Introduction
Polycystic ovary syndrome (PCOS) was originally described by Stein and Leventhal in 1935 as a reproductive disorder characterized by oligo-amenorrhea, hirsutism, and polycystic ovary morphology.1 Thirty years ago, it was first reported that women with PCOS had hyperinsulinemia.2 Subsequent research indicated that PCOS was associated with a unique disorder of insulin action, as well as defects in insulin secretion, and together these abnormalities conferred a substantially increased risk of glucose intolerance.3 Although the clinical manifestations of PCOS are heterogeneous, the hallmarks of the syndrome remain anovulation, androgen excess, and insulin resistance. Moreover, each of these features of the syndrome is responsible for the promotion of hypertension in this population. Therefore, therapy for hypertension should be targeted at treatment of these underlying abnormalities.
Diagnostic Criteria
The diagnostic criteria for PCOS have been a source of controversy. Outside the US, the diagnosis has been based on the presence of polycystic ovary morphology (PCO) by ovarian ultrasound examination; affected women are then further stratified based on ovulatory status. However, the finding that approximately 25% of normal women in many series can have PCO led investigators in the US to focus on the biochemical features of the syndrome for diagnostic criteria.4-5 The 1990 NIH-NICHD conference on PCOS proposed what have become known as the NIH diagnostic criteria: hyperandrogenism (clinical and/or biochemical) and chronic anovulation with the exclusion of specific disorders of the ovary, adrenal and pituitary (Table 1).4 PCO by ultrasound was not included as a criterion because of the lack of diagnostic specificity of this finding.6 Ultrasonographically detected polycystic ovaries can be present in women with normal ovulation and sex hormone levels, whereas women with all the endocrine features of PCOS can have normal ovarian morphology by ultrasound exam.6-7 The NIH criteria have been used to diagnose PCOS in the majority of the studies of hypertension in affected women.
Table 1.
Diagnostic Criteria for PCOSa
| NIH Criteriab | Hyperandrogenism/Hyperandrogenemia Chronic anovulation |
| Rotterdam Criteria | Two of the following: hyperandrogenism/hyperandrogenemia, chronic anovulation, polycystic ovaries |
| Androgen Excess Society criteria | Hyperandrogenism/Hyperandrogenemia Infrequent or irregular ovulation OR regular ovulation and polycystic ovaries |
All criteria include the exclusion of other medical conditions, including thyroid or pituitary dysfunction, androgen–secreting tumors, Cushing’s syndrome, or congenital adrenal hyperplasia
NIH criteria developed with National Institute of Child Health and Human Development
In 2003, an international conference in Rotterdam reassessed the diagnostic criteria for PCOS and proposed revised criteria that included PCO.8 The Rotterdam criteria require two of three of the following findings: hyperandrogenism, chronic anovulation, PCO (Table 1). Thus, these criteria would include all those women with PCOS by NIH criteria. However, the Rotterdam criteria also include women with hyperandrogenism and ovulatory cycles who would not be considered to have PCOS by NIH criteria. Since most ovulatory women with PCO have hyperandrogenism and increased LH levels, this additional group of women with PCOS according to Rotterdam criteria are analogous to the ovulatory PCO women identified by non-US investigators on the basis of ovarian ultrasound morphology. There are studies to suggest that ovulatory women with PCO are not insulin resistant compared to anovulatory women with PCO,9 although recent studies suggest they may have milder metabolic abnormalities.10 Therefore, it is unclear whether these women should be grouped with those with the classic anovulatory form of the disorder. Recently, the Androgen Excess Society (AES) developed diagnostic criteria for PCOS based on the feature of androgen excess, endorsing the NIH criteria but also recommending that women with regular ovulation and polycystic ovaries on ultrasound be included as a PCOS phenotype.11
Epidemiology
PCOS is one of the most common endocrine disorders affecting women of reproductive age.12 A recent Australian study examined the prevalence of PCOS in a retrospective birth cohort employing the NIH, Rotterdam, and AES diagnostic criteria.13 These data revealed prevalence based on NIH diagnostic criteria of 8.7 ± 2.0%, less than the 11.9 ± 2.4% and 10.2 ± 2.2% using Rotterdam and AES criteria, respectively. Prevalence estimates of PCOS among populations worldwide employing the NIH diagnostic criteria have been reported as approximately 6% in the Southeastern U.S.,14 Spain,15 the Mediterranean,16 and Mexico.17 Although studies have reported no statistically significant differences in the PCOS prevalence between black and white women,14, 18 there are data suggesting an increased prevalence of PCOS among Hispanic19 and Mexican-American17 women compared to other racial and ethnic groups. Nonetheless, PCOS is likely underdiagnosed in clinical practice so the use of chart based data in prevalence estimate derivations is problematic.
Pathophysiology
The biochemical reproductive phenotype in PCOