Abstract
The voltage-gated Kv1.3 and the calcium-activated KCa3.1 potassium channel modulate many calcium-dependent cellular processes in immune cells, including T-cell activation and proliferation, and have therefore been proposed as novel therapeutic targets for immunomodulation. Kv1.3 is highly expressed in CCR7− effector memory T cells and is emerging as a target for T-cell mediated diseases like multiple sclerosis, rheumatoid arthritis, type-1 diabetes mellitus, allergic contact dermatitis, and psoriasis. KCa3.1 in contrast is expressed in CCR7+ naïve and central memory T cells, as well as in mast cells, macrophages, dedifferentiated vascular smooth muscle cells, fibroblasts, vascular endothelium, and airway epithelium. Given this expression pattern, KCa3.1 is a potential therapeutic target for conditions ranging from inflammatory bowel disease, multiple sclerosis, arthritis, and asthma to cardiovascular diseases like atherosclerosis and post-angioplasty restenosis. Results from animal studies have been supportive of the therapeutic potential of both Kv1.3 and KCa3.1 blockers and have also not shown any toxicities associated with pharmacological Kv1.3 and KCa3.1 blockade. To date, two compounds targeting Kv1.3 are in preclinical development but, so far, no Kv1.3 blocker has advanced into clinical trials. KCa3.1 blockers, on the other hand, have been evaluated in clinical trials for sickle cell anemia and exercise-induced asthma, but have so far not shown efficacy. However, the trial results support KCa3.1 as a safe therapeutic target, and will hopefully help enable clinical trials for other medical conditions that might benefit from KCa3.1 blockade.
Keywords: immunosuppression, potassium channel, Kv1.3, KCa3.1, TRAM-34, PAP-1
INTRODUCTION
Since the 1950s, ion channels in excitable cells such as myocytes and neurons have been extensively studied because of their importance in propagating electrical impulses. However, the importance of ion channels in non-excitable lymphocytes was not recognized until 1984, when DeCoursey et al. [DeCoursey et al. 1984] and Matteson et al. [Matteson and Deutsch 1984] discovered a voltage-gated potassium channel, later identified to be Kv1.3 [Grissmer et al. 1990], in human T cells. In the early 1990s, a second potassium channel, the calcium-activated potassium channel KCa3.1 was found in human and murine T and B cells [Grissmer et al. 1993; Logsdon et al. 1997; Mahaut-Smith and Schlichter 1989; Partiseti et al. 1992]. Studies since then have demonstrated that both channels help maintain a negative membrane potential, which is necessary to sustain the driving force for calcium entry, and therefore indirectly modulate many of the calcium-dependent cellular processes in T cells (for an extensive review see: [Cahalan and Chandy 2009]). The increase in cytosolic calcium concentration following T-cell receptor activation by antigen allows the nuclear factor of activated T cells (NFAT) to translocate to the nucleus and initiate transcription, ultimately leading to cytokine secretion and T cell proliferation (Figure 1). However, if potassium efflux through Kv1.3 and KCa3.1 is blocked, the T cell membrane depolarizes. Calcium influx through the CRAC (calcium-release activated calcium) channel is consequently reduced and T cell activation prevented [Cahalan and Chandy 2009]. Kv1.3 and KCa3.1 channel blockers have therefore been proposed as novel means for therapeutic immunosuppression [Chandy et al. 2004].
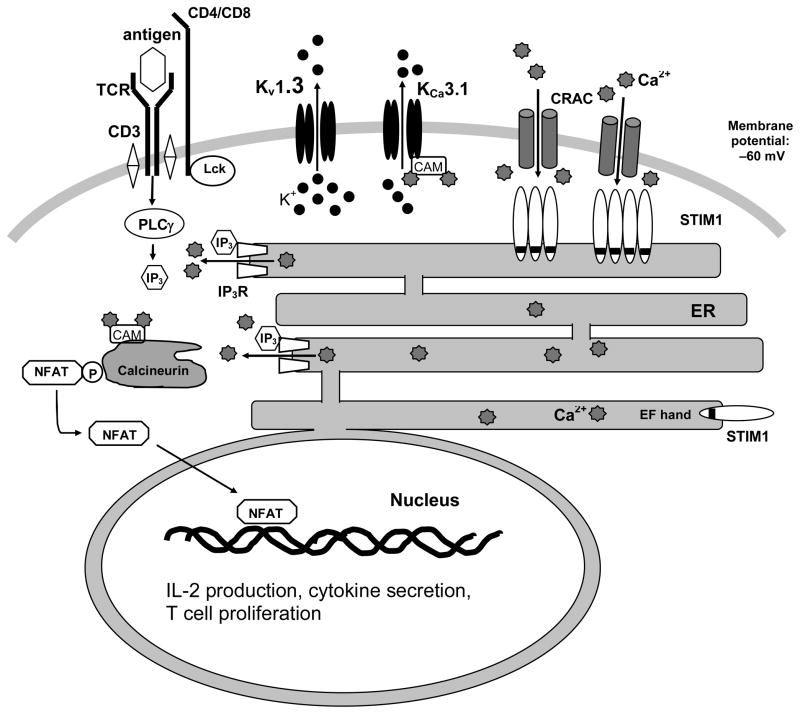
Figure 1.
The potassium channels Kv1.3 and KCa3.1 facilitate calcium signaling during T cell activation. Antigen presentation on major histocompatibility complex (MHC) class II molecules to the T-cell receptor-CD3 complex activates phospholipase C (PLC-γ), which produces inositol-1,4,5-triphosphate (IP3). IP3 binds to the IP3 receptor (IP3R) on the endoplasmatic reticulum (ER) membrane, which opens to release Ca2+ stored in the ER. When the EF-hand containing stromal interaction molecule 1 (STIM1), located on the ER membrane, senses depletion of internal Ca2+, it activates CRAC (Ca2+ release activate Ca2+) channels, located on the plasma membrane, to allow extracellular Ca2+ influx, which both depolarizes the cell membrane and increases cytosolic Ca2+ concentration. Kv1.3 channels and KCa3.1 channels then open in response to the membrane depolarization and increase in calcium concentration, respectively, and maintain the driving force for Ca2+ entry through CRAC channels. Ultimately, the rise in cytosolic Ca2+ activates the phosphatase calcineurin, leading to dephosphorylation of the transcription factor nuclear factor of activated T cells (NFAT), enabling its translocation into the nucleus.
Unlike the calcineurin inhibitors cyclosporine and tacrolimus, Kv1.3 and KCa3.1 blockers are usually viewed as immunomodulators rather than general immunosuppressants because Kv1.3 and KCa3.1 blockers preferentially suppress specific T or B cell subsets [Chandy et al. 2004; Wulff et al. 2003; Wulff et al. 2004]. For example, human T cells can be categorized into three subsets: naïve T cells, central memory T cells (TCM), and effector memory T cells (TEM) [Sallusto et al. 1999]. Naïve T cells, expressing both the chemokine receptor CCR7 and also the phosphatase, CD45RA, migrate to lymphoid organs and require a strong stimulus for activation. As naïve T cells are repeatedly challenged with the same antigen, they will differentiate into increasingly reactive memory T cells. TCM cells still express CCR7 and home to lymphoid organs, but have a lower activation threshold than naïve T cells [Geginat et al. 2001; Sallusto et al. 1999]. In contrast, CCR7− TEM cells home to sites of inflammation and have an even lower activation threshold [Sallusto et al. 1999]. The expression levels of Kv1.3 and KCa3.1 change in parallel with this change in T cell differentiation state. KCa3.1 expression is high in naïve and TCM cells, which are therefore susceptible to KCa3.1 blockers [Ghanshani et al. 2000]. Kv1.3, in contrast, is overexpressed in TEM cells, rendering TEM cells highly responsive to Kv1.3 blockers [Beeton et al. 2006; Wulff et al. 2003]. Therefore, specific blockers for these two channels do not globally inhibit all T cells, but rather selectively inhibit specific sub-populations [Chandy et al. 2004]. Kv1.3 and KCa3.1 blockers might consequently offer a more targeted approach to immunosuppression, distinct from the calcineurin inhibitors and from each other.
