Abstract
Several fungi present high tolerance to toxic metals and some are able to transform metals into metal–oxalate complexes. In this study, the ability of Beauveria bassiana to produce copper oxalates was evaluated. Growth performance was tested on various copper-containing media. B. bassiana proved highly resistant to copper, tolerating concentrations of up to 20 g L−1, and precipitating copper oxalates on all media tested. Chromatographic analyses showed that this species produced oxalic acid as sole metal chelator. The production of metal–oxalates can be used in the restoration and conservation of archeological and modern metal artifacts. The production of copper oxalates was confirmed directly using metallic pieces (both archeological and modern). The conversion of corrosion products into copper oxalates was demonstrated as well. In order to assess whether the capability of B. bassiana to produce metal–oxalates could be applied to other metals, iron and silver were tested as well. Iron appears to be directly sequestered in the wall of the fungal hyphae forming oxalates. However, the formation of a homogeneous layer on the object is not yet optimal. On silver, a co-precipitation of copper and silver oxalates occurred. As this greenish patina would not be acceptable on silver objects, silver reduction was explored as a tarnishing remediation. First experiments showed the transformation of silver nitrate into nanoparticles of elemental silver by an unknown extracellular mechanism. The production of copper oxalates is immediately applicable for the conservation of copper-based artifacts. For iron and silver this is not yet the case. However, the vast ability of B. bassiana to transform toxic metals using different immobilization mechanisms seems to offer considerable possibilities for industrial applications, such as the bioremediation of contaminated soils or the green synthesis of chemicals.
Keywords: Beauveria bassiana, metal immobilization, oxalates, bioremediation, conservation science
Introduction
Fungi play an essential role in the bioweathering of rocks and minerals, and especially in the transformation of metals, which are present in large quantities in mineral components. This transformation regulates metal bioavailability through either solubilization or immobilization (Gadd, 2007). The capability to accumulate and precipitate metals has biotechnological applications and can be exploited for the bioremediation of metal-polluted soils and waste treatment (Gadd, 2010).
Leaching of metals by fungi is the result of different mechanisms promoted by proton efflux or metabolites with chelating properties. Metal immobilization can also occur through others processes of reductive metal precipitation such as the synthesis of metallic nanoparticles (Gadd, 2007). Among the metabolites with chelating properties, some of the most common are carboxylic acids and in particular oxalic acid. In previous studies, different species of fungi were studied for their production of oxalic acid and transformation of metal-containing minerals (Sayer and Gadd, 1997; Gharieb et al., 2004). This includes a few papers published on the precipitation of toxic metals as metal–oxalates, as for example copper oxalates by entomopathogenic fungi such as Beauveria species (Fomina et al., 2005) and iron oxalates by ectomycorrhizal fungi (Landeweert et al., 2001).
On the other hand, there is a growing interest for the synthesis of inorganic materials by biological means because these are more environmental friendly processes. Novel applications of this are the use of microorganisms for corrosion control or protection of stone monuments, which were recently illustrated in literature (Cappitelli et al., 2006; Zuo, 2007). In the field of conservation, this could represent an innovative treatment for archeological and artistic metal artifacts. This will be in contrast to the treatments currently employed such as the application of organic protective coatings, which simply create a barrier against aggressive environments in a non-selective way.
As part of the Biological patinA for arcHaeological and Artistic Metal ArtefactS (BAHAMAS) project, a novel approach based on inorganic treatments addressing specific corrosion features is envisaged for copper, iron, and silver. These substrates are widely represented in cultural heritage artwork and face several problems of active corrosion. The research activities foreseen aim at creating protective fungal patinas by the conversion of existing corrosion products into more stable and less soluble compounds while maintaining the surface’s physical appearance. The color of the oxalate layer created will be different according to the treated metal substrate and is expected to be green, red–brown, and white on the respective copper/bronze, iron, and silver substrates. As part of this project, the aim of the present study was to evaluate the ability of Beauveria bassiana to tolerate and transform copper, iron, and silver compounds into stable compounds, such as metal–oxalate complexes. The performance of B. bassiana was compared with four other phylogenetically related soil fungal species in order to establish whether B. bassiana is exceptionally promising for this biotechnological application.
Materials and Methods
Strains
Beauveria bassiana was isolated from copper-contaminated vineyard soil (Bevaix Abbey, Neuchâtel, Switzerland). The Bevaix abbey vineyard soil (Neuchâtel, Switzerland) was treated for more than a century with copper-based pesticide agents and is highly contaminated with Cu (total concentration in soil reaches 350 ppm). Trichophyton mentagrophytes (MUCL 9823), Trichophyton rubrum (MUCL 11954), Arthroderma quadrifidum (MUCL 9822), and Geomyces pannorum (MUCL 151) were provided from the culture collection BCCM/MUCL of the University of Louvain (Belgium). The five strains are deposited in the culture collection of the Microbiology Laboratory (University of Neuchatel).
