Abstract
Mitochondrial dysfunction is a prominent feature of Alzheimer's disease (AD) brain. Our prior studies demonstrated reduced mitochondrial number in susceptible hippocampal neurons in the brain from AD patients and in M17 cells overexpressing FAD-causing APP mutant (APPswe). In the current study, we investigated whether alterations in mitochondrial biogenesis contribute to mitochondrial abnormalities in AD. Mitochondrial biogenesis is regulated by the PGC-1α-NRF-TFAM pathway. Expression levels of PGC-1α, NRF 1, NRF 2, and TFAM were significantly decreased in both AD hippocampal tissues and APPswe M17 cells, suggesting a reduced mitochondrial biogenesis. Indeed, APPswe M17 cells demonstrated decreased mitochondrial DNA/nuclear DNA ratio, correlated with reduced ATP content, and decreased cytochrome C oxidase activity. Importantly, overexpression of PGC-1α could completely rescue while knockdown of PGC-1α could exacerbate impaired mitochondrial biogenesis and mitochondrial deficits in APPswe M17 cells, suggesting reduced mitochondrial biogenesis is likely involved in APPswe-induced mitochondrial deficits. We further demonstrated that reduced expression of p-CREB and PGC-1α in APPswe M17 cells could be rescued by cAMP in a dose-dependent manner, which could be inhibited by PKA inhibitor H89, suggesting that the PKA/CREB pathway plays a critical role in the regulation of PGC-1α expression in APPswe M17 cells. Overall, our study demonstrated that impaired mitochondrial biogenesis likely contributes to mitochondrial dysfunction in AD.
Keywords: Alzheimer's disease, mitochondrial biogenesis, mitochondrial transcription factor A (TFAM), nuclear respiratory factors (NRF), proliferator-activated receptor gamma coactivator 1alpha (PGC-1a)
Introduction
Alzheimer's disease (AD), the most common neurodegenerative disorder of the elderly, is characterized by senile plaques, neurofibrillary tangles, and progressive death of neuronal cells at selective brain regions (Smith 1998). The molecular mechanisms underlying the pathogenesis of AD are still unclear. Mitochondrial dysfunction is a prominent and early feature of AD (Zhu et al. 2006) with reduced energy metabolism as one of the best documented early abnormalities in AD (Blass 2000). Key mitochondrial enzymes of oxidative metabolism (i.e., cytochrome C oxidase, KGDHC, and PDHC) are deficient in AD (Manczak et al. 2004, Qin et al. 2009). Early deficits in synaptic mitochondria were found in an AD mouse model in a recent study (Du et al. 2010). In addition, damaged mitochondrial DNA (mtDNA) including DNA mutation and DNA defects are also found to be involved in AD (Lin et al. 2002). Both amyloid precursor protein (APP) and amyloid-β (Aβ) locate in mitochondria (Calkins et al. 2011, Anandatheerthavarada et al. 2003, Caspersen et al. 2005, Chen & Yan 2010, Hansson Petersen et al. 2008, Manczak et al. 2006) and Aβ not only contributes to significant oxidative damage of mtDNA, but also leads to impaired mtDNA gene expression (de la Monte et al. 2000, Brooks et al. 2007). More recent studies suggest abnormal mitochondrial dynamics including excessive mitochondrial fragmentation and abnormal mitochondrial distribution plays a critical role in mitochondrial dysfunction in AD (Wang et al. 2008a, Wang et al. 2009, Wang et al. 2008b, Manczak et al. 2011, Su et al., Wang et al.).
During the life cycle of mitochondria, mitochondrial biogenesis plays an essential role in maintaining mitochondrial homeostasis to meet the physiological needs of eukaryotic cells. However, the status of this important aspect of mitochondrial life and function in AD is unclear (Onyango et al. 2010). The factors regulating mitochondrial biogenesis include nuclear respiratory factor 1 (NRF 1) and nuclear respiratory factor 2 (NRF 2), which control the nuclear genes to encode mitochondrial protein, and mitochondrial transcription factor A (TFAM), which drives transcription and replication of mtDNA. The expression of NRF 1, NRF 2, and TFAM are regulated by peroxisome proliferation activator receptor gamma-coactivator 1α (PGC-1α) (Wu et al. 1999), the master regulator of mitochondrial biogenesis. A recent study showed reduced PGC-1α levels in AD (Qin et al. 2009), implicating an impaired mitochondrial biogenesis signaling in AD. Interestingly, our prior studies demonstrated reduced mitochondrial number in susceptible hippocampal neurons in the brain from AD patients (Hirai et al. 2001) and in M17 cells overexpressing familial AD (FAD)-causing APP mutant (Wang et al. 2008b). To explore whether a reduced mitochondrial biogenesis contributes to reduced mitochondria and mitochondrial dysfunction in AD, we investigated the profile of mitochondrial biogenesis signals in AD cases and in M17 cells overexpressing FAD-causing APP mutant and how they may affect mitochondrial function.
Materials and Methods
Brain Tissue
Brain tissue from the hippocampal and cerebellum regions was obtained postmortem using approved IRB protocol from AD (n= 15; ages=65-91; postmortem interval=3.6±0.4 hr) and control (n= 14; ages=53-91; postmortem interval=3.3±0.5 hr) patients, as assessed on clinical and pathological criteria established by CERAD and an NIA consensus panel (Khachaturian 1985, Mirra et al. 1991).
Cell Culture, Transfection, and Treatment
M17, a widely used human neuroblastoma cell line, was maintained in Opti-MEM medium (Invitrogen, Eugene, OR), supplemented with 5% fetal bovine serum and 1% penicillin-streptomycin, in a humid incubator with 5% CO2 at 37°C. The cell lines stably expressing human Swedish mutation (K670M/N671L) APP695 or control vector were described in the previous research (Wang et al. 2008b). The micro-RNA (miR) RNA interference (RNAi) vector was generated via the pcDNA 6.2-GW/EmGFP-miR constructs (Invitrogen). 50 μg/ml blasticidin (Invivogene biotech, Eugene, OR) was used for PGC-1α knockdown stable line selection. The control vectors and plasmids of PGC-1α were reported in previous studies respectively (Lerin et al. 2006, Puigserver et al. 1998). Transfection was performed using lipofectamine 2000 following the instructions (Invitrogen). Stable cell lines were maintained with 100 μg/ml of geneticin, 200 μg/ml of hygromycin or 25 μg/ml of blasticidin. APPswe cells were treated and incubated for 48 h with 0.5, 1, 5, 10 mM cAMP. Cells were also stimulated by cAMP at 5 mM, with or without 10 μM PKA inhibitor H89 (Cell Signaling, Beverly, MA).
Real time PCR to detect mtDNA copy number
mtDNA copy number was measured by real-time PCR method using the Step one plus Real-Time PCR System (Applied Biosystems, Carlsbad, CA) with the SYBR Green detection method. Total intracellular DNA in different cell lines was extracted using QIAamp DNA mini kit (QIAGEN, Germantown, MD) following the manufacturer's instruction. Each real-time PCR (20 μl total volume) contained 3 μl of template DNA (50 μg), 10 μl of 2 × SYBR Green Real-time PCR Master (Applied Biosystems), 1 μl of each of the forward and reverse primers and 5 μl of ultrapure water. The primers for a subunit of human electron transport chain used for mtDNA amplification were: forward, 5′- CAAACCTACGCCAAAATCCA-3′ and reverse, 5′-GAAATGA ATGAGCCTACAGA-3′ (Kukidome et al. 2006, Xu et al. 2009). Primers for Human nuclear 18S, used for internal control, were: forward, 5′-ACGGACCAGAGCGAAAGCA-3′ and reverse, 5′-GACATCTAAGGGCATCACAGAC-3′. Relative amounts of mtDNA and nDNA copy numbers were compared. The ddCt (mtDNA to 18S) represents the mtDNA copy number in a cell.
Immunoblot
Brain samples from hippocampus and cerebellum of AD and age-matched control or cells were lysed with 1× cell lysis buffer (Cell Signaling), plus 1 mM PMSF (Sigma, St. Louis, MO) and Protease Inhibitor Cocktail (Sigma). Bicinchoninic acid assay (Pierce, Rockford, IL) was used to determine protein concentration. Proteins were separated by 10% SDS-PAGE and were electrotransferred to Immobilon-P (Millipore, Billerica, MA). After blocking with10% non-fat dry milk, transferred membrane was incubated with primary and secondary antibodies (Zhu et al. 2000). The following antibodies were used in this study: PGC-1α (C terminal) from Calbiochem, San Diego, CA; NRF 1, NRF 2α, NRF 2β, TFAM, and cytochrome B from Santa Cruz Biotechnology, Santa Cruz, CA; Two close bands were recognized by NRF2β antibody which represented the two splice variants that differ in their C-termini. Lamin A/C, p-CREB, and CREB from Cell Signaling; PKA RI, PKA RIIβ, and PKAc from BD Labs, Franklin Lakes, NJ; actin from Chemicon, Atlanta, GA. Blots were developed with Immobilon Western Chemiluminescent HRP Substrate (Millipore) or the immunoCruz (Santa Cruz Biotechnology).
Cell nuclear protein extraction
Cell nuclear proteins were extracted using Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Rockford, IL) according to the instructions.
ATP and cytochrome C oxidase activity
ATP levels were measured by the ATP Determination Kit (Invitrogen), which was described in the previous study (Wang et al. 2008b). Cytochrome C oxidase activity was determined using the cytochrome C oxidase activity kit (Sigma) following the instructions.