The therapeutic potential of Kv1.3 blockers for the management of T-cell mediated autoimmune diseases
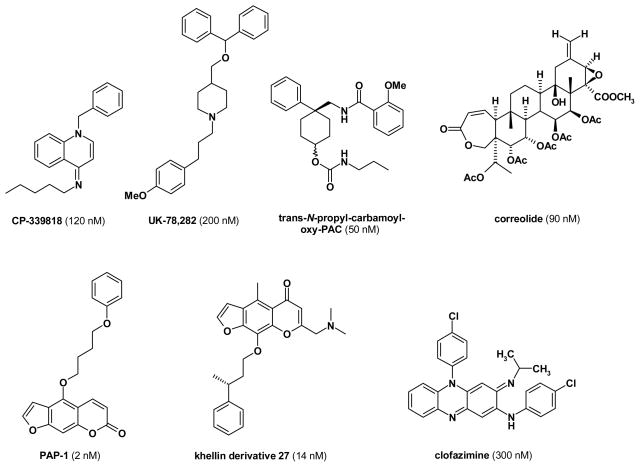
Like the 40 other voltage-gated potassium channels in the human genome [Harmar et al. 2009], Kv1.3 is a tetramer, with each subunit consisting of 6 transmembrane segments. The 5th and 6th transmembrane segments form the ion conduction pore, while the S4 segment contains the voltage-sensor. Membrane depolarization, detected by the voltage-sensor, induces a conformational change and opens the Kv1.3 channel pore, allowing potassium to efflux from the cell to offset depolarization. Because of its important role in T cell activation, Kv1.3 has a relatively well-studied pharmacology. The channel is blocked by a large number of venom-derived peptides such as the scorpion toxins margatoxin [Garcia-Calvo et al. 1993], charybdotoxin [Price et al. 1989] and OSK1 [Mouhat et al. 2005] or the sea anemone toxin Stichodactyla helianthus (ShK) toxin [Tudor et al. 1996]. Several pharmaceutical companies and academic groups have also attempted to discover small molecule inhibitors for Kv1.3 (see Figure 2 for structures). Before they seem to have abandoned their Kv1.3 programs, Pfizer and Merck have been pursuing Kv1.3 for immunosuppression in the 1990s and identified dihydroquinoline, piperidine, nortriterpene- or benzamide type Kv1.3 blockers exemplified by CP-339818 [Hill et al. 1995; Michne et al. 1995; Nguyen et al. 1996], UK-78,282 [Burgess et al. 1997; Hanson et al. 1999], correolide [Goetz et al. 1998; Koo et al. 1999; Felix et al. 1999; Hanner et al. 1999] and trans-N-propyl-carbamoyloxy-PAC [Miao et al. 2003; Schmalhofer et al. 2002]. Later identified small molecule Kv1.3 blockers are the phenoxyalkoxypsoralens PAP-1 by our laboratory [Schmitz et al. 2005], a series of related khellin and khellinone derived compounds [Baell et al. 2004; Flynn et al. 2009; Flynn et al. 2008a; Flynn et al. 2008b; Harvey et al. 2006] and the “old” leprosy drug clofazimine [Ren et al. 2008].
Figure 2.
Chemical structures of Kv1.3 blockers.
As mentioned above, Kv1.3 is the predominant potassium channel in TEM cells [Beeton et al. 2006; Wulff et al. 2003]. Upon activation, TEM cells increase Kv1.3 expression from approximately 300 channels per cell in the resting state to 1500–2000 channels per cell in activated cells. Unlike naïve and TCM cells, TEM cells do not express the chemokine receptor CCR7, and therefore do not localized to lymphoid organs. Instead, TEM cells directly target to sites of inflammation [Kivisakk et al. 2004; Matheu et al. 2008; Rus et al. 2005], where they play an immediate effector function, secreting interferon-γ, interleukin-2, and tumor necrosis factor-α, and have been reported to be involved in the pathogenesis of T-cell mediated autoimmune diseases, such as multiple sclerosis (MS), rheumatoid arthritis (RA), type-1 diabetes, and psoriasis.
MS is an autoimmune condition affecting the central nervous system, characterized by inflammation, demyelination and axon degeneration [Lassmann et al. 2007; Steinman 2001]. It is associated with a high morbidity, and patients can suffer from a wide range of neurologic symptoms that include optic neuritis, nystagmus, sensory impairment, gait imbalance, paraparesis, paraplegia, bowel and bladder dysfunction, and sexual dysfunction. MS is oftentimes treated with potent immunosuppressants and immunomodulators, such as methylprenisolone, methotrexate, cyclophosphamide and more recently natalizumab and fingolimod [Aharoni 2010]. As a chronic condition, MS requires life-long treatment, which becomes complicated by the adverse side-effects and risks associated with the prolonged use of immunosuppressants. Therefore, there is a need for better MS treatments and Kv1.3 blockers might be one possible therapeutic option. Myelin-antigen reactive T cells in the blood and infiltrating T cells in postmortem brain sections of MS patients have been shown to be predominantly Kv1.3highCCR7− TEM cells [Rus et al. 2005; Wulff et al. 2003]. Kv1.3 was further validated as a potential target for MS by the observation that Kv1.3 blocking peptides like kaliotoxin, ShK and several ShK derivatives effectively treat both monophasic adoptive-transfer and chronic-relapsing experimental autoimmune encephalomyelitis (EAE) in rats [Beeton et al. 2001a; Beeton et al. 2005; Beeton et al. 2001b; Matheu et al. 2008]. Based on these encouraging results Airmid (Airmid.com) and Kineta Inc. (Kinetabio.com) are currently performing preclinical studies with ShK derivatives [Beeton et al. 2005; Pennington et al. 2009] as injectables for the treatment of MS, while efforts are ongoing to prolong the short half-life of OSK1 by conjugating these 35 amino acid peptides to Fc antibody fragments [Sullivan et al. 2006].
Another potential therapeutic application of Kv1.3 blockers is rheumatoid arthritis (RA), which is an autoimmune condition that affects the joints with erosion of cartilage and bone, ultimately resulting in joint deformation [Scott et al. 2010]. T cells isolated from the synovial fluid of patients with RA are mainly Kv1.3highCCR7− TEM cells [Beeton et al. 2006]. In comparison, T cells from osteoarthritis patients are primarily Kv1.3lowCCR7+ naïve T cells and TCM cells. In the pristane-induced arthritis model using Dark Agoti rats, 21 days of treatment with ShK-L5-amide significantly decreased the number of affected joints and reduced the severity of radiological and histopathological findings [Beeton et al. 2006].
Type-1 diabetes, which is characterized by autoimmune destruction of the insulin-producing pancreatic beta cells eventually leading to absolute insulin deficiency [van Belle et al.], is another autoimmune disease that might respond to pharmacologic Kv1.3 blockade. Beeton et al. {2006] reported that insulin and GAD-65 reactive T cells in the blood of children with new-onset type I diabetes are predominantly Kv1.3highCCR7− TEM cells [Beeton et al. 2006]. In contrast, islet-antigen reactive T cells obtained from type-2 diabetic patients or healthy controls are mostly Kv1.3lowCCR7+ naïve T cells and TCM cells. The same study reported that the Kv1.3 blocker, PAP-1 delays diabetes onset and reduces diabetes incidence in type-1 diabetes prone BioBreeding Worchester (DP-BB/Wor) rats, which spontaneously develop type-1 diabetes mellitus. Furthermore, histological analysis demonstrated both an increase in the number of intact islets and a decrease in intra-islet T cell and macrophage infiltration. Interestingly, ShK-L5, the peptidic Kv1.3 blocker, was also tested in the DP-BB/Wor rat diabetes model, but was less effective than PAP-1 presumably because the more lipophilic PAP-1 was able to concentrate in the pancreas better. Like PAP-1, ShK-L5 delayed diabetes onset, but it did not reduce diabetes incidence at day-110 of age [Wulff and Pennington 2007].