Media and culture conditions
Agar malt extract medium (MA; 15 g L−1 agar and 12 g L−1 malt in distilled water) was autoclaved at 121°C, 25 min L−1. Different metal compounds were added to the medium for the specific experiments (Table 1). Brochantite Cu4(OH)6(SO4)4 and atacamite Cu2Cl(OH)3 were synthesized according to the literature (Sharkey and Lewin, 1971; Tanaka et al., 1991). Cuprite Cu2O, hematite Fe2O3, and silver nitrate AgNO3 were purchased from Fluka (purum). Magnetite Fe3O4 and iron (III) oxide FeOOH were supplied by Aldrich (99.99%) and acanthite Ag2S by Riedel-de Haën (pure). The different metal compounds were sterilized with an UV-rays exposure of 30 min and added to the media after autoclaving. When biocides were used, these were added after autoclaving to the melted malt agar media at a temperature lower than 60°C. Cultures were incubated (unless otherwise stated) at room temperature in the dark.
Table 1.
Composition of the different media used for the growth rates measurements.
| Medium | Composition |
|---|---|
| MA | 12 g.L−1 malt + 15 g.L−1 agar |
| MABRO | MA + 5g.L−1 brochantite |
| MACUP | MA + 5g.L−1 cuprite |
| MAACAN | MA + 5g.L−1 acanthite |
| MAHE | MA + 5g.L−1 hematite |
| MAMA | MA + 5g.L−1 magnetite |
| MAIOH | MA + 5g.L−1 iron (III) oxide |
Growth performance test
Pre-cultures onto MA petri dishes incubated at room temperature were used as inocula. Square fungal plugs of 5 mm side were taken out with a sterilized cork borer and placed onto new Petri dishes containing the different modified solid media summarized in Table 1. The media (two or three replicates for each composition) were incubated at room temperature for 18 days (8 days for iron) and the mycelia growth diameters measured. The growth rates were determined measuring the radial growth diameters of the mycelium each 3–4 days in two directions at 90° angles (Trinci, 1971; Griffin et al., 1974; Prosser, 1994). MA medium was used as a control.
Organic acid analysis
Beauveria bassiana was grown at room temperature for 21 days on two different malt agar media that contained 15 g L−1 agar and 8 or 12 g L−1 malt in distilled water (three replicates for each composition). Cultures were filtrated on 0.2 μm Whatman paper and cell filtrates were lyophilized and suspended in 100 μL deionized water. The organic acids excretion was analyzed using high performance liquid chromatography (HPLC) with a UV–VIS DAD detector. A calibration was established using 11 different organic acids as standards. An Agilent HPLC 1100 series controller was used with an ion-exchange column (H+ form, Benson, Reno, NV, USA) and sulfuric acid (20 mM) as eluent. Forty-five microliters portions of triplicate samples were eluted for 20 min at 7 mL min−1. UV–VIS absorbance spectra of the acid function C = O were collected between 210 and 400 nm.
Samples
Two copper washers (W33 and W34), one Roman bronze (Cu/Sn) alloy coin (C1), and one copper coin from France (Louis XIII Double Tournois, C2) were used to evaluate the copper oxalate formation in a liquid medium. Two copper roof sheets (R1 and R2, 0.1 cm × 1.5 cm × 2 cm) were used for evaluate the copper oxalates formation in a solid medium. Two iron washers (W37 and W38) and two iron nails (N58 and N59) were used for evaluate the iron oxalates formation in a liquid medium. The experiments with solid media were performed on four window hinges (Fe1–Fe4) and four iron screws (Fe6–Fe9). The silver oxalates formation was evaluated on a one Swiss Franc coin (Ag 83.5%/Cu 16.5% alloy) C3 from 1966.
Formation of metal–oxalates on samples
The different objects were not sterilized and placed in 85-mm diameter Petri dishes. For copper, a malt medium containing 12 g L−1 malt was used for the cultures in liquid medium on samples W33, W34, C1, and C2 (immersion <2 mm). For assays in solid medium cultures with copper, MA, and MABRO media (Table 1) were put onto the copper roof sheets R1 and R2 and their shape adapted to the size of the samples. The thickness of the solid media was 2 mm. Finally B. bassiana was inoculated under a laminar flow hood using aliquots of spores suspended in water. Conidies were collected from cultures of B. bassiana onto MA petri dishes and suspended in deionized water in order to obtain enough conidies per square millimeter of the samples surface (at least 200 conidies for each Petri dish). The copper oxalates formation was evaluated during 3 weeks through optical microscopy observations and FTIR spectroscopy analysis every week. An identical protocol was followed for iron. Solid media MA, MAHE, MAMA, and MAIOH were used on samples Fe1–Fe4 and Fe6–Fe9. Liquid medium (malt 12 g L−1) was used on iron nails N58 and N59 and on iron washers W37 and W38. The precipitation of silver oxalates was evaluated on the sample C3 using a MA medium. The experimental protocol was the same as the one developed for copper.
Formation of silver nanoparticles
The experimental protocol was adapted from the literature (Vigneshwaran et al., 2007; Birla et al., 2009; Ingle et al., 2009). Conidia were collected from cultures of B. bassiana onto MA petri dishes and suspended in deionized water in order to obtain a final concentration of 8 × 105 spores mL−1. Two milliliters aliquots of B. bassiana spores were used to inoculate 250 ml Schott flasks each containing 100 mL of a malt medium (12 g L−1) and the flasks were placed under agitation (125 rpm) for 150 h in dark at room temperature. The culture was filtered on an 11-μm Whatman filter (previously sterilized under UV for 40 min) in order to separate mycelium and cell filtrate. The mycelium (in 24.5 mL deionized water) and 24.5 mL cell filtrate were inoculated each in 250 mL Schott flasks. Silver nitrate was added to obtain a final concentration of 6 mM Ag+ ions. The flasks were then incubated at room temperature under agitation (125 rpm) for 72 h. Controls were prepared in the same way but adding 24.5 mL deionized water instead.
Characterization of transformation products
Optical microscopy
Bright field observations were performed using an Leica DMR optical microscope equipped with fixed oculars of 10× and objectives with different magnifications (5, 10, 40, and 100×). Photomicrographs were recorded with a Nikon DXM1200 digital camera directly connected to the microscope and controlled through the ACT-1 software.
Scanning electron microscopy and energy dispersive spectroscopy
A Tescan Mira LMU environmental scanning electron microscope, equipped with an energy dispersive X-ray analyzer was used to observe the crystals formed by B. bassiana. The samples were lyophilized, covered with carbon and gold layers, and finally observed in the secondary electron mode at an acceleration voltage of 15 kV and a working distance of 5–25 mm.
FTIR microscopy
A Biorad Excalibur spectrometer coupled with a Varian UMA500 FTIR microscope fitted with an MCT detector cooled by liquid nitrogen was used for FTIR measurements. The measurements were made either in reflection mode or in transmission mode using a micro diamond compression cell. All spectra were acquired in the range 4000–650 cm−1, at a spectral resolution of 4 cm−1. A total of 64 scans were recorded and the resulting interferogram averaged. Data collection and post-run processing were carried out using Varian resolutions Pro™ software.
X-ray diffraction
A calibration was established using mixtures of dihydrated copper oxalate (CuC2O4·2H2O) and quartz with exact proportion. The cultures of B. bassiana in MABRO, MATA, and MACUP media were dried at 60°C and then ground to a homogenous powder with particle size <40 μm. For each Cu(II) concentration, one sample of 800 mg has been pressed (20 bars) in a powder holder covered with a blotting paper and analyzed by XRD, using a SCINTAG 2000 Diffractometer, Thermo, Ecublens, Switzerland. Fixed analysis conditions were as follows: wave length: 1.5406 Å Cu Kα; generator power: 45 kV and 40 mA; emitting slits: 2, 4 mm; receiving slits: 0.5, 0.3 mm; continuous 2θ scan from 1° to 65°; step size: 0.02°; scan rate 1.00/min; acquisition step size (chopper increment; 0.03° 2θ).
UV–VIS spectroscopy
Two-milliliter aliquots of cell filtrate were centrifuged at 10,000 g for 10 min and rinsed three times with deionized water. Mycelium was centrifuged at 10,000 g for 10 min, rinsed three times with deionized water and homogenized with a ultrasonic bath. Sodium chloride was added in order to precipitate the remaining silver (I) ions, which would interfere with the measurements. A UV–VIS Thermo Genesis 10 s spectrometer was used and absorbance spectra were recorded between 300 and 800 nm with 1 nm resolution.
Statistical analysis
All statistical analyses were performed using R software. For experimental results on solid media with iron compounds, the significance (p < 0.05) of differences among growth rates was evaluated using one-way ANOVA for parametric data and a complementary Tukey’s post hoc test.
Results and Discussion
Effect of different copper compounds on growth and precipitation of copper oxalates in B. bassiana
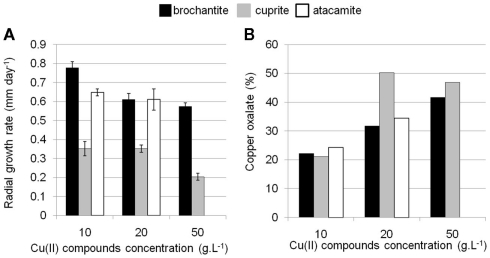
In order to test the ability of B. bassiana to tolerate and immobilize copper through the precipitation of copper oxalates, this strain was exposed to Cu(II) present in different chemical forms (Cu oxide, hydroxysulfate, and hydroxychloride). Growth performance was compared with a control medium containing only a carbon source but no Cu(II) (MA medium). In MA, B. bassiana had the highest growth rate (3.9 mm day−1) compared to copper-enriched solid media, which significantly altered growth rates. On medium supplemented with increasing concentrations of cuprite (Cu oxide, MACUP medium) growth rates decreased to 0.35 ± 0.06 mm day−1 at 10 and to 0.35 ± 0.03 mm day−1 at 20 g L−1 and reduced to 0.20 ± 0.03 mm day−1 at 50 g L−1 (Figure 1A). Likewise, growth rates severely decreased for media containing brochantite (Cu hydroxysulfate, MABRO medium; Figure 1A). Despite the elevated toxicity of chlorides for fungi, this strain developed on atacamite (Cu hydroxychloride, MATA medium) with concentrations of Cu chloride up to 20 g L−1 and had a radial growth of 0.60 ± 0.10 mm day−1. However, B. bassiana growth was fully inhibited at 50 g L−1 of atacamite (Figure 1A). The lowest B. bassiana growth was on MACUP media and could be related to the higher solubility of cuprite in water respect to brochantite and atacamite (Cramer et al., 2002).
Figure 1.
Ability of B. bassiana to tolerate and form copper oxalates on different soil media containing cuprite (MACUP), brochantite (MABRO), and atacamite (MATA). (A) Radial growth rates on solid media amended with Cu(II) concentrations ranging from 10 to 50 g L−1. The bars reflect mean values of three replicates. Error bars indicate SEs of the means. (B) Copper oxalates semi-quantitative estimation calculated from the XRD measurements and expressed in percentage of the dry weight of treated mycelium.
After 18 days of incubation, green concentric ring patterns were observed on the surface of the MACUP and MABRO Petri dishes, suggesting the solubilization of copper and its transformation into copper oxalates within Liesegang rings. Such patterns are formed when reaction, diffusion, and precipitation processes interact together (Henisch, 1988). After 3 months of incubation, the amount of copper oxalates precipitated by B. bassiana was estimated by XRD analysis (Figure 1B). In general, the amount of copper oxalates increased with the amount of copper available in the medium. With 10 g L−1 of copper, the amount of copper represented 22.5% of the total weight and rose to 38.9% with 20 g L−1 and 44.3% with 50 g L−1. The results illustrated that in presence of more copper, B. bassiana precipitated a larger quantity of copper oxalates. This feature together with the stimulation of oxalic acid production was already reported for other fungal species and in particular for brown rot basidiomycetes (Clausen and Green, 2003).
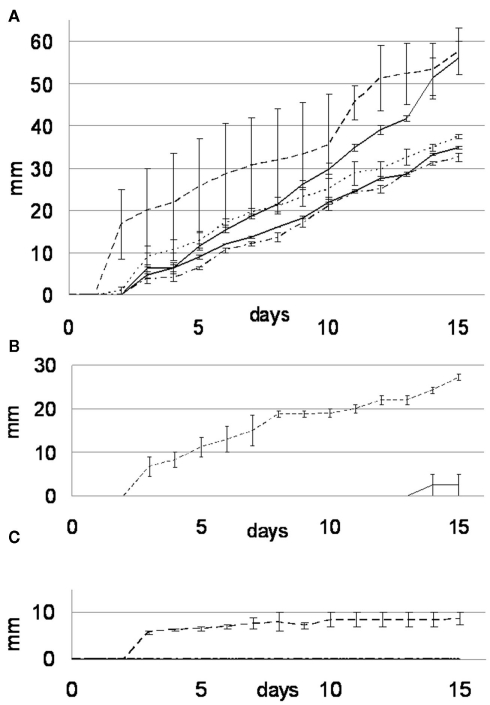
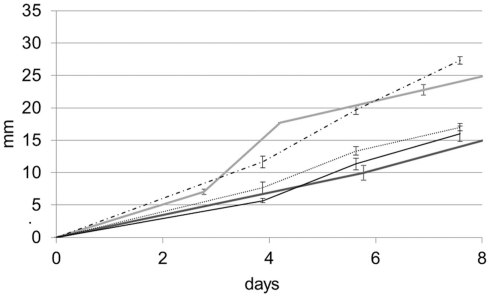
Beauveria bassiana was isolated from vineyard soil that was treated for centuries with copper-based pesticide agents. It certainly developed a resistance toward high concentrations of copper ions, as suggested by the results presented below. It is worth mentioning that B. bassiana is part of the entomopathogenic fungi group, whose copper resistance is already reported in the literature (Baath, 1991). In order to test whether the ability of B. bassiana to tolerate copper is exceptional, four other soil fungi were tested for their ability to tolerate and immobilize copper through the precipitation of copper oxalates. In control medium (MA), B. bassiana grew at a slightly faster rate (3.94 ± 0.35 mm day−1) than A. quadrifidum and G. Pannorum, which had rates of 3.8 ± 0.26 and 2.47 ± 0.06 mm day−1 respectively (Figure 2A). The two Trichophyton strains (T. mentagrophytes and T. rubrum) grew with the lowest rates (2.29 ± 0.06 and 1.51 ± 0.04 mm day−1, respectively). However, the Cu protection mechanisms of strains others than B. bassiana seemed limited, as they were not able to grow on MABRO or MACUP media (Figures 2B,C). On MABRO, only A. quadrifidum (0.26 ± 0.26 mm day−1) developed marginally (Figure 2B). On MACUP none of the four other strains was able to grow (Figure 2C).
Figure 2.
Growth rate curves for the MA (A), MABRO (B), and MACUP (C) media. Beauveria bassiana (dash line), Trichophyton mentagrophytes (black line), Trichophyton rubrum (dash-dot line), Arthroderma quadrifidum (gray line), and Geomyces pannorum (dot line). Error bars indicate SEs of the means.
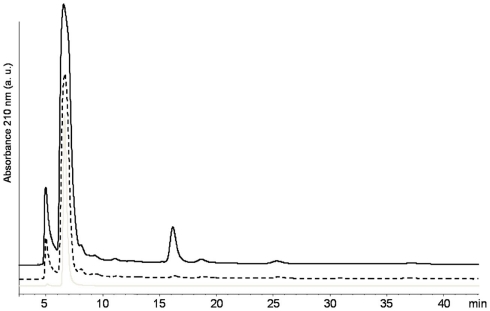
Regarding the immobilization mechanism used by B. bassiana, HPLC analyses combined with UV–VIS spectroscopy revealed that this strain excreted considerable amounts of oxalic acid and none of the other organic acids evaluated (Figure 3). The concentration of oxalic acid produced varied at different concentrations of malt. In 8 g L−1 of malt, 23.3 kg m−3 day−1 of oxalic acid was produced. This value increased to 31.6 kg m−3 day−1 when the concentration of malt was increased to 12 g L−1. Based on these values, the theoretical total production of oxalic acid after 21 days of incubation, would be of 489 kg m−3 on solid medium containing 8 g L−1 of malt and 663 kg m−3 with 12 g L−1 of malt. For comparison, in Aspergillus niger, which is the most important fungi studied for production of oxalic acid, production is up to 10 times lower considering the values reported for wild (3.7 kg m−3 day−1) or mutant (9.7 kg m−3 day−1) strains. In addition, A. niger excreted also citric and gluconic acid (Cameselle et al., 1998; Rymowicz and Lenart, 2003).
Figure 3.
High performance liquid chromatography separation spectra for B. bassiana cultures on MA medium with 8 g L−1 (black line) and 12 g L−1 (dash line) of malt together with the HPLC reference spectra of oxalic acid (gray line).
In addition, the characterization of copper oxalates formed by B. bassiana was further performed by means of ESEM observations, XRD, and FTIR measurements (Joseph et al., 2011). These data completed the work here presented. ESEM observations allowed characterizing the crystals as rounded foil-like concretions, which were identified as moolooite (CuC2O4·nH2O with n < 1), a natural hydrated copper oxalate by XRD and FTIR measurements on the three media MACUP, MABRO, and MATA.
Application of oxalate biopatinas on copper/bronze samples
The precipitation of copper oxalates presented here has a potential application in the field of art conservation science. In fact, on outdoor exposed bronzes, copper oxalates are identified but not associated with the phenomenon of cyclical corrosion (Graedel et al., 1987). Instead they form green protective patinas on the bronze surface, which are highly insoluble and chemically stable even in acidic atmospheres (Marabelli and Mazzeo, 1993). Thus, considering the production of copper oxalates demonstrated in B. bassiana prompted the evaluation of the formation of a homogenous layer of copper oxalates on corroded artifacts.
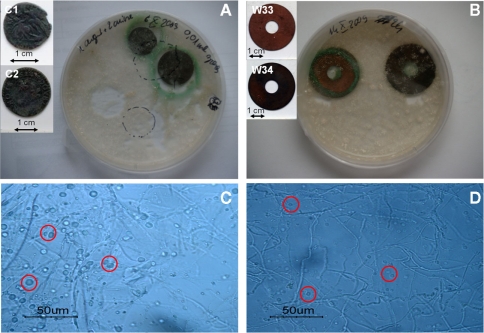
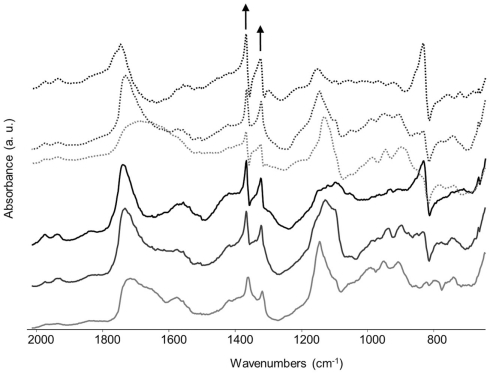
After 3 weeks of incubation of B. bassiana in liquid medium in the presence of several copper-containing objects, the formation of copper oxalates was observed on the different samples (Figure 4). The green copper oxalates were distributed around the sample either on the edge of the sample (W33 and W34) or in the surrounding medium (samples C1 and C2; Figures 4A,B). Such application procedure is therefore unsuitable for the purpose of creating a homogeneous and adherent layer of copper oxalates on the samples. We designed another application procedure where the fungi can be directly inoculated on a solid medium that has been previously poured onto the samples. Copper roof sheets were used for this optimized application procedure. Optical microscopy observations on the R1 and R2 coupons have shown that either within MA or MABRO media, copper oxalates crystals formed and were already visible after 7 days of incubation (Figures 4C,D). Moreover, a higher amount of copper oxalates appeared to be formed in MA medium (Figure 4C) respect to MABRO (Figure 4D), and suggested that the addition of copper to the medium did not increase the copper oxalates precipitation. The surface of the treated samples was characterized by FTIR measurements (Figure 5). The results showed that the original patina composed of brochantite is gradually transformed into copper oxalates and that the conversion is all but complete on the surface areas where B. bassiana had grown.
Figure 4.
Optimization of the treatment of copper objects with B. bassiana. (A,B) Aspect of samples immersed in liquid medium inoculated with B. bassiana cultures after 3 weeks incubation. Objects depicted: (A) C1 and C2, (B) W33, and W34. Embedded images present samples before treatment. (C,D) Optical microscopy observations of MA (C) and MABRO (D) medium after 7 days incubation of B. bassiana with R1 and R2 samples respectively. Red circles indicate the presence of copper oxalates.
