Abstract
Vascular integrity or the maintenance of blood vessel continuity is a fundamental process regulated, in part, by the endothelial glycocalyx and cell-cell junctions. Defects in endothelial barrier function are an initiating factor in several disease processes including atherosclerosis, ischemia/reperfusion, tumor angiogenesis, cancer metastasis, diabetes, sepsis and acute lung injury. The glycosaminoglycan, hyaluronan (HA), maintains vascular integrity through endothelial glycocalyx modulation, caveolin-enriched microdomain regulation and interaction with endothelial HA binding proteins. Certain disease states increase hyaluronidase activity and reactive oxygen species (ROS) generation which break down high molecular weight HA to low molecular weight fragments causing damage to the endothelial glycocalyx. Further, these HA fragments can activate specific HA binding proteins upregulated in vascular disease to promote actin cytoskeletal reorganization and inhibition of endothelial cell-cell contacts. This review focuses on the crucial role of HA in vascular integrity and how HA degradation promotes vascular barrier disruption.
Keywords: Endothelial permeability, glycocalyx, caveolin-enriched microdomain, actin cytoskeleton, CD44, HABP2, versican, TLR2, TLR4, caveolin-1
Introduction
Vascular integrity (i.e. the maintenance of blood vessel continuity) is required for normal cardiovascular homeostasis [1, 2]. Several mechanisms regulate basal vascular integrity including the endothelial glycocalyx, a meshwork of hyaluronan (HA), proteoglycans, glycolipids and proteins between the vascular luminal space and the endothelial cell (EC) surface, endothelial cell-cell junctions which are controlled by tight junctions, adherens junctions and caveolin-enriched microdomains (CEM) [1-9]. Certain pathologies induce degradation of the glycocalyx and disruption of EC-EC junctions causing leakage of fluids and proteins into the underlying tissue [1, 2, 10-15].
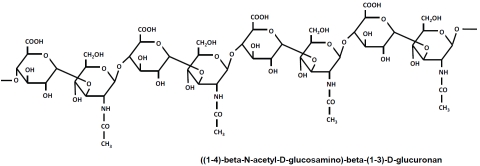
The major non-sulfated glycosaminoglycan in most tissues, hyaluronan (HA), plays a fundamental role in the maintenance of vascular integrity [4, 16-28]. HA is composed of a linear repeat of disaccharide units consisting of D-glucuronic acid and N-acetylglucosamine [19-22] (Figure 1). The major form of HA in vivo, high molecular weight HA (HMW-HA), has a molecular weight >1 million Da. HMW-HA exhibits a random coil structure that can expand in aqueous solutions [23-25]. Aqueous HMW-HA is highly viscous and elastic, properties which contribute to its filtering functions in the glycocalyx [26-28]. HA is a dynamic molecule with a high rate of metabolism. In humans, the turnover rate for HA is 5 grams per day of the 15 total grams in the body [29]. The majority of HA in the vasculature is incorporated into the endothelial glycocalyx and the extracellular matrix of the underlying tissue [17, 27, 28, 30]. The levels of soluble HA are low in normal human plasma due to rapid removal by the liver and kidneys [29].
Figure 1.
The Chemical Structure of hyaluronan (HA). HA is composed of linear repeating disaccharide units consisting of D-glucuronic acid and N-acetylglucosamine [19].
In EC, as in other cell types, HA is synthesized by hyaluronan synthases (HAS) [31]. The three main HAS (HAS1, HAS2 and HAS3) differ in the Km values for their substrates (D-glucuronic acid and N-acetylglucosamine) leading to differential rates of hyaluronan synthesis and secretion from the plasma membrane [32]. HAS1 and HAS2 produce HA with a molecular weight > 500 kDa and HAS3 produces < 500 kDa HA [31]. HAS2 deletion results in embryonic lethality due to cardiac developmental defects and vascular abnormalities, effects which are rescued by addition of exogenous HMW-HA [33,34].
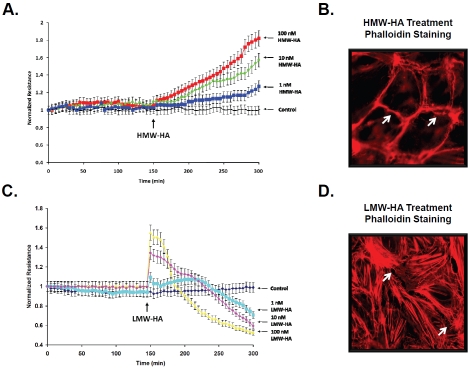
HA is degraded in certain disease states by hyaluronidases and ROS to produce lower molecular weight fragments (<500 kDa) (24). There are six hyaluronidase genes encoding HYAL-1,2,3,4, PHYAL1 (a pseudogene) and PH-20 [35,36]. HYAL enzymes have different cellular localization and optimal pH activity which can lead to generation of different sized HA fragments [35, 37-39]. Degradation of HA in the vasculature occurs in multiple pathological conditions [24, 39, 40]. Our laboratory has demonstrated that HMW-HA (∼1 million Da) promoted Rac1-dependent cortical actin formation and EC barrier enhancement while low molecular weight HA (LMW-HA, ∼2,500 Da) induced RhoA-dependent actin stress fiber formation and disruption of the EC barrier in vitro [18] (Figure 2). The differential mechanisms of HA's regulation of vascular integrity in normal and disease states are discussed below.
Figure 2.
Hyaiuronan Regulation of Endotheiiai Barrier Function and the Actin Cytoskeieton. HMW-HA (∼1 million Da) induces a dose-dependent increase in human pulmonary microvascuiar EC barrier function (A) and promotes cortical actin ring formation (B). The arrows indicate areas of cortical actin associated with EC contacts. In contrast, LMW-HA (∼2,500 Da) promotes a biphasic response resulting in EC barrier disruption (C) and actin stress fiber formation (D). The arrows indicate gap formations between ECs. This research was originally published in The Journal of Biological Chemistry (Singleton et al., J. Biol. Chem., 2006, 10;281(45):34381-93) © the American Society for Biochemistry and Molecular Biology [18].