Kv1.3 blockers might also be useful for the treatment of inflammatory skin conditions like psoriasis and allergic contact dermatitis (ACD). Psoriasis is a chronic inflammatory skin disease that oftentimes presents as erythematous papules or plaques with a characteristic silvery scale [Nestle et al. 2009]. ACD in contrast is a skin hypersensitivity reaction to allergens or irritants [Saint-Mezard et al. 2004]. CD8+ TEM cells are thought to play a major role in both ACD and psoriasis, although the pathology of psoriasis has also been shown to involve CD4+ T cells, dendritic cells and keratinocytes [Gudjonsson et al. 2004]. While ACD itself is a very common condition, it is also a useful model system to evaluate potential therapies for psoriasis. Since the small molecule Kv1.3 blocker PAP-1 is lipophilic (Figure 2) it could potentially be developed into a topical treatment for psoriasis and ACD. In an ACD animal model, female Lewis rats were first sensitized to oxazolone and then challenged with local application of oxazolone onto the ear 7 days post-sensitization. PAP-1 treatment effectively suppressed oxazolone-induced inflammation, inhibited CD8+ T cell and macrophage infiltration and reduced production of the inflammatory cytokines interferon-γ, interleukin-2, and interleukin-17 [Azam et al. 2007]. Another study reported that in severe combined immunodeficient (SCID) mice direct injection of the peptide ShK into transplanted skin grafts from psoriasis patients reduced inflammatory responses in a subset of responding grafts [Gilhar et al.]. Similar promising observations have been made in delayed-type hypersensitivity (DTH), which is mediated by CD4+ TEM cells [Soler et al. 2003]. While scientists at Merck demonstrated that correolide and margatoxin prevent DTH in mini-pigs [Koo et al. 1999; Koo et al. 1997], our own group showed that PAP-1 reduces DTH in rats [Schmitz et al. 2005]. As demonstrated by elegant in vivo two-photon imaging experiments, Kv1.3 blockers suppress DTH and other TEM responses by preventing TEM cell reactivation and inhibiting their migration in inflamed tissue [Matheu et al. 2008].
Based on the aforementioned studies and experiments demonstrating that Kv1.3 blockers can also prevent anti-glomerular basement membrane glomerulonephritis in rats [Hyodo et al.] and skin transplant rejection in immunodeficient mice reconstituted with human T cells [Ren et al. 2008], there is a cogent argument for the use of Kv1.3 inhibitors as effective immunomodulators in the management of T-cell mediated autoimmune diseases, especially since Kv1.3 appears to be a relatively safe therapeutic target. Although the channel is also expressed in macrophages [Mackenzie et al. 2003; Vicente et al. 2003] osteoclasts [Arkett et al. 1994], platelets [Maruyama 1987], microglia [Khanna et al. 2001; Schlichter et al. 1996], adipocytes [Pappone and Ortiz-Miranda 1993], oligodendrocytes [Chittajallu et al. 2002], and the olfactory bulb [Fadool and Levitan 1998; Fadool et al. 2004], a 28-day and a 6-month toxicity study with PAP-1, and a 28-day toxicity study with ShK-L5 in male and female rats found no histopathological changes, and no hematological or serum chemistry changes [Azam et al. 2007; Beeton et al. 2006]. In keeping with the observation made in two-photon imaging experiments that Kv1.3 blockers have no effect on homing to or motility in lymph nodes of naive and TCM cells, Kv1.3 blockers also did not delay influenza virus clearance in rats [Matheu et al. 2008]. Additionally, PAP-1 was studied for 28 days in rhesus macaques with no evidence for any changes in blood chemistry or hematology [Pereira et al. 2007]. The treatment did not prevent the development of a protective TCM-mediated immune response to nasal flu vaccination. However, since TEM cells are involved in suppressing chronic viral infections, PAP-1 treatment was associated with a PCR detectable CMV virus reactivation, which did not result in any symptoms [Pereira et al. 2007].
KCa3.1 as a therapeutic target for immunosuppression and inflammation
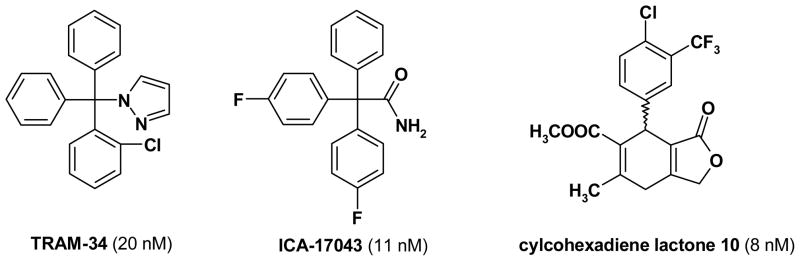
KCa3.1 resembles Kv1.3 in having 6 transmembrane segments, but instead of being activated by voltage, the calcium-activated KCa3.1 channel opens in response to increases in intracellular calcium [Joiner et al. 1997; Logsdon et al. 1997]. This “calcium-gating” is mediated by calmodulin, which is tightly bound to the channel’s intracellular C-terminus [Fanger et al. 1999]. Similar to Kv1.3, KCa3.1 has a relatively well-developed pharmacology. The channel is inhibited by the scorpion toxins maurotoxin, charybdotoxin and the charybdotoxin derivative ChTX-Glu32 [Castle et al. 2003; Kharrat et al. 1996; Rauer et al. 2000]. It further has three potent and selective small molecule blockers. Our own group designed the small molecule KCa3.1 blocker TRAM-34 [Wulff et al. 2000], while scientists at Icagen Inc. developed the fluorinated triphenyl acetamide ICA-17043 [Ataga et al. 2006], and chemists at Bayer identified a class of 4-phenyl pyrans and related compounds exemplified by the cylcohexadiene lactone 10 [Mauler et al. 2004]. For structures please see Figure 3 and for an extensive review of the medicinal chemistry of KCa3.1 see [Wulff et al. 2007].
Figure 3.
Chemical structures of KCa3.1 blockers.
In contrast to Kv1.3, which is highly expressed in TEM cells, KCa3.1 expression is important in naïve T cells and TCM cells. Both naïve T cells and TCM cells, when activated, transcriptionally up-regulate KCa3.1 from less than 20 KCa3.1 channels per cell to approximately 500 channels per cell, without a change in Kv1.3 expression [Ghanshani et al. 2000]. Genetic deletion of KCa3.1 in mice resulted in decreased T cell receptor-stimulated calcium influx and IL-2 production in undifferentiated Th0 cells, but did not interfere with CD4 T cell differentiation. However, calcium influx and cytokine production remained impaired in KCa3.1−/− Th1 and Th2 CD4 T cells, while T-regulatory and Th17 functions were found to be normal in these mice [Di et al. 2010]. In addition to T cells, KCa3.1 channels are also expressed in innate immune cells like mast cells [Duffy et al. 2001], macrophages [Hanley et al. 2004], microglia [Khanna et al. 2001], and in non-immune cell types, including dedifferentiated proliferating smooth muscles [Kohler et al. 2003; Neylon et al. 1999], vascular endothelium [Grgic et al. 2009a], fibroblasts [Pena and Rane 1999], and airway epithelium [McCann et al. 1990]. The role of KCa3.1 in these other cell types is similar to its role in T lymphocytes; KCa3.1 essentially affects cell activation and proliferation through its modulation of calcium influx by maintaining a negative membrane potential [Wulff and Castle 2010]. In contrast to Kv1.3 blockers (see above), KCa3.1 blockers are therefore thought to be more useful in suppressing more acute immune responses but have also been evaluated in T-cell mediated diseases like inflammatory bowel disease (IBD), EAE, RA and asthma. However, because of the channel’s diverse expression pattern in these other cell types, KCa3.1 blockade has also been studied in cardiovascular conditions such as atherosclerosis and restenosis.