Figure 5.
Reflectance FTIR spectra (2000–650 cm−1) obtained from the surface of the samples R1 and R2 after 1 (gray), 2 (dark gray), or 3 (black) weeks of incubation of B. bassiana on MA (continuous lines) or MABRO (dash lines) medium, respectively. The arrows indicate the characteristic absorbance bands of copper oxalates, whose heights increased with time.
Application on iron and silver samples
Considering the encouraging results obtained for copper-containing objects, the ability of B. bassiana to precipitate iron oxalates, form silver oxalates, or to reduce silver compounds to elemental silver was evaluated as well. To our knowledge this is the first time that such an attempt is conducted.
Iron oxalates formation
The ability of B. bassiana to transform and immobilize iron was tested on different iron compounds. Growth rates were significantly different (F = 52.18, df = 4, p = 0.00001) for all tested media. Among the different iron-containing media, B. bassiana demonstrated to have a much higher growth on MAIOH than on the other two media tested (Figure 6). These results were in agreement with the fact that iron is essential to fungi and not as toxic as copper. Even after 45 days of incubation, no crystal formation was observed within the media.
Figure 6.
Growth rate curves for the B. bassiana cultures on MA (gray line) MABRO (dark gray line), MAHE (black line), MAMA (dot line), and MAIOH (dash-dot line) media. Error bars indicate SEs of the means.
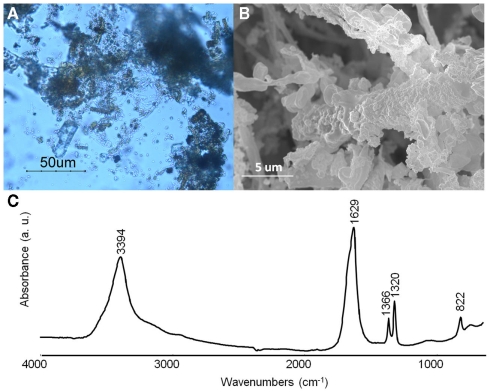
Since our primarily interest is to obtain iron oxalate layers over art objects, a first attempt was made with iron samples on different solid media. After 2 months of incubation with MAHE, some hyphae adopted a red coloration under optical microscopy observations (data not shown). However contamination did not allow an accurate evaluation for all other media and further experiments will be therefore be foreseen with the use of antibiotics in order to obtain a selective medium for B. bassiana. Follow up experiments performed on iron samples (N58, N59, W37, and W38) in liquid media gave very promising results: after a few weeks, hyphae around the samples presented a red coloration. Optical microscopy showed that hyphae close to the objects accumulated a dark red aggregate and seemed completely incrusted with red crystals (Figure 7A). The crystals were characterized by SEM observations and FTIR measurements. SEM observations allowed observing the encrustation of crystals around hyphae (Figure 7B). Further, FTIR analyses showed the presence of characteristics absorbance bands at 1366 (νsC–O), 1320 (νsC–O), and 822 (δC–O) cm−1 (Figure 7C), which can be attributed to iron oxalates (D’Antonio et al., 2009). Based on these results, further test should be performed to lessen the amount of water used for example a spray for the aspersion of the culture over the pieces.
Figure 7.
Beauveria bassiana cultures on MA medium with iron sample W38. (A) Optical microscopy observations. (B) Secondary electron image. (C) Transmittance FTIR spectrum (4000–650 cm−1) obtained from hyphae incrusted in red crystals.
Silver oxalates and nanoparticles precipitation
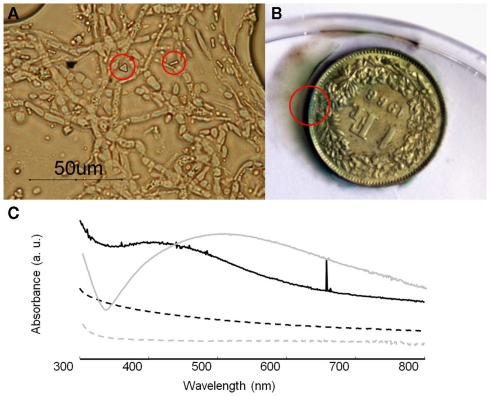
For the growth performance of B. bassiana on silver compounds the highest growth rate was obtained in MAACAN medium (3.4 mm day−1). Optical observations showed the presence of few rectangular and bipyramidal crystals (3–4 μm length; Figure 8A). The same crystal morphology was reported in the literature for silver oxalate aggregates (Boldyrev, 2002; Pourmortazavi et al., 2008), but due to the small amount of crystals observed, any attempt to characterize these crystals with SEM–EDS and FTIR microscopy was not possible. However, the mechanism for silver immobilization in B. bassiana seemed similar to that for copper, as the same extracellular precipitation was observed. It is worth saying that most silver artifacts are based on a copper–silver alloy and a green patina will therefore be formed by the precipitation of copper oxalates as for sample C3 (Figure 8B). In these cases, the esthetic value of the objects will thus be altered, as a color change of the object’s surface is not acceptable as far as conservation ethics are concerned. Hence, the formation of an eventual homogeneous and compact layer of silver oxalates appeared problematic for the conservation of silver artifacts.
Figure 8.
Growth of B. bassiana on silver compounds. (A) Optical microscopy observations of B. bassiana cultures on MAACAN medium with rectangular and bipyramidal crystals indicated by red circles. (B) Sample C3 (copper–silver alloy) with copper oxalates formed around the area indicated by a red circle. (C) UV–visible absorbance spectra of mycelium (black line) and cell filtrate (dash line) containing silver nanoparticles. As control the reference spectra for mycelium (gray line) and cell filtrate (gray dash line) were included.
Therefore a different conservation strategy against the tarnishing of silver artifacts based on the synthesis of silver nanoparticles was tested. As reported in the literature, some fungi are able to transform silver nitrate into silver metal nanoparticles (Rai et al., 2009). This may represent an innovative conservation method where tarnished areas could be reverted back to metal silver. The ability of B. bassiana to synthesize Ag0 nanoparticles was tested either in liquid medium or within a cell filtrate (Figure 8C). After 3 days of incubation, UV–VIS spectroscopy analysis showed a characteristic absorbance band at 420 nm, which was absent in the controls and can be attributed to the presence of Ag0 nanoparticles due a Plasmon resonance phenomenon. Comparing the mycelium and cell filtrate spectra, the peak height is much higher in this latter confirming the synthesis of metal nanoparticles by a still unknown extracellular enzyme (Ingle et al., 2009). This simple and eco-friendly biosynthesis of silver nanoparticles by B. bassiana seemed very promising in particular in the field of nanotechnology, where such materials are studied for their antimicrobial properties.
Conclusion and Perspectives
In this study, the ability of B. bassiana to grow in presence of Cu ions and to form copper oxalate crystals on different media was highlighted. Moreover, the possibilities offered by the use of B. bassiana as an innovative conservation approach were demonstrated. In fact, the results of the HPLC analysis showed the specific production of oxalic acid. This feature will allow us to create oxalate patinas without the formation of other carboxylates, as it could be the case for other fungal strains producing a combination of carboxylic acids. In addition, the precipitation of iron oxalates and silver nanoparticles was reported for the first time. The different crystal aggregates were characterized by either SEM, XRD measurements, or UV–VIS spectroscopy. Further FTIR analysis permitted to observe the gradual conversion of copper sulfates into oxalates and to identify iron oxalates. B. bassiana is a cosmopolite fungi, which has been widely applied as insecticide following eco-friendly processes. Therefore, safety concerns are minimal and this will allow the development of an in situ application kit for conservators–restorers.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research has been partially carried out with the support of the European Union, within the VII Framework Program (Contract: BAHAMAS, PIEF-GA-2009-252759, 2010-2012). Authors acknowledge Mr. T. Adatte and Dr. P. Vonlanthen (Institute of geology and paleontology, University of Lausanne, Switzerland) for providing X-ray diffraction and SEM–EDS facilities, respectively and for their assistance during experiments. Authors are also grateful to the analytical service of the Faculty of Sciences, University of Neuchâtel and in particular Dr. A. Vallat for assistance during experiments.
References
- Baath E. (1991). Tolerance of copper by entomogenous fungi and the use of copper-amended media for isolation of entomogenous fungi from soil. Mycol. Res. 95, 1140–1142 10.1016/S0953-7562(09)80562-5 [DOI] [Google Scholar]
- Birla S. S., Tiwari V. V., Gade A. K., Ingle A. P., Yadav A. P., Rai M. K. (2009). Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett. Appl. Microbiol. 48, 173–179 10.1111/j.1472-765X.2008.02510.x [DOI] [PubMed] [Google Scholar]
- Boldyrev V. V. (2002). Thermal decomposition of silver oxalate. Thermochim. Acta 388, 63–90 10.1016/S0040-6031(02)00044-8 [DOI] [Google Scholar]
- Cameselle C., Bohlmann J. T., Núñez M. J., Lema J. M. (1998). Oxalic acid production by Aspergillus niger. Bioprocess Biosyst. Eng. 19, 247–252 [Google Scholar]
- Cappitelli F., Zanardini E., Ranalli G., Mello E., Daffonchio D., Sorlini C. (2006). Improved methodology for bioremoval of black crusts on historical stone artworks by use of sulfate-reducing bacteria. Appl. Environ. Microbiol. 72, 3733–3737 10.1128/AEM.72.5.3733-3737.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen C. A., Green F. (2003). Oxalic acid overproduction by copper-tolerant brown-rot basidiomycetes on southern yellow pine treated with copper-based preservatives. Int. Biodeterior. Biodegradation 51, 139–144 10.1016/S0964-8305(02)00098-7 [DOI] [Google Scholar]
- Cramer S., Matthes S., Covino B., Bullard S., Holcomb G. (2002). “Environmental factors affecting the atmospheric corrosion of copper,” in Outdoor Atmospheric Corrosion (ASTM International), ed. Townsend H. (Philadelphia, PA: ASTM; ), 254–264 [Google Scholar]
- D’Antonio M. C., Wladimirsky A., Palacios D., Coggiolaa L., González-Baró A. C., Baran E. J., Mercader R. C. (2009). Spectroscopic investigations of iron(II) and iron(III) oxalates. J. Braz. Chem. Soc. 20, 445–450 10.1590/S0103-50532009000300006 [DOI] [Google Scholar]
- Fomina M., Hillier S., Charnock J. M., Melville K., Alexander I. J., Gadd G. M. (2005). Role of oxalic acid over excretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microbiol. 71, 371–381 10.1128/AEM.71.1.371-381.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd G. M. (2007). Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 111, 3–49 10.1016/j.mycres.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Gadd G. M. (2010). Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156, 609–643 10.1099/mic.0.037143-0 [DOI] [PubMed] [Google Scholar]
- Gharieb M. I., Ali M. I., El-Shoura A. A. (2004). Transformation of copper oxychloride fungicide into copper oxalate by tolerant fungi and the effect of nitrogen source on tolerance. Biodegradation 15, 49. 10.1023/B:BIOD.0000009962.48723.df [DOI] [PubMed] [Google Scholar]
- Graedel T. E., Nassau K., Franey J. P. (1987). Copper patinas formed in the atmosphere – I. Introduction. Corros. Sci. 27, 639. 10.1016/0010-938X(87)90047-3 [DOI] [Google Scholar]
- Griffin D. H., Timberlake W. E., Cheney J. C. (1974). Regulation of macromolecular synthesis, colony development, and specific growth rate of Achlya bisexualis during balanced growth. J. Gen. Microbiol. 80, 381–388 [Google Scholar]
- Henisch H. (1988). Crystals in Gels and Liesegang Rings: In vitro Veritas. Cambridge: Cambridge University Press; [DOI] [PubMed] [Google Scholar]
- Ingle A., Rai M., Gade A., Bawaskar M. (2009). Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanopart. Res. 11, 2079–2085 10.1007/s11051-008-9573-y [DOI] [Google Scholar]
- Joseph E., Simon A., Prati S., Wörle M., Job D., Mazzeo R. (2011). Development of an analytical procedure for evaluation of the protective behaviour of innovative fungal patinas on archaeological and artistic metal artefacts. Anal. Bioanal. Chem. 399, 2899–2907 10.1007/s00216-010-4279-2 [DOI] [PubMed] [Google Scholar]
- Landeweert R., Hoffland E., Finlay R. D., Kuyper T. W., Van Breemen N. (2001). Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. (Amst.) 16, 248–254 10.1016/S0169-5347(01)02122-X [DOI] [PubMed] [Google Scholar]
- Marabelli M., Mazzeo R. (1993). La corrosione dei bronzi esposti all’aperto: problemi di caratterizzazione. La Metallurgia Italiana 85, 247–254 [Google Scholar]
- Pourmortazavi S. M., Hajimirsadeghi S. S., Kohsari I., Fareghi Alamdari R., Rahimi-Nasrabadi M. (2008). Determination of the optimal conditions for synthesis of silver oxalate nanorods. Chem. Eng. Technol. 31, 1532–1535 10.1002/ceat.200800090 [DOI] [Google Scholar]
- Prosser J. I. (1994). “Kinetics of filamentous growth and branching,” in Growing Fungus, eds Gow N. A. R., Gadd G. M. (London: Chapman & Hall; ), 301–318 [Google Scholar]
- Rai M., Yadav A., Gade A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27, 76–83 10.1016/j.biotechadv.2009.04.025 [DOI] [PubMed] [Google Scholar]
- Rymowicz W., Lenart D. (2003). Oxalic acid production from lipids by a mutant of Aspergillus niger at different pH. Biotechnol. Lett. 25, 955–958 10.1023/A:1024082130677 [DOI] [PubMed] [Google Scholar]
- Sayer J. A., Gadd G. M. (1997). Solubilization and transformation of insoluble inorganic metal compounds to insoluble metal oxalates by Aspergillus niger. Mycol. Res. 101, 653–661 10.1017/S0953756296003140 [DOI] [Google Scholar]
- Sharkey J. B., Lewin S. Z. (1971). Conditions governing the formation of atacamite and paratacamite. Am. Mineral. 56, 181–194 [Google Scholar]
- Tanaka H., Kawano M., Koga N. (1991). Thermogravimetry of basic copper(II) sulphates obtained by titrating NaOH solution with CuSO4 solution. Thermochim. Acta 182, 281. 10.1016/0040-6031(91)80012-8 [DOI] [Google Scholar]
- Trinci A. P. J. (1971). Influence of the width of the peripheral growth zone on the radial growth rate of fungal colonies on solid media. J. Gen. Microbiol. 67, 325–344 [Google Scholar]
- Vigneshwaran N., Kathe A. A., Varadarajan P. V., Nachane R. P., Balasubramanya R. H. (2007). Silver-protein (core-shell) nanoparticle production using spent mushroom substrate. Langmuir 23, 7113–7117 10.1021/la063627p [DOI] [PubMed] [Google Scholar]
- Zuo R. (2007). Biofilms: strategies for metal corrosion inhibition employing microorganisms. Appl. Microbiol. Biotechnol. 76, 1245–1253 10.1007/s00253-007-1130-6 [DOI] [PubMed] [Google Scholar]