Abstract
During trascatheter aortic valve implantation (TAVI) the native valve is not removed but crushed. Thus, a slight prosthesis insufficiency is not uncommon and has been reported in about 70% of patients for both available types of percutaneous valves. However, the definition of clinically “significant” valve regurgitation is not fully established yet. In most cases, aortic insufficiency is mild and clinical acceptable, however, severe insufficiency can occur. Paravalvular insufficiency is usually prevalent, and it may be the consequence of prosthesis/patient mismatch due to an undersizing of the implanted device or to an incomplete expansion of the prosthesis stent frame, or also to incorrect site of prosthesis implantation. Thus, an accurate assessment of the aortic valve annulus before TAVI is mandatory in order to select the optimal size of the valve. The presence of large calcium burden or bicuspid valve as well as the correct implantation of the device are other key determinants of final valve insufficiency. When severe regurgitation is present, an integration of hemodynamic, angiographic, transthoracic and TEE data is necessary to tailor the best clinical decision on a per-patient basis.
Keywords: Valvular leak, transcatheter aortic valve implantation
Introduction
A 77-year man with high-risk severe aortic stenosis (EuroSCORE 25.61%) was referred for Transcatheter Aortic Valve Implantation (TAVI). Aortic annulus was 24 mm at transesophageal echocardiogram; 24.4 mm (sagittal) and 25.7 mm (coronal) at angioTC assessment. Peripheral vessels were suitable for transfemoral approach. Thus a 26 mm transfemoral Edwards Sapien XT valve implantation was planned (Figure 1A). After valve deployment, a relevant aortic regurgitation was detected (Figure 1B). What further evaluation and treatment is indicated?
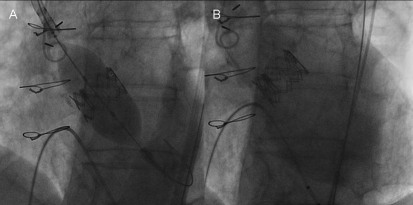
Figure 1.
PVL after TAVI. Figure shows a 26-mm transfemoral Edwards Sapien XT valve implantation (Panel A) and a severe valve regurgitation after prosthesis implantation (Panel B).
Epidemiology of Valvular Leak after TAVI
Percutaneous TAVI has extended our ability to treat patients with severe symptomatic aortic stenoses being no or poor candidates for open heart surgery [1]. At the present time two different devices are available, one self-expandable, porcine pericardial leaflets, and nitinol frame, and the other one balloon-expandable, bovine pericardial leaflets, and Cobalt-Chromium frame. Both devices have shown good performance once implanted, with transvalvular gradient and effective orifice area that are respectively lower and higher than that observed in surgical valves [2-8]. However, TAVI may result in severe complications, starting with vascular injuries, cerebral or peripheral embolisation, pericardial effusion, ventricular rupture, conduction abnormalities, aortic dissections and aortic regurgitation.
Regurgitation originating between the prosthetic sewing ring and the native valve annulus is a well-known phenomenon reported as paravalvular, perivalvular and peri/ or para prosthetic leaks. In surgical series, the occurrence of paravalvular leak (PVL) has been reported in very few cases, being 2% and 8% after a bioprosthe-sis or mechanical prosthesis implantation, respectively [9]. Thus, it does not represent a main issue in the context of surgical aortic valve replacement and is not addresses in the current guidelines for valvular heart disease [9-10].
In contrast to surgical aortic valve replacement, during TAVI procedure the native valve is not removed but crushed. Thus, a slight aortic insufficiency is not uncommon and has been reported in about 70% of patients for both available types of percutaneous valves [11-14]. On the other hand, a moderate regurgitation has been observed in up to 40% of the cases [11-12]. In most cases, aortic insufficiency is clinical acceptable, however, severe insufficiency can occur and although current reports mention treatment of such severe insufficiencies only very briefly [3, 7], the problem is not trivial because the underlying pathophysiology can be very different (see below). It should be also acknowledged that discrepancies in the prevalence of valve regurgitation after TAVI [2-7,15-16] are mainly due to the absence of standardized definition and protocols to detect (i.e angiography versus echocardiography) and score (qualitative or semiquantitative methods) the leak. Moreover the definition of clinically “significant” PVL is not fully established because of the lack of evidence exploring the pathophysiology of the valvular leak and its impact on outcome. More recently the Valve Academic Research Consortium provided a consensus aimed to standardize definitions on technical and clinical end-points in TAVI procedure [17]. Nevertheless, trying to estimate and quantify the valve regurgitation they adopted arbitrarily a standard classification that is commonly used to describe regurgitation in native valve.