Inflammatory bowel disease (IBD) is an inclusive term that describes a broad group of chronic relapsing intestinal inflammatory disorders [Engel and Neurath 2010]. It can present with abdominal pain, diarrhea, gastrointestinal bleeding and/or hemorrhage, weight loss, and vomiting, in addition to a variety of extraintestinal symptoms. Furthermore, chronic IBD has been associated with colon cancer [Kulaylat and Dayton 2010]. IBD includes two major forms, Crohn’s disease (CD) and ulcerative colitis (UC). Both CD and UC are thought to involve CD4+ T cells, with CD traditionally being considered a Th1 CD4+ disease and UC a Th2 CD4+ disease. Since KCa3.1 is expressed in CD4+ T cells, where it has a crucial role in T cell activation, KCa3.1 has been proposed as a novel treatment for IBD. This hypothesis was tested in two different mouse models of IBD. In one experiment, CD4+CD25−CD45RBhigh T cells from either wild-type mice or KCa3.1−/− mice were transferred into a Rag2−/− recipient [Di et al. 2010]. This adoptive transfer model of IBD is characterized by mucosal infiltration of immune cells and symptoms of chronic diarrhea and weight loss. Results showed that Rag2−/− mice that were injected with wild-type cells lost more weight than the mice that received KCa3.1−/− T cells. Histological evaluation of the colons corroborated the weight loss data and showed significantly less inflammatory cell infiltration and tissue destruction in the Rag2−/− mice that received Kca3.1−/− T cells [Di et al. 2010]. In another set of experiments, bowel inflammation was induced with trinitrobenzene sulfonic acid (TNBS). Mice that were treated with the KCa3.1 inhibitor TRAM-34 had significantly less inflammation macroscopically and by histological analysis than vehicle treated control mice. Interestingly, in a recent study with CD patients in Australia and New Zealand, a KCa3.1 (KCNN4) single nucleotide polymorphism has been associated with illeal CD [Simms et al. 2010].
The KCa3.1 blockers, TRAM-34 and ICA-17043 have further been reported to be effective in MOG-peptide induced EAE in mice [Reich et al. 2005] and in collagen antibody induced rheumatoid arthritis [Chou et al. 2008]. However, in both cases, effects on microglia or macrophages are likely to have participated since both models involve a strong innate immune system component. For example, in cultured microglia KCa3.1 blockade has been reported to reduce respiratory burst, iNOS induction, nitric oxide production [Kaushal et al. 2007; Khanna et al. 2001; Schilling et al. 2004], while in organotypic hippocampal slices, which better reflect in vivo conditions, TRAM-34 has been found to reduce microglia mediated neuronal killing [Maezawa et al. 2011]. Additional evidence for a neuroprotective role for KCa3.1 blockers comes from the observation that both cylcohexadiene lactone and triarylmethane type KCa3.1 inhibitors reduce brain edema and infarct volume caused by traumatic brain injury in rats [Mauler et al. 2004].
Asthma is a chronic obstructive airway disorder that can cause a myriad of respiratory symptoms like wheezing, chest-tightness, and chronic cough, often exacerbated by allergies, cold or warm temperatures, and physical activity. It is pathologically characterized by inflammation, bronchial hyper-responsiveness, and chronic airway remodeling. Essentially, there are recurrent injuries to the airway, followed by an impaired healing response by the airway epithelium, with production of inflammatory cytokines and profibrogenic growth factors, leading to more inflammation, bronchial hyper-responsiveness and chronic airway remodeling. Amongst the changes noted, there is smooth muscle hypertrophy and hyperplasia, fibroblast proliferation, and increase subepithelial collagen deposition. KCa3.1 might be relevant as a target for attenuating some of the pathological processes that occur in asthma based on its expression in many of the cell types implicated in asthma: the airway epithelium, proliferating smooth muscle cells, vascular endothelium, fibroblasts, mast cells, macrophages, and Th2 cells (see [Bradding and Wulff 2009] for a review). Specifically, KCa3.1 appears to play a critical role in immediate allergic reactions, like anaphylaxis, based on the observations that KCa3.1 knockout mice have less severe systemic anaphylactic reactions [Shumilina et al. 2008], and that pharmacological KCa3.1 blockade dampens mast cell degranulation [Cruse et al. 2006; Duffy et al. 2004]. KCa3.1 also appears to be an important modulator of bronchial hyper-responsiveness and airway remodeling; inhibition with TRAM-34 attenuates both vascular and airway smooth muscle proliferation [Kohler et al. 2003; Shepherd et al. 2007], while charybdotoxin has long been known to reduce FGF-induced fibroblast proliferation [Pena et al. 2000]. In an unilateral uretral obstruction model of kidney fibrosis, KCa3.1 deletion as well as pharmacological blockade of KCa3.1 significantly reduced renal fibrosis in mice and rats [Grgic et al. 2009c], suggesting that KCa3.1 blockers might have similar effects on airway fibrotic changes and remodelling. In a sheep allergen challenge model of asthma, ICA-17043 indeed was able to reduce the increase in airway resistance and hyper-reactivity [Robinette et al. 2008] and based on these encouraging results, Icagen Inc. subsequently initiated a small Phase II allergen challenge study in patients with allergic asthma. Two weeks of treatment with ICA-17043 at 40 mg/day reduced the inflammatory marker exhaled nitric oxide as well as late allergen mediated increases in airway resistance. Unfortunately, in a second proof-of-concept Phase II trial, in which the effect of ICA-17043 on exercise induced asthma was examined, no improvement in lung function was observed following 4 weeks of treatment (Icagen Inc. www.icagen.com). Taken together, these studies overall support the hypothesis that KCa3.1 blockers might be useful in the management of asthma [Bradding and Wulff 2009], but suggest that clinical trials should be longer-term and that emphasis should be placed on evaluation of airway remodeling.
Because of the involvement of KCa3.1 in the proliferation of dedifferentiated vascular smooth muscle cells in addition to its proposed role in inflammation, KCa3.1 has also been studied in cardiovascular diseases like post-angioplasty restenosis and atherosclerosis. In a rat model of balloon angioplasty Köhler et al. demonstrated that 6 weeks of TRAM-34 treatment significantly reduces neointimal smooth muscle hyperplasia (restenosis) in the carotid artery [Kohler et al. 2003]. These observations were later translated into the porcine coronary overstretch injury model, which very closely mimics post-angioplasty injury seen in humans. In this case, direct administration of TRAM-34 to the coronary vessel wall through a drug-coated balloon catheter during angioplasty decreased restenosis and smooth muscle phenotypic changes [Tharp et al. 2008].
Atherosclerosis is a prevalent cardiovascular condition characterized by accumulation of lipids and cholesterol in the arterial wall with concomitant activation and proliferation of vascular smooth muscle cells (VSMCs), inflammatory monocytes/macrophages, and T lymphocytes. During this vascular remodeling process, VSMCs dedifferentiate from a contractile state into a proliferative state and migrate to form a fibrous cap overlying the lipid core. Inflammation occurs with T lymphocyte and macrophage activation, ultimately leading to endothelial cell injury and destruction. In keeping with its known expression in proliferating VSMCs [Kohler et al. 2003], macrophages [Hanley et al. 2004], and T lymphocytes [Ghanshani et al. 2000], KCa3.1 expression has been found to be elevated in the VSMCs, macrophages, and T lymphocytes isolated from atherosclerotic lesions in ApoE−/− mice [Toyama et al. 2008], a commonly used animal model of atherosclerosis. To explore the therapeutic potential of KCa3.1 blockers in the management of atherosclerosis, Toyama et al. [2008] therefore tested two KCa3.1 blockers, TRAM-34 and clotrimazole, in ApoE−/− mice. KCa3.1 blockade proved to be an effective novel therapeutic strategy, with the data showing that pharmacological KCa3.1 blockade suppressed VSMC proliferation and migration, decreased macrophage and T lymphocyte infiltration into atherosclerotic plaques, and thereby significantly reducing atherosclerosis development [Toyama et al. 2008].