HA regulation of the endothelial glycocalyx
The endothelial glycocalyx is a negatively charged “mesh” of membrane glycoproteins, proteoglycans and glycosaminoglycans (including HA) which is located on the luminal side of the endothelium in all blood vessels {8, 13, 15, 27, 41]. Endothelial glycocalyx thickness varies with vessel size and can range from 0.5 μm in capillaries up to 4.5 μm in the carotid arteries [8]. Newly synthesized HA may be incorporated in to the glycocalyx as it is extruded from the cell membrane and then bound by CD44 or other HA-binding proteins. There are several novel techniques currently utilized to determine the contribution of HA to the EC glycocalyx including fluorescent correlation spectroscopy and atomic force microscopy [14, 41-44]. The endothelial glycocalyx also incorporates serum proteins such as albumin, fibrinogen and extracellular superoxide dismutase [15]. The glycocalyx has a number of important vasculoprotective functions in vivo, including a) regulation of vascular permeability, b) modulation of leukocyte rolling and adhesion, c) transduction of shear stress leading to NO release and d) inhibition of coagulation [13, 14, 45-48].
Vascular permeability
The glycocalyx can be described as a molecular sieve along the capillary wall, with pore size dependent on the spacing between the glycocalyx fibers. Proteins, peptides and even lipids may penetrate this “sieve” to various degrees thereby establishing a dynamic equilibrium between components in the flowing blood and those retained within the glycocalyx. According to this model proposed by Adamson et al., an almost protein-free space should exist beneath the outer face of the glycocalyx next to the luminal surface of the endothelial cell as plasma is forced outwards hydrostatically, but proteins are retained or excluded from the glycocalyx [49]. Because the fluid passing thorough the glycocalyx is therefore extremely low in protein, an inwardly directed oncotic gradient will be generated across the glycocalyx limiting net out-flow of filtrate towards the interstitial space [15, 50-52]. Degradation of the coronary glycocalyx with hyaluronidase leads to myocardial edema in perfused rat hearts [53]. Gao and Lipowsky also investigated the effects of hyaluronidase treatment on glycocalyx permeability in post-capillary venules in the rat [27]. They reported that although heparinase, chondroitinase and hyaluronidase treatment all decreased the thickness of the glycocalyx, only treatment with hyaluronidase and chrondroitinase increased the diffusion of FITC to the sublayer of the glycocalyx [27]. This indicates that HA and chrondroitin (CN) contribute a significantly greater amount to glycocalyx permeability than HS, and may indicate HS is more concentrated in the upper portion of the glycocalyx while HA and CN contribute to a denser sublayer adjacent to the EC.
Leukocyte rolling and adhesion
The dimensions of the glycocalyx are such that it can sterically hinder firm attachment of leukocytes and platelets to the endotheliai cells [52, 54-56]. Disruption or shedding of the glycocalyx leads to increased leukocyte adhesion [56, 57]. Inhibition of hyaluronan synthesis using 4-methylumbelliferone (4-MU) in the ApoE deficient mouse led to decreased glycocalyx formation and increased adhesion of leukocytes in the carotid artery which ultimately led to increased artherosclerosis [58]. HA fragments, which may be released with glycocalyx disruption, act as pro-inflammatory molecules [59].
Shear stress
HA is an important factor in mechanosensing and mediating nitric oxide (NO) release within the endothelium [60-62]. Mochizuki et al., compared NO levels in isolated canine femoral arteries before and after hyaluronidase perfusion to degrade the HA component of the glycocalyx [60]. The NO production rate increased linearly with perfusion rate before enzyme treatment; and they observed a significant decrease in the rate of NO production following hyaluronidase treatment (0.084 to 0.009 nmol/ml) [60]. However acetylcholine-induced NO production was unaffected [63]. A later study by Pahakis et al., reported that removal of hyaluronan, heparan sulfate or sialic acid but not chondroitin sulfate could individually block shear induced NO production in primary bovine endothelial cells [61]. However in vitro studies using human vascular umbilical endothelial cells demonstrated that shear stress leads to an increase in hyaluronan (but not heparan sulfate) incorporation into the glycocalyx and the media of cultured cells [30]. Taken together, these studies would seem to indicate that hyaluronan is a key component in transducing shear stress leading to NO production in the endothelium.
Inhibition of coagulation
Closely linked to its role in leukocyte adhesion and shear stress mechanosensing is the role of the glycocalyx in inhibiting coagulation. The glycocalyx harbors a wide range of proteins involved in coagulation, fibrinolysis and haemostasis including antithrombin III, thrombo-modulin and tissue factor pathway inhibitor which all help to maintain an anti-thrombotic environment [8]. However, damage to the glycocalyx results in a loss of these factors (and also exposes the endothelium to platelet adhesion) and is believed to be a first step in the development of a pro-thrombotic environment [8].
Glycocalyx recovery after injury
Although there is currently no data available on rates of glycocalyx recovery in humans, a study using mouse models following hyaluronidase or TNF-α treatment indicates that it can take up to seven days for full reconstitution of the glycocalyx to occur [42]. Mulivor and Lipowsky have demonstrated that an infusion of pertussis toxin, which inhibits G-protein stimulated shedding of glycosaminoglycan chains could significantly attenuate glycocalyx loss in response to either ischemia/reperfusion or N-formylmethionyl-leucyl-phenylalanine (fMLP) administration [12]. A number of studies have used a perfusion of exogenous HMW-HA to restore the glycocalyx following degradation with by hyaluronidase [28] or in response to ischemia/reperfusion injury [64]. In contrast, HA degradation products can induce ROS production (and vice versa), a crucial factor in glycocalyx degradation in numerous vascular disease processes [40, 64, 65]. Perhaps most promising from a clinical standpoint, Nieuwdrop et al., have used an infusion of the antioxidant NAC to protect again hyperglycemic induced glycocalyx shedding, which further indicates a role for ROS in glycocalyx disruption [14].
HA regulation of endothelial caveolin-enriched microdomain dynamics
In endothelial cells, as in many other cell types, there are specialized cholesterol- and sphingolipid/glycolipid-enriched microdomains called lipid rafts which have been implicated in numerous cellular functions [66-69]. EC contain a subset of lipid rafts termed caveolin-enriched micro-domains (CEM) which are 50 to 100 nm plasma membrane microdomains containing the scaffolding protein, caveolin-1 [6, 18, 69, 70]. We and others have demonstrated that CEM are important regulators of vascular integrity. Caveolin-1 knockout mice do not have CEM (caveolae) formation in EC and exhibit microvascular hyper-permeability [71-74].
