Abstract
The recent development of transcatheter aortic valve implantation (TAVI) for severe aortic stenosis (AS) treatment offers a viable option for high-risk patient categories. Our aim is to evaluate whether 2D strain and strain rate can detect subtle improvement in global and regional LV systolic function immediately after TAVI. 2D conventional and 2D strain (speckle analysis) echocardiography was performed before, at discharge and after three months in thirty three patients with severe AS. After TAVI, we assessed by conventional echocardiography an immediate reduction of transaortic peak pressure gradient (p<0.0001), of mean pressure gradient (p<0.0001) and a concomitant increase in aortic valve area (AVA: 1.08±0.31 cm2/m2; p<0.0001). 2D longitudinal systolic strain showed a significant improvement in all patients, both at septal and lateral level, as early as 72 h after procedure (septal: -14.2±5.1 vs -16.7±3.7%, p<0.001; lateral: -9.4±3.9 vs -13.1±4.5%, p<0.001; respectively) and continued at 3 months follow-up (septal: -18.1±4.6%, p<0.0001; lateral: -14.8±4.4%, p<0.0001; respectively). Conventional echocardiography after TAVI proved a significant reduction of LV end-systolic volume and of LV mass with a mild improvement of LV ejection fraction (EF) (51.2±11.8 vs 52.9±6.4%; p<0.02) only after three months. 2D strain seems to be able to detect subtle changes in LV systolic function occurring early and late after TAVI in severe AS, while all conventional echo parameters seem to be less effective for this purpose. Further investigations are needed to prove the real prognostic impact of these echocardiographic findings.
Keywords: Transcatheter aortic valve implantation, left ventricular function, 2D strain
Introduction
Patients with severe aortic stenosis (AS) are subjected to long-standing pressure overload of left ventricle (LV), leading to LV hypertrophy and dysfunction [1]. At the early stage of disease, when ejection fraction (EF) is still preserved, subclinical systolic abnormalities that may contribute to symptoms can be underestimated by conventional echocardiography. However it would be clinically helpful to detect early subtle effects of aortic valve replacement on LV systolic function [2, 3].
Myocardial deformation imaging with determination of myocardial strain and strain rate derived from tissue Doppler echocardiography has been proposed as a reliable mean for the detection of any clinical and subclinical regional LV dysfunction in several diseases [4, 5].
However, this technique presents the disadvantage of being considerably angle dependent [6]. The recent development of two-dimensional (2D) strain based on speckle tracking of 2D grayscale images overcomes this limitation [7-10]. The application of these indexes to AS and its clinical significance have not still been fully established [2, 11, 12].
Recent studies have demonstrated that transcatheter aortic valve implantation (TAVI) offers a viable and “less invasive” option for the treatment of critical aortic stenosis patients at high risk with conventional surgery [13, 14]. This technique, in fact, can decrease LV elevated afterload in patients with AS, acutely reducing transaortic pressure gradients. However, its effects on LV systolic function are currently unknown.
Our aim is to evaluate whether 2D strain and strain rate are sensitive enough to detect subtle improvement in regional LV systolic function immediately after TAVI.
Materials and methods
Patient population
From September 2007 to December 2010 we consecutively selected 50 patients who underwent successful TAVI with third-generation self-expanding CoreValve prosthesis (Medtronic, CV Luxembourg S.a.r.l.) at the Cardiac Thoracic and Vascular Department of the University of Pisa. Inclusion criteria for TAVI were the following: 1) severe native AS with an area <1 cm2 or <0.6 cm2/m2 and age 80 years or a logistic Euro-SCORE of 15% or age 65 years and at least one of the following complications: liver cirrhosis, pulmonary insufficiency (forced expiratory volume in one second <1l), previous cardiac surgery, pulmonary hypertension 60 mmHg, porcelain aorta, recurrent pulmonary embolus, right ventricular insufficiency, thoracic burning sequelae with contraindication for open chest surgery, history of mediastinum radiotherapy, severe connective tissue disease with contraindication for surgery, or cachexia (body mass index <18 kg/m2). 2) Echocardiographic aortic valve annulus diameter 20 and 27 mm. 3) ascending aorta diameter <45 mm at the sinotubular junction [15, 16]. Patients with a prior pace-maker or requiring definitive pacemaker implantation after TAVI and those with a myocardial scar were excluded from the study. All patients gave written informed consent for the procedure.
Preinterventional morphological patient screening included transthoracic as well as transesophageal echocardiography, computed tomographic angiography, and invasive cardiac evaluation with coronary angiogram and left ventriculography [17, 18]. Patients baseline operative risk was estimated by the logistic Eu-roSCORE and the STS score [19, 20].
Device description and procedure
The CoreValve aortic valve prosthesis consists of a trileaflet bioprosthetic porcine pericardial tissue valve, which is mounted and sutured in a self-expanding nitinol stent. Further details of the device have already been described in previous studies [14, 16] Vascular access was obtained either by percutaneous approach through the common femoral artery with pre-implantation of a vascular closure device (Prostar XL, Abbott Vascular, Abbott Park, IL), or by surgical cut down of the subclavian artery [21]. The procedure was performed with the patient under local anesthesia with a mild systemic sedative treatment, according to patients needs [22] [23]. Valvuloplasty with a 22 mm or 25 mm balloon (NuCLEUS™ PTV, NuMED Inc., Hopkinton, NY) under rapid pacing at 180 bpm was performed before CoreValve deployment. The prosthesis was then deployed retrograde over a stiff guide wire placed in the left ventricle, under fluoroscopic guidance. After the placement, this kind of valve starts to work immediately.
Two valve size of 26 and 29 mm expanded diameters were available. Aspirin (100 mg daily) and clopidogrel (300 mg oral load, followed by 75 mg daily) were given at least 3 days before the procedure; aspirin was continued indefinitely, while clopidogrel was administered for 3 to 6 months. Acute procedural success was defined as as the adequate technical placement of the valve within the aortic root with absence of periprocedural major adverse cardiovascular and cerebral events in the first 48 h after device implantation [13].
Clinical follow-up and transthoracic echocardiography were performed after 72 h and 3 months after device implantation.
Pre-and post-operative conventional 2D Color Doppler Echocardiography
All echocardiographic measurements were performed using a commercially available ultrasound system (Vivid 7, General Electric Healthcare, Milwaukee, WI) equipped with a harmonic 4.0-MHz variable-frequency phased-array transducer. The end-diastolic LV diameter (LVEDD), end-systolic LV diameter (LVESD), end-diastolic thickness of ventricular septum (EDSth) and end-diastolic thickness of LV posterior wall (EDPWth) were measured by M-mode echocardiography. Left ventricular mass (LVM) was calculated with the corrected formula of the American Society of Echocardiography and was indexed for body surface area (LVMbs) and height (LVMh) (h 2.7).
LV mass index was determined by dividing the LV mass measure by the body surface area (g/ m2) (LMVbs) and by height (LVMh) (h 2.7). LV end -diastolic volume (EDV) and end-systolic volume (ESV) were calculated by the apical 2- and 4-chamber views using a modified Simpson's method. LV ejection fraction was calculated as ejection fraction (EDV-ESV)/EDV *100. Transmitral flow and LV outflow velocity patterns were obtained by the apical long-axis view with the pulsed Doppler method.
Transaortic peak velocity was measured by continuous wave Doppler echocardiography and pressure gradient was calculated using the simplified Bernoulli equation. The aortic valve area was obtained by the continuity equation method and was normalized for the body surface area to find the aortic valve area index.
Diastolic function was assessed by measuring peak velocities of the E wave (early diastole), the A wave (late diastole), the deceleration time of the E wave, and the E' wave (average of early diastolic lateral and septal mitral annulus velocity) [24, 25]. In particular, we considered the ratio (E/E') between transmitral early diastolic flow velocity (E) and early velocity of mitral annulus motion (E') as expression of left ventricular diastolic pressure [26].
Pre-and post-operative Two-Dimensional Strain Imaging
We acquired LV short-axis view at the mid level and LV 4-chamber view using a high frame rate (80 frames/sec). The mid short-axis view contained the papillary muscles. At each plane, 3 consecutive cardiac cycles were acquired at end expiration breath holding and stored digitally on a hard disk for off line analysis. Image analysis was performed off line on a PC workstation using custom analysis software (Echopac PC, Version 6.0.X, GE Healthcare, Fairfield, CT). The LV endocardial border of the end-systolic frame was manually traced, automatically creating a region of interest including the entire transmural wall for all patients with the software selecting natural acoustic markers moving with the tissue [27, 28].
2D LV strain and strain rate were measured using a dedicated software package. In the present study, longitudinal strain and strain rate were assessed at the mid lateral and septal walls on the apical 4-chamber view. In fact, the mid segments were both little influenced by basal and apical torsion and better reflected the deformation phenomenon. Circumferential and radial strain and strain rate were assessed in the 6 LV walls on the parasternal LV short-axis view at the chordae tendineae level and their average values were used for comparison [29]. The intra-class correlation coefficient (ri) was calculated according to Bland and Altman's procedure[30]. Nearly three values of 2D Strain were sampled for each patient and for each segment: the correlation coefficient (ri) was 0.88.
Statistical analysis
Categorical variables were presented as frequencies and were compared by chi-square test. Continuous variables were presented as mean ± standard deviation. The comparison of a single group over different points of time was achieved by the analysis of variance for repeated measures. Linear regression analysis was made to test the correlation between the variations, before and after procedure, of functional and structural ultrasonic parameters. A p value <0.05 was considered statistically significant.
Results
Patient baseline characteristics
Baseline clinical and echocardiographic data are shown in Table 1. Mean age was 82.4±5.9 years with a mean EuroScore of 19.3%. According the echocardiographic test, all patients had severe AS, with AVA averaging between 0.32±0.20 cm2/m2. Peak aortic pressure gradient and mean pressure gradient were 86.2 ± 22.5 mmHg and 53.2 ± 15.2 mmHg respectively. LV ejection fraction averaged between 51.8±11.8 and it was lower than 45% only in 3 patients.
Table 1.
Data of the clinical and echocardiographic variables at baseline
| (n=50) | |
|---|---|
| Age, years (mean ± SD) | 82.4±5.9 |
| Logistic EuroScore, % | 19.3±11.4 |
| Female gender, n (%) | 27 (54%) |
| Body surface area, m2 (mean± SD) | 1.77±0.18 |
| Body mass index, kg/m2 | 24.2±3.7 |
| PAS, mmHg (mean± SD) | 131.2±15.2 |
| PAD, mmHg (mean± SD) | 71.2±12.1 |
| Prior coronary artery disease, n(%) | 5 (10%) |
| Prior cerebral ischemic events, n(%) | 5 (10%) |
| Peripheral vascular disease, n(%) | 10 (20%) |
| Severe lung disease, n(%) | 6 (12%) |
| Diabetes mellitus, n(%) | 15 (30%) |
| Hypertension, n(%) | 28 (56%) |
| NYHA class II | 20 (40%) |
| NYHA class III-IV | 30 (60%) |
| LVEDD, cm (mean± SD) | 5.2 ± 0.5 |
| LVEDV, ml (mean± SD) | 103.1 ± 40.8 |
| LV ejection fraction, % (mean± SD) | 51.2±11.8 |
| Peak aortic jet velocity, m/sec (mean± SD) | 4.5±0.7 |
| Peak pressure gradient, mmHg (mean± SD) | 86.2 ± 22.5 |
| Mean pressure gradient, mmHg (mean± SD) | 53.2 ±15.2 |
| Aortic valve area indexed, cm2/m2 (mean± SD) | 0.32 ± 0.20 |
LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; NYHA, New York Heart Association.
Acute procedural and follow-up results
Acute device success was achieved in all patients (100%). In 2 patients a suboptimal placement of the prosthesis with remaining aortic regurgitation had to be corrected by implantation of a second CoreValve prosthesis (prosthesis in prosthesis). Neither aortic dissection nor procedural coronary flow impairment was observed in the entire study population. Thirty-one (63%) patients received a 26-mm valve and 19 (37%) patients received a 29-mm valve.
There were no immediate changes in aortic systolic and diastolic pressure or in heart rate, whereas LV systolic and LV end diastolic pressures significantly decreased after TAVI (163.2±26.5 vs 131.3±31.2 mmHg, p<0.0001 and 17.1±4.8 vs 12.3±4.3 mmHg, p<0.0001 respectively). Overall mortality at 90 days was 14% (n=7). Five deaths occurred peri procedurally: 3 patients died of cardiogenic shock (baseline ejection fraction <45%), 1 cardiac tamponade and 1 patient died after a major stroke. There were 2 non-cardiac deaths occurring after 30 days. Furthermore, at 3 months follow-up patients in class NYHA I was 90% and in class II were 10%.
Pre-and post-operative conventional 2D Color Doppler Echocardiography
Standard echocardiographic parameters before and after TAVI are shown in Table 2. Early after procedure (72 h), transaortic peak pressure gradient assessed by echocardiography was significantly reduced (18.6±8.3 mmHg; p <0.0001), as mean pressure gradient (11.4±5.9 mmHg;p<0.0001) with concomitant increase in AVA (1.08±0.31 cm2/m2;p<0.0001). LV ejection fraction improved with a mild significance only at 3 months follow-up (51.2±11.8 vs 52.9±6.4%;p<0.02). Obviously LVM, LVMh and LVMbs reduced significantly at three months follow-up (p<0.0001) (Table 2). No LV diastolic function index showed improvement early or/ and late except for E/E' ratio that significantly improved, both at septal and lateral level, only after 3 months follow-up (septal: 20.1±8.1 vs 15.3±5.6; p<0.0001 and lateral: 17.8±7.4 vs 14.2±4.1; p<0.0001, respectively).
Table 2.
Echo-Doppler conventional parameters
| Baseline (n=50) Mean ± SD | 72 h after pAVI (n=45) Mean ± SD | Follow-up (n=43) Mean ± SD | p< | |
|---|---|---|---|---|
| Echo-Doppler Parameters | ||||
| LVEDD (cm) | 5.21 ±0.52 | 5.12 ±0.48 | 5.09 ± 0.49 | >0.2 |
| LVESD (cm) | 3.31 ±0.71 | 3.29 ±0.62 | 3.01 ±0.61° | 0.008 |
| Fractional shortening (%) | 36.2 ± 12.1 | 36.4 ±9.5 | 39.8 ± 11.2 | >0.2 |
| LVEDV (ml) | 103.1 ±40.81 | 107.3 ±40.1 | 94.8 ±26.2 | >0.2 |
| LV ejection fraction (%) | 51.2±11.8 | 52.1 ±8.1 | 52.9 ±6.4* | 0.02 |
| LVM (g) | 305.8 ±47.9 | 304.4 ±46.7 | 279.1 ±55.4° | 0.0001 |
| LVMh(g/m2.7) | 81.4 ± 19.8 | 81.1 ±21.0 | 70.4 ±19.6° | 0.0001 |
| LVMbs(g/m2) | 176.9 ±30.1 | 177.7 ±24.4 | 163.5 ±31.4° | 0.0001 |
| Peak aortic jet velocity, m/sec (m/sec) | 4.5±0.7 | 1.9 ±0.6° | 2.2 ±0.4° | 0.0001 |
| Peak pressure gradient (mmHg) | 86.2 ±22.5 | 18.6 ±8.3° | 18.9 ± 7.1° | 0.0001 |
| Mean pressure gradient (mmHg) | 53.2 ±15.2 | 11.4 ±5.9° | 11.8 ±5.4° | 0.0001 |
| Aortic valve area (cm2/m2) | 0.32 ±0.20 | 1.08 ±0.31° | 1.12 ±0.38° | 0.0001 |
| (E), cm/s | 0.71±0.31 | 0.74±0.36 | 0.81±0.34 | >0.2 |
| E/A | 0.66±0.24 | 0.83±0.61 | 0.92±0.62 | >0.2 |
| PW TDI E/Es | 20.1 ±8.1 | 21.4 ±7.8 | 15.3 ±5.6° | 0.0001 |
| PW TDI E/El | 17.8 ±7.4 | 18.1 ±6.4 | 14.2 ±4.1° | 0.0001 |
LVEDD, left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVM: left ventricular mass; LVMh: left ventricular mass indexed for height; LVMbs: left ventricular mass indexed for body-surface; E: early diastolic transmitral velocity; E/A: ratio between early diastolic transmitral velocity (E) and late diastolic transmitral velocity (A); PW E/Es: ratio between early diastolic transmitral flow velocity (E) and early velocity of septal mitral anulus motion (Es); PW E/El: ratio between early diastolic transmitral flow velocity (E) and early velocity of lateral mitral anulus motion (El).
Pre-and post-operative Two-Dimensional Strain Imaging
The improvement of longitudinal systolic 2D strain was significant both at septal and lateral level already 72 h after procedure (septal: -14.2±5.1 vs -16.7±3.7%, p<0.001; lateral: -9.4±3.9 vs -13.1±4.5%, p<0.001; respectively) and continued at 3 months follow-up (septal:-18.1±4.6%, p<0.0001; lateral:-14.8±4.4%, p<0.0001; respectively) (Table 3).
Table 3.
Left ventricular longitudinal, radial and circumferential strain values assessed by speckle tracking
| Baseline (n=50) Mean ± SD | 72 h after pAVI (n=45) Mean ± SD | Follow-up (n=43) Mean ± SD | P< | |
|---|---|---|---|---|
| LV Longitudinal 2D strain (%) | ||||
| Segments | ||||
| Mid septal | -14.2 ±5.1 | -16.7 ±3.7° | -18.1 ±4.6° | 0.0001 |
| Mid lateral | -9.4 ± 3.9 | -13.1 ±4.5° | -14.8 ±4.4° | 0.0001 |
| LV Radial 2D strain | ||||
| Segments | ||||
| Anterior | 27.9 ± 13.6 | 29.2 ± 15.1 | 45.1 ±16.7° | 0.0001 |
| Anteroseptal | 25.9 ± 12.7 | 28.9 ± 14.6 | 41.9 ±17.9° | 0.0001 |
| Septal | 26.1 ± 13.4 | 31.3 ± 14.3 | 45.2 ±19.5° | 0.0001 |
| Inferior | 25.8 ± 12.6 | 32.5 ± 16.6 | 48.1 ±18.3° | 0.0001 |
| Posterior | 27.4 ± 15.4 | 32.8 ± 15.9 | 49.8 ±16.3° | 0.0001 |
| Lateral | 28.9 ± 14.9 | 33.1 ± 14.4 | 50.1 ±15.8° | 0.0001 |
| LV Circumferential 2D strain (%) | ||||
| Segments | ||||
| Anterior | -21.5 ± 8.9 | -21.8 ± 7.6 | -25.8 ±8.9° | 0.007 |
| Anteroseptal | -16.1 ±6.8 | -15.9 ± 6.1 | -19.5 ± 7.4° | 0.001 |
| Septal | -12.1 ±5.1 | -13.9 ± 5.5 | -16.7 ± 7.2° | 0.001 |
| Inferior | -11.4 ±4.7 | -13.4 ± 6.2 | -15.9 ± 7.1° | 0.001 |
| Posterior | -13.1 ± 7.1 | -14.8 ± 7.2 | -19.6 ±6.1° | 0.0001 |
| Lateral | -17.2 ±8.5 | -18.3 ± 6.9 | -25.1 ±8.4° | 0.0001 |
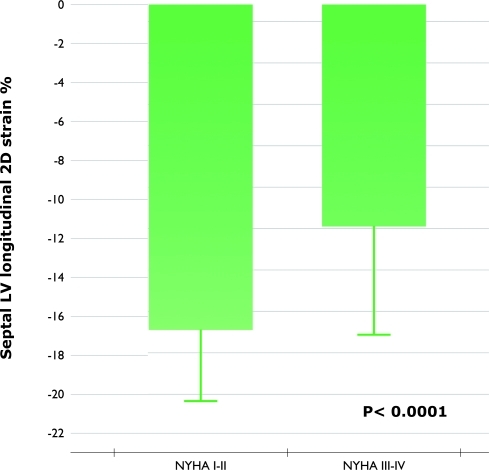
Baseline patients in NYHA classes III to IV had a significant lower longitudinal systolic strain at septal level compared to patients in NYHA classes I to II (-16.8±3.3% vs 11.7±5.1%, p<0.0001; respectively) (Figure 1).
Figure 1.
Baseline longitudinal systolic strain at septal level of patients in NYHA classes III to IV compared to patients in NYHA classes I to II.
Furthermore we found a mild significant correlation between the improvement of NYHA class after three months follow-up and the improvement of longitudinal systolic strain at septal level (r=0.37; p<0.02).
Early and late diastolic longitudinal 2D strain rate didn't show significant improvement at 72 h after procedure and at 3 months follow up.
Systolic longitudinal 2D strain rate, instead, significantly improved at septal and lateral level 72 h after procedure (-0.8±0.3 vs -0.9±0.3 sec -1, p<0.001 and -0.7±0.3 vs -0.9±0.4 sec -1, p<0.001; respectively) and 3 months later (-1.1±0.3 sec -1, p<0.0001 and -0.8±0.3 sec -1, p<0.0001; respectively).
Only after 3 months we observed a significant improvement of radial and circumferential strain at each segment with the concomitant regression of LVM (Table 3). No parameter of radial and circumferential 2D strain rate showed significant changes at 72 h after procedure and at 3 months follow up.
Correlation between strain values and AS parameters before and after TAVI
We analyzed the relationship between preoperative strain parameters and AS severity parameters in all patients and we found a significant correlation between LV longitudinal strain at septal and lateral level and AVA (Figure 2). Furthermore, already early after the procedure, we detected a significant correlation between the improvement of LV longitudinal strain both at septal and lateral level (Δ LV longitudinal strain) and the increase of aortic valve area (Δ AVA) (Figure 3).
Figure 2.
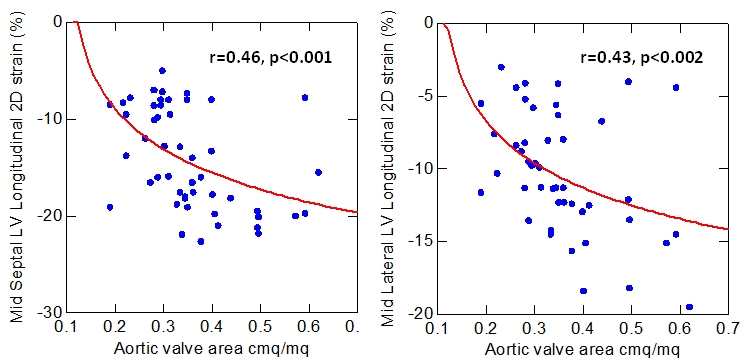
Baseline Correlation between LV longitudinal strain at septal and lateral level and aortic valve area.
Figure 3.
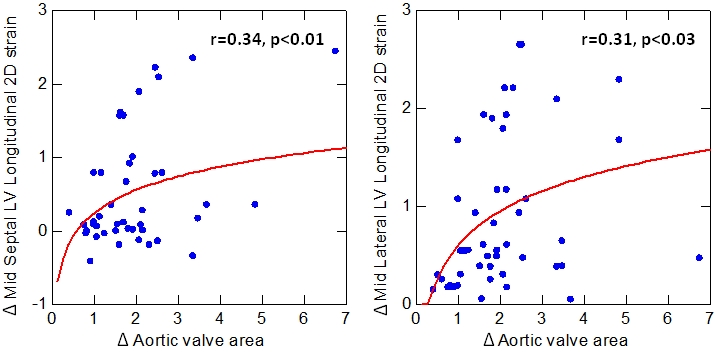
Correlation between the early improvement of LV longitudinal strain (baseline-72 h) both at septal and lateral level (Δ LV longitudinal strain) and the increase of aortic valve area (baseline-72 h) (Δ AVA).
Discussion
The main findings of the present study include: 1. A significant improvement of aortic valve function after the procedure as documented by the reduction of transprosthetic gradients whit the concomitant increase of AVA both early and follow-up; 2. A Significant improvement of systolic longitudinal 2 D strain early and after 3 months even if LVEF mildly improved only at three months follow up; 3. A Significant improvement of radial and circumferential 2D strain only after 3 months with a concomitant reduction of LVM; 4. The significant correlation between the improvement of LV longitudinal strain at septal level and the improvement of haemodynamic parameters early after the procedure.
The excessive pressure overload in AS causes an increase of systolic wall stress that determines a significant left ventricular concentric hypertrophy. In the evolution of AS, patients frequently develop global LV dysfunction evidenced by low EF. However, at the early stage of disease, when EF is still preserved, subclinical myocardial dysfunction can be detected in the form of myocytes hypertrophy and reactive interstitial fibrosis [31, 32]. In fact we found a significant correlation between baseline LV longitudinal strain at septal and lateral level and AVA (Figure 2).
Conventional echocardiography is an appropriate instrument to detect global LV dysfunction while tissue Doppler imaging, in particular strain and strain rate imaging, can better detect subtle systolic myocardial function damage before global LV dysfunction occurrence [2, 33].
Although aortic valve replacement has a favorable impact on LV remodeling with an immediate afterload decrease, in the present study LVEF showed a mild improvement only after three months follow (p<0.02). That might be correlated to the preserved baseline LV EF (51.2% ± 11.8). Doppler and strain imaging are preferred for the analysis of early subtle changes in systolic function after aortic valve replacement when EF is preserved [2, 3]. In fact, in our study longitudinal systolic strain shows an early significant improvement (confirmed even after 3 months follow up) after TAVI both at septum and lateral wall level even when the EF has not improved. Furthermore the early variation of longitudinal strain was significantly related to the increase of AVA (Figure 3).
Radial and circumferential systolic strain demonstrates a late significant improvement both with ESV and LVM reduction and EF improvement.
Our findings confirm that the hemodynamic improvement determined by TAVI induces early LV systolic functional improvement detected only by longitudinal 2D strain and late structural significant modification such as LV hypertrophy regression and LVEF improvement detected also by conventional echocardiography [3, 34, 35].
The actual possibility to analyze by 2D strain ventricular function in all three deformation components [36] (longitudinal, radial and circumferential) allows to discover the complex physiopathology modification induced by TAVI, with an acute afterload reduction [3].
Only after three months, we observed a significant improvement of radial and circumferential strain, in parallel to a significant decrease of E/ E' ratio, indirect expression of left ventricular end diastolic pressure. The chronic reduction of afterload determined by TAVI could further induce a significant reduction of LV mass, expressing the LV inverse remodeling [34].
In the present study, therefore, the early LV longitudinal strain improvement could represent a response to an acute reduction of afterload deriving from TAVI procedure; the late improvement both of radial and circumferential strain, instead, could be caused by the significant reduction of left ventricular hypertrophy and the concomitant decrease of collagen content.
The clinical relevance of our findings is that longitudinal 2D strain pre e post procedure allows selecting patients who manifest an improvement of myocardial deformability post procedure and those who do not show any improvement that, consequently, will surely need a further attention and therapeutic support.
Study limitations
The low number of patients and the short follow-up represent a limitation for the study together with the new methodology used. Furthermore since the analysis has been done on AS patients with normal EF or mildly reduced EF, it is even more difficult to assess early improvements of LV function based on the variation of conventional echocardiographic parameters.
The present data have been obtained by the implantation of one specific type of valve prosthesis (Corevalve). Even if we do not expect any difference, these data would need to be replicated with other types of valve prosthesis, such as Edwards-SAPIEN valve. Further studies are needed to confirm the results.
Conclusions
The 2D strain technology can early detect the subtle improvement of global and regional LV systolic function immediately after TAVI while all conventional echo parameters prove to be less effective for this purpose. Only after three months follow up, it is possible to observe a significant LVM reduction and a mild EF improvement.
Acknowledgments
We thank Dr Giovanna Lastrucci for editorial assistance.
References
- 1.Orsinelli DA, Aurigemma GP, Battista S, Krendel S, Gaasch WH. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis. A high risk subgroup identified by preoperative relative wall thickness. J Am Coll Cardiol. 1993;22:1679–1683. doi: 10.1016/0735-1097(93)90595-r. [DOI] [PubMed] [Google Scholar]
- 2.Iwahashi N, Nakatani S, Kanzaki H, Hasegawa T, Abe H, Kitakaze M. Acute improvement in myocardial function assessed by myocardial strain and strain rate after aortic valve replacement for aortic stenosis. J Am Soc Echocardiogr. 2006;19:1238–1244. doi: 10.1016/j.echo.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 3.Bauer F, Eltchaninoff H, Tron C, Lesault PF, Agatiello C, Nercolini D, Derumeaux G, Cribier A. Acute improvement in global and regional left ventricular systolic function after percutaneous heart valve implantation in patients with symptomatic aortic stenosis. Circulation. 2004;110:1473–1476. doi: 10.1161/01.CIR.0000134961.36773.D6. [DOI] [PubMed] [Google Scholar]
- 4.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue doppler echocardiography in patients with al (primary) cardiac amyloidosis. Circulation. 2003;107:2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 5.Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003;41:611–617. doi: 10.1016/s0735-1097(02)02869-3. [DOI] [PubMed] [Google Scholar]
- 6.Storaa C, Aberg P, Lind B, Brodin LA. Effect of angular error on tissue doppler velocities and strain. Echocardiography. 2003;20:581–587. doi: 10.1046/j.1540-8175.2003.01135.x. [DOI] [PubMed] [Google Scholar]
- 7.Langeland S, Wouters PF, Claus P, Leather HA, Bijnens B, Sutherland GR, Rademakers FE, D'Hooge J. Experimental assessment of a new research tool for the estimation of two-dimensional myocardial strain. Ultrasound Med Biol. 2006;32:1509–1513. doi: 10.1016/j.ultrasmedbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E, Abraham TP, Belohlavek M. Two-dimensional strain–a doppler-independent ultrasound method for quantitation of regional deformation: Validation in vitro and in vivo. J Am Soc Echocardiogr. 2005;18:1247–1253. doi: 10.1016/j.echo.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Becker M, Bilke E, Kuhl H, Katoh M, Kramann R, Franke A, Bucker A, Hanrath P, Hoffmann R. Analysis of myocardial deformation based on pixel tracking in two dimensional echocardiographic images enables quantitative assessment of regional left ventricular function. Heart. 2006;92:1102–1108. doi: 10.1136/hrt.2005.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vannan MA, Pedrizzetti G, Li P, Gurudevan S, Houle H, Main J, Jackson J, Nanda NC. Effect of cardiac resynchronization therapy on longitudinal and circumferential left ventricular mechanics by velocity vector imaging: Description and initial clinical application of a novel method using high-frame rate b-mode echocardiographic images. Echocardiography. 2005;22:826–830. doi: 10.1111/j.1540-8175.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- 12.Poulsen SH, Sogaard P, Nielsen-Kudsk JE, Egeblad H. Recovery of left ventricular systolic longitudinal strain after valve replacement in aortic stenosis and relation to natriuretic peptides. J Am Soc Echocardiogr. 2007;20:877–884. doi: 10.1016/j.echo.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, Sauren B, Mohr FW, Walther T, Zickmann B, Iversen S, Felderhoff T, Cartier R, Bonan R. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding corevalve prosthesis: Device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50:69–76. doi: 10.1016/j.jacc.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, Sinhal A, Carere RG, Munt B, Ricci D, Ye J, Cheung A, Lichtenstein SV. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116:755–763. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- 15.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A. Guidelines on the management of valvular heart disease: The task force on the management of valvular heart disease of the european society of cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 16.Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW. Percutaneous implantation of the corevalve self-expanding valve prosthesis in high-risk patients with aortic valve disease: The sieg-burg first-in-man study. Circulation. 2006;114:1616–1624. doi: 10.1161/CIRCULATIONAHA.106.639450. [DOI] [PubMed] [Google Scholar]
- 17.Chin D. Echocardiography for transcatheter aortic valve implantation. Eur J Echocardiogr. 2009;10:i21–29. doi: 10.1093/ejechocard/jen245. [DOI] [PubMed] [Google Scholar]
- 18.Tops LF, Wood DA, Delgado V, Schuijf JD, Mayo JR, Pasupati S, Lamers FP, van der Wall EE, Schalij MJ, Webb JG, Bax JJ. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2008;1:321–330. doi: 10.1016/j.jcmg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Leontyev S, Walther T, Borger MA, Lehmann S, Funkat AK, Rastan A, Kempfert J, Falk V, Mohr FW. Aortic valve replacement in octogenarians: Utility of risk stratification with euroscore. Ann Thorac Surg. 2009;87:1440–1445. doi: 10.1016/j.athoracsur.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 20.Berman M, Stamler A, Sahar G, Georghiou GP, Sharoni E, Brauner R, Medalion B, Vidne BA, Kogan A. Validation of the 2000 bernstein-parsonnet score versus the euroscore as a prognostic tool in cardiac surgery. Ann Thorac Surg. 2006;81:537–540. doi: 10.1016/j.athoracsur.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Petronio AS, De Carlo M, Bedogni F, Marzocchi A, Klugmann S, Maisano F, Ramondo A, Ussia GP, Ettori F, Poli A, Brambilla N, Saia F, De Marco F, Colombo A. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the corevalve revalving system. Circ Cardiovasc Interv. 2010;3:359–366. doi: 10.1161/CIRCINTERVENTIONS.109.930453. [DOI] [PubMed] [Google Scholar]
- 22.Behan M, Haworth P, Hutchinson N, Trivedi U, Laborde JC, Hildick-Smith D. Percutaneous aortic valve implants under sedation: Our initial experience. Catheter Cardiovasc Interv. 2008;72:1012–1015. doi: 10.1002/ccd.21777. [DOI] [PubMed] [Google Scholar]
- 23.Covello RD, Maj G, Landoni G, Maisano F, Michev I, Guarracino F, Alfieri O, Colombo A, Zangrillo A. Anesthetic management of percutaneous aortic valve implantation: Focus on challenges encountered and proposed solutions. J Cardiothorac Vasc Anesth. 2009;23:280–5. doi: 10.1053/j.jvca.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, Lam PK, Sanderson JE. Peak early diastolic mitral annulus velocity by tissue doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol. 2003;41:820–826. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 25.Giorgi D, Di Bello V, Pedrinelli R, Bertini A, Talini E, Dell'Omo G, Mengozzi G, Palagi C, Dell'Anna R, Mariani M. Ultrasonic tissue characterization and doppler tissue imaging in the analysis of left ventricular function in essential arterial hypertension: A preliminary study. Echocardiography. 2002;19:187–198. doi: 10.1046/j.1540-8175.2002.00187.x. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 27.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda T, Baba H, Akasaka T, Akiyama M, Neishi Y, Tomita J, Sukmawan R, Koyama Y, Watanabe N, Tamano S, Shinomura R, Komuro I, Yoshida K. Assessment of regional myocardial strain by a novel automated tracking system from digital image files. J Am Soc Echocardiogr. 2004;17:1234–1238. doi: 10.1016/j.echo.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Perk G, Tunick PA, Kronzon I. Non-doppler twodimensional strain imaging by echocardiography–from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234–243. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derumeaux G, Mulder P, Richard V, Chagraoui A, Nafeh C, Bauer F, Henry JP, Thuillez C. Tissue doppler imaging differentiates physiological from pathological pressure-overload left ventricular hypertrophy in rats. Circulation. 2002;105:1602–1608. doi: 10.1161/01.cir.0000012943.91101.d7. [DOI] [PubMed] [Google Scholar]
- 32.Di Bello V, Giorgi D, Viacava P, Enrica T, Nardi C, Palagi C, Grazia Delle Donne M, Verunelli F, Mariani MA, Grandjean J, Dell'Anna R, Di Cori A, Zucchelli G, Romano MF, Mariani M. Severe aortic stenosis and myocardial function: Diagnostic and prognostic usefulness of ultrasonic integrated backscatter analysis. Circulation. 2004;110:849–855. doi: 10.1161/01.CIR.0000138930.12773.41. [DOI] [PubMed] [Google Scholar]
- 33.Kowalski M, Herbots L, Weidemann F, Breithardt O, Strotmann J, Davidavicius G, D'Hooge J, Claus P, Bijnens B, Herregods MC, Sutherland GR. One-dimensional ultrasonic strain and strain rate imaging: A new approach to the quantitation of regional myocardial function in patients with aortic stenosis. Ultrasound Med Biol. 2003;29:1085–1092. doi: 10.1016/s0301-5629(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 34.Jilaihawi H, Jeilan M, Spyt T, Chin D, Logtens E, Kovac J. Early regression of left ventricular wall thickness following percutaneous aortic valve replacement with the corevalve bioprosthesis. J Invasive Cardiol. 2009;21:151–15. [PubMed] [Google Scholar]
- 35.Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O, Ramondo A, Ussia G, Wenaweser P, Windecker S, Laborde JC, de Jaegere P, Serruys PW. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 fr) corevalve revalving system: Results from the multicentre, expanded evaluation registry 1-year following ce mark approval. EuroIntervention. 2008;4:242–249. doi: 10.4244/eijv4i2a43. [DOI] [PubMed] [Google Scholar]
- 36.Abraham TP, Dimaano VL, Liang HY. Role of tissue doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]