Mechanisms of valvular leaks
The aortic insufficiency after TAVI might be classified taking into account the type of regurgitation, its mechanism, and the etiology (Table 1). The vast majority of valve regurgitation is due to PVL, intraprosthetic or combined leaks being 6% and 17%, respectively [18]. The underlying pathophysiology of the leaks can be very different as shown in Figure 2. Paravalvular insufficiency may derive from a prosthesis/patient mismatch due to an undersizing of the implanted device or to an incomplete expansion of the prosthesis stent frame, or also to incorrect site of prosthesis implantation (i.e too low or too high; Figure 2). On the other hand, intravalvular insufficiency might be due to the opening failure of the prosthesis' leaflets, to leaflets damage occurring during the crimping manoeuvre or during the implantation phase (i.e postdilatation) or again to a prosthesis/patient mismatch because of an incorrect sizing of the valve.
Table 1.
Aortic Regurgitation after TAVI
| Classification | Mechanism | Ethiology |
|---|---|---|
| Incorrect size | ||
| 1. Prosthesis/patient mismatch | Calcium | |
| Paravalvular (PVL) | 2. Incomplete valve adherence to aortic annulus | Bicuspid |
| 3. Low/high implantation of the valve | LVOT/Aorta angle | |
| Technique of implantation | ||
| Valvular | 1. Prosthesis/patient mismatch | Incorrect size |
| 2. Valvular damage | I o II valve dysfunction |
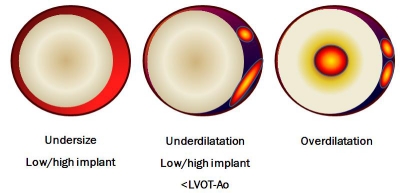
Figure 2.
Valvular leak mechanisms. Incomplete valve adherence is due to prosthesis-patient mismatch or to incorrect site of implantation, resulting in large paravalvular leak (left panel); asymmetrical coverage of native aortic root may be due to incomplete prosthesis expansion because of remaining material of the native valve (mid panel). In right panel is shown an intra-valvular leak secondary to inappropriate overexpansion of the valve.
Differently from surgical series, the direct sizing of native aortic annulus is not possible during TAVI, thus an accurate assessment of the latter is mandatory during the screening phase of the patient. It is noteworthy that the selection of the prosthesis that matches properly with the native aortic annulus is paramount to final procedural success, as the goal is to displace the native valve leaflets and deploy the device within the valve annulus. The native annulus should be identified with the virtual basal ring below the nadir of the aortic cusps, which provides the right plane of the annulus, that is not the anatomic ventriculo-aortic junction [19]. Different methods are used to assess aortic annulus measurement: echocardiogram (transthoracic or transesophageal), angiography, and computed tomography (Figure 3). There is not a gold standard for annulus measurement, and a multimodal assessment is strongly recommended [20], since the aortic annulus is not circular but might be ovoid in shape (Figure 4).
Figure 3.
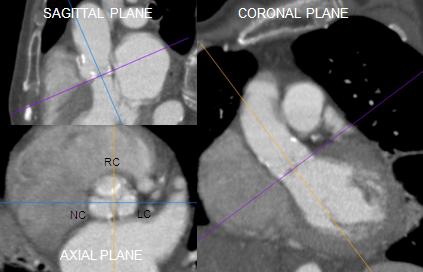
Pre-TAVI assessment. Annu-lus evaluation by using different imaging showing that significant inter-test disagreement might exist because different anatomical planes are explored by MDCT, TTE and TEE respectively . The combined assessment may reduce the chance for error and provide a more comprehensive definition of the annular size and shape in patients considered for TAVI.
