurine depletion with 3% β -alanine in drinking water (i.e., from conception to weaning) produced increased sympathetic activity and mean arterial pressure in male offspring on a high (5%) glucose diet and in female offspring on a normal as well as high glucose diet at 7-8 weeks of age. The authors concluded that prenatal taurine deficiency alters arterial pressure control of adult offspring, leading to enhancement of blood pressure in both male and female rats, partially being gender specific. Despite the gender-related differences, the observation on the effect of prenatal taurine deficiency appears to be consistent with the observed trend of blood pressure changes, emphasizing the important role of endogenous taurine in the regulation of blood pressure even at the prenatal stage.
Summary and general comments
In all of the animal models of hypertension described, taurine supplementation has been shown to produce antihypertensive effects. As outlined above, the genesis of hypertension in these animal models is multifactorial and complex. Although it is not entirely clear for the most part, several mechanisms can be proposed for the effect of taurine in these animal models.
It should be noted that earlier reports conjectured that hypertension was generally associated with deficiency of taurine. Accordingly, attempts were made to relate the various forms of hypertension to decreased taurine contents in plasma and certain tissues. However, no consistent information was reported in most reports. For instance, the levels of taurine in plasma and liver were found to be similar in both SHR and control rats although the levels were reduced in SHRSP [58]. In addition, taurine supplementation was unable to change plasma taurine in any of the animal groups.
While one study found increased taurine levels in certain areas of the brain of the SHRSP after taurine treatment, another group reported no difference in the taurine contents of these brain areas across the three models [59]. In addition, Yamamoto et al. [35] reported a slight decrease in plasma taurine in SHR at 15 weeks of age in contrast to the increase observed by Jones [60] at the age of 8 weeks. On the other hand, other investigators showed diminished plasma taurine concentrations in SHR and FHR in relation to the severity of hypertension [61,62]. Although these data may suggest dose-related antihypertensive effects of taurine in these models, the mechanism behind the reduction is unknown. There is a possibility that some of the inconsistencies in taurine levels are related to differences in assay methodologies. Overall, the reported results suggest the need for further investigations with consideration of more appropriately designed experiments and measurement of taurine contents in specific target tissues of interest. In support of the latter, there are reports of increased release of taurine from aortic wall of the SHR in association with decreased uptake and content [20].
The findings of the animal studies suggest that taurine exerts its antihypertensive effects by various mechanisms. Aside from the CNS, depending upon the animal model, the effect of taurine has been shown to be associated with (a) a reduction of catecholamine (E, NE) levels in plasma and/or tissues (ie., sympathetic activity), (b) enhanced natriuresis/diuresis/urinary volume and kallikrein secretion, (c) antioxidation and maintenance/augmentation of NO, (d), normalization of HbAA and cation transport, (e) modulation of activity of the renin-angiotensin-aldosterone system and (f) minimization of serum levels of cytokine, endothelin and other vasoactive mediators. Some of these observations have found to be consistent with the results obtained using taurine deficient animals. While lowering blood pressure in hypertensive rats, oral taurine supplementation does not seem to affect blood pressure of healthy animals. Although some of the above effects of taurine could be linked indirectly to its action in the CNS, there are effects specifically mediated via the CNS. These include (a) regulation of the activity of hypothalamic neurons that secrete vasopressin, (b) modulation of baroreflex, (c) augmentation of hypothalamic opioid activity, and (d) attenuation of local renin-angiotensin system. Surprisingly, many of the CNS-mediated effects of taurine are observed in both hypertensive and normotensive rats. The possible sites of action of taurine as a hypotensive agent are shown in Figure 1.
Figure 1.
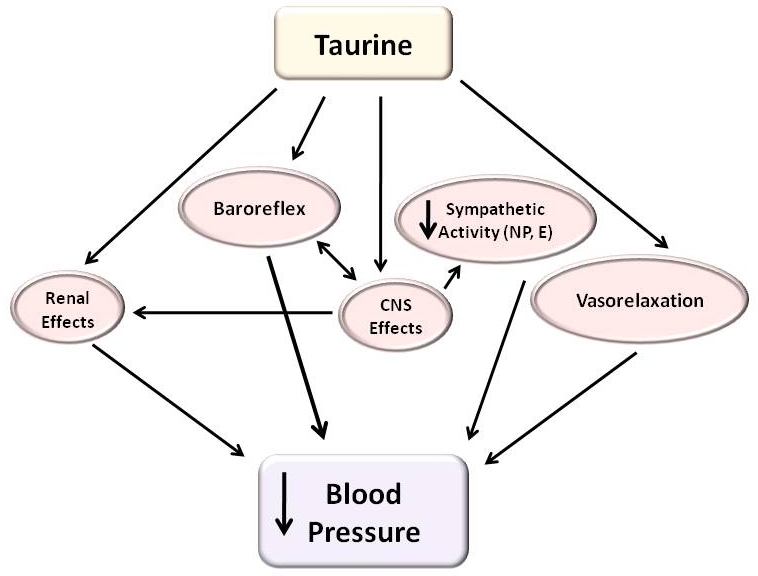
Possible sites of action of taurine as a hypotensive agent (refer to text for details).
As described above, although both oral and centrally administered taurine causes a reduction in blood pressure particularly in hypertensive rats, the specific mechanisms involved are not entirely clear, particularly at a molecular level. As a result, there is no a unifying concept that can explain the different observations. This is a relevant subject that requires further investigation.
Effects of taurine on isolated vascular tissues, vascular smooth muscle cells and endothelial cells
Although administered taurine (orally and centrally) as well as endogenous taurine have been demonstrated to induce a reduction in blood pressure, particularly in the setting of hypertension by the various mechanisms described, from this observation alone it is not defined whether or not the compound produces direct relaxant effects on the vasculature. In an attempt to address this problem, the consequences of direct interaction of taurine with the vascular system have been the focus of investigation, albeit to a limited extent, by different groups of investigators using different experimental approaches. Accordingly, the effects of taurine on isolated vascular tissue, vascular smooth muscle cells and endothelial cells were studied in a more direct fashion under in vitro conditions. This approach provided information based on the in vivo vascular action of taurine (i.e., in taurine supplemented and deficient animals) or on the direct effect of the compound on isolated vascular preparations in vitro.
In vitro studies on isolated vascular tissues based on in vivo effects of taurine
Vascular effects of taurine have been examined using isolated vascular preparations from taurine supplemented animals. The major findings of these studies are presented briefly.
Our group determined the effects of chronic taurine supplementation on the reactivity of the rat aorta in male WKY rats given 1% oral taurine in drinking water for 7-8 weeks [63]. Endothelium-intact or mechanically-denuded aortic ring preparations from control and the taurine-supplemented rats were suspended in standard tissue baths containing oxygenated Krebs solution at 37° C for isometric tension measurements. Contractile responses to NE and potassium chloride (KCl) were attenuated in rings from taurine-treated rats as compared to controls both in the absence and presence of endothelium. However, the magnitude of attenuation was greater in endothelium-intact tissues contracted with NE. Acetylcholine (ACh)-induced relaxation responses were augmented in endothelium-intact vessels from rats supplemented with taurine compared to the responses of control preparations. Relaxation of the aortas from taurine-treated and control rats in response to sodium nitroprusside (SNP) were not different from each other. These results suggest that chronic taurine treatment attenuates vascular contractility in rats in a nonspecific manner and this effect is partly mediated via the endothelium.
Assessing the vascular effects of 3% taurine in drinking water for 5 weeks on blood vessels reactivity in rormotensive and hypertensive animals, Li et al.[64] found that the contractile responses of mesenteric arteries from taurine-treated SHRSP but not control WKY rats to NE were markedly decreased. In contrast, the responses of the arteries from either the hypertensive or normotensive animals to angiotensin II or KCl were not altered by taurine treatment. These results are generally in line with the anti-hypertensive effects of taurine observed in hypertensive animals supplemented with oral taurine. These experiments, however, do not provide information whether or not the effects of taurine were mediated via the vascular smooth muscle or endothelium, indicating the need for further studies in this direction.
In addition, chronic oral taurine administration (50 or 100 mg/kg for 10 weeks) prevented impairment of endothelium-dependent relaxation of aortas from streptozotocin (STZ)-induced diabetic and hypercholestrolemic mice to Ach and A23187, without affecting blood glucose [65]. In another group of STZ-induced diabetic rats treated with 1% taurine for 6 weeks, the preservation of endothelium-dependent aortic relaxation was associated with a reduction of oxidative stress and oxLDL, and downregulation of cell adhesion molecule-1 (ICAM-1) and lectin-like oxLDL receptor-1 (LOX-1) expression [66]. Li et al. [67] also reported that oral taurine supplementation as 1% in drinking water for 2-6 weeks prevented functional impairment of the vascular endothelium of sciatic nerves from STZ -induced diabetic rats. These different findings from diabetic animal studies provide evidence for the vasoprotective effect of taurine in diabetes, mediated via its action on the endothelium. Chronic taurine treatment (50 mg/kg ip for 21 days) of rats given nicotine was reported to reverse impaired contractile and relaxant responses of aortas to PE and Ach, respectively, along with normalization of endogenous gluththione (GSH) levels, lipid peroxidation and myloperoxidase (MPO) activities [68]. This effect of taurine has been suggested to be related to its action against oxidative damage of the rat aorta caused by nicotine. Studies performed by Zulli et al [69] on left main coronary arteries of rabbits fed high-fat diet, with and without methionine and/or 2.2% taurine, also demonstrated the beneficial effects of taurine as manifested by reduced/normalized intima to media ratio, atherosclerosis and endothelial apoptosis. This was associated with improvements in coronary artery wall pathology along with decreased plasma total homocysteine, methionine, apoptosis, and CCAAT/enhancer binding protein homologous protein [70]. In brief, the coronary artery data elucidate the antiapoptotic and antiatherogenic properties of systemically administered taurine, possibly mediated, at least in part, via normalization of endoplasmic reticulum stress.
Studies on direct in vitro effects of taurine on isolated vascular tissues
Major findings of the direct effects of taurine on vascular tissue preparations from healthy and diseased animals under in vitro conditions are briefly reviewed in this section.
Ristori and Verdetti, [70], using isolated tissue bath experiments, demonstrated that direct application of taurine (1 mM) caused a reduction of NE and KCl-induced contraction of rat aortas. This effect of taurine was found to be partly dependent on the presence of the endothelium, but independent of intracellular calcium. In addition, Franconi et al. [71] reported that intraluminal administration of taurine (10-80 mM) inhibited the contractile responses of another vascular tissue, the rabbit ear artery, to KCl but not NE. A more recent investigation by another group showed that the direct application of taurine (20-80 mM) produced concentration-dependent relaxation of rat aortic rings precontracted by both PE and KCl [72]. The relaxation was inhibited by tetraethylamonium (TEA), a non-selective potassium channel blocker but not by L-NAME, indomethacin, 4-aminopyridine, glibenclamide, barium chloride or iberiotoxin. Preincubation of tissue with 20-60 mM taurine inhibited the contraction induced by PE, without affecting the basal tone [72]. The inhibitory effect of taurine on PE-induced contraction of isolated renal and mesenteric arterial rings was also attenuated by TEA. Overall, the findings of these experiments revealed that taurine directly relaxes or inhibits KCl- and PE-induced contraction of various rat arteries and the mechanism may involve opening of potassium channels. Further studies by the same group also showed that taurine induces a direct vasorelaxant effect on precontracted porcine coronary arteries or inhibits the arteries from contracting in response to different contractile agents [73]. The activation of KIR, KATP and KCa channels was suggested to be involved in the taurine-induced effects on the porcine coronary arteries. In addition, depending on the degree of the background muscle tone, taurine was also reported to either cause further contraction or relaxation of the rat aorta by directly acting on NE- and KCl -induced contractile responses [74]. Accordingly, when vascular tone was excessively low, taurine promoted vasoconstriction, while it induced vasorelaxation when the evoked contraction was relatively high. Thus, it was suggested that taurine may modulate vascular wall tone to maintain blood flow, an indication of the homeostatic role it may play in the function of blood vessels [74].
The results of direct vascular effects of taurine were also reported for blood vessel preparations from hypertensive and diabetic animals.
In vitro exposure of mesenteric arteries from SHRSP but not control WKY rats to 10 mM taurine for 15 min has been shown to exert a selective inhibition of contraction to NE (Li et al., 1996) [64]. This effect of taurine is consistent with its in vivo effect found in SHRSP by the same investigators. Taurine was also studied for its effect on the activity of the sympathetic nervous system in hypertension by measuring pressor responses of perfused mesenteric arteries from SHR to electrical stimulation [75]. Addition of 3% taurine to perfusate suppressed NE overflow and pressor responses induced by electrical stimulation of the mesenteric preparation, with greater suppressive effects produced in the SHR than the WKY control rats. These data suggest that taurine may lower blood pressure in hypertension, at least in part, via suppression of NE release from peripheral sympathetic nerves [75].
More recently, our laboratory was involved in the study of the in vitro effect of taurine on the reactivity of aortas from STZ-induced diabetic rats of 12-14 weeks duration to NE and Ach [76]. In these studies, control and diabetic aortas were incubated in isolated tissue baths with 10 mM taurine for 2 hr before adding drugs. In endothelium-denuded tissues that were not incubated with taurine, the contractile responses of the diabetic aortas to NE, but not KCl, were enhanced compared to control responses. With 2 hr incubation with taurine, the augmented contractile responses of the diabetic aortas to NE were attenuated to control levels. This effect of taurine was associated with a reduction in calcium mobilization and protein kinase C (PKC) activation. In the absence of taurine, endothelium-dependent relaxation induced by Ach was also attenuated in aortas from diabetic rats. Incubation of endothelium-intact aortic tissues with taurine reduced the inhibitory effect of diabetes on Ach-mediated vasorelaxation. Similar to the effects of diabetes, treatment of non-diabetic rat aortic rings with high concentration of glucose (45 mM) for 3 hr caused enhancement of contraction of the vascular smooth muscle to PE and impairment of endothelium-mediated vasorelaxation to Ach, as compared to control responses. Co-incubation of the tissues with 5-10 mM taurine concentration-dependently reduced the alterations in both contraction and relaxation caused by high glucose in responses to PE and Ach. Overall, our data suggest that taurine prevents or ameliorates diabetes-induced vascular reactivity alterations involving both the smooth muscle and endothelium; similar observations have been reported for the vasculature of hypertensive animals by other investigators [76].
Using the fructose-fed insulin resistance diabetic rat model, Xue et al (2008) [77] determined the direct vascular effect of taurine on contraction of aortic rings to KCl and PE. It was found that while taurine (20-80 mM) relaxed contraction of the rings from control rats, it enhanced the contraction of tissues from insulin resistance rats. The taurine-induced enhancement of contraction observed in the insulin resistance aortas was endothelium-dependent, but the relaxation in control tissues was endothelium-independent. TEA-sensitive K(+) channels may be involved in both the contraction and relaxation responses to taurine [77]. It is clear that the responses of the aortas from the insulin resistance rats to taurine were opposite to those of the aortas from STZ-diabetic rats described above, but the responses of the control groups were similar in both cases [76]. The variation in responsiveness of the diabetic aortas to taurine is likely to be related to differences in the animal models used in the two studies, demonstrating the selectivity of vascular effects of taurine on the basis of disease conditions.
In vitro studies using vascular tissues from taurine deficient animals
We investigated the effect of taurine deficiency on vascular reactivity in vitro in order to determine the role of endogenous taurine in the modulation of vascular functionality [16,78]. Accordingly, we studied the reactivity of aortic ring preparations from rats depleted of taurine with 3% β-alanine in drinking water for 3 weeks. As expected, contractile responses of endothelium-denuded aortas from taurine-deficient rats to NE and KCl were enhanced compared with control responses. In addition, the sensitivity of the endothelium-denuded aortas to SNP was attenuated by taurine deficiency. Similarly, taurine deficiency reduced the relaxant responses of endothelium-intact aortic rings elicited by Ach, and this effect was associated with decreased NO production. On the other hand, incubation of rat aortic tissue with a high dose of β-alanine (40 mM for 30 min) in vitro did not affect its reactivity to vasoactive agents, indicating lack of direct vascular effect of β-alanine per se.[54]. Taken together, the results of our experiments with β-alanine-treated rats suggest that taurine deficiency augments contractility but attenuates relaxation of vascular smooth muscle in a nonspecific manner [16,63]. These observations may be of relevance to our subsequent demonstration that taurine deficiency accelerates the development of salt-induced hypertension in the uninephroctomized rats [56]. Impairment of endothelium-dependent responses, which is at least in part associated with reduced NO generation, may contribute to the attenuation of vasorelaxtion. These data generally support the notion that endogenous taurine has an inhibitory effect on the basal tone or evoked contraction of blood vessels and this effect involves the release of NO from the endothelium. These observations, together with our previous findings obtained using taurine supplemented rats, further emphasizes the relevance of endogenous taurine as a modulator of vascular functionality [16,63].
We subsequently assessed the reactivities of both aortas and mesenteric arteries from β-alanine-treated rats (3% in drinking water for 3 weeks) to adenosine receptor agonists [78]. In both endothelium-intact and denuded aortas, taurine deficiency diminished relaxation caused by 2-chloroadenosine (CAD) and 5'-N-ethyl-carboxyamineoadenosine (NECA), whose effects are known to be mediated via A2A receptor activation. The endothelium-dependent responses were attenuated by the NO synthase inhibitor L-NAME in both groups. However, the inhibitory effect of L-NAME was less marked in the β-alanine-treated group, further indicating that the effect of taurine deficiency was linked to a reduction in NO generation. As in the aortas, CAD produced both endothelium-dependent and -independent relaxation in the rat superior mesenteric artery, and both tissue responses were inhibited by β-alanine treatment, suggesting that not only similar responses can be produced by a given adenosine receptor agonist in different vascular beds, but also β-alanine treatment modulates these responses. On the other hand, N6-yclopentyladenosine (CPA)-induced aortic relaxation was found to be endothelium-independent and this was not altered by taurine deficiency. These results indicate that endogenous taurine deficiency causes differential inhibitory effects on adenosine receptor-mediated vasorelaxation, depending upon the type of agonist used. The implication of this observation is that endogenous taurine has selective modulatory role in adenosine receptor-mediated vascular responsiveness, with no tissue related differences in its effect [78].
Effects of taurine on vascular smooth muscle cells
Vascular smooth muscle cells (VSMCs) play a central role in the function of blood vessels. These cells determine the contractile ability and lumen size of blood vessels among other functional roles. These functions of VSMCs can be influenced variably by the effects of vasoactive mediators and pathophysiological conditions [79]. However, despite their important role, only limited information is available in the literature regarding the effect of taurine on VSMCs per se. The presence and functionality of a TAUT system have been clearly shown in VSMCs derived from rat aortas [23,26,80] and it is likely that the system is also present in other VSMCs. The available information on the effect of taurine on VSMCs is mainly related to taurine's inhibitory effects on cell proliferation, antioxidation and atheroscrerosis [80].
Taurine, as low as 0.3 mM, has been shown to inhibit the proliferation of rat aortic VSMCs in culture, as monitored by measurement of cell counts and rate of DNA synthesis, which was determined by [3H]thymidine incorporation into DNA [23,26,80]. Protein content of these VSMCs was also found to be decreased by 30 mM taurine. On the other hand, [3H]leucine incorporation into newly synthesized protein was not affected by the 30 mM taurine, indicating that taurine does not inhibit protein synthesis or survivability of VSMCs but rather reduces total protein content by inhibiting cellular proliferation [26,80,81].
In another investigation, taurine (5, 10, and 20 mmol/L) antagonized the effects of homocysteine on ROS (H2O2 and O2-) generation and antioxidant enzyme (SOD and catalase) activities in rat VSMCs in vitro [81]. Also, lysophosphatidic acid (LPA)-induced release of calcium from cultured VSMCs cells and the migration and proliferation of the cells have been observed to be inhibited by taurine in a concentration-dependent manner [82]. LPA is a lipid component in atherosclerotic plaques and is considered to have an important role in the development of atherosclerosis. It has thus been suggested that LPA-mediated effect is one of the mechanisms for the antiatherosclerotic action of taurine. Taurine has also been shown to attenuate the progression of atherosclerosis by its ability to lower serum lipids and reduce the oxidation of LDL, as well as by reducing the risk of arterial thrombus formation via inhibition of platelet aggregation [82].
The reported effects of taurine on VSMCs are processes of “normalization” which can be beneficial in conditions of vascular abnormalities such as atherosclerosis. Some of these effects are in line with the observations made in relation to taurine's effects on vascular reactivity and blood pressure. Further studies using VSMCs are relevant since they can provide information which otherwise would be difficult to be obtained using intact vascular tissues. From such studies, signaling processes and molecular mechanisms involved in the interactions between taurine and VSMCs can be unveiled more reliably.
Effects of taurine on vascular endothelial cells
The endothelium is a mono-layer of cells that line the lumen of blood vessels. It provides an interface between circulating blood and underlying vascular smooth muscle. In addition to serving as a physical barrier between blood and tissues, the endothelium is involved in multiple other important functions including regulation of vascular tone, blood coagulation, cell growth, and platelet and leukocyte adhesion [83]. Therefore, maintaining endothelial health is important for the proper functioning of the cardiovascular system.
Besides the endothelium-dependent effects shown in intact vascular preparations, there are also reports, albeit limited, that assessed the effects of taurine on endothelial cells per se. The relevance of the endothelium for the vascular action of taurine is in part indicated by the demonstration of 3H-taurine staining of endothelial cells [21,22]. Accordingly, taurine (0.125 -2.5 mg/ml for 48 hr and 14 days) has also been shown to prevent high-glucose-induced human umbilical vein endothelial cells (HUVEC) apoptosis as determined by DNA fragmentation [83,84]. These results were correlated with attenuation of high glucose-mediated increased reactive oxygen species (ROS) and intracellular (IC) calcium concentration by taurine. This suggests that the anti-apoptosis effect of taurine was mediated via ROS inhibition and IC calcium stabilization. In rats made hyperglycemic with the administration of glucose, 200 mg/kg taurine treatment for 5 days prevented endothelial cell apoptosis and ICAM-1 expression [85]. These findings indicate that the anti-apoptotic effect of taurine is associated with its anti-inflammatory effect. Consistent with this, exposure of HUVEC to taurine (0.5-2.5 mg/ml for 20 hr) was also shown to protect endothelial dysfunction induced by hyperglycemia and/or oxLDL through down-regulation of apoptosis and ICAM-1 expression [85,86]. This antiin-flamatory effect of taurine is proposed to be beneficial for preventing the development of atherosclerosis and angiopathies in diabetes. Furthermore, apoptosis and impairment of function of HUVEC caused by sodium arsenate could be attenuated by taurine (0.5 mg/ml at 0 and 6 hr) [86]. In these cells, taurine also reduced PMN-mediated necrosis and A23187-induced IC calcium elevation and cell death. It was suggested that these effects of taurine are the results of its antioxidant activity and modulation of IC calcium, which prevent cell dysfunction and death. Studies by Tan et al. [87] demonstrated that ox-LDL-induced increased levels of lactate dehydrogenase (LDH), asymmetric dimethylarginine (ADMA), TNF-alpha and malondialdehyde (MDA, but decreased NO level and LDH activity in HUVEC could be attenuated by taurine (1 or 5 μg/ml). These results suggest that taurine protects endothelial dysfunction caused by ox-LDL and this effect is related to enhanced ADMA level (an endogenous NO synthase inhibitor) by increased lactate dehydrogenase activity. Also, increased release of endothelin-1 and inhibition of NO production by HUVEC incubated in monocyte-conditioned medium from smokers were found to be attenuated by oral taurine supplementation [88]. This beneficial effect of taurine was partly attributed to an upregulation of NO synthase expression. Although, more or less, consistent and potentially useful results have been reported by investigators, more needs to be done to fill existing knowledge gaps in this area. Such an effort may include investigations on endothelial cells from different vascular sources and the signaling and molecular mechanisms that may be involved in the action of taurine under normal and pathological conditions.
Summary and general comments
The studies on isolated vascular preparations provide more specific information on the effect of taurine on the vasculature, independent of other endogenous influencing factors. The overall observation is that taurine produces biological effects on vascular tissues, with or without the endothelium, VSMCs and endothelial cells by different mechanisms under normal and “pathological” conditions. Figure 2 illustrates the major sites involved in the vascular action of taurine, as reported in the literature.
Figure 2.
Vascular effects of taurine based on experimental observations (refer to text for details).
Experiments with vascular tissue preparations from taurine supplemented animals have indicated that taurine exerts effects on the vasculature. Relative to the effects of direct application of taurine under in vitro conditions, the in vivo administration of the supplement seems to produce more chronic and practical outcomes. Such effects of taurine may be associated with alterations in cellular processes in the vasculature that are manifested by alerted tissue responsiveness. The mechanism(s) for these taurine-induced alterations in cellular processes remains to be investigated. Both the vascular smooth muscle and endothelium have been shown to be responsive to the in vivo exposure to taurine. Thus, vascular tissues from both healthy and diseased (primarily hypertensive, diabetic and atherosclerotic) animals treated with taurine have been observed to respond with vasorelaxation. However, tissues from diseased animals seem to be more responsive to the effects of taurine, suggesting that the compound is more effective under conditions of greater stress. This discovery is supported, for instance, by the fact that taurine is ineffective in inhibiting basal tones of isolated vessel preparations while it relaxes contracted vessels or that taurine deficiency or supplementation does not affect baseline blood pressure. The exact reason for this differential observation is unclear (i.e., under healthy and pathological conditions). In most cases, the vasorelaxant effects of taurine seen in abnormal vessels is associated with improvements of other related factors, including lipid profiles, ROS levels, intimal thickness and NO release. It is likely that taurine's effects on these factors contribute to its action on vascular reactivity.
The literature also shows that taurine with direct in vitro application, almost always causes vasorelatation, and, in certain cases, this response is endothelium-dependent. This is considered an acute effect of taurine. In this respect, taurine also produces similar effects on vascular reactivity, as observed with the in vivo (chronic) administration. In some vascular preparations, the acute (in vitro) effect of taurine is related to the opening (stimulation) of K+ channels (***). Similar to its in vivo effects, direct application of taurine also resulted in greater vasorelaxation in blood vessels from SHR, SHRSP and STZ-diabetic rats. In the SHR, the vascular effect was associated with inhibition of sympathetic activity by taurine.
The in vivo and in vitro (direct) effects of taurine observed in isolated vascular tissues are consistent (albeit in the opposite directions) with the results obtained using blood vessel preparations from taurine deficient animals. The information from both experimental approaches provides support for the antihypertensive effect of taurine reported for different groups of animals. In other words, direct inhibition of vascular smooth muscle contractility and enhancement of endothelium-dependent vasorelaxation that may, at least partly, be associated with the release of NO and opening of K+ channels contribute to this effect of taurine. In addition, the in vitro data are also in line with the vasoprotective effects of taurine in diabetes and atherosclerosis. However, despite these additional insights from isolated tissue experiments, the vascular action of taurine is incompletely understood, requiring further investigations, particularly with regard to the mechanisms involved and their relationships with the in vivo effects of the supplement in whole animal.
Considering effects on vascular tissue components, it has been reported that taurine inhibits rat aortic VSMC proliferation and migration and this effect is, at least in part, linked to inhibition of ROS and IC calcium release. These results are more consistent with the antiatherosclerotic effects of taurine reported in other studies. However, as there are no reports regarding the effects of taurine on VSMCs from other vascular sources, it is not known if this information is applicable to all smooth muscle cells. In addition, either under normal or pathological conditions, no studies have been reported relating the effect of taurine seen in VSMCs to its effects on vascular reactivity and blood pressure. With regard to endothelial cells, taurine has been shown to inhibit glucose- and/or oxLDL- induced enhancement in apoptosis, ROS, IC calcium, ICAM-1 expression, TNF-alpha and ADMA in HU-VEC, while increasing NO production. Furthermore, HUVEC apoptosis and cell death caused by sodium arsenate and PMN were suppressed by taurine. The supplement also reduced endothelin-1 release and increased NO generation in HUVEC incubated in monocyte-conditioned medium from smokers. It is clear that most of the above endothelial cell studies used HUVEC and are linked to diabetes and atherosclerosis. As with VSMCs, there is a dearth of information on many other aspects, including effects of taurine on endothelial cells from other vascular sources, details of the mechanisms that may be involved in the action of taurine and the relevance of effects on endothelial cells to its overall in vivo action.
Human studies on effects of taurine related to the vasculature
There is limited information on the effects of taurine in humans in relation to the vasculature and most of this information is from trials in hypertensive patients. Oral taurine (6 g/day) supplementation given to essential hypertensive patients on a salt-restricted diet was reported to alleviate the symptoms of hypertension after 6 weeks of treatment- with reduced systolic, diastolic and mean arterial blood pressure [89]. It was demonstrated that this effect of taurine was associated with augmented renal kallikrein-kinin and prostaglandin systems. Similar reductions in systolic, diastolic and mean arterial blood pressure were observed in another group of hypertensive patients treated with 6 g/day taurine only for 7 days [90]. As expected, no changes were observed in blood pressure in patients with placebo. Consistent with animal studies, taurine also produced no effects in normotensive subjects [90]. In general agreement with the above studies, other researchers also found that oral taurine given at a dose of 3 g/day for 8 weeks decreased both systolic and diastolic blood pressure in about 65% of hypertensive cases [91]; the reason for the lack of effect on unresponsive patients is unknown.
In addition, taurine also induced beneficial effects in diabetic patient with regard to the vasculature. Accordingly, young type 1 diabetic patients given oral taurine supplementation for 2 weeks displayed reversal of both arterial stiffness and brachial artery reactivity as assessed by flow-mediated dilatation (FMD) [92]. From this observation, taurine was proposed to have the potential for long-term treatment of diabetic patients, particularly the progression towards atherosclerosis and related cardiovascular diseases.
Overall, these limited human studies are consistent with the observations made in normotensive, hypertensive and diabetic animals. It is likely that at least some of the mechanisms described in animal studies may also apply to humans. However, the human studies need to be expanded to include a more complete assessment based on animal studies.
Hypothetical mechanisms for direct vascular effects of taurine relevant to acute vasorelaxant and antihypertensive effects
Compared to most amino acids, taurine is unique in its physiochemical properties and biological activities. This is believed to be related to its distinct feature emanating from the presence of sulfonic group in lieu of carboxylic group, which is common in most amino acids. Taurine mostly remains free rather than becoming incorporated into peptides or proteins and behaves as a zwitterions [1]. Along with its relatively strong hydrophilic nature, such character contributes to taurine's ability to participate in osmoregulation. By virtue of its chemical nature, taurine has also been shown to act as a free radical scavenger and as an antioxidant [1,4,68,81]. Further, taurine chloramines, which is formed by the reaction of taurine with the highly toxic hypochlorous acid, serves as a cellular signaling molecule that can downregulate the expression of inflammatory mediators while upregulating the expression of eNOS [68,93]. In addition, intracellular taurine interacts electrostatically with polar groups of membrane phospholipids, with possible effects on membrane permeability and fluidity, which in turn influences the susceptibility of the structures and functions of membrane-bound proteins to covalent modification and modulation [1,4,8]. We hypothesize that the above-noted properties of taurine provide at least a partial mechanistic explanation for the observed direct vasorelaxant and antihypertensive effects of taurine.