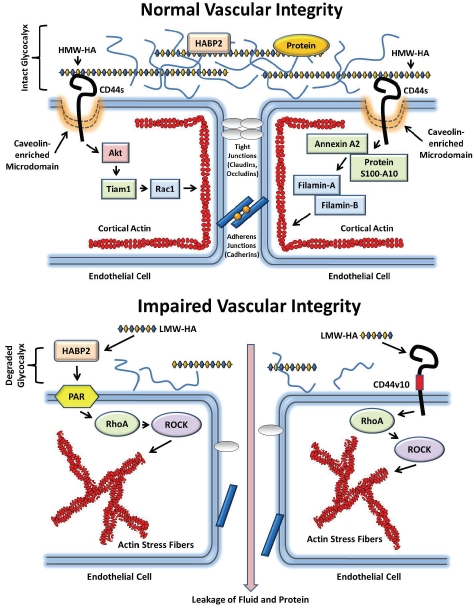
CEM are crucial for HA regulation of vascular integrity [4, 18]. Our previous published data indicate that HMW-HA recruited CEM containing the HA binding protein, CD44, and actin cytoskeletal regulatory proteins to areas of EC-EC contact [4, 18] (Figure 3). Abolishing CEM formation by cholesterol depletion (MβCD) or silencing caveolin-1 expression blocked HMW-HA-mediated human pulmonary microvascular EC Rac1 activation, cortical actin formation and barrier enhancement in vitro [18]. In addition, we observed that HMW-HA protection from LPS-induced pulmonary vascular hyper-permeability was blocked in the caveolin-1 knockout mouse [4]. These data indicate the crucial regulatory role of caveolin-1 and CEM in HMW-HA-mediated enhancement of vascular integrity.
Figure 3.
Hyaluronan Regulation of Normal and Impaired Vascular Integrity. High molecular weight hyaluronan (HMW-HA), the major non-sulfated glycosaminoglycan in the body, maintains vascular integrity through endothelial glycoca-lyx modulation, caveolin-enriched microdomain (CEM) regulation and interaction with endothelial HA binding proteins (upper panel). In the glycocalyx, HMW-HA interacts with proteoglycans (including versican) and glycoproteins to form a negatively charged “mesh” located on the luminal side of the endothelium in all blood vessels [8]. This glycocalyx regulates vascular permeability and incorporates serum proteins such as albumin, fibrinogen and extracellular super-oxide dismutase [15]. HMW-HA binds to and inhibits the EC barrier disrupting activity of the extracellular serine protease HABP2 [130]. In addition, HMW-HA binds to the transmembrane receptor, CD44s (standard form), in CEM which results in Akt-mediated Tiaml activation and Racl-GTP formation Ieadingto cortical actin formation and strengthening of EC-EC contacts [18]. Further, HMW-HA recruits several other actin regulatory proteins to CEM including annexin A2, protein S100-A10, filamin-A and filamin-B which enhance cortical actin formation and vascular integrity [4]. In disease states such as atherosclerosis, ischemia/reperfusion, tumor angiogenesis, cancer metastasis, diabetes, sepsis and acute lung injury, there is impaired vascular integrity (lower panel). Damage to the endothelium generates reactive oxygen species (ROS) and hyaiuronidase activation lead to generation of low molecular weight HA fragments (LMW-HA) [17, 28, 100]. In addition to CD44vlO (variant 10) ligation, LMW-HA binds to and activates HABP2 which induces protease-activated receptor (PAR) activation in EC [18. 130]. These events promote RhoA-GTP formation and stimulation of rho kinase (ROCK) activity Ieadingto actin stress fiber formation and EC barrier disruption [18, 130]. Disruption of the endothelium promotes leakage of fluid and protein into the underlying tissue [1, 2, 6, 106, 134].
HA involvement in vascular disease
In certain vascular disease states, HMW-HA is degraded by hyaluronidases and ROS to low molecular weight fragments [20]. The differential activities of HMW-HA and its degradation products on vascular integrity are due to changes in endothelial glycocalyx dynamics, regulation of EC-EC contacts and CEM dynamics and the relative expression of specific HA-binding proteins in normal and disease states which are discussed below.
Atherosclerosis
The initiating step in atherosclerosis is EC barrier dysfunction followed by accumulation of low density lipoproteins, cholesterol and monocytes to form a plaque [75-77]. Subsequently, there is augmented EC barrier dysfunction and vascular smooth muscle cell proliferation eventually leading to plaque rupture and thrombosis [78].
HA regulates EC barrier function and atherosclerosis [18, 58, 79, 80]. Nagy et al., demonstrated that inhibition of HA synthesis with 4-methylumbelliferone (4-MU) in pro-atherosclerotic (apoE knockout) mice resulted in glycocalyx damage and accelerated atherosclerosis [58].
The main receptor for HA, CD44, and the HA-binding proteoglycan, versican, are upregulated in atherosclerotic lesions [79, 81, 82]. When CD44 knockout mice are bred with apoE knockout mice, there is a 50-70% reduction in aortic lesions [82]. Since CD44 is expressed in multiple cell types, the role of endothelial CD44 in HA regulation of vascular integrity with atherosclerosis remains to be determined.
Ischemia/Reperfusion
Ischemia/reperfusion injury leads to tissue damage caused when a blood supply is returned to a tissue/organ after a period of disrupted blood flow (ischemia) [83-85]. Reperfusion of previously ischemic tissue induces reactive oxygen species (ROS) production, activates inflammatory and blood coagulation cascade responses and increases microvascular permeability [84]. Ischemia/reperfusion is important in numerous processes including cardiac arrest, stroke and organ transplantation [84].
Ischemia/reperfusion injury can stimulate shedding of the glycocalyx [12]. Although the exact mechanism triggering ischemia/reperfusion glycocalyx disruption is unknown, it is believed that increased free radical production along with TNF-α and mast cell degranulation may combine to induce shedding and enzymatic degradation of the glycocalyx [10, 51, 52, 86]. In addition, HA and CD44 expression are upregulated in the microvascular endothelium during ischemia/reperfusion [87, 88].
Toll-like receptors (TLR) have been implicated in the pathology observed in ischemia/reperfusion [89-93]. TLR2 and TLR4, expressed in many cell types including EC, can bind hyaluronan fragments [20, 94]. Zanotti et al., demonstrated that treatment of human pulmonary microvascular EC monolayers with a competitive TLR4 inhibitor protected against simulated ischemia/reperfusion-induced actin cytoskeletal reorganization and gap formation [95]. However, the role of TLRs in HA regulation of vascular integrity during ischemia/reperfusion remains to be determined.
Diabetes
Diabetes refers to a group of metabolic diseases involving hyperglycemia, either caused by insufficient insulin production (Type I diabetes) or because of decreased cellular responses to insulin referred to as insulin resistance (Type 2 diabetes) [96, 97]. Hyperglycemia damages vascular integrity through direct and indirect effects resulting in EC barrier disruption [45, 46, 97-99].
Hyperglycemia has also been shown to stimulate shedding of the glycocalyx 45, 46, 100]. Increased levels of plasma HA and decreased glycocalyx volume were observed in healthy volunteers following a 6 hour glucose infusion [45]. Increased plasma concentrations of HA and hyaluronidase have also been detected in type 1 diabetes patients compared to matched controls [80, 100]. In addition, administration of HMW-HA reduced the pathology observed in diabetic (Cg-m+/+Lepr(db)) mice [101].
CD44 (HA-binding receptor) plays a substantial role in diabetes. In animal models of diabetes, CD44 expression is increased [101, 102]. In the transfer model of NOD mice (Type 1 diabetes model), treatment of mice with CD44 antibodies or hyaluronidase induced resistance to insulin-dependent diabetes mellitus (IDDM) [103, 104].
Sepsis
Sepsis refers to a systemic microbial infection characterized by inflammation, activation of the blood coagulation cascade, blood stagnation and clot formation leading to hypoxia and organ failure [11, 105]. Recently, defects in EC barrier function have been suggested as a causative factor in sepsis pathology [106].
Systemic inflammatory response syndrome (SIRS) and sepsis promote damage to the glycocalyx resulting in increased circulating levels of HA, increased inflammatory response and interstitial edema [107]. Increased levels of glycocalyx components in the blood positively correlated with mortality in these conditions [11, 107].
Toll-like receptors (TLR) are crucial in the host response to sepsis [108-112]. TLR sense exogenous and endogenous danger-associated molecular motifs called pathogen-associated molecular patterns (PAMPs) [113-115]. EC mainly express TLR2 and TLR4 [116, 117]. Besides bacterial lipoprotein and lipopolysaccharide, TLR2/4 can bind to a variety of other PAMPs including hyaluronan fragments [20, 94]. Interestingly, Muto et al., 2009 demonstrated that 200-500 kDa HA and CD44 suppress TLR4-mediated septic responses in mice [118].
Acute lung injury
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are the leading causes of death in pediatric and adult critical care patients with a mortality rate of ∼40% [119, 120]. An important feature of ALI is endothelial barrier disruption resulting in leakage of fluids, proteins and inflammatory cells into alveoli with consequent pulmonary edema [121].
The main receptor for HA in EC, CD44, is a type 1 transmembrane glycoprotein that undergoes alternative exon splicing between exons 5 and 15 leading to a tandem insertion of one or more variant exons (v1-v10, or exons 6 through exons 14) within the membrane proximal region of the extracellular domain [122, 123]. We have demonstrated that human pulmonary microvascular EC express the CD44 isoforms, CD44s (standard form) and CD44v10 [18]. In vitro models of pulmonary EC barrier function indicate that HMW-HA (∼1 million Da) activates CD44s signaling and promotes barrier enhancement through its interaction with the S1P1 receptor and activation of Rac1 signaling leading to cortical actin formation while HA fragments (∼2.5 KDa) activate CD44v10 signaling and induce barrier disruption via the S1P3 receptor and RhoA-mediated actin stress fiber formation [18] (Figure 3).
We have demonstrated that targeted deletion of CD44 in the mouse pulmonary vasculature increases basal leakiness in the lungs [4]. Further, intravenous administration of HMW-HA protects against pulmonary vascular leakiness in a CD44- and caveolin-1 dependent manner in a mouse model of LPS-induced ALI [4].
Lipopolysaccharide (LPS) is a potent endotoxin from Gram-negative bacteria that, when administered intratracheally, produces an inflammatory reaction which includes disruption of the EC barrier and consequent leakage of fluid, protein and immune cells into lung airspaces [16, 124, 125]. Recently, it has been demonstrated by our laboratory and others that CD44 knockout mice have increased BAL protein and HA concentration and exaggerated inflammatory cell recruitment of both macrophages and neutrophils with LPS-induced lung injury [6, 126]. CD44 knockout mice also have increased NF-κB nuclear translocation and cytokine production [126]. These data suggest that CD44 limits the in vivo response to LPS and prevents excessive tissue damage.
Toll-like receptor 4 (TLR4) is expressed in multiple cell types including EC and can bind hyaluronan fragments in addition to other ligands including LPS [94, 124]. Inhibition of TLR4 in animal models and TLR4 loss-of-function mutations in humans protect against LPS-induced lung injury [111, 127, 128] Interestingly, CD44 deficient mice have decreased expression of negative regulators of TLR including IL-1R-associated kinase M (IRAK-M), Toll-interacting protein (Tollip) and TNFα-induced protein 3 (A20) [129].
Although mainly produced in the liver, we and others have demonstrated that the pulmonary endothelium expresses the extracellular HA-binding serine protease, HABP2, which is upregulated with lung injury [130-132]. HABP2 promotes LPS- and LMW-HA-mediated human pulmonary endothelial cell barrier disruption through a mechanism that involves protease-activated receptors (PAR) [130]. Conversely, HMW-HA inhibits HABP2 activation (Figure 3). We determined the contribution of vascular HABP2 to lung injury in mice by inhibiting HABP2 through intravenous administration of HABP2 siRNA and observed attenuation of LPS-induced acute lung injury [130]. In addition, vascular inhibition of HABP2 expression attenuates another mouse model of lung injury with pulmonary vascular hyper-permeability, ventilator-induced lung injury, demonstrating an important role of HABP2 in the regulation of vascular integrity [130].