Results
Mitochondrial biogenesis proteins are reduced in hippocampal tissues from AD patients
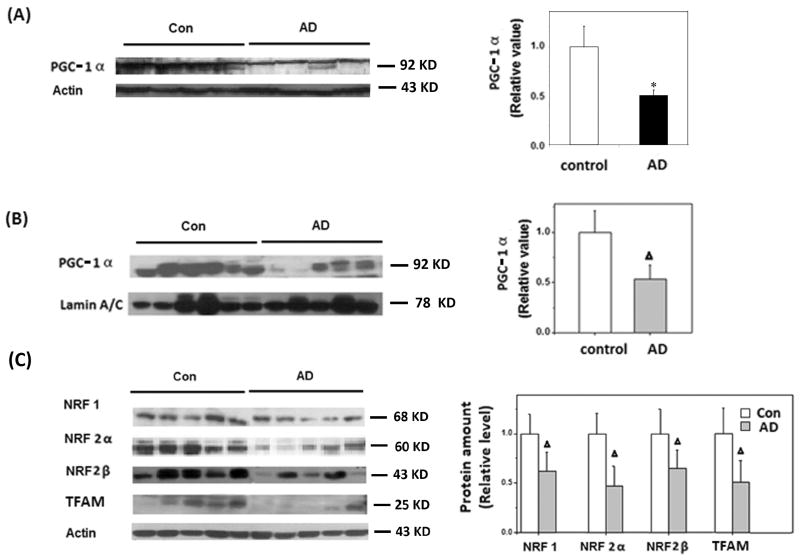
Since mitochondrial biogenesis is regulated by the PGC-1α-NRF 1-TFAM pathway, we first measured the expression level of PGC-1α in hippocampal tissues. Real-time PCR studies of 15 AD and 10 age-matched controls suggested a 50% decrease in PGC-1α mRNA levels in AD (not shown). Immunoblot analysis also revealed significantly reduced total protein levels of PGC-1α in AD brains (Figure 1A). Since PGC-1α is a transcription factor, we also measured the nuclear levels of PGC-1α protein. Immunoblot of nuclear fraction revealed significantly reduced PGC-1α levels in AD brain compared to age-matched controls, suggesting this pathway may be impaired in AD brain (Fig. 1B). Indeed, further immunoblot analysis of proteins downstream to PGC-1α, i.e., NRF 1, NRF 2α, NRF 2β, and TFAM, confirmed significant reduction in the levels of all four downstream proteins in AD brain compared to age-matched normal controls: the levels of NRF 1, NRF 2α, NRF 2β, and TFAM were reduced by 38.2% (p<0.01), 53.3% (p<0.01), 40.7% (p<0.01), and 43% (p<0.01), respectively in AD samples (Fig. 1C). However, in contrast to that in hippocampus, no significant decrease in any of these proteins was found in cerebellum, the unaffected brain region, from AD patients (not shown). These data suggest a reduced mitochondrial biogenesis in affected brain regions in AD.
Figure 1.
Mitochondrial biogenesis proteins were reduced in AD hippocampus. A) Immunoblot and quantification analysis revealed that total protein levels of PGC-1α were significantly reduced in AD hippocampus (n=8) compared to controls (n=7) (*p<0.05, Student's t test); Actin was used as an internal loading control. B) Immunoblot and quantification analysis revealed that the nuclear levels of PGC-1α were significantly reduced in AD hippocampus (n=10) compared to age-matched controls (n=11) (Δp<0.01, Student's t test); Lamin A/C was used as an internal loading control. C) Representative immunoblotting and quantification analysis revealed that the levels of NRF 1/NRF 2α/NRF 2β/TFAM were significantly reduced in AD hippocampus (n=10) compared to age-matched controls (n=11) (Δp<0.01, Student's t test); Actin was used as an internal loading control. Equal protein amounts (25 μg) were loaded.
Reduced mitochondrial biogenesis causes mitochondrial dysfunction in PGC-1α knockdown cells
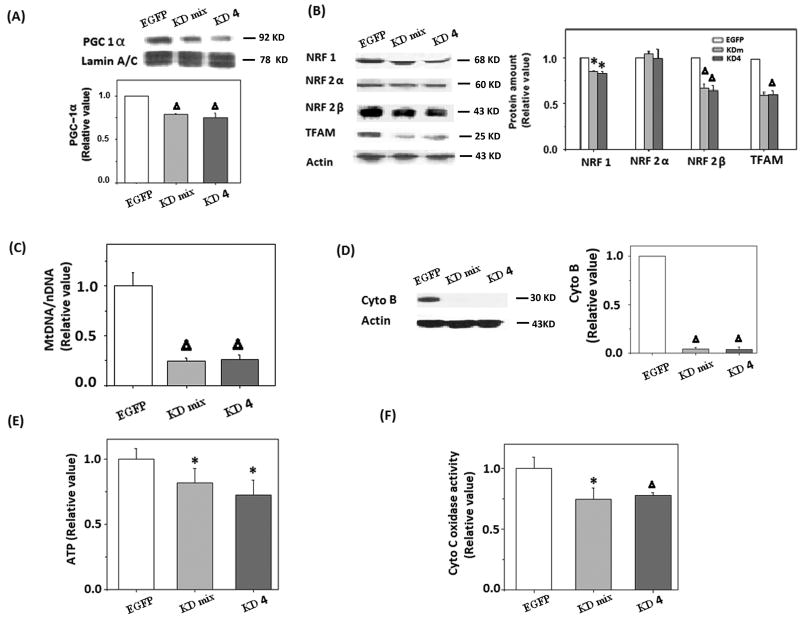
We hypothesize that reduced mitochondrial biogenesis contributes to mitochondrial dysfunction in AD neurons. To study the effect of reduced PGC-1α and downstream effectors on mitochondrial function in neuronal cells, a mixed clone (KDm) and a single clone, clone 4 (KD4), of M17 cells stably transfected with PGC-1α miR RNAi were established (Fig. 2A). Immunoblot analysis confirmed reduced expression of PGC-1α in both cell lines. The levels of NRF 1, NRF 2β, and TFAM were also reduced in both PGC-1α KDm and KD4 cell lines, whereas the level of NRF 2α was constant (Fig. 2B).
Figure 2.
Downregulation of PGC-1α led to reduced mitochondrial biogenesis proteins and mitochondrial content in M17 cells. A) Representative immunoblot results of PGC-1α expression in PGC-1α Knockdown mixed clone (KDm), single clone4 (KD4) and control cells (EGFP), Lamin A/C was used as an internal loading control. B) Representative immunoblot and quantification analysis revealed that NRF 1, NRF 2β, and TFAM were significantly reduced (*p<0.05, Δp<0.01, Student's t test) in both PGC-1α KDm and PGC-1α KD4 cells. C) Mitochondrial-to-nuclear DNA ratios were determined by quantitative real-time PCR and are expressed relative to control cells (Δp<0.01, Student's t test). D) Representative immunoblot and quantification analysis revealed that the level of cytochrome B was significantly reduced (Δp<0.01, Student's t test) in PGC-1α knockdown cells. E) ATP level in cells was determined by firefly luciferase, a significant reduction of ATP level was found in PGC-1α KDm and PGC-1α KD4 cells (Δp<0.01, Student's t test). F) Cytochrome C oxidase activity was significantly reduced in PGC-1α KDm and PGC-1α KD4 cells (Δp<0.01, Student's t test).
To directly determine whether mitochondrial biogenesis is affected, we measured mtDNA relative to nuclear DNA (mtDNA/nDNA) by real-time PCR and found that mtDNA/nDNA was significantly reduced in PGC-1α KDm and KD4 cells (Fig. 2C). Moreover, reduced expression level of mitochondrial protein, cytochrome B, was also found in these cells (Fig. 2D).
Mitochondria are the major source of ATP. We investigated the effect of reduced PGC-1α on ATP production by firefly fluorescence assay and found reduced ATP levels in PGC-1α KDm and KD4 cells compared to EGFP controls (Fig. 2E), which correlates with an obvious reduction of cytochrome C oxidase activity in these cells (Fig. 2F), suggesting that impaired mitochondrial biogenesis negatively impacts mitochondrial function in neurons.
Impaired mitochondrial biogenesis in APPswe M17 cells
Mutations in the APP gene including the Swedish mutation (APPswe) cause FAD. We previously demonstrated that overexpression of APPswe causes significant decrease in mitochondrial number and a slight but significant increase in mitochondrial size (Wang et al. 2008b). In the current study, we investigated whether impaired mitochondrial biogenesis could contribute to decreased mitochondrial number in APPswe M17 cells.
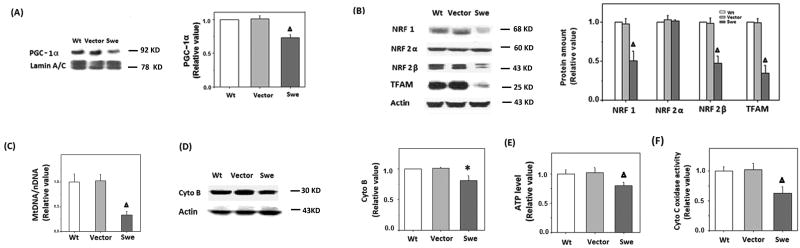
Compared to wild-type (WT) or vector-control M17 cells, APPswe M17 cells demonstrated significantly reduced levels of nuclear PGC-1α protein (Fig. 3A). Immunoblot and quantification analysis also revealed that the protein levels of NRF 1, NRF 2β, and TFAM were reduced by more than 50% in APPswe cells compared to WT and vector-control M17 cells (p<0.01), whereas the level of NRF 2α did not change significantly (Fig. 3B).
Figure 3.
The expression of human APP swedish mutation caused reduced expression of mitochondrial biogenesis proteins and reduced mitochondrial content. A) Immunoblot and quantification analysis revealed that the nuclear levels of PGC-1α were significantly reduced in APPswe cells (n=10, Δp<0.01, Student's t test). B) Representative immunoblot and quantification analysis revealed that NRF 1, NRF 2β, and TFAM were significantly reduced in APPswe M17 cells (Δp<0.01, Student's t test). C) Mitochondrial-to-nuclear DNA ratios were determined by quantitative real-time PCR and were expressed relative to control cells (Δp<0.01, Student's t test). D) Representative immunoblot and quantification analysis revealed that the level of cytochrome B was significantly reduced (*p<0.05, Student's t test) in APPswe cells. E) ATP level in cells was determined by firefly luciferase, a significant reduction of ATP level was found in APPswe cells (Δp<0.01, Student's t test). F) Cytochrome C oxidase activity was significantly reduced in APPswe cells (Δp<0.01, Student's t test).
Consistent with a reduced mitochondrial biogenesis signaling, we also found that mtDNA/nDNA ratio (Fig. 3C), along with expression level of mitochondrial protein, cytochrome B (Fig. 3D), was significantly reduced in APPswe M17 cells. Functionally, APPswe M17 cells displayed significant reduced ATP level and cytochrome C oxidase activity compared to WT and vector-control M17 cells (Fig. 3E, F).