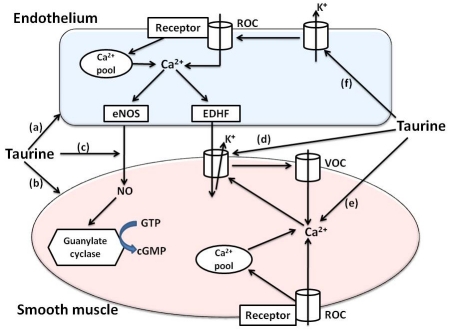
One likely hypothesis that can be attributed to the vasorelaxant effect of taurine is its role as an osmoregulatory agent. As an organic osmolyte, taurine is intimately involved in regulatory volume decrease and regulatory volume increase that cells experience when exposed to osmotic swelling and osmotic shrinkage, respectively. The volume changes are usually associated with alterations in either extracellular or intracellular electrolyte/ionic concentrations, which can be caused by bioactive substances that induce membrane permeability changes. The regulatory volume decrease is linked to taurine efflux while taurine uptake is linked to regulatory volume increase [22]. Nonetheless, such changes in cell volume affect a whole host of cellular processes including ion exchangers and transporters as well as osmosensitive signaling pathways. It is thus hypothesized that the osmoregulatory effect of taurine impacts signaling pathways that regulate vascular function (and blood pressure) and this may involve effects on endothelial cells and/or VSMCs (Figure 3). Clearly, this concept needs to be verified with experimental evidence.
Figure 3.
Proposed mechanisms for direct vasorelaxant effects of taurine. Taurine is proposed to act as a vasorelaxant by different mechanisms as described below (refer to text for further details): (a) and (b) acting as an osmoregulator on endothelial and vascular smooth muscle cells with consequential effects on osmosensitive signaling pathways;(c) preserving and enhancing the production of NO by acting as an antioxidant and increasing eNOS expression; (d) activating K+ channels in vascular smooth muscle cells resulting in a reduction in Ca2+I; (e) reducing vascular smooth muscle cell Ca2+I by other potential mechanisms; (f) activating K+ channels in endothelial cells thereby reinforcing Ca2+entry via ROC. Abbreviation: EDHF, endothelial derived hyperpolarizing factor; NO, nitric oxide; eNOS, endothelial nitric oxide synthase; ROC, receptor-operated channel; VOC, voltage-operated channel.
The modulatory role of taurine on membrane permeability and fluidity is proposed to influence the structures/morphologies and functions of a range of membrane-bound proteins such as receptors, transport proteins, ion channels, G-proteins and effector enzymes [1,8,94]. In the vasculature, this effect can be expressed by altered responsiveness of endothelial cells and/or VSMCs to vasoactive agents. For instance, the taurine-induced NO release from the vascular endothelium, the opening of smooth muscle K+ channels and the reduction in IC calcium associated with vasorelaxation are likely to be linked to this modulatory role of taurine (Figure 3). The extent to which this mechanism plays a role in vascular function and regulation of blood pressure awaits further investigation.
The proposed role of taurine as an antioxidant and promoter of eNOS expression seems to be more important in the increased production as well as preservation of NO released from endothelial cells; this contributes to vasorelaxation and hypotension associated with taurine (Figure 3). However, the exact mechanism(s) how this process occurs is not yet clear.
An important consideration for the above proposed research on the role of tauine in the vasculature relates to the recently introduced TAUT knockout mouse [95]. This animal model can serve as a valuable experimental tool whereby animals are subjected to various stresses that affect vascular function and blood pressure and relevant outcome measures determined. Nonetheless, similar to other genetically modified animal models, caution is warranted in interpretation of findings from the TAUT knockout mouse because of compensatory changes that may accompany loss of taurine [95,96]. To our knowledge, vascular effects of TAUT knockout have not been determined in any animal model.
Overall summary and conclusion
Review of the literature generally indicates that taurine exerts vascular effects by acting at different target sites and by various mechanisms. Oral taurine supplementation induces hypotension in different animal models of hypertension through both central and peripheral effects. This is partly supported by the demonstration of vasorelaxant responses elicited by taurine in isolated vascular tissue preparations. Results of isolated tissue studies further provide evidence that taurine, following oral administration, improves vascular relaxation, intimal thickening, endothelial apoptosis, oxidative stress and inflammation associated with diabetes and various related vascular disorders. Taurine also acts as an anti-proliferative and antioxidant agent in VSMCs. In endothelial cells, taurine variably inhibits apoptosis, inflammation, oxidative stress and cell death, while increasing NO generation. Oral taurine supplementation alleviates the symptoms of hypertension in hypertensive human patients and reverses arterial stiffness and brachial artery reactivity in type 1 diabetic patients.
It is thus evident that taurine administration is effective as antihypertensive in both experimental models of hypertension and in human hypertensive patients. This effect involves, among other factors, direct vasorelaxation (endothelium-dependent and/or independent) by taurine, which is also implicated to be beneficial for diabetes-induced angiopathy. Although a certain level of understanding exists regarding the vasorelaxant effect of taurine, the mechanism(s) involved is not entirely clear, particularly in disease conditions that impair the vasculature. Other vascular effects of taurine that occur more specifically in VSMCs or endothelial cells are also likely to be associated with beneficial effects against related vascular pathologies. Besides the limitations in the sources of vascular cells investigated, the mechanism(s) for the reported effects is also incompletely understood. The significant knowledge gap on the role of taurine in the vasculature suggests the need for further research in experimental models and ultimately human subjects. Additional studies to address these issues may lead to the development of therapeutic or diet-based strategies to reduce the burdens of vascular disease.
Acknowledgments
The studies from the authors' laboratories were supported by the Combined Intramural Grant Program of the Georgia Health Sciences University Research Institute (WA) and Taisho Pharmaceutical Company of Japan (MSM).
References
- 1.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Hanson SH. The role of taurine in diabetes and the development of diabetes complications. Diabetes Metabolism. Research and Reviews. 2001;17:330–346. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 3.Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208:19–25. doi: 10.1016/j.atherosclerosis.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SJ, Ramesh C, Gupta H, Lee W. Taurine diabetes interaction: from involvement to protection. J Biol Regul Homeost Agents. 2007;21:63–77. [PubMed] [Google Scholar]
- 5.Tappaz ML. Taurine biosynthetic enzymes and taurine transporter: molecular identification and regulations. Neurochem Res. 2004;29:83–96. doi: 10.1023/b:nere.0000010436.44223.f8. [DOI] [PubMed] [Google Scholar]
- 6.Gupta RC, Seki Y, Yosida J. Role of taurine in spinal cord injury. Curr Neurovasc Res. 2006;3:225–235. doi: 10.2174/156720206778018776. [DOI] [PubMed] [Google Scholar]
- 7.Lubec B, Yahua Z, Pertti S, Pentti T, Kitzmüller E, Lubec G. Distribution and disappearance of the radiolabeled carbon derived from L-arginine and taurine in the mouse. Life Sci. 1997;60:2373–2381. doi: 10.1016/s0024-3205(97)00297-x. [DOI] [PubMed] [Google Scholar]
- 8.McCarty MF. Complementary vascular-protective actions of magnesium and taurine: a rationale for magnesium taurate. Medical Hypothesis. 1996;46:89–100. doi: 10.1016/s0306-9877(96)90007-9. [DOI] [PubMed] [Google Scholar]
- 9.Militante JD, Lombardini JB, Schaffer SW. The role of taurine in the pathogenesis of the car-diomyopathy of insulin-dependent diabetes mellitus. Cardiovasc Res. 2000;46:393–402. doi: 10.1016/s0008-6363(00)00025-0. [DOI] [PubMed] [Google Scholar]
- 10.Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23:381–393. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- 11.McCarty MF. Exploiting complementary therapeutic strategies for the treatment of type II diabetes and prevention of its complications. Med Hypotheses. 1997;49:143–152. doi: 10.1016/s0306-9877(97)90219-x. [DOI] [PubMed] [Google Scholar]
- 12.Gu W, Yang YZ, He MX. A study on combination therapy of Western and traditional Chinese medicine of acute viral myocarditis] Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996;16:713–716. [PubMed] [Google Scholar]
- 13.Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17(Suppl 1):S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parcell S. Sulfur in human nutrition and application in medicine. Alternative Medicine Review. 2002;7:22–44. [PubMed] [Google Scholar]
- 15.Korang K, Milakofsky L, Hare TA, Hofford JM, Vogel WH. Levels of taurine, amino acids and related compounds in plasma, vena cava, aorta and heart of rats after taurine administration. Pharmacology. 1996;52:263–270. doi: 10.1159/000139391. [DOI] [PubMed] [Google Scholar]
- 16.Abebe W, Mozaffari MS. Taurine depletion alters vascular reactivity in rats. Can J Physiol Pharmacol. 2003;81:903–909. doi: 10.1139/y03-088. [DOI] [PubMed] [Google Scholar]
- 17.Allo SN, Bagby L, Schaffer SW. Taurine depletion, a novel mechanism for cardioprotection from regional ischemia. Am J Physiol. 1997;273:H1956–61. doi: 10.1152/ajpheart.1997.273.4.H1956. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffari MS, Azuma J, Patel C, Schaffer SW. Renal excretory responses to saline load in the taurine depleted and the taurine-supplemented rat. Biochem Pharmacol. 1997;54:619–24. doi: 10.1016/s0006-2952(97)00213-x. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffari MS, Borke JL. Taurine in subman-dibular gland of the rat: effect of muscarinic drugs. J Histochem Cytochem. 2002;50:527–32. doi: 10.1177/002215540205000409. [DOI] [PubMed] [Google Scholar]
- 20.Shi YR, Bu DF, Qi YF, Gao L, Jiang HF, Pang YZ, Tang CS, Du JB. Dysfunction of myocardial taurine transport and effect of taurine supplement in rats with isoproterenol-induced myocardial injury. Acta Pharmacol Sin. 2002;23:910–918. [PubMed] [Google Scholar]
- 21.Terauchi A, Nakazaw A, Johkura K, Yan L, Usuda N. Immunohistochemical localization of taurine in various tissues of the mouse. Amino Acids. 1998;15:151–160. doi: 10.1007/BF01345288. [DOI] [PubMed] [Google Scholar]
- 22.Alfieri RR, Cavazzoni A, Petronini PG, Bonelli MA, Caccamo AE, Borghetti AF, Wheeler KP. Compatible osmolytes modulate the response of porcine endothelial cells to hypertonicity and protect them from apoptosis. J Physiol. 2002;540:499–508. doi: 10.1113/jphysiol.2001.013395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Tenner TE, Lombardini JB. Inhibition of rat vascular smooth muscle cell proliferation by taurine and taurine analogues. Biochem Pharmacol. 1999;57:1331–1339. doi: 10.1016/s0006-2952(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 24.Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, Ganapathy V. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem J. 1994;300:893–900. doi: 10.1042/bj3000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian X, Vinnakota S, Edwards C, Sarkar HK. Molecular characterization of taurine transport in bovine aortic endothelial cells. Biochim Biophys Acta. 2000;1509:324–334. doi: 10.1016/s0005-2736(00)00315-1. [DOI] [PubMed] [Google Scholar]
- 26.Liao XB, Zhou XM, Li JM, Tan ZP, Liu LM, Zhang W, Tan H, Lu Y, Yuan LQ. Taurine transporter is expressed in vascular smooth muscle cells. Amino Acids. 2007;33:639–643. doi: 10.1007/s00726-006-0486-8. [DOI] [PubMed] [Google Scholar]
- 27.Bkaily G, Haddad G, Benchekroun JT, Pothier P, Wang S, Sperelakis N. Modulation of Ca2+ and Na+ transport by taurine in heart and vascular smooth muscle. Adv Exp Med Biol. 1996;403:263–273. doi: 10.1007/978-1-4899-0182-8_28. [DOI] [PubMed] [Google Scholar]
- 28.Horie R, Yamori Y, Nara Y, Sawamura M, Mano M. Effect of sulphur amino acids on the development of hypertension and atherosclerosis in stroke-prone spontaneously hypertensive rats. J Hypertens Suppl. 1987;5:S223–5. [PubMed] [Google Scholar]
- 29.Trachtman H, Del Pizzo R, Rao P, Rujikarn N, Sturman JA. Am J Taurine lowers blood pressure in the spontaneously hypertensive rat by a catecholamine independent mechanism. Am. J Hypertens. 1989;2:909–12. doi: 10.1093/ajh/2.12.909. [DOI] [PubMed] [Google Scholar]
- 30.Abe M, Shibata K, Matsuda T, Furukawa T. Inhibition of hypertension and salt intake by oral taurine treatment in hypertensive rats. Hypertension. 1987;10:383–389. doi: 10.1161/01.hyp.10.4.383. [DOI] [PubMed] [Google Scholar]
- 31.Meldrum MJ, Tu R, Patterson T, Dawson R, Jr, Petty T. The effect of taurine on blood pressure, and urinary sodium, potassium and calcium excretion. Adv Exp Med Biol. 1994;359:207–15. doi: 10.1007/978-1-4899-1471-2_21. [DOI] [PubMed] [Google Scholar]
- 32.Dawson R, Jr, Liu S, Jung B, Messina S, Eppler B. Effects of high salt diets and taurine on the development of hypertension in the stroke-prone spontaneously hypertensive rat. Amino Acids. 2000;19:643–665. doi: 10.1007/s007260070014. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto K, Tabei R, Fukushima M, Nosaka S, Tamori Y, Ichijima K, Haebara H, Matsumoto, Maruyama T, Suzuki Y, Tamegai M. Further observations of the development of spontaneously hypertensive rats. Jap Circ J. 1996;30:703–716. doi: 10.1253/jcj.30.703. [DOI] [PubMed] [Google Scholar]
- 34.Fujita T, Sato Y. The antihypertensive effect of taurine in DOCA-salt rats. J Hypertens Suppl. 1984;2:S563–5. [PubMed] [Google Scholar]