Figure 4.
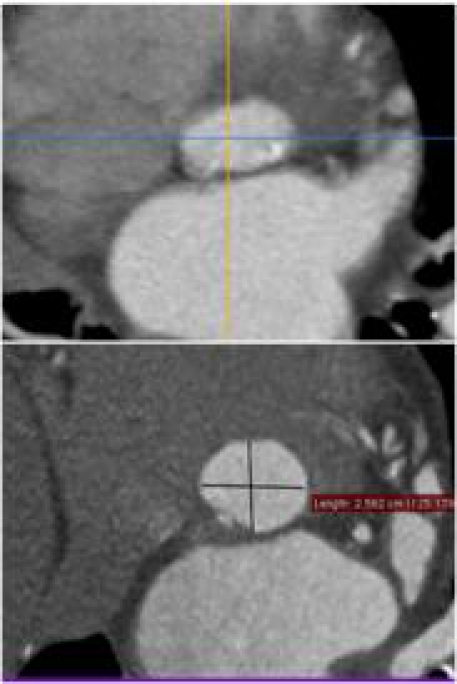
Aortic Valve and annulus morphology. Aortic annulus is a complex 3D structure. The annulus is often oval shaped, as has been observed by MDCT.
Tops et al. [21] reported that the annulus had an oval configuration in approximately 50% of patients evaluated for TAVI, with a mean difference between coronal and sagittal measurements of 3.0 ± 1.9 mm. An oval configuration of the annulus was also noted by Delgado et al. [22], who reported a significant difference between mean coronal (25.1 ± 2.4 mm) and sagittal (22.9 ± 2.0 mm) measurements in 53 patients with severe aortic stenosis. This oval geometry of the annulus has been previously underappreciated on imaging but has been well described in the surgical literature. There have been a number of published methods for measurement of the aortic annulus on MDCT, but it is important to consider that different techniques might explore different plane and thus provide different dimensions of the annulus. For instance, the sagittal diameter of annulus as assessed by CT relate to the annulus diameter assessed by transesophageal echo in long-axis view, on the other hand, the coronal CT diameter matchs with the measurements of the annulus assessed by angiography. The assumption of a circular geometry of the aortic annulus and the left ventricular outflow tract might lead to significant underestimation of their “true” cross-sectional area by use of two-dimensional assessment. Therefore multiple measurements in different planes are recommended. To this regard, the clinical use of 3D echocardiogram may improve pre-TAVI evaluation, when compared to 2D echo, by the assessment of the aortic annulus in 3 planes. Moreover, a balloon- based annular measurement is an adjunctive tool to select the proper valve size. In fact, intraoperative evaluation of aortic regurgitation during balloon inflation for valvuloplasty increases the accuracy of the optimal device size selection [23].
Although, the selection of the correct sizing of the device is of utmost importance to avoid valve insufficiency, it is not the only issue. The presences of large calcium burden or unidentified bicuspid valve as well as incorrect implantation of the device are other key determinants of final valve insufficiency. Moreover the presence of a bicuspid valve should be taken into consideration, because of its association to asymmetrical stent frame expansion [24]. To note, this condition is frequently observed also in the elderly, accounting for up to 38% of patients as reported in surgical series [25].
The incorrect expansion of the frame is the second more frequent mechanism of paravalvular insufficiency [24,26]. For that reason, the pre-TAVI assessment should consider the analysis of the calcium burden and distribution (Figure 5), Although the calcium provides an anchorage to the stent frame, it may also interfere with the complete and symmetrical frame expansion. Para-valvular insufficiency due to incomplete valve adherence to the aortic annulus because of remaining material of the native valve or a prosthesis-patient mismatch may be overcome by post-dilatation [13],which is normally discouraged or by implantation of a plug device [27-28] or in less severe cases by “faithful watching”. It has been reported in case also the possibility of a further expansion of the self-expanding Core-Valve during the days following the implantation resulting in a decrease of the regurgitation [29], but data are aneddoctical.