Manipulation of PGC-1α expression modulates mitochondrial biogenesis and dysfunction in APPswe M17 cells
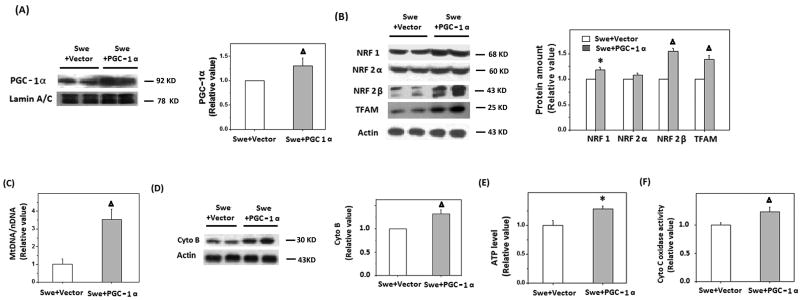
In order to assess whether PGC-1α reduction played a causal role in the abnormalities in mitochondrial biogenesis and function in APPswe M17 cells, we investigated whether overexpression of PGC-1α rescues APPswe M17 cells. APPswe M17 cells were transiently transfected with PGC-1α plasmid. 48 h after transfection, expression of mitochondrial biogenesis proteins and mitochondrial parameters were determined. Overexpression of PGC-1α was confirmed by immunoblot (Fig. 4A). Indeed, expression of the downstream proteins including NRF 1, NRF 2β, and TFAM was also significantly increased (*p<0.05, Δp<0.01, Student's t test) in APPswe cells overexpressing PGC-1α (Fig. 4B). Consistently, mtDNA/nDNA (Fig. 4C) and expression of cytochrome B (Fig. 4D), were also significantly increased to the level comparable to WT M17 cells, suggesting the restoration of mitochondrial biogenesis. Most importantly, the overexpression of PGC-1α led to the rescue of mitochondrial function as reflected by increased ATP level (Fig. 4E) along with increased cytochrome C oxidase activity (Fig. 4F) in APPswe M17 cells.
Figure 4.
Overexpression of PGC-1α could rescue the impaired mitochondrial biogenesis signal in APPswe cells. A) Representative immunoblot and quantification analysis showed that the expression of PGC-1α increased significantly after transient transfection with PGC-1α construct. B) Representative immunoblot and quantification analysis revealed that the levels of NRF 1, NRF 2β, and TFAM were significantly increased (*p<0.05, Δp<0.01, Student's t test). C) Real time PCR revealed that mtDNA was significantly increased after transfection with PGC-1α for 48 h (Δp<0.01, Student's t test). D) Representative immunoblot analysis revealed that cytochrome B was significantly increased (Δp<0.01, Student's t test) after cells was transfected with PGC-1α for 48 h. E) ATP levels in cells were determined by firefly luciferase after transfection with PGC-1α for 48 h, and a significant increase of ATP level was found (Δp<0.01, Student's t test). F) Cytochrome C oxidase activity was measured 48 h after transfection with PGC-1α, and the results indicated that cytochrome C oxidase activity was significantly increased compared to control vector transfection (Δp<0.01, Student's t test).
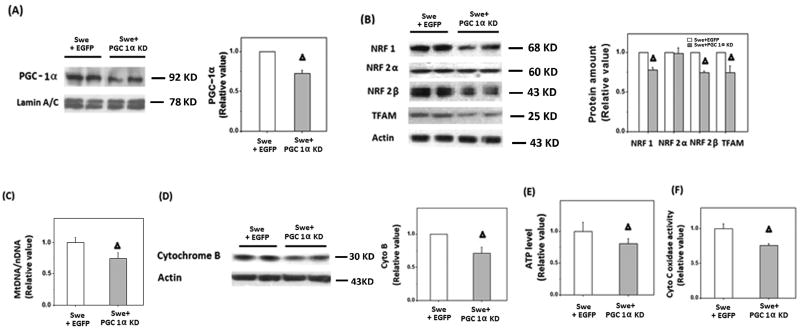
APPswe M17 cells were also transfected with the miR RNAi of PGC-1α. Reduced expression of PGC-1α was confirmed by immunoblot (Fig. 5A). As expected, downregulation of PGC-1α led to significantly reduced levels of NRF 1, NRF 2β, and TFAM (Fig. 5B) along with further reduction in mtDNA/nDNA ratio (Fig. 5C), and decreased cytochrome B levels (Fig. 5D) in APPswe M17 cells. Functionally, this led to further reduction in ATP content (Fig. 5E) as well as cytochrome C oxidase activity (Fig. 5F).
Figure 5.