- 35.Yamamoto J, Akabene S, Yoshimi H, Makai M, Ikeda M. Effects of taurine on stress-evoked hemodynamic and plasma catecholamine changes in spontaneously hypertensive rats. Hypertension. 1985;7:913–922. doi: 10.1161/01.hyp.7.6.913. [DOI] [PubMed] [Google Scholar]
- 36.Kuwahara M, Kawaguchi T, Ito K, Tsubone H. Effects of taurine on cardiovascular and autonomic nervous functions in cold exposed rats. Adv Exp Med Biol. 2009;643:533–540. doi: 10.1007/978-0-387-75681-3_55. [DOI] [PubMed] [Google Scholar]
- 37.Ideishi M, Miura S, Sakai T, Sadaguri M, Misumi Y, Arakawa K. Taurine amplifies renal kallikrein and prevents salt induced hypertension in Dahl rats. J Hypertens. 1994;12:653–661. [PubMed] [Google Scholar]
- 38.Ji Y, Tao L, Xu HL, Rao MR. Effects of taurine and enalapril on blood pressure, platelet aggregation and the regression of left ventricular hypertrophy in two-kidney-one-clip renovascular hypertensive rats. Yao Xue Xue Bao. 1995;30:886–890. [PubMed] [Google Scholar]
- 39.Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol. 1999;77:749–754. [PubMed] [Google Scholar]
- 40.Nandhini AT, Thirunavukkarasu V, Anuradha CV. Taurine modulates kallikrein activity and glucose metabolism in insulin resistant rats. Amino Acids. 2002;22:27–38. doi: 10.1007/s726-002-8199-3. [DOI] [PubMed] [Google Scholar]
- 41.Nandhini AT, Thirunavukkarasu V, Anuradha CV. Taurine modifies insulin signaling enzymes in the fructose fed insulin resistant rats. Diabetes Metab. 2005;31:337–44. doi: 10.1016/s1262-3636(07)70202-1. [DOI] [PubMed] [Google Scholar]
- 42.Rahman MM, Park HM, Kim SJ, Go HK, Kim GB, Hong CU, Lee YU, Kim SZ, Kim JS, Kang HS. Taurin prevents hypertension and increases exercise capacity in rats with fructose-induced hypertension. Am J Hypertens. 2011;24:574–581. doi: 10.1038/ajh.2011.4. [DOI] [PubMed] [Google Scholar]
- 43.Harada H, Kitazaki K, Tsujino T, Watarai Y, Iwata S, Nonaka H, Hayashi T, Takeshita T, Morimoto K, Yokoyama M. Oral taurine supplementation prevents the development of ethanol -induced hypertension in rats. Hypertens Res. 2000;23:277–284. doi: 10.1291/hypres.23.277. [DOI] [PubMed] [Google Scholar]
- 44.Hu J, Xu X, Yang J, Wu G, Sun C, Lv Q. Anti-hypertensive effect of taurine in rat. Adv Exp Med Biol. 2009;643:75–80. doi: 10.1007/978-0-387-75681-3_8. [DOI] [PubMed] [Google Scholar]
- 45.Kulthinee S, Wyss JM, Jirakulsomchok D, Roysommuti S. High sugar intake exacerbates cardiac reperfusion injury in perinatal taurine depleted adult rats. J Biomed Sci. 2010;17(Suppl 1):S22. doi: 10.1186/1423-0127-17-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roysommuti S, Malila P, Lerdweeraphon W, Jirakulsomchok D, Wyss JM. Perinatal taurine exposure alters renal potassium excretion mechanisms in adult conscious rats. J Biomed Sci. 2010;17(Suppl 1):S29. doi: 10.1186/1423-0127-17-S1-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussy N, Deleuze C, Pantaloni A, Desarménien MG, Moos F. Agonistic action of taurine on gly-cine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol. 1997;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muratani H, Ferrario CM, Averill DB. Ventrolateral medulla in spontaneously hypertensive rats: role of angiotensin II. Am J Physiol. 1993;264:R388–395. doi: 10.1152/ajpregu.1993.264.2.R388. [DOI] [PubMed] [Google Scholar]
- 49.Yang CP, Lin MT. Amino acids injected into the cerebroventricular system induce an enhancement of reflex bradycardia in the rat. Neuro-pharmacology. 1983;22:919–922. doi: 10.1016/0028-3908(83)90141-7. [DOI] [PubMed] [Google Scholar]
- 50.Singewald N, Kouvelas D, Chen F, Philippu A. The release of inhibitory amino acids in the hypothalamus is tonically modified by impulses from aortic baroreceptors as a consequence of blood pressure fluctuations. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:348–355. doi: 10.1007/pl00005061. [DOI] [PubMed] [Google Scholar]
- 51.Mozaffari MS, Schaffer DJ. Taurine modulates arginine vasopressin-mediated regulation of renal function. Cardiovasc Pharmacol. 2001;37:742–750. doi: 10.1097/00005344-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Guo LJ, Athineos P. Effects of hemodynamic changes on taurine release from posterior hypothalamus of freely moving rats. Zhongguo Yao Li Xue Bao. 1995;16:405–408. [PubMed] [Google Scholar]
- 53.Yang CP, Liu HJ, Chou LS, Lin MT. Effect of intra-raphe injection of amino acids on cardiovascular function in rats. Chin J Physiol. 1992;35:143–149. [PubMed] [Google Scholar]
- 54.Mozaffari MS, Abebe W. Cardiovascular responses of the taurine-depleted rat to vasoactive agents. Amino Acids. 2000;19:625–634. doi: 10.1007/s007260070012. [DOI] [PubMed] [Google Scholar]
- 55.Zhu DN, Moriguchi A, Mikami H, Higaki J, Ogihara T. Central amino acids mediate cardiovascular response to angiotensin II in the rat. Brain Res Bull. 1998;45:189–197. doi: 10.1016/s0361-9230(97)00338-9. [DOI] [PubMed] [Google Scholar]
- 56.Mozaffari MS, Patel C, Abdelsayed R, Schaffer SW. Accelerated NaCl-nduced hypertension in taurine- deficient rat: role of renal function. Kidney Int. 2006;70:329–337. doi: 10.1038/sj.ki.5001503. Jul. [DOI] [PubMed] [Google Scholar]
- 57.Roysommuti S, Suwanich A, Jirakulsomchok D, Wyss JM. Prenatal taurine depletion increases susceptibility to adult sugar-induced hypertension in rats. Adv Exp Med Biol. 2009;643:123–133. doi: 10.1007/978-0-387-75681-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nara Y, Yamori Y, Lovenberg W. Effect of f dietary taurine on blood pressure in spontaneously hypertensive rats. Biochem Pharmacol. 1978;27:2689–2692. doi: 10.1016/0006-2952(78)90043-6. [DOI] [PubMed] [Google Scholar]
- 59.Thant M, Yamori Y, Okamoto K. Baroreceptor function revealed by acute sinoaortic denervation in spontaneously hypertensive rats. Jpn Circ J. 1969;33:501–507. doi: 10.1253/jcj.33.501. [DOI] [PubMed] [Google Scholar]
- 60.Jones MR. Free amino acid pools in the spontaneously hypertensive rat: a longitudinal study. J Nutr. 1988;118:579–587. doi: 10.1093/jn/118.5.579. [DOI] [PubMed] [Google Scholar]
- 61.Tanabe Y, Urata H, Kiyonaga A, Ikeda M, Tanaka H, Shindo M, Arakawa K. Changes in serum concentrations of taurine and other amino acids in clinical antihypertensive exercise therapy. Clin Exp Hypertens A. 1989;11:149–165. doi: 10.3109/10641968909035297. [DOI] [PubMed] [Google Scholar]
- 62.Nandhini TA, Anuradha CV. Inhibition of lipid peroxidation, protein glycation and elevation of membrane ion pump activity by taurine in RBC exposed to high glucose. Clinica Chimica Acta. 2003;336:129–135. doi: 10.1016/s0009-8981(03)00337-1. [DOI] [PubMed] [Google Scholar]
- 63.Abebe W, Mozaffari MS. Effect of chronic taurine treatment on reactivity of rat aorta. Amino Acids. 2000;19:615–623. doi: 10.1007/s007260070011. [DOI] [PubMed] [Google Scholar]
- 64.Li N, Sawamura M, Nara Y, Ikeda K, Yamori Y. Direct inhibitory effects of taurine on norepi-nephrine-induced contraction in mesenteric artery of stroke-prone spontaneously hypertensive rats. Adv. Exp. Med. Biol. 1996;403:257–262. doi: 10.1007/978-1-4899-0182-8_27. [DOI] [PubMed] [Google Scholar]
- 65.Katama K, sugiura M, Kojima S, Kasuya Y. Restoration of endothelium-dependent relaxation in both hypercholesterolemia and diabetes by chronic taurine. Eur. J. Pharmacol. 1996;303:47–53. doi: 10.1016/0014-2999(96)00094-5. [DOI] [PubMed] [Google Scholar]