Figure 5.
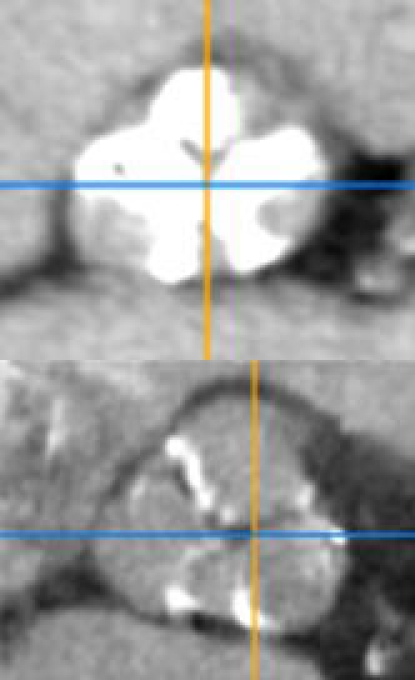
Two clinical examples of different calcium burden and distribution.
The third mechanism of paravalvular insufficiency is the misemployment of the valve in the aortic annulus, such as too-high or too-low implantation. To this regard, some differences between the two transcatheter valves should be highlighted. The implantation of the balloon-expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) provides a “one-shot” procedure, thus deserve careful evaluation of the device position before definite deployment. A proper rapid pacing during the deployment phase is necessary to ensure a steady and accurate device delivery, that may further with enhanced using a “two steps” inflation of the balloon for an improved control of the valve position. The “first step” consists in an incomplete inflation of the balloon expandable valve followed by an angiographic assessment of the proper position of the valve, and only then “second step” by a complete deployment of the device. On the other hand dedicated imaging assistance workstation, such as real-time an-giography-based C-THV (Paieon Inc, NY, USA), may help to optimize the device implantation, especially during the learning curve period.
On the opposite, the self-expandable Medtronic CoreValve device (Medtronic, Minneapolis, Minnesota, USA) can be released step-by-step, thus allowing, at least in theory, a more controlled release of the valve during its deployment. Indeed, also with this valve, the appropriate positioning might be challenging in particular anatomic subset such as the case of angulated ascending aorta (Figure 6). To this regard, the angle between the aortic root axis and left ventricular outflow tract, has been shown to be a major predictor of paravalvular leak after Core-Valve implantation, together with the depth of implantation [9]. Moreover, although minimal late repositioning or adjustment are possible in case of too low implantation of the valve by snaring and pulling the device upward [30,31], we should notice that this trick is technically difficult, and may also threaten the patient because of the risk of valve dislocation and stroke. To this regard, a para-valvular insufficiency due to too low implantation of both the types of valve might be succeeded by implantation of the second, the so-called “valve-in-valve”, that will be lower or higher according to the position of the first implanted valve. This procedure results frequently in a significant reduction of the valve insufficiency as reported by others [2,32].
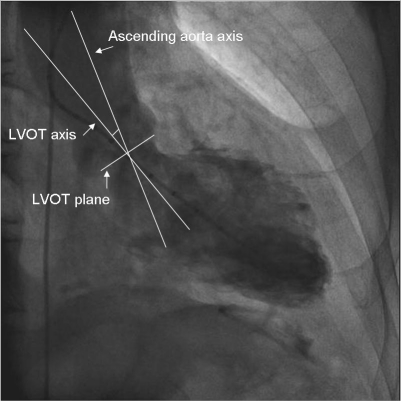
Figure 6.
Clinical example of evaluation of the angle between the ascending aorta axis and left ventricular outflow tract axis.
Clinical implications
Clinical implications of valvular leak are not fully established. Although, in most cases, aortic insufficiency is stable during follow-up [7,11, 33] and clinical acceptable, severe insufficiency can occur. More recently, some authors have shown that even less than severe valvular leak might be related to higher 1-year mortality after TAVI [7]. When severe regurgitation is present, a multimodality imaging for an accurate assessment of the underlying pathophysiology of the valve regurgitation is of utmost importance to select the adequate therapy.