Concluding remarks
Hyaluronan (HA) plays a crucial role in the maintenance and enhancement of vascular integrity. HA maintains vascular integrity through endothelial glycocalyx modulation, caveolin-enriched microdomain regulation and interaction with endothelial HA binding proteins [4, 18, 28, 130]. Defects in vascular integrity are a causative factor in several disease processes including atherosclerosis, ischemia/reperfusion, tumor angiogenesis, cancer metastasis, diabetes, sepsis and acute lung injury [1, 2, 106]. In disease states such as diabetes and sepsis, increase hyaluronidase activity and reactive oxygen species (ROS) generation which break down HMW-HA to LMW fragments causing damage to the endothelial glycocalyx [17, 20, 80, 107]. HA fragments can activate specific HA binding proteins upregulated in vascular disease including CD44, HABP2, TLR2 and TLR4 to promote actin cytoskeletal reorganization and inhibition of endothelial cell-cell contacts [18, 20, 94, 130, 133](Figure 3). CD44 also regulates the EC barrier-enhancing ability of other agents including hepatocyte growth factor (HGF) [6]. In addition to EC, vascular integrity can be regulated by other cell types including vascular smooth muscle cells and pericytes which are beyond the scope of this review. Further, disruption of vascular integrity is crucial for other disease processes including tumor angiogenesis and cancer metastasis. HA plays an important role in these processes and is described elsewhere [122, 134-141]. The ability of exogenously administered HMW-HA to restore damaged glycocalyx function and enhance EC barrier integrity make it a novel potential therapeutic strategy for diseases with defects in vascular integrity [4, 17, 142, 143].
Acknowledgments
Dr. Patrick A. Singleton was supported in part by the American Heart Association National Scientist Development Grant 0730277N, the American Lung Association National Biomedical Research Grant RG-75229-N and NIH NHLBI grant R01-HL 095723.
Disclosures
Dr. Lennon has no conflict of interest. Dr. Singleton is a provisional patent holder involving applications of hyaluronan with the University of Chicago and has not received any financial gain.
References
- 1.Murakami M, Simons M. Regulation of vascular integrity. J Mol Med. 2009;87:571–582. doi: 10.1007/s00109-009-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20 II-3-10. [PubMed] [Google Scholar]
- 4.Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lenno FE, Moreno-Vinasco L, Garcia JG. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol. 2010;299:L639–651. doi: 10.1152/ajplung.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singleton PA, Moreno-Vinasco L, Sammani S, Wanderling SL, Moss J, Garcia JG. Attenuation of Vascular Permeability by Methylnaltrexone: Role of mOP-R and S1P3 Transactivation. Am J Respir Cell Mol Biol. 2007;37:222–231. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- 6.Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JG. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J Biol Chem. 2007;282:30643–30657. doi: 10.1074/jbc.M702573200. [DOI] [PubMed] [Google Scholar]
- 7.Tesfamariam B, DeFelice AF. Endothelial injury in the initiation and progression of vascular disorders. Vascul Pharmacol. 2007;46:229–237. doi: 10.1016/j.vph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv: Euro J Physiol. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1179–1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 10.Bruegger D, Rehm M, Abicht J, Paul JO, Stoeckelhuber M, Pfirrmann M, Reichart B, Becker BF, Christ F. Shedding of the endothelial glycocalyx during cardiac surgery: onpump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2009;138:1445–1447. doi: 10.1016/j.jtcvs.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 11.Henrich M, Gruss M, Weigand MA. Sepsisinduced degradation of endothelial glycocalix. ScientificWorldJournal. 2010;10:917–923. doi: 10.1100/tsw.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulivor AW, Lipowsky HH. Inflammationand ischemia-induced shedding of venular glycocalyx. American journal of physiology. Heart and circulatory physiology. 2004;286:H1672–1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 13.Nieuwdorp M, Meuwese MC, Vink H, Hoekstra JB, Kastelein JJ, Stroes ES. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr Opin Lipidol. 2005;16:507–511. doi: 10.1097/01.mol.0000181325.08926.9c. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg BM, Nieuwdorp M, Stroes ES, Vink H. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep. 2006;58(Suppl):75–80. [PubMed] [Google Scholar]
- 15.Van Teeffelen JW, Brands J, Stroes ES, Vink H. 2007 Endothelial glycocalyx: sweet shield of blood vessels. Trends in Cardiovasc Med. 2007;17:101–105. doi: 10.1016/j.tcm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Bannerman DD, Goldblum SE. Direct effects of endotoxin on the endothelium: barrier function and injury. Lab Invest. 1999;79:1181–1199. [PubMed] [Google Scholar]
- 17.Wheeler-Jones CP, Farrar CE, Pitsillides AA. Targeting hyaluronan of the endothelial glycocalyx for therapeutic intervention. Curr Opin Investig Drugs. 2010;11:997–1006. [PubMed] [Google Scholar]
- 18.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 19.Almond A. Hyaluronan. Cell Mol Life Sci. 2007;64:1591–1596. doi: 10.1007/s00018-007-7032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olczyk P, Komosinska-Vassev K, Winsz-Szczotka K, Kuznik-Trocha K, Olczyk K. [Hyaluronan: structure, metabolism, functions, and role in wound healing] Postepy Hig Med Dosw (Online) 2008;62:651–659. [PubMed] [Google Scholar]
- 22.Wang A, de la Motte C, Lauer M, Hascall V. Hyaluronan matrices in pathobiological processes. Febs J. 2011 doi: 10.1111/j.1742-4658.2011.08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott JE, Heatley F. Biological properties of hyaluronan in aqueous solution are controlled and sequestered by reversible tertiary structures, defined by NMR spectroscopy. Biomacromolecules. 2002;3:547–553. doi: 10.1021/bm010170j. [DOI] [PubMed] [Google Scholar]
- 24.Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921–1943. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 25.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 26.Furlan S, La Penna G, Perico A, Cesaro A. Hyaluronan chain conformation and dynamics. Carbohydr Res. 2005;340:959–970. doi: 10.1016/j.carres.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. doi: 10.1016/j.mvr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Amer J Physiology. 1999;277:H508–514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 29.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 30.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–452. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 31.Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem. 2007;282:36777–36781. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 32.Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB 2002; Life. 54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 33.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronanaugmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 34.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern R, Kogan G, Jedrzejas MJ, Soltes L. The many ways to cleave hyaluronan. Biotechnol Adv. 2007;25:537–557. doi: 10.1016/j.biotechadv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Hofinger ES, Hoechstetter J, Oettl M, Bernhardt G, Buschauer A. Isoenzyme-specific differences in the degradation of hyaluronic acid by mammalian-type hyaluronidases. Gly coconj J. 2008;25:101–109. doi: 10.1007/s10719-007-9058-8. [DOI] [PubMed] [Google Scholar]