- 66.Wang LJ, Yu YH, Zhang LG, Wang Y, Niu N, Li Q, Guo LM. Taurine rescues vascular endothelial dysfunction in streptozocin-induced diabetic rats: correlated with downregulation of LOX-1 and ICAM-1 expression on aortas. Eur J Pharmacol. 2008;597:75–80. doi: 10.1016/j.ejphar.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 67.Li F, Abatan OI, Kim H, Burnett D, Larkin D, Obrosova IG, Stevens MJ. Tauine reverses neurological and neurovascular deficits in Zucker diabetic fatty rats. Neurobiol Dis. 2006;22:669–676. doi: 10.1016/j.nbd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Sener G, Ozer Sehirli A, Ipçi Y, Cetinel S, Cikler E, Gedik N, Alican I. Taurine treatment protects against chronic nicotine-induced oxidative changes. Fundam Clin Pharmacol. 2005;19:155–164. doi: 10.1111/j.1472-8206.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 69.Zulli A, Lau E, Wijaya BP, Jin X, Sutarga K, Schwartz GD, Learmont J, Wookey PJ, Zinellu A, Carru C, Hare DL. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension. 2009;53:1017–1022. doi: 10.1161/HYPERTENSIONAHA.109.129924. [DOI] [PubMed] [Google Scholar]
- 70.Ristori MT, Verdetti J. Effects of taurine on rat aorta in vitro. Fundam Clin Pharmacol. 1991;5:245–258. doi: 10.1111/j.1472-8206.1991.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 71.Fanconi F, Giotti A, Manzini S, Martini F, Stendardi I, Zilliti L. The effect of taurine on High potassium- and noradrenaline-induced contraction in rabbit ear artery. Br. J. Pharmacol. 1982;75:605–612. doi: 10.1111/j.1476-5381.1982.tb09180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niu LG, Zhang MS, Liu Y, Xue WX, Liu DB, Zhang J, Liang YQ. Vasorelaxant effect of taurine is diminished by tetraethylammonium in rat isolated arteries. Eur J Pharmacol. 2008;580:169–174. doi: 10.1016/j.ejphar.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Niu L, Zhang W, Cui L, Zhang X, Liang Y, Zhang M. Effect of taurine on contractions of the porcine coronary artery. Pharmacol Rep. 2009;61:681–689. doi: 10.1016/s1734-1140(09)70120-3. [DOI] [PubMed] [Google Scholar]
- 74.Nishida S, Satoh H. vascular modulation of rat aorta by taurine. Adv Exp Med Biol. 2009;643:37–46. doi: 10.1007/978-0-387-75681-3_4. [DOI] [PubMed] [Google Scholar]
- 75.Hano T, Kasano M, Tomari H, Iwane N. Taurine suppresses pressor response through the inhibition of sympathetic nerve activity and the improvement in baro-reflex sensitivity of spontaneously hypertensive rats. Adv Exp Med Biol. 2009;643:57–63. doi: 10.1007/978-0-387-75681-3_6. [DOI] [PubMed] [Google Scholar]
- 76.Abebe W. Effects of taurine on the reactivity of aortas from diabetic rats. Life Sci. 2008;82(5-6):279–89. doi: 10.1016/j.lfs.2007.11.012. Jan 30. [DOI] [PubMed] [Google Scholar]
- 77.Xue W, Zhang M, Li J, Wu D, Niu L, Liang Y. Effect of taurine on aortic rings isolated from fructose-fed insulin resistance Sprague-Dawley rat are changed. Cardiovasc Drugs Ther. 2008;22:461–8. doi: 10.1007/s10557-008-6124-9. [DOI] [PubMed] [Google Scholar]
- 78.Abebe W, Mozaffari MS. Effect of taurine deficiency on adenosine receptor-mediated relaxation of the rat aorta. Vascular Pharmacology. 2003;40:219–228. doi: 10.1016/j.vph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Chang L, Zhao J, Xu J, Jiang W, Tang CS, Qi YF. Effects of taurine and homocysteine on calcium homeostasis and hydrogen peroxide and superoxide anions in rat myocardial mitochondria. Clin Exp Pharmacol Physiol. 2004;31:237–243. doi: 10.1111/j.1440-1681.2004.03983.x. [DOI] [PubMed] [Google Scholar]
- 80.Ozsarlak-Sozer G, Kerry Z, Gokce G, Oran I, Topcu Z. Oxidative stress in relation to telomere length maintenance in vascular smooth muscle cells following balloon angioplasty. J Physiol Biochem. 2011;67:35–42. doi: 10.1007/s13105-010-0046-2. [DOI] [PubMed] [Google Scholar]
- 81.Chang L, Xu JX, Zhao J, Pang YZ, Tang CS, Qi YF. Taurine antagonized oxidative stress injury induced by homocysteine in rat vascular smooth muscle cells. Acta Pharmacol Sin. 2004;25:341–346. [PubMed] [Google Scholar]
- 82.Kaneyuki U, Ueda S, Yamagishi S, Kato S, Fujimura T, Shibata R, Hayashida A, Yoshimura J, Kojiro M, Oshima K, Okuda S. Pitavastatin inhibits lysophosphatidic acid-induced proliferation and monocyte chemoattractant protein-1 expression in aortic smooth muscle cells by suppressing Rac-1-mediated reactive oxygen species generation. Vascul Pharmacol. 2007;46:286–292. doi: 10.1016/j.vph.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 83.Charreau B. Molecular regulation of endothelial cell activation: novel mechanisms and emerging targets. Curr Opin Organ Transplant. 2011;16:207–213. doi: 10.1097/MOT.0b013e3283446c52. [DOI] [PubMed] [Google Scholar]
- 84.Wu QD, Wang JH, Fennessy F, Redmond HP, Hayes DB. Taurine prevents high-glucose induced human vascular endothelial cell apoptosis. Am J Physiol. 1999;277:C1229–C1238. doi: 10.1152/ajpcell.1999.277.6.C1229. [DOI] [PubMed] [Google Scholar]
- 85.Casey RG, Joyce CG, Hayes DJB. Taurine attenuates acute hyperglycemia-induced endothelial cell apoptosis, leucocyte-endothlial cell interactions and cardiac dysfunction. J Vasc Res. 2007;44:31–39. doi: 10.1159/000097893. [DOI] [PubMed] [Google Scholar]
- 86.Wang JH, Redmond HP, Watson RW, Condron C, Bouchier-Hayes D. The beneficial effect of taurine on the prevention of human endothelial cell death. Shock. 1996;6:331–8. doi: 10.1097/00024382-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 87.Tan B, Jiang DJ, Huang H, Jia SJ, Jiang JL, Hu CP, Li YJ. Taunine acts against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Vascul Pharmacol. 2007;46:338–345. doi: 10.1016/j.vph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 88.Fennessy EM, Monelet MB, Wang JH, Kelly CJ, Bouchier-Hayes DJ. Taurine and Vitamin C modify monocyte and endothelial dysfunction in young smokers. Circulation. 2003;107:410–415. doi: 10.1161/01.cir.0000046447.72402.47. [DOI] [PubMed] [Google Scholar]
- 89.Kohashi N, Katori R. Decrease of urinary taurine in essential hypertension. Jpn Heart J. 1983;24:91–102. doi: 10.1536/ihj.24.91. [DOI] [PubMed] [Google Scholar]
- 90.Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation. 1987;75:525–532. doi: 10.1161/01.cir.75.3.525. [DOI] [PubMed] [Google Scholar]
- 91.Ogawa M, Takahara A, Ishijima M, Tazaki S. Decrease of plasma sulfur amino acids in essential hypertension. Jpn Circ J. 1985;49:1217–24. doi: 10.1253/jcj.49.1217. [DOI] [PubMed] [Google Scholar]
- 92.Moloney MA, Casey RG, O'Donnell DH, Fitzgerald P, Thompson C, Bouchier-Hayes DJ. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diab Vasc Dis Res. 2010;7:300–10. doi: 10.1177/1479164110375971. [DOI] [PubMed] [Google Scholar]
- 93.Egan BM, Abdih H, Kelly CJ, Condron C, Bouchier-Hayes DJ. Effect of of intravenous taurine on endotoxin induced acute lung injury in sheep. Eur J Surg. 2001;167:575–80. doi: 10.1080/110241501753171164. [DOI] [PubMed] [Google Scholar]
- 94.Adams DJ. Ionic channels in vascular endothelial cells. Trends Cardiovasc Med. 1994;4:18–26. doi: 10.1016/1050-1738(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 95.Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, Schaffer SW. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. Fujio Y, Azuma J. J Mol Cell Cardiol. 2008;44:927–37. doi: 10.1016/j.yjmcc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 96.Kuramoto N, Takizawa T, Takizawa T, Matsuki M, Morioka H, Robinson JM, Yamanishi K. Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase. J Clin Invest. 2002;109:243–50. doi: 10.1172/JCI13563. [DOI] [PMC free article] [PubMed] [Google Scholar]