The exact diagnosis may be troublesome because angiography gives only the severity of the insufficiency but not always the underlying pathophysiology, whereas echocardiography may be difficult due to interference of the metal of the prosthesis with the ultrasound as well as underestimation of the severity of aortic insufficiency in the case of paravalvular leaks. Therefore, an integration of all available data, that is, hemodynamic data, post-implantation angiography, auscultation, transthoracic, and TEE, as well as the clinical course is necessary to make a good judgement [10]. Finally, there will be a learning curve for exact judgement of post-implantation aortic insufficiency in TAVI.
Case discussion and conclusion
The cause of the severe regurgitation was a too low implantation leading to paravalvular leakage through the uncovered cell of the prosthesis, as assessed by transesophageal echo. The left ventricular end diastolic pressure was 22 mmHg and the pulse pressure 130/40 mmHg. In this case the regurgitation cannot be overcome by post-dilatation that we commonly discourage in case of balloon expandable prosthesis. Thus, a second valve was implanted into the first valve with a complete resolution of the leak (Figure 7A and B). This case shows the importance to determine, by multimodality approach, the degree and the pathophysiology of the aortic regurgitation after TAVI procedures to select the most adequate therapy and to avoid “oculo-regurgitation” reflex.
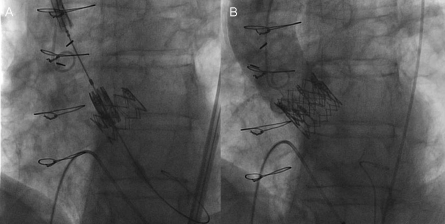
Figure 7.
Valve-in-Valve procedure to overcome a severe regurgitation after a “too low” valve implantation (Panel A). The Aortic angiography after second valve implantation confirmed the resolution of valve regurgitation (Panel B).
References
- 1.Vahanian A, Alfieri O, Al-Attar N, Antunes M, Bax J, Cormier B, Cribier A, De Jaegere P, Fournial G, Kappetein AP, Kovac J, Ludgate S, Maisano F, Moat N, Mohr F, Nataf P, Piérard L, Pomar JL, Schofer J, Tornos P, Tuzcu M, van Hout B, Von Segesser LK, Walther T, European Association of Cardio-Thoracic Surgery; European Society of Cardiology; European Association of Percutaneous Cardiovascular Interventions Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2008;29:1463–70. doi: 10.1093/eurheartj/ehn183. [DOI] [PubMed] [Google Scholar]
- 2.Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O. Thirty-Day Results of the SAPIEN aortic bio-prosthesis european outcome (SOURCE) Registry: a european registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62–69. doi: 10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 3.Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, Iung B, Donzeau-Gouge P, Tribouilloy C, Debrux JL, Pavie1 A, Gueret P, on behalf of the FRANCE Registry Investigators Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–7. doi: 10.1093/eurheartj/ehq261. [DOI] [PubMed] [Google Scholar]
- 4.Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, Hambrecht R, Sack S, Hauptmann KE, Richardt G, Figulla HL, Senges J, on behalf of the German Transcatheter Aortic Valve Interventions—Registry Investigators Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. 2011;32:198–204. doi: 10.1093/eurheartj/ehq339. [DOI] [PubMed] [Google Scholar]
- 5.Rodés-Cabau J, Webb JC, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochellière R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk. J Am Coll Cardiol. 2010;55:1080–90. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O, Ramondo A, Ussia G, Wenaweser P, Windecker S, Laborde JC, de Jaegere P, Serruys PW. Procedural and 30-day outcome following transcatheter aortic valve implantation using the third generation (18Fr) CoreValve Revalving System: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. Eurointervention. 2008;4:242–9. doi: 10.4244/eijv4i2a43. [DOI] [PubMed] [Google Scholar]