- 37.Akca H, Tani M, Hishida T, Matsumoto S, Yokota J. Activation of the AKT and STAT3 pathways and prolonged survival by a mutant EGFR in human lung cancer cells. Lung Cancer. 2006;54:25–33. doi: 10.1016/j.lungcan.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Stern R. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology. 2003;13:105R–115R. doi: 10.1093/glycob/cwg112. [DOI] [PubMed] [Google Scholar]
- 39.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwdorp M, Meuwese MC, Mooij HL, Ince C, Broekhuizen LN, Kastelein JJ, Stroes ES, Vink H. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J Applied Physiol. 2008;104:845–852. doi: 10.1152/japplphysiol.00440.2007. [DOI] [PubMed] [Google Scholar]
- 42.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res. 2009;104:1318–1325. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens AP, Hlady V, Dull RO. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Amer J Physiol Lung Cell Mol Physiol. 2007;293:L328–335. doi: 10.1152/ajplung.00390.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Callaghan R, Job KM, Dull RO, Hlady V. Stiffness and heterogeneity of the pulmonary endothelial glycocalyx measured by atomic force microscopy. Amer J Physiol Lung Cell Mol Physiol. 2011 doi: 10.1152/ajplung.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes E S, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 46.Perrin RM, Harper SJ, Bates DO. A role for the endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem Biophys. 2007;49:65–72. doi: 10.1007/s12013-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chappell D, Jacob M, Hofmann-Kiefer K, Rehm M, Welsch U, Conzen P, Becker BF. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res. 2009;83:388–396. doi: 10.1093/cvr/cvp097. [DOI] [PubMed] [Google Scholar]
- 48.Drake-Holland AJ, Noble MI. The important new drug target in cardiovascular medicine–the vascular glycocalyx. Cardiovasc & Dematol Disord Drug Targets. 2009;9:118–123. doi: 10.2174/187152909788488708. [DOI] [PubMed] [Google Scholar]
- 49.Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557:889–907. doi: 10.1113/jphysiol.2003.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curry FR. Microvascular solute and water transport. Microcirculation. 2005;12:17–31. doi: 10.1080/10739680590894993. [DOI] [PubMed] [Google Scholar]
- 51.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 52.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 53.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 54.Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34:133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 55.Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 56.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282–1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 57.Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for plateletendothelial cell adhesion. Circulation. 2000;101:1500–1502. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 58.Nagy N, Freudenberger T, Melchior-Becker A, Rock K, Ter Braak M, Jastrow H, Kinzig M, Lucke S, Suvorava T, Kojda G, Weber AA, Sorgel F, Levkau B, Ergun S, Fischer JW. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation. 2010;122:2313–2322. doi: 10.1161/CIRCULATIONAHA.110.972653. [DOI] [PubMed] [Google Scholar]
- 59.Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 60.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shearinduced endothelium-derived nitric oxide release. Amer J Physiol Heart Circ Physiol. 2003;285:H722–726. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 61.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumagai R, Lu X, Kassab GS. Role of glycocalyx in flow-induced production of nitric oxide and reactive oxygen species. Free Rad Biol & Med. 2009;47:600–607. doi: 10.1016/j.freeradbiomed.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Endo A, Nagashima K, Kurose H, Mochizuki S, Matsuda M, Mochizuki N. Sphingosine 1-phosphate induces membrane ruffling and increases motility of human umbilical vein endothelial cells via vascular endothelial growth factor receptor and CrkII. J Biol Chem. 2002;277:23747–23754. doi: 10.1074/jbc.M111794200. [DOI] [PubMed] [Google Scholar]
- 64.Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Amer Physiol Heart Circ Physiol. 2006;290:H2247–2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 65.Casalino-Matsuda SM, Monzon ME, Day AJ, Forteza RM. Hyaluronan fragments/CD44 mediate oxidative stress-induced MUC5B upregulation in airway epithelium. Am J Respir Cell Mol Biol. 2009;40:277–285. doi: 10.1165/rcmb.2008-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ushio-Fukai M. Localizing NADPH oxidasederived ROS. Sci STKE. 2006 doi: 10.1126/stke.3492006re8. re8. [DOI] [PubMed] [Google Scholar]
- 67.Pendyala S, Usatyuk P, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH Oxidase in Vascular Endothelium: The Role of Phospholipases, Protein Kinases, and Cytoskeletal Proteins. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li XA, Everson WV, Smart EJ. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med. 2005;15:92–96. doi: 10.1016/j.tcm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 70.Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-Mediated Transactivation of the S1P1 Receptor in Caveolin-Enriched Microdomains Regulates Endothelial Barrier Enhancement by Oxidized Phospholipids. Circ Res. 2009;104:978–986. doi: 10.1161/CIRCRESAHA.108.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 72.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. (2007) Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (-/-) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-name, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 74.Wunderlich C, Schober K, Schmeisser A, Heerwagen C, Tausche AK, Steinbronn N, Brandt A, Kasper M, Schwencke C, Braun-Dullaeus RC, Strasser RH. The adverse cardiopulmonary phenotype of caveolin-1 deficient mice is mediated by a dysfunctional endothelium. J Mol Cell Cardiol. 2008;44:938–947. doi: 10.1016/j.yjmcc.2008.02.275. [DOI] [PubMed] [Google Scholar]
- 75.Gebbers JO. Atherosclerosis, cholesterol, nutrition, and statins–a critical review. Ger Med Sci. 2007;5 Doc04. [PMC free article] [PubMed] [Google Scholar]
- 76.Xiangdong L, Yuanwu L, Hua Z, Liming R, Qiuyan L, Ning L. Animal models for the atherosclerosis research: a review. Protein Cell. 2011;2:189–201. doi: 10.1007/s13238-011-1016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 78.Lendon C, Born GV, Davies MJ, Richardson PD. Plaque fissure: the link between atherosclerosis and thrombosis. Nouv Rev Fr Hematol. 1992;34:27–29. [PubMed] [Google Scholar]
- 79.Karangelis DE, Kanakis I, Asimakopoulou AP, Karousou E, Passi A, Theocharis AD, Triposkiadis F, Tsilimingas N B, Karamanos NK. Glycosaminoglycans as key molecules in atherosclerosis: the role of versican and hyaluronan. Curr Med Chem. 2010;17:4018–4026. doi: 10.2174/092986710793205354. [DOI] [PubMed] [Google Scholar]
- 80.Nieuwdorp M, Holleman F, de Groot E, Vink H, Gort J, Kontush A, Chapman MJ, Hutten BA, Brouwer CB, Hoekstra JB, Kastelein JJ, Stroes ES. Perturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosis. Diabetologia. 2007;50:1288–1293. doi: 10.1007/s00125-007-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bot PT, Pasterkamp G, Goumans MJ, Strijder C, Moll FL, de Vries JP, Pals ST, de Kleijn DP, Piek JJ, Hoefer IE. (2010) Hyaluronic acid metabolism is increased in unstable plaques. Eur J Clin Invest. 2010;40:818–827. doi: 10.1111/j.1365-2362.2010.02326.x. [DOI] [PubMed] [Google Scholar]
- 82.Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, Yeh C, Secreto A, Assoian RK, Rader DJ, Pure E. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J Clin Invest. 2001;108:1031–1040. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walke S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 84.de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39:481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 85.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337–345. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bruegger D, Rehm M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Conzen P, Becker BF. Exogenous nitric oxide requires an endothelial glycocalyx to prevent postischemic coronary vascular leak in guinea pig hearts. Critical care. 2008;12 doi: 10.1186/cc6913. R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rouschop KM, Roelofs JJ, Claessen N, da Costa Martins P, Zwaginga JJ, Pals ST, Weening JJ, Florquin S. Protection against renal ischemia reperfusion injury by CD44 disruption. J Am Soc Nephrol. 2005;16:2034–2043. doi: 10.1681/ASN.2005010054. [DOI] [PubMed] [Google Scholar]
- 88.Kocak B, Orug T, Turhan N, Ozcay N, Gonenc F. CD44 expression in renal ischemiareperfusion injury in rats. Int Urol Nephrol. 2009;41:791–794. doi: 10.1007/s11255-009-9542-0. [DOI] [PubMed] [Google Scholar]
- 89.Eckle T, Eltzschig HK. Toll-like receptor signaling during myocardial ischemia. Anesthesiology. 2011;114:490–492. doi: 10.1097/ALN.0b013e31820a4d78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang W, Tang W, Geng Q, Xu X. Inhibition of Toll-like Receptor 4 With Vasoactive Intestinal Peptide Attenuates Liver Ischemia-Reperfusion Injury. Transplant Proc. 2011;43:1462–1467. doi: 10.1016/j.transproceed.2011.01.191. [DOI] [PubMed] [Google Scholar]
- 91.Mersmann J, Koch A, Tran N, Zimmermann R, Granja TF, Larmann J, Herzog C, Theilmeier G, Bornstein SR, Kirschning CJ, Zacharowski K. Toll-like receptor 2 signaling triggers fatal arrhythmias upon myocardial ischemiareperfusion. Critical Care Med. 2010;38:1927–1932. doi: 10.1097/CCM.0b013e3181ef455b. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Abarbanell AM, Herrmann JL, Weil BR, Poynter J, Manukyan MC, Crisostomo PR, Meldrum DR. Toll-like receptor signaling pathways and the evidence linking toll-like receptor signaling to cardiac ischemia/ reperfusion injury. Shock. 2010;34:548–557. doi: 10.1097/SHK.0b013e3181e686f5. [DOI] [PubMed] [Google Scholar]
- 93.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 94.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 95.Zanotti G, Casiraghi M, Abano JB, Tatreau JR, Sevala M, Berlin H, Smyth S, Funkhouser WK, Burridge K, Randell SH, Egan TM. Novel critical role of Toll-like receptor 4 in lung ischemia-reperfusion injury and edema. Am J Physiol Lung Cell Mol Physiol. 2009;297:L52–63. doi: 10.1152/ajplung.90406.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Serne EH, de Jongh RT, Eringa EC, Ijzerman RG, de Boer MP, Stehouwer CD. Microvascular dysfunction: causative role in the association between hypertension, insulin resistance and the metabolic syndrome? Essays Biochem. 2006;42:163–176. doi: 10.1042/bse0420163. [DOI] [PubMed] [Google Scholar]
- 97.Triggle CR, Ding HA. Review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens. 2010;4:102–115. doi: 10.1016/j.jash.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 98.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 99.Wei X, Schneider JG, Shenouda SM, Lee A, Towler DA, Chakravarthy MV, Vita JA, Semenkovich CF. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J Biol Chem. 2011;286:2933–2945. doi: 10.1074/jbc.M110.193037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Campo GM, Avenoso A, Micali A, Nastasi G, Squadrito F, Altavilla D, Bitto A, Polito F, Rinaldi M G, Calatroni A, D'Ascola A, Campo S. High-molecular weight hyaluronan reduced renal PKC activation in genetically diabetic mice. Biochim Biophys Acta. 2010;1802:1118–1130. doi: 10.1016/j.bbadis.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Lucarini G, Zizzi A, Aspriello SD, Ferrante L, Tosco E, Lo Muzio L, Foglini P, Mattioli-Belmonte M, Di Primio R, Piemontese M. Involvement of vascular endothelial growth factor, CD44 and CD133 in periodontal disease and diabetes: an immunohistochemical study. J Clin Periodontol. 2009;36:3–10. doi: 10.1111/j.1600-051X.2008.01338.x. [DOI] [PubMed] [Google Scholar]
- 103.Naor D, Nedvetzki S, Walmsley M, Yayon A, Turley EA, Golan I, Caspi D, Sebban LE, Zick Y, Garin T, Karussis D, Assayag-Asherie N, Raz I, Weiss L, Slavin S. CD44 involvement in autoimmune inflammations: the lesson to be learned from CD44-targeting by antibody or from knockout mice. Ann N Y Acad Sci. 2007;1110:233–247. doi: 10.1196/annals.1423.025. [DOI] [PubMed] [Google Scholar]
- 104.Weiss L, Botero-Anug AM, Hand C, Slavin S, Naor D. CD44 gene vaccination for insulin-dependent diabetes mellitus in non-obese diabetic mice. Isr Med Assoc J. 2008;10:20–25. [PubMed] [Google Scholar]
- 105.Tyml K. Critical role for oxidative stress, platelets, and coagulation in capillary blood flow impairment in sepsis. Microcirculation. 2011;18:152–162. doi: 10.1111/j.1549-8719.2010.00080.x. [DOI] [PubMed] [Google Scholar]
- 106.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002011. 88ps25. [DOI] [PubMed] [Google Scholar]
- 107.Berg S. Hyaluronan turnover in relation to infection and sepsis. J Intern Med. 1997;242:73–77. doi: 10.1046/j.1365-2796.1997.00177.x. [DOI] [PubMed] [Google Scholar]
- 108.Jung K, Lee JE, Kim HZ, Kim HM, Park BS, Hwang SI, Lee JO, Kim SC, Koh GY. Tolllike receptor 4 decoy, TOY, attenuates gramnegative bacterial sepsis. PloS one. 2009;4 doi: 10.1371/journal.pone.0007403. e7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wittebole X, Castanares-Zapatero D, Laterre PF. Toll-like receptor 4 modulation as a strategy to treat sepsis. Med Inflamm. 2010 doi: 10.1155/2010/568396. 568396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou L, Feng Y, Chen YJ, Si R, Shen S, Zhou Q, Ichinose F, Scherrer-Crosbie M, Chao W. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Critical care medicine. 2010;38:1335–1342. doi: 10.1097/CCM.0b013e3181d99e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 112.Freudenberg MA, Tchaptchet S, Keck S, Fejer G, Huber M, Schutze N, Beutler B, Galanos C. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiol. 2008;213:193–203. doi: 10.1016/j.imbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 114.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 115.Triantafilou M, Triantafilou K. The dynamics of LPS recognition: complex orchestration of multiple receptors. J Endotoxin Res. 2005;11:5–11. doi: 10.1179/096805105225006641. [DOI] [PubMed] [Google Scholar]
- 116.Fan J, Frey RS, Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112:1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shin HS, Xu F, Bagchi A, Herrup E, Prakash A, Valentine C, Kulkarni H, Wilhelmsen K, Warren S, Hellman J. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J Immunol. 2011;186:1119–1130. doi: 10.4049/jimmunol.1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muto J, Yamasaki K, Taylor KR, Gallo RL. Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol Immunol. 2009;47:449–456. doi: 10.1016/j.molimm.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Devaney J, Contreras M, Laffey JG. Clinical Review: Gene-based therapies for ALI/ARDS: where are we now? Critical Care. 2011;15:224. doi: 10.1186/cc10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bajwa EK. Acute lung injury: time to find a way that works. Respir Care. 2011;56:714–715. doi: 10.4187/respcare.01350. [DOI] [PubMed] [Google Scholar]
- 121.Singleton PA, Garcia JG. Stretching the search for therapeutic targets in acute lung injury. Transl Res. 2008;152:255–256. doi: 10.1016/j.trsl.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 122.Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–259. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirano H, Screaton GR, Bell MV, Jackson DG, Bell JI, Hodes RJ. CD44 isoform expression mediated by alternative splicing: tissuespecific regulation in mice. Int Immunol. 1994;6:49–59. doi: 10.1093/intimm/6.1.49. [DOI] [PubMed] [Google Scholar]
- 124.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 125.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hollingsworth JW, Li Z, Brass DM, Garantziotis S, Timberlake SH, Kim A, Hossain I, Savani RC, Schwartz DA. CD44 regulates macrophage recruitment to the lung in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol. 2007;37:248–253. doi: 10.1165/rcmb.2006-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith LS, Kajikawa O, Elson G, Wick M, Mongovin S, Kosco-Vilbois M, Martin TR, Frevert CW. Effect of Toll-like receptor 4 blockade on pulmonary inflammation caused by mechanical ventilation and bacterial endotoxin. Exp Lung Res. 2008;34:225–243. doi: 10.1080/01902140802022492. [DOI] [PubMed] [Google Scholar]
- 128.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 129.Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, Bucala R, Noble PW. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–2475. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- 130.Mambetsariev N, Mirzapoiazova T, Mambetsariev B, Sammani S, Lennon FE, Garcia JG, Singleton PA. (2010) Hyaluronic Acid binding protein 2 is a novel regulator of vascular integrity. Arterioscler Thromb Vasc Biol. 2010;30:483–490. doi: 10.1161/ATVBAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wygrecka M, Markart P, Fink L, Guenther A, Preissner KT. Raised protein levels and altered cellular expression of factor VII activating protease (FSAP) in the lungs of patients with acute respiratory distress syndrome (ARDS) Thorax. 2007;62:880–888. doi: 10.1136/thx.2006.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mu E, Liu X, Chen S, Zhi L, Li X, Xu X, Ma X. Changes in factor VII-activating protease in a bleomycin-induced lung injury rat model and its influence on human pulmonary fibroblasts in vitro. International J Mol Med. 2010;26:549–555. doi: 10.3892/ijmm_00000498. [DOI] [PubMed] [Google Scholar]
- 133.Pure E, Assoian RK. Rheostatic signaling by CD44 and hyaluronan. Cell Signal. 2009;21:651–655. doi: 10.1016/j.cellsig.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecularweight hyaluronan. Int J Cancer. 1997;71:251–256. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 135.Gaffney J, Matou-Nasri S, Grau-Olivares M, Slevin M. Therapeutic applications of hyaluronan. Mol Biosyst. 2010;6:437–443. doi: 10.1039/b910552m. [DOI] [PubMed] [Google Scholar]
- 136.Gao F, Liu Y, He Y, Yang C, Wang Y, Shi X, Wei G. Hyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesis. Matrix Biol. 2010;29:107–116. doi: 10.1016/j.matbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 137.Pardue EL, Ibrahim S, Ramamurthi A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis. 2008;4:203–214. doi: 10.4161/org.4.4.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Slevin M, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J Biol Chem. 2002;277:41046–41059. doi: 10.1074/jbc.M109443200. [DOI] [PubMed] [Google Scholar]
- 139.Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- 140.Chen Z, Zhuo W, Wang Y, Ao X, An J. Downregulation of layilin, a novel hyaluronan receptor, via RNA interference, inhibits invasion and lymphatic metastasis of human lung A549 cells. Biotechnol Appl Biochem. 2008;50:89–96. doi: 10.1042/BA20070138. [DOI] [PubMed] [Google Scholar]
- 141.Monz K, Maas-Kuck K, Schumacher U, Schulz T, Hallmann R, Schnaker EM, Schneider SW, Prehm P. Inhibition of hyaluronan export attenuates cell migration and metastasis of human melanoma. Journal Cell Biochem. 2008;105:1260–1266. doi: 10.1002/jcb.21925. [DOI] [PubMed] [Google Scholar]
- 142.Yadav AK, Mishra P, Agrawal GP. An insight on hyaluronic acid in drug targeting and drug delivery. J Drug Target. 2008;16:91–107. doi: 10.1080/10611860802095494 . [DOI] [PubMed] [Google Scholar]
- 143.Lennon FE, Singleton PA. The Role of Hyaluronan and Hyaluronan Binding Proteins in Lung Pathobiology. Am J Physiol Lung Cell Mol Physiol. 2011 doi: 10.1152/ajplung.00071.2010. May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]