Transfection with miR RNAi of PGC-1α exacerbated the impaired mitochondrial biogenesis in APPswe cells. A) Representative immunoblotting and quantification analysis showed that the expression of PGC-1α significantly reduced after transient transfection with miR RNAi of PGC-1α. B) Representative immunoblotting and quantification analysis revealed that the levels of NRF 1, NRF 2 and TFAM were significantly reduced after transient transfection (*p<0.05, Δp<0.01, Student's t test). C) Real time PCR revealed that mtDNA was significantly reduced after transient transfection with miR RNAi of PGC-1α for 48 h compared with EGFP control cells (Δp<0.01, Student's t test). D) Representative immunoblotting and quantification analysis revealed cytochrome B was significantly reduced (Δp<0.01, Student's t test) after cells was transfected with miR RNAi of PGC-1α for 48 h. E) Firefly luciferase assay showed that ATP level displayed a significant decrease after transient transfection with miR RNAi of PGC-1α for 48 h in APPswe cells (Δp<0.01, Student's t test). F) Cytochrome C oxidase activity was significantly decreased after transfection with miR RNAi of PGC-1α in APPswe cells (Δp<0.01, Student's t test).
PKA/CREB pathway plays a critical role in the reduced expression of PGC-1α in APPswe cells
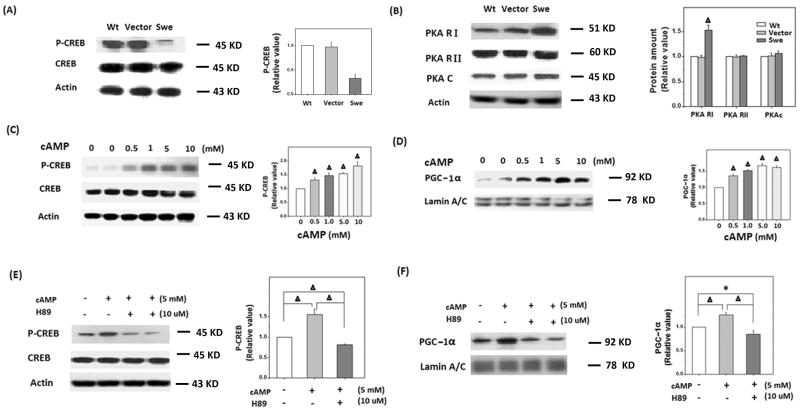
The expression of PGC-1α is regulated by transcription factor CREB (Lonard & O'Malley 2006). Indeed, we found that the level of CREB phosphorylated at Ser133 was significantly decreased in APPswe M17 cells (Fig. 6A), suggesting reduced activity of CREB in these cells. CREB can be phosphorylated and activated by the PKA pathway. PKA is composed of catalytic subunits (C) and regulatory subunits (R, including RI and RII), with the regulatory units blocking the catalytic centers of the catalytic units. Indeed, immunoblotting and quantification analysis revealed significantly higher levels of PKA RI but no changes in catalytic subunit in APPswe cells (Fig. 6B), suggesting that reduced PKA activity may be involved in the decreased CREB phosphorylation/activation and PGC-1α in APPswe M17 cells. To corroborate the role of PKA, we investigated the effect of cAMP, a PKA activator, on the phosphorylation of CERB and expression of PGC-1α in APPswe M17 cells. APPswe M17 cells were harvested after being treated with various concentrations of cAMP for 48 h and CREB and PGC-1α levels were determined by immunoblot. Indeed, in the concentration range used in our study, cAMP induced a dose-dependent increase in the levels of both p-CREB and PGC-1α in APPswe M17 cells (Fig. 6C, D). Furthermore, cAMP-induced phosphorylation of CREB (Fig. 6E) and increased expression of PGC-1α (Fig. 6F) were completely abolished by 10 μM H-89, a specific PKA inhibitor, thus demonstrating that PKA played a critical role in the reduced expression of p-CREB and PGC-1α in APPswe M17 cells.
Figure 6.
PKA/CREB pathway played a critical role in the reduced expression of PGC-1α in APPswe cells. A) Immunoblotting and quantification analysis revealed that the level of p-CREB was significantly reduced in APPswe cells (Δp<0.01, Student's t test). B) Immunoblotting analysis revealed that the level of PKA RI was significantly increased in APPswe cells, while the levels of PKA RIIβ and PKAc remained constant (Δp<0.01, Student's t test). C, D) Immunoblotting experiment and quantification analysis revealed that cAMP stimulated the expression of p-CREB and PGC-1α in a dose-dependent manner (Δp<0.01, Student's t test), actin and Lamin A/C were used as an internal controls; E, F) the increased level of p-CREB and PGC-1α induced by cAMP (5 mM) was significantly inhibited by PKA inhibitor H89 (10 μM) in APPswe cells (Δp<0.01, Student's t test).
Discussion
Mitochondria dysfunction plays an important role in AD, but the mechanism is still unclear (Reddy & Beal 2008, Reddy & Beal 2005, Swerdlow 2009, Swerdlow et al. 2010, Zhu et al. 2004). In this study, we demonstrated significant alterations in the expression pattern of proteins involved in mitochondrial biogenesis in hippocampal tissues from AD brain compared to age matched control brain. We found that the expression levels of NRF 1, NRF 2, and TFAM along with nuclear levels of PGC-1α are significantly reduced in AD hippocampus, suggesting that mitochondrial biogenesis is likely impaired in AD. Consistent with this notion, we also found reduced expression levels of PGC-1α, NRF 1, NRF 2, and TFAM along with significantly reduced mtDNA/nDNA ratio and mitochondrial contents in M17 cells overexpressing FAD-causing the APPswe mutant. Importantly, overexpression of PGC-1α almost completely restored, while knockdown of PGC-1α exacerbated, mitochondrial biogenesis deficits and mitochondrial dysfunction in APPswe M17 cells, suggesting reduced mitochondrial biogenesis likely contributes to mitochondrial dysfunction. Moreover, we further demonstrated reduced PKA/CREB signaling in APPswe M17 cells and specific activation of this pathway restored PGC-1α levels and suggest that the impaired PKA/CREB signaling plays an important role in the reduction of PGC-1α and mitochondrial biogenesis in APPswe M17 cells.