- 7.Tamburino C, Capodanno D, Ramando A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Malsano F, Marzocchi A, Poli A, Antoniucci D, Napodano M, De Carlo M, Fiorina C, Ussia GP. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 8.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewisw JS, Smith SC, Jr, Hiratzka LF, Hunt SA, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 Guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and Society of Thoracic Surgeons. Circulation. 2006;114:84. [Google Scholar]
- 9.Clavel MA, Webb JC, Pibarot P, Altwegg L, Dumont E, Thompson C, De Larochellière R, Doyle D, Masson, Bergeron S, Bertrand OF, Rodés-Cabau J. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol. 2009;53:1883–1891. doi: 10.1016/j.jacc.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 10.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–68. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 11.Sherif MA, Abdel-Wahab M, Stocker B, Geist V, Richardt D, Tolg R, Richardt G. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the Medtronic CoreValve bioprosthesis. J Am Coll Cardiol. 2010;56:1623–9. doi: 10.1016/j.jacc.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Detaint D, Lepage L, Himbert D, Brochet E, Messika-Zeitoun D, Iung B, Vahanian A. Determinants of significant paravalvular regurgitation after transcatheter aortic valve implantation: impact of device and annulus discongruence. J Am Coll Cardiol Intv. 2009;2:821–27. doi: 10.1016/j.jcin.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Grube E, Buellesfeld L, Mueller R, Sauren B, Zickmann B, Nair D, Beucher H, Felderhoff T, Iversen S, Gerckens U. Progress and Current Status of Percutaneous Aortic Valve Replacement: Results of Three Device Generations of the CoreValve Revalving System. Circulation Cardiovasc Interv. 2008;1:167–175. doi: 10.1161/CIRCINTERVENTIONS.108.819839. [DOI] [PubMed] [Google Scholar]
- 14.Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein S, Lee M, Masson JB, Thompson C, Moss R, Carere R, Munt B, Nietlispach F, Humphries K. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation. 2009;119:3009–16. doi: 10.1161/CIRCULATIONAHA.108.837807. [DOI] [PubMed] [Google Scholar]
- 15.Lefevre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schachinger V, De Bruyne B, Eltchaninoff H, Thielmann M, Himbert D, Romano M, Serruys P, Greinecker JW, on behalf of the PARTNER EU Investigator Group One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–157. doi: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godino C, Maisano F, Montorfano M, Latib A, Chieffo A, Michev I, Al-Lamee R, Bande M, Mussardo M, Arioli F, Ielasi A, Cioni M, Taramasso M, Arendar I, Grimaldi A, Spagnolo P, Zangrillo A, La Canna G, Alfieri O, Colombo A. Outcomes after transcatheter aortic valve implantation with both Edwards-SAPIEN and Core-Valve devices in a single center: the Milan experience. J Am Coll Cardiol Intv. 2010;3:1110–21. doi: 10.1016/j.jcin.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel MA, Petersen J, Popma JJ, Takkenberg JJ, Vahanian A, van Es GA, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32:205–17. doi: 10.1093/eurheartj/ehq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer F, Lemercier M, Zajarias A, Tron C, Eltchaninoff H, Cribier A. Immediate and long-term echocardiographic findings after transcatheter aortic valve implantation for the treatment of aortic stenosis: the Cribier-Edwards/ Edwards-Sapien valve experience. J Am Soc Echocardiogr. 2010;23:370–6. doi: 10.1016/j.echo.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RH. The surgical anatomy of the aortic root. MMCTS. doi: 10.1510/mmcts.2006.002527.