Like Kv1.3, KCa3.1 seems to be a relatively safe therapeutic target. Genetic deletion of KCa3.1 results in benign phenotypes. Two independently generated KCa3.1−/− mice populations are both viable, without gross abnormalities in any major organs, and are able to produce normal litter sizes [Begenisich et al. 2004; Si et al. 2006]. There are a few mild phenotypic findings in KCa3.1−/− mice: an impaired volume regulation in erythrocytes and lymphocytes [Begenisich et al. 2004], a reduced endothelial-derived hyperpolarizing factor (EDHF) response with a concomitant mild increase in blood pressure [Si et al. 2006], as well as subtle erythrocyte macrocytosis and progressive splenomegaly [Grgic et al. 2009b]. Studies of KCa3.1 blockers also show a safe toxicity profile. There were no changes in blood chemistry, hematology, or necropsy of major organs found with daily administration of TRAM-34 at 120 mg/kg in a 28-day toxicity study in mice and rats [Toyama et al. 2008]. ICA-17043 which was in clinical trials for sickle cell anemia and asthma, also appears safe [Ataga and Stocker 2009]. The Phase III trial for sickle cell anemia showed no associated toxicities but was terminated in 2007 because of lack of efficacy in reducing sickling crisis despite the fact that ICA-17043 improved haemolysis and indicators of erythrocyte survival [Ataga et al. 2011].
DISCUSSION
Given the requirement for long-term treatment in many T-cell mediated autoimmune diseases and other chronic inflammatory conditions, there is an unmet need for alternative immunomodulating drugs that are effective, yet safe for prolonged use. As has been put forth in this review, selective blockers for the potassium channels Kv1.3 and KCa3.1 might constitute useful additions to our therapeutic toolbox. Kv1.3 and KCa3.1 are important for calcium influx during T cell activation. In a sense, Kv1.3 and KCa3.1 blockers, resemble calcineurin inhibitors by disrupting calcium signaling and thus dampening T cell activation. However, Kv1.3 and KCa3.1 blockers offer a more selective approach to immunosuppression because they preferentially suppress certain T cell subpopulations, thus allowing a more targeted therapeutic approach; Kv1.3 blockers preferentially affect CCR7−TEM cells and are therefore particularly suited for the treatment of autoimmune diseases like MS, RA and psoriasis. KCa3.1 blockers in contrast target naïve and TCM T cells, but also affect the activation, migration and proliferation of macrophages, microglia, mast cells, dedifferentiated vascular smooth muscle cells and their therapeutic potential therefore extends beyond purely T-cell driven diseases to such conditions as asthma, restenosis, atherosclerosis, and fibrosis.
To date, Kv1.3 and KCa3.1 blockers have both shown encouraging results in multiple, disease-relevant in vitro and in vivo studies, and the time now seems right to finally translate more than 20 years of work on the physiological and pathophysiological role of these channels into clinical trials. In this respect, it is of course disappointing that ICA-17043 failed in trials for sickle cell anemia and more recently in exercise induced asthma. However, these trials have at least shown the field that targeting KCa3.1 is safe and well tolerated and now provide encouragement for potential clinical trials with KCa3.1 blockers for IBD, airway remodeling in asthma, prevention of coronary restenosis after angioplasty, atherosclerosis and potentially neuroinflammation associated with MS and other neurological disorders. It should also be mentioned here that the archetypal KCa3.1 inhibitor clotrimazole, which served as the chemical template for both TRAM-34 and ICA-17043, was reported to improve the symptoms of rheumatoid arthritis in a small clinical trial in the 1980s [Wojtulewski et al. 1980].
For Kv1.3 no compound has reached clinical trials yet, but several pharmaceutical companies including Amgen, Solvay/Abbott) and Bionomics are active in the Kv1.3 area ([Wulff et al. 2009]) and Airmd (Airmid.com) and Kineta Inc. (Kinetabio.com) are currently performing preclinical studies with ShK derivatives and are expected to be in Phase-1 human trials by the end of 2011 with an injectable for MS.
Acknowledgments
Funding/Support Information: This publication was made possible by Grant Number UL1 RR024146 from the National Center for Research for Medical Research to JL, and the American Recovery and Reinvestment Act Stimulus Fund (NIH R01GM076063-04S1 to HW).
Footnotes
Conflict of Interest: HW is an inventor on several University of California patents claiming PAP-1 and TRAM-34 for immunosuppression. She is also a scientific founder of Airmid, a start-up company that is aiming to develop Kv1.3 blockers as immunosuppressants.
References
- Aharoni R. Immunomodulatory drug treatment in multiple sclerosis. Expert Rev Neurother. 2010;10:1423–1436. doi: 10.1586/ern.10.117. [DOI] [PubMed] [Google Scholar]
- Arkett SA, Dixon J, Yang JN, Sakai DD, Minkin C, Sims SM. Mammalian osteoclasts express a transient potassium channel with properties of Kv1.3. Receptors Channels. 1994;2:281–293. [PubMed] [Google Scholar]
- Ataga KI, Orringer EP, Styles L, Vichinsky EP, Swerdlow P, Davis GA, Desimone PA, Stocker JW. Dose-escalation study of ICA-17043 in patients with sickle cell disease. Pharmacotherapy. 2006;26:1557–1564. doi: 10.1592/phco.26.11.1557. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Reid M, Ballas SK, Yasin Z, Bigelow C, James LS, Smith WR, Galacteros F, Kutlar A, Hull JH, Stocker JW. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043) Br J Haematol. 2011;153:92–104. doi: 10.1111/j.1365-2141.2010.08520.x. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Stocker J. Senicapoc (ICA-17043): a potential therapy for the prevention and treatment of hemolysis-associated complications in sickle cell anemia. Expert Opin Investig Drugs. 2009;18:231–239. doi: 10.1517/13543780802708011. [DOI] [PubMed] [Google Scholar]
- Azam P, Sankaranarayanan A, Homerick D, Griffey S, Wulff H. Targeting effector memory T cells with the small molecule Kv1.3 blocker PAP-1 suppresses allergic contact dermatitis. J Invest Dermatol. 2007;127:1419–1429. doi: 10.1038/sj.jid.5700717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell JB, Gable RW, Harvey AJ, Toovey N, Herzog T, Hansel W, Wulff H. Khellinone derivatives as blockers of the voltage-gated potassium channel Kv1.3: synthesis and immunosuppressive activity. J Med Chem. 2004;47:2326–2336. doi: 10.1021/jm030523s. [DOI] [PubMed] [Google Scholar]
- Beeton C, Barbaria J, Giraud P, Devaux J, Benoliel A, Gola M, Sabatier J, Bernard D, Crest M, Beraud E. Selective Blocking of Voltage-Gated K+ Channels Improves Experimental Autoimmune Encephalomyelitis and Inhibits T Cell Activation. J Immunol. 2001a;166:936–944. doi: 10.4049/jimmunol.166.2.936. [DOI] [PubMed] [Google Scholar]
- Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA, Chandy KG. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol. 2005;67:1369–1381. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Beraud E. Selective Blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci USA. 2001b;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, Leehealey CJ, BSA, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- Bradding P, Wulff H. The K+ channels KCa3.1 and Kv1.3 as novel targets for asthma therapy. Br J Pharmacol. 2009;157:1330–1339. doi: 10.1111/j.1476-5381.2009.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess LE, Kock K, Cooper K, Biggers MS, Ramchandani M, Smitrovich JH, Gilbert EJ, Bruns MJ, Mather RJ, Donovan CB, Hanson DC. The SAR of UK-78,282: a novel blocker of human T cell Kv1.3 potassium channels. Bioorg Med Chem Lett. 1997;7:1047–1052. [Google Scholar]
- Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle NA, Lodon DO, Creech C, Fajloun Z, Stocker JW, Sabatier J-M. Maurotoxin - a potent inhibitor of the intermediate conductance Ca2+-activated potassium channel. Mol Pharmacol. 2003;63:409–418. doi: 10.1124/mol.63.2.409. [DOI] [PubMed] [Google Scholar]
- Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. Potassium channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Chen Y, Wang H, Yuan X, Ghiani CA, Heckman T, CJM, Gallo V. Regulation of Kv1 subunit expression in oligodendrocyte progentitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci USA. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CC, Lunn CA, Murgolo NJ. KCa3.1: target and marker for cancer, autoimmune disorder and vascular inflammation? Expert Rev Mol Diagn. 2008;8:179–187. doi: 10.1586/14737159.8.2.179. [DOI] [PubMed] [Google Scholar]
- Cruse G, Duffy SM, Brightling CE, Bradding P. Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax. 2006;61:880–885. doi: 10.1136/thx.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Di L, Srivastava S, Zhdanova O, Ding Y, Li Z, Wulff H, Lafaille M, Skolnik EY. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci USA. 2010;107:1541–1546. doi: 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SM, Berger P, Cruse G, Yang W, Bolton SJ, Bradding P. The K+ channel iKCA1 potentiates Ca2+ influx and degranulation in human lung mast cells. J Allergy Clin Immunol. 2004;114:66–72. doi: 10.1016/j.jaci.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Duffy SM, Lawley WJ, Conley EC, Bradding P. Resting and activation-dependent ion channels in human mast cells. J Immunol. 2001;167:4261–4270. doi: 10.4049/jimmunol.167.8.4261. [DOI] [PubMed] [Google Scholar]
- Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571–583. doi: 10.1007/s00535-010-0219-3. [DOI] [PubMed] [Google Scholar]
- Fadool DA, Levitan IB. Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J Neurosci. 1998;18:6126–6137. doi: 10.1523/JNEUROSCI.18-16-06126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron. 2004;41:389–404. doi: 10.1016/s0896-6273(03)00844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger CM, Ghanshani S, Logsdon NJ, Rauer H, Kalman K, Zhou J, Beckingham K, Chandy KG, Cahalan MD, Aiyar J. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J Biol Chem. 1999;274:5746–5754. doi: 10.1074/jbc.274.9.5746. [DOI] [PubMed] [Google Scholar]
- Felix JP, Bugianesi RM, Schmalhofer WA, Borris R, Goetz MA, Hensens OD, Bao JM, Kayser F, Parsons WH, Rupprecht K, Garcia ML, Kaczorowski GJ, Slaughter RS. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, Kv1.3. Biochemistry. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- Flynn B, Baell JB, Chaplin JH, Gill GS, Grobelyn DW, Harvey AJ, Mould JA, Paul D. 2009043117. Bionomics Limited; Australia: WO. 2009 Novel aryl potassium channel blockers and uses thereof.
- Flynn B, Baell JB, Harvey AJ, Chaplin JH, Paul D, Mould JA. WO2008040057. Bionomics Limited; Australia: 2008a Novel benzofuran potassium channel blockers and uses thereof.
- Flynn B, Baell JB, Harvey AJ, Chaplin JH, Paul D, Mould JA. 2008040058. Bionomics Limited; Australia: WO. 2008b Novel chromenone potassium channel blockers and uses thereof.
- Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ, Garcia ML. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem. 1993;268:18866–18874. [PubMed] [Google Scholar]
- Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Cahalan MD, Chandy KG. Up-regulation of the IKCa1 potassium channel during T-cell activation: Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Bergman R, Assay B, Ullmann Y, Etzioni A. The beneficial effect of blocking Kv1.3 in the psoriasiform SCID mouse model. J Invest Dermatol. 2011;131:118–124. doi: 10.1038/jid.2010.245. [DOI] [PubMed] [Google Scholar]
- Goetz MA, Hensens OD, Zink DL, Borris RP, Morales F, Tamayo-Castillo G, Slaughter RS, Felix J, Ball RG. Potent Nor-triterpenoid blockers of the voltage-gated potassium channel Kv1.3 from Spachea correae. Tetrahedron Lett. 1998;39:2895–2898. [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009a;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Paschen S, Kaistha A, Busch C, Si H, Kohler K, Elsasser HP, Hoyer J, Kohler R. Disruption of the Gardos channel (KCa3.1) in mice causes subtle erythrocyte macrocytosis and progressive splenomegaly. Pflugers Arch. 2009b;458:291–302. doi: 10.1007/s00424-008-0619-x. [DOI] [PubMed] [Google Scholar]
- Grgic I, Kiss E, Kaistha BP, Busch C, Kloss M, Sautter J, Muller A, Kaistha A, Schmidt C, Raman G, Wulff H, Strutz F, Grone HJ, Kohler R, Hoyer J. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc Natl Acad Sci USA. 2009c;106:14518–14523. doi: 10.1073/pnas.0903458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S, Dethlefs B, Wasmuth JJ, Goldin AL, Gutman GA, Cahalan MD, Chandy KG. Expression and chromosomal localization of a lymphocyte K+ channel gene. Proc Natl Acad Sci USA. 1990;87:9411–9415. doi: 10.1073/pnas.87.23.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Cahalan MD. Calcium-activated potassium channels in resting and activated human T lymphocytes. Expression levels, calcium dependence, ion selectivity, and pharmacology. J Gen Physiol. 1993;102:601–630. doi: 10.1085/jgp.102.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, Musset B, Renigunta V, Limberg SH, Dalpke AH, Sus R, Heeg KM, Preisig-Muller R, Daut J. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc Natl Acad Sci USA. 2004;101:9479–9484. doi: 10.1073/pnas.0400733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Schmalhofer WA, Green B, Bordallo C, Liu J, Slaughter RS, Kaczorowski GJ, Garcia ML. Binding of correolide to Kv1 family potassium channels. J Biol Chem. 1999;274:25237–25244. doi: 10.1074/jbc.274.36.25237. [DOI] [PubMed] [Google Scholar]
- Hanson DC, Nguyen A, Mather RJ, Rauer H, Koch K, Burgess LE, Rizzi JP, Donovan CB, Bruns MJ, Canniff PC, Cunningham AC, Verdries KA, Mena E, Kath JC, Gutman GA, Cahalan MD, Grissmer S, Chandy KG. UK-78,282, a novel piperidine compound that potently blocks the Kv1.3 voltage-gated potassium channel and inhibits human T cell activation. Br J Pharmacol. 1999;126:1707–1716. doi: 10.1038/sj.bjp.0702480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Hills RA, Rosser EM, Jones M, Buneman OP, Dunbar DR, Greenhill SD, Hale VA, Sharman JL, Bonner TI, Catterall WA, Davenport AP, Delagrange P, Dollery CT, Foord SM, Gutman GA, Laudet V, Neubig RR, Ohlstein EH, Olsen RW, Peters J, Pin JP, Ruffolo RR, Searls DB, Wright MW, Spedding M. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucleic Acids Res. 2009;37:D680–685. doi: 10.1093/nar/gkn728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AJ, Baell JB, Toovey N, Homerick D, Wulff H. A new class of blockers of the voltage-gated potassium channel Kv1.3 via modification of the 4- or 7-position of khellinone. J Med Chem. 2006;49:1433–1441. doi: 10.1021/jm050839v. [DOI] [PubMed] [Google Scholar]