Previously we found a slight but significant reduction in mitochondrial number in hippocampal neurons from biopsied AD brain (Hirai et al. 2001). Similarly, significantly reduced mitochondrial number was also reported in M17 cells overexpressing the APPswe mutant (Wang et al. 2008b). Although alterations in mitochondrial dynamics and/or mitophagy may contribute to changes in mitochondrial number and mass (Moreira et al. 2007), changes in mitochondrial biogenesis could also impact such mitochondrial parameters. Given that PGC-1α is the key convergent component of the complex regulation pathways of mitochondrial biogenesis (Wenz 2009), such a notion is further supported by the recent finding that PGC-1α expression decreases in the AD brain as a function of dementia (Qin et al. 2009). However, given the pleotrophic roles of PGC-1α, it remains to be determined whether and how the mitochondrial biogenesis signaling is altered and whether such alterations contribute to mitochondrial dysfunction in AD. In this study, we not only confirmed reduced nuclear PGC-1α in AD brain, but further demonstrated decreased expression of NRF 1, NRF 2, and TFAM, factors downstream of PGC-1α in AD brain and AD cell models, suggesting that the mitochondrial biogenesis signaling is indeed impaired in AD. A reduced mitochondrial biogenesis is further indicated by reduced mtDNA/nDNA ratio and reduced levels of critical mitochondrial contents in APPswe M17 cells. Consistent with our prior studies, APPswe M17 cells demonstrated mitochondrial dysfunction as reflected by reduced ATP production. To explore the causal relationship between mitochondrial biogenesis deficits and mitochondrial dysfunction, we overexpressed PGC-1α in APPswe M17 cells which almost completely restored the expression of NRF 1, NRF 2, and TFAM and mtDNA/nDNA ratio and mitochondrial contents, indeed, this also resulted in the attenuation of mitochondrial dysfunction in APPswe M17 cells. In fact, another group also reported that overexpression of TFAM could protect SH-SY5Y cells from Aβ-induced mitochondrial dysfunction (Xu et al. 2009). On the other hand, knockdown of PGC-1α exacerbated mitochondrial biogenesis deficits and mitochondrial dysfunction in APPswe M17 cells. Overall, these data suggest that impaired mitochondrial biogenesis is likely involved in the mitochondrial dysfunction and play an important role in the pathogenesis of AD. In this regard, it is notable that genes encoding proteins involved in mitochondrial biogenesis have been associated with AD. For example, TFAM locates on chromosome 10, on which several genes were reported to be linked with sporadic AD (Blacker et al. 2003). The moderate possible risk of AD associated with TFAM genotypes and haplotype was found (Gunther et al. 2004). Evidence also showed that variations in TFAM were involved in the pathogenesis of sporadic late-onset AD in the Han Chinese population (Zhang et al. 2011).
Increased mitochondrial biogenesis through PGC-1α activation has been suggested to be a potential therapeutic approach for mitochondrial disorders in cell and animal models of mitochondrial disease (Srivastava et al. 2009, Wenz et al. 2008). Our study supports PGC-1α as a potential therapeutic target to increase mitochondrial biogenesis and improve mitochondrial function in AD. Indeed, such a notion is supported by a recent study suggesting that SIRT1 and resveratrol, a SIRT1-activating molecule, confers significant neuroprotection in a mouse model of AD and tauopathies likely through inhibition of PGC-1α acetylation and promotion of PGC-1α activity (Kim et al. 2007). In this regard, it is important to understand how PGC-1α levels are regulated in APPswe M17 cells. The proximal promoter of PGC-1α contains a functional CREB binding site and PGC-1α levels are regulated by the activity of CREB (Herzig et al. 2001). We found that although the overall levels of CREB remain unchanged, the level of active CREB phosphoryated at Ser133 is significantly reduced in APPswe M17 cells, correlating with reduced PGC-1α expression in these cells. Furthermore, we demonstrated increased levels of PKA regulatory subunits in APPswe M17 cells, suggesting a likely reduced PKA activity in these cells. The fact that the treatment of PKA activator, cAMP, led to increased p-CREB and PGC-1α in a dose-dependent manner which could be completely abolished by specific PKA inhibitor H89 suggesting that the PKA/CREB pathway plays an important role in reduced expression of PGC-1α in APPswe M17 cells. Indeed, treatment of H89 could lead to further decreased levels of p-CREB and PGC-1α in APPswe M17 cells. Perhaps, not coincidentally, CREB protein level and phosphorylation level are also decreased in AD and a reduction in phosphorylation of CREB resulting from inactivation of PKA has also been demonstrated in the postmortem brains of patients with AD (Yamamoto-Sasaki et al. 1999), suggesting that reduced PKA/CREB signaling may also be responsible for reduced PGC-1α expression in AD brain. Therefore, it may be desirable to activate the PKA/CREB signaling pathway as therapeutics to increase the levels or activity of PGC-1α to improve mitochondrial biogenesis and mitochondrial function in AD. Nevertheless, it must be cautioned that PKA is also involved in the hyperphosphorylation of tau protein in AD. Therefore, the activation of PKA to stimulate PGC-1α-dependent mitochondrial biogenesis may cause complication.
Overall, our study demonstrated reduced mitochondrial biogenesis signaling in AD brain and AD cell model. Induction of mitochondrial biogenesis alleviates mitochondrial dysfunction. Based on these data, we suggest that impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in AD and enhancing mitochondrial biogenesis may represent a potential pharmacologic approach for the treatment of AD.
Acknowledgments
This work was supported by the Dr. Robert M. Kohrman Memorial Fund, the National Institutes of Health (AG031852 to X.W.Z.), and the Alzheimer's Association (IIRG-10-173471 to G.P.).
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid-β
- APP
amyloid precursor protein
- FAD
familial AD
- miR
micro-RNA
- mtDNA
mitochondrial DNA
- TFAM
mitochondrial transcription factor A
- nDNA
nuclear DNA
- NRF
nuclear respiratory factor
- PGC-1α
peroxisome proliferation activator receptor gamma-coactivator 1α
- RNAi
RNA interference
- WT
wild-type
Footnotes
Dr. Zhu was a consultant for and received grant support from Medivation. Dr. Perry is a consultant for Takeda Pharmaceuticals and Neurotez and owns stock options in Neurotez and Voyager. No other authors have any disclosures.
References
- Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacker D, Bertram L, Saunders AJ, et al. Results of a high-resolution genome screen of 437 Alzheimer's disease families. Hum Mol Genet. 2003;12:23–32. doi: 10.1093/hmg/ddg007. [DOI] [PubMed] [Google Scholar]
- Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer's syndrome. Ann N Y Acad Sci. 2000;924:170–183. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Lynch PJ, Ingle CC, Hatton A, Emson PC, Faull RL, Starkey MP. Gene expression profiles of metabolic enzyme transcripts in Alzheimer's disease. Brain Res. 2007;1127:127–135. doi: 10.1016/j.brainres.2006.09.106. [DOI] [PubMed] [Google Scholar]
- Calkins M, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Chen JX, Yan SS. Role of mitochondrial amyloid-beta in Alzheimer's disease. J Alzheimers Dis. 2010;20 2:S569–578. doi: 10.3233/JAD-2010-100357. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Luong T, Neely TR, Robinson D, Wands JR. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer's disease. Lab Invest. 2000;80:1323–1335. doi: 10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C, von Hadeln K, Muller-Thomsen T, et al. Possible association of mitochondrial transcription factor A (TFAM) genotype with sporadic Alzheimer disease. Neurosci Lett. 2004;369:219–223. doi: 10.1016/j.neulet.2004.07.070. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, Alikhani N, Behbahani H, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukidome D, Nishikawa T, Sonoda K, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer's disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- Lonard DM, O'Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Siedlak SL, Wang X, et al. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta. 2010;1802:228–234. doi: 10.1016/j.bbadis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Diaz F, Iommarini L, Aure K, Lombes A, Moraes CT. PGC-1alpha/beta induced expression partially compensates for respiratory chain defects in cells from patients with mitochondrial disorders. Hum Mol Genet. 2009;18:1805–1812. doi: 10.1093/hmg/ddp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wang X, Bonda D, Perry G, Smith M, Zhu X. Abnormal mitochondrial dynamics--a novel therapeutic target for Alzheimer's disease? Mol Neurobiol. 41:87–96. doi: 10.1007/s12035-009-8095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009;17:737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20 2:S265–279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener Dis. 7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol. 2008a;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008b;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T. PGC-1alpha activation as a therapeutic approach in mitochondrial disease. IUBMB Life. 2009;61:1051–1062. doi: 10.1002/iub.261. [DOI] [PubMed] [Google Scholar]
- Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Xu S, Zhong M, Zhang L, et al. Overexpression of Tfam protects mitochondria against beta-amyloid-induced oxidative damage in SH-SY5Y cells. FEBS J. 2009;276:3800–3809. doi: 10.1111/j.1742-4658.2009.07094.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Sasaki M, Ozawa H, Saito T, Rosler M, Riederer P. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res. 1999;824:300–303. doi: 10.1016/s0006-8993(99)01220-2. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yu JT, Wang P, Chen W, Wu ZC, Jiang H, Tan L. Mitochondrial transcription factor A (TFAM) polymorphisms and risk of late-onset Alzheimer's disease in Han Chinese. Brain Res. 2011;1368:355–360. doi: 10.1016/j.brainres.2010.10.074. [DOI] [PubMed] [Google Scholar]
- Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, Hirai K, Smith MA. Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis. 2006;9:147–153. doi: 10.3233/jad-2006-9207. [DOI] [PubMed] [Google Scholar]
- Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:880–888. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Perry G, Aliev G. Mitochondrial failures in Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2004;19:345–352. doi: 10.1177/153331750401900611. [DOI] [PMC free article] [PubMed] [Google Scholar]