- 20.Messika-Zeitoun D, Serfaty JM, Brochet E, Ducrocq G, Lepage L, Detaint D, Hyafil F, Himbert D, Pasi N, Laissy JP, Iung B, Vahanian A. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol. 2010;55:186–94. doi: 10.1016/j.jacc.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 21.Tops LF, Wood DA, Delgado V, Schuijf JD, Mayo JR, Pasupati S, Lamers FP, van der Wall EE, Schalij MJ, Webb JG, Bax JJ. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. J Am Coll Cardiol Cardiovasc Imaging. 2008;1:321–30. doi: 10.1016/j.jcmg.2007.12.006. May. [DOI] [PubMed] [Google Scholar]
- 22.Delgado V, Ng AC, van de Veire NR, van der Kley F, Schuijf JD, Tops LF, de Weger A, Tavilla G, de Roos A, Kroft LJ, Schalij MJ, Bax JJ. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J. 2010;31:1114–23. doi: 10.1093/eurheartj/ehq018. [DOI] [PubMed] [Google Scholar]
- 23.Babaliaros VC, Junagadhwalla Z, Lerakis S, Thourani V, Liff D, Chen E, Vassiliades T, Chappell C, Gross N, Patel A, Howell S, Green JT, Veledar E, Guyton R, Block PC. Use of balloon aortic valvuloplasty to size the aortic annulus before implantation of a balloon-expandable transcatheter heart valve. J Am Coll Cardiol Intv. 2010;3:114–8. doi: 10.1016/j.jcin.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Zegdi R, Ciobotaru V, Noghin M, et al. Is it reasonable to treat all calcified stenotic valves with a valved stent? Results from a human anatomic study in adults. J Am Coll Cardiol. 2008;51:579–84. doi: 10.1016/j.jacc.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–5. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 26.Masson JB, Kovac J, Schuler G, Ye J, Cheung A, Kapadia S, Tuzcu ME, Kodali S, Leon MB, Webb JG. Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. J Am Coll Cardiol Intv. 2009;2:811–20. doi: 10.1016/j.jcin.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Hammerstingl C, Werner N, Nickenig G. Symptomatic paravalvular leakage after mechanical aortic valve replacement in a critically ill patient: why not just “plug” the hole? Eur J Echocardiogr. 2009;10:576–578. doi: 10.1093/ejechocard/jep018. [DOI] [PubMed] [Google Scholar]
- 28.Yaron Shapira, Rafael Hirsch, Ran Kornowski, David Hasdai, Abid Assali, Mordehay Vaturi, Horst Sievert, Ralph Hein, Alexander Battler, Alex Sagie. Percutaneous Closure of Perivalvu-lar Leaks with Amplatzer® Occluders: Feasibility, Safety and Short-Term Results. J Heart Valve Dis. 2007;16:305–313. [PubMed] [Google Scholar]
- 29.Grube E, Buellesfeld L, Mueller R, Sauren B, Zickmann B, Nair D, Beucher H, Felderhoff T, Iversen S, Gerckens U. Progress and Current Status of Percutaneous Aortic Valve Replacement: Results of Three Device Generations of the CoreValve Revalving System. Circ Cardiovasc Interv. 2008;1:167–175. doi: 10.1161/CIRCINTERVENTIONS.108.819839. [DOI] [PubMed] [Google Scholar]
- 30.Latib A, Michev I, Laborde JC, Montorfano M, Colombo A. Post-implantation repositioning of the CoreValve percutaneous aortic valve. J Am Coll Cardiol Intv. 2010;3:119–21. doi: 10.1016/j.jcin.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Ussia GP, Barbanti M, Immè S, Scarabelli M, Mulè M, Cammalleri V, Aruta P, Pistritto AM, Capodanno D, Deste W, Di Pasqua MC, Tamburino C. Management of implant failure during transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;76:440–9. doi: 10.1002/ccd.22595. [DOI] [PubMed] [Google Scholar]
- 32.Ussia GP, Barbanti M, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, Poli A, Napodano M, Tamburino C. The valve-in-valve technique for treatment of aortic bioprosthesis malposition an analysis of incidence and 1-year clinical outcomes from the italian CoreValve registry. J Am Coll Cardiol. 2011;57:1062–8. doi: 10.1016/j.jacc.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Rajani R, Kakad M, Khawaja MZ, Lee L, James R, Saha M, Hildick-Smith D. Paravalvular regurgitation one year after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;75:868–72. doi: 10.1002/ccd.22399. [DOI] [PubMed] [Google Scholar]