- Hill RJ, Grant AM, Volberg W, Rapp L, Faltynek C, Miller D, Pagani K, Baizman E, Wang S, Guiles JW, et al. WIN 17317-3: novel nonpeptide antagonist of voltage-activated K+ channels in human T lymphocytes. Mol Pharmacol. 1995;48:98–104. [PubMed] [Google Scholar]
- Hyodo T, Oda T, Kikuchi Y, Higashi K, Kushiyama T, Yamamoto K, Yamada M, Suzuki S, Hokari R, Kinoshita M, Seki S, Fujinaka H, Yamamoto T, Miura S, Kumagai H. Voltage-gated potassium channel Kv1.3 blocker as a potential treatment for rat anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol. 2010;299:F1258–1269. doi: 10.1152/ajprenal.00374.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci USA. 1997;94:11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal V, Koeberle PD, Wang Y, Schlichter LC. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J Neurosci. 2007;27:234–244. doi: 10.1523/JNEUROSCI.3593-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Roy L, Zhu X, Schlichter LC. K+ channels and the microglial respiratory burst. Am J Physiol Cell Physiol. 2001;280:C796–806. doi: 10.1152/ajpcell.2001.280.4.C796. [DOI] [PubMed] [Google Scholar]
- Kharrat R, Mabrouk K, Crest M, Darbon H, Oughideni R, Martin-Eauclaire MF, Jacquet G, el Ayeb M, Van Rietschoten J, Rochat H, Sabatier JM. Chemical synthesis and characterization of maurotoxin, a short scorpion toxin with four disulfide bridges that acts on K+ channels. Eur J Biochem. 1996;242:491–498. doi: 10.1111/j.1432-1033.1996.0491r.x. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B, Wujek J, Ravid R, Staugaitis SM, Lassmann H, Ransohoff RM. Expression of CCR7 in multiple sclerosis: Implications for CNS immunity. Ann Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- Kohler R, Wulff H, Eichler I, Kneifel M, Neumann D, Knorr A, Grgic I, Kampfe D, Si H, Wibawa J, Real R, Borner K, Brakemeier S, Orzechowski HD, Reusch HP, Paul M, Chandy KG, Hoyer J. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108:1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- Koo GC, Blake JT, Shah K, Staruch MJ, Dumont F, Wunderler D, Sanchez M, McManus OB, Sirotina-Meisher A, Fischer P, Boltz RC, Goetz MA, Baker R, Bao J, Kayser F, Rupprecht KM, Parsons WH, Tong XC, Ita IE, Pivnichny J, Vincent S, Cunningham P, Hora D, Jr, Feeney W, Kaczorowski G, et al. Correolide and derivatives are novel immunosuppressants blocking the lymphocyte Kv1.3 potassium channels. Cell Immunol. 1999;197:99–107. doi: 10.1006/cimm.1999.1569. [DOI] [PubMed] [Google Scholar]
- Koo GC, Blake JT, Talento A, Nguyen M, Lin S, Sirotina A, Shah K, Mulvany K, Hora D, Jr, Cunningham P, Wunderler DL, McManus OB, Slaughter R, Bugianesi R, Felix J, Garcia M, Williamson J, Kaczorowski G, Sigal NH, Springer MS, Feeney W. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J Immunol. 1997;158:5120–5128. [PubMed] [Google Scholar]
- Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. J Surg Oncol. 2010;101:706–712. doi: 10.1002/jso.21505. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem. 1997;272:32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- Mackenzie AB, Chirakkal H, North RA. Kv1.3 potassium channels in human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2003;285:L862–868. doi: 10.1152/ajplung.00095.2003. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Zimin P, Wulff H, Jin LW. A-beta oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J Biol Chem. 2011;286:3693–3706. doi: 10.1074/jbc.M110.135244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Schlichter LC. Ca2+-activated K+ channels in human B lymphocytes and rat thymocytes. J Physiol (Lond) 1989;415:69–83. doi: 10.1113/jphysiol.1989.sp017712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. A patch-clamp study of mammalian platelets and their voltage-gated potassium current. J Physiol (Lond) 1987;391:467–485. doi: 10.1113/jphysiol.1987.sp016750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson DR, Deutsch C. K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984;307:468–471. doi: 10.1038/307468a0. [DOI] [PubMed] [Google Scholar]
- Mauler F, Hinz V, Horvath E, Schuhmacher J, Hofmann HA, Wirtz S, Hahn MG, Urbahns K. Selective intermediate-/small-conductance calcium-activated potassium channel (KCNN4) blockers are potent and effective therapeutics in experimental brain oedema and traumatic brain injury caused by acute subdural haematoma. Eur J Neurosci. 2004;20:1761–1768. doi: 10.1111/j.1460-9568.2004.03615.x. [DOI] [PubMed] [Google Scholar]
- McCann JD, Matsuda J, Garcia M, Kaczorowski G, Welsh MJ. Basolateral K+ channels in airway epithelia. I. Regulation by Ca2+ and block by charybdotoxin. Am J Physiol. 1990;258:L334–342. doi: 10.1152/ajplung.1990.258.6.L334. [DOI] [PubMed] [Google Scholar]
- Miao S, Bao J, Garcia ML, Goulet JL, Hong XJ, Kaczorowski G, Kayser F, Koo GC, Kotliar A, Schmalhofer W, Shah K, Sinclair PJ, Slaughter RS, Springer MS, Staruch MJ, Tsou NN, Wong F, Parsons WH, Rupprecht K. Benzamide derivatives as blockers of the Kv1.3 ion channel. Bioorg Med Chem Lett. 2003;13:1161–1164. doi: 10.1016/s0960-894x(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Michne W, Guiles J, Treasurywala A, Castonguay L, Weigelt C, Oconnor B, Volberg W, Grant A, Chadwick C, Krafte D. Novel inhibitors of potassium ion channels on human T lymphocytes. J Med Chem. 1995;38:1877–1883. doi: 10.1021/jm00011a007. [DOI] [PubMed] [Google Scholar]
- Mouhat S, Visan V, Ananthakrishnan S, Wulff H, Andreotti N, Grissmer S, Darbon H, De Waard M, Sabatier JM. K+ channel types targeted by synthetic OSK1, a toxin from Orthochirus scrobiculosus scorpion venom. Biochem J. 2005;385:95–104. doi: 10.1042/BJ20041379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molceular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between KCa channel diversity and smooth muscle cell function. Circ Res. 1999;85:e33–e43. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- Nguyen A, Kath JC, Hanson DC, Biggers MS, Canniff PC, Donovan CB, Mather RJ, Bruns MJ, Rauer H, Aiyar J, Lepple-Wienhues A, Gutman GA, Grissmer S, Cahalan MD, Chandy KG. Novel nonpeptide agents potently block the C-type inactivated conformation of Kv1.3 and suppress T cell activation. Mol Pharmacol. 1996;50:1672–1679. [PubMed] [Google Scholar]
- Pappone PA, Ortiz-Miranda SI. Blockers of voltage-gated K channels inhibit proliferation of cultured brown fat cells. Am J Physiol. 1993;264:C1014–1019. doi: 10.1152/ajpcell.1993.264.4.C1014. [DOI] [PubMed] [Google Scholar]
- Partiseti M, Choquet D, Diu A, Korn H. Differential regulation of voltage- and calcium-activated potassium channels in human B lymphocytes. J Immunol. 1992;148:3361–3368. [PubMed] [Google Scholar]
- Pena TL, Chen SH, Konieczny SF, Rane SG. Ras/MEK/ERK Up-regulation of the fibroblast KCa channel FIK is a common mechanism for basic fibroblast growth factor and transforming growth factor-beta suppression of myogenesis. J Biol Chem. 2000;275:13677–13682. doi: 10.1074/jbc.275.18.13677. [DOI] [PubMed] [Google Scholar]
- Pena TL, Rane SG. The fibroblast intermediate-conductance KCa channel, FIK, as a prototype for the cell growth regulatory function of the IK channel family. J Membr Biol. 1999;172:249–257. doi: 10.1007/s002329900601. [DOI] [PubMed] [Google Scholar]
- Pennington MW, Beeton C, Galea CA, Smith BJ, Chi V, Monaghan KP, Garcia A, Rangaraju S, Giuffrida A, Plank D, Crossley G, Nugent D, Khaytin I, Lefievre Y, Peshenko I, Dixon C, Chauhan S, Orzel A, Inoue T, Hu X, Moore RV, Norton RS, Chandy KG. Engineering a stable and selective peptide blocker of the Kv1.3 channel in T lymphocytes. Mol Pharmacol. 2009;75:762–773. doi: 10.1124/mol.108.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LE, Villinger F, Wulff H, Sankaranarayanan A, Raman G, Ansari AA. Pharmacokinetics, toxicity, and functional studies of the selective Kv1.3 channel blocker 5-(4-phenoxybutoxy)psoralen in rhesus macaques. Exp Biol Med (Maywood) 2007;232:1338–1354. doi: 10.3181/0705-RM-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M, Lee SC, Deutsch C. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1989;86:10171–10175. doi: 10.1073/pnas.86.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauer H, Lanigan MD, Pennington MW, Aiyar J, Ghanshani S, Cahalan MD, Norton RS, Chandy KG. Structure-guided transformation of charybdotoxin yields an analog that selectively targets Ca2+-activated over voltage-gated K+ channels. J Biol Chem. 2000;275:1201–1208. doi: 10.1074/jbc.275.2.1201. [DOI] [PubMed] [Google Scholar]
- Reich EP, Cui L, Yang L, Pugliese-Sivo C, Golovko A, Petro M, Vassileva G, Chu I, Nomeir AA, Zhang LK, Liang X, Kozlowski JA, Narula SK, Zavodny PJ, Chou CC. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 2005;35:1027–1036. doi: 10.1002/eji.200425954. [DOI] [PubMed] [Google Scholar]
- Ren YR, Pan F, Parvez S, Fleig A, Chong CR, Xu J, Dang Y, Zhang J, Jiang H, Penner R, Liu JO. Clofazimine inhibits human Kv1.3 potassium channel by perturbing calcium oscillation in T lymphocytes. PLoS ONE. 2008;3:e4009. doi: 10.1371/journal.pone.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette L, Abraham WM, Bradding P, Krajewski J, Antonio B, Shelton T, Bannon AW, Rigdon G, Krafte D, Wagoner K, Castle NA. Senicapoc (ICA-17043), a potent and selective KCa3.1 K+ channel blocker, attenuates allergen-induced asthma in sheep. 15th International Conference of the Inflammation Research Association; Chantilly, VA. 2008. [Google Scholar]
- Rus H, Pardo CA, Hu L, Darrah E, Cudrici C, Niculescu T, Niculescu F, Mullen KM, Allie R, Guo L, Wulff H, Beeton C, Judge SI, Kerr DA, Knaus HG, Chandy KG, Calabresi PA. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci USA. 2005;102:11094–11099. doi: 10.1073/pnas.0501770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, Nicolas JF. Allergic contact dermatitis. Eur J Dermatol. 2004;14:284–295. [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schilling T, Stock C, Schwab A, Eder C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglia migration. Eur J Neurosci. 2004;19:1469–1474. doi: 10.1111/j.1460-9568.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- Schlichter LC, Sakellaropoulos G, Ballyk B, Pennefather PS, Phipps DJ. Properties of K+ and Cl− channels and their involvement in proliferation of rat microglial cells. Glia. 1996;17:225–236. doi: 10.1002/(SICI)1098-1136(199607)17:3<225::AID-GLIA5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schmalhofer WA, Bao J, McManus OB, Green B, Matyskiela M, Wunderler D, Bugianesi RM, Felix JP, Hanner M, Linde-Arias A-R, Ponte CG, Velasco L, Koo G, Staruch MJ, Miao S, Parsons WH, Rupprecht K, Slaughter RS, Kaczorowski GJ, Garcia ML. Identification of a new class of inhibitors of the voltage-gated potassium channel, Kv1.3, with immunosuppressant properties. Biochemistry. 2002;18:7781–7794. doi: 10.1021/bi025722c. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hansel W, Wulff H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- Shepherd MC, Duffy SM, Harris T, Cruse G, Schuliga M, Brightling CE, Neylon CB, Bradding P, Stewart AG. KCa3.1 Ca2+ activated K+ channels regulate human airway smooth muscle proliferation. Am J Respir Cell Mol Biol. 2007;37:525–531. doi: 10.1165/rcmb.2006-0358OC. [DOI] [PubMed] [Google Scholar]
- Shumilina E, Lam RS, Wolbing F, Matzner N, Zemtsova IM, Sobiesiak M, Mahmud H, Sausbier U, Biedermann T, Ruth P, Sausbier M, Lang F. Blunted IgE-mediated activation of mast cells in mice lacking the Ca2+-activated K+ channel KCa3.1. J Immunol. 2008;180:8040–8047. [Google Scholar]
- Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Kohler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Simms LA, Doecke JD, Roberts RL, Fowler EV, Zhao ZZ, McGuckin MA, Huang N, Hayward NK, Webb PM, Whiteman DC, Cavanaugh JA, McCallum R, Florin TH, Barclay ML, Gearry RB, Merriman TR, Montgomery GW, Radford-Smith GL. KCNN4 gene variant is associated with ileal Crohn’s Disease in the Australian and New Zealand population. Am J Gastroenterol. 2010;105:2209–2217. doi: 10.1038/ajg.2010.161. [DOI] [PubMed] [Google Scholar]
- Soler D, Humphreys TL, Spinola SM, Campbell JJ. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood. 2003;101:1677–1682. doi: 10.1182/blood-2002-07-2348. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Sullivan JK, JGM, Miranda LP, Nguyen HQ, Walker KW, Hu S, Gegg CV, McDonough SI. 2006116156. WO. 2006 Amgen. Fusion proteins of toxin peptides with linkers and IgG and their use as therapeutic agents.
- Tharp DL, Wamhoff BR, Wulff H, Raman G, Cheong A, Bowles DK. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscler Thromb Vasc Biol. 2008;28:1084–1089. doi: 10.1161/ATVBAHA.107.155796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, Pratt PF, Hatoum OA, Gutterman DD, Harder DR, Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor JE, Pallaghy PK, Pennington MW, Norton RS. Solution structure of ShK toxin, a novel potassium channel inhibitor from a sea anemone. Nat Struct Biol. 1996;3:317–320. doi: 10.1038/nsb0496-317. [DOI] [PubMed] [Google Scholar]
- van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, Soler C, Solsona C, Celada A, Felipe A. Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J Biol Chem. 2003;278:46307–46320. doi: 10.1074/jbc.M304388200. [DOI] [PubMed] [Google Scholar]
- Wojtulewski JA, Gow PJ, Walter J, Grahame R, Gibson T, Panayi GS, Mason J. Clotrimazole in rheumatoid arthritis. Ann Rheum Dis. 1980;39:469–472. doi: 10.1136/ard.39.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, Chandy KG. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Castle NA. Therapeutic potential of KCa3.1 blockers: recent advances and promissing trends. Expert Rev Clin Pharmacol. 2010;3:385–396. doi: 10.1586/ecp.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Knaus HG, Pennington M, Chandy KG. K+ channel expression during B-cell differentiation: implications for immunomodulation and autoimmunity. J Immunol. 2004;173:776–786. doi: 10.4049/jimmunol.173.2.776. [DOI] [PubMed] [Google Scholar]
- Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small-and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem. 2007;14:1437–1457. doi: 10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Haensel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proc Natl Acad Sci USA. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Pennington M. Targeting effector memory T-cells with Kv1.3 blockers. Curr Opin Drug Discov Devel. 2007;10:438–445. [PubMed] [Google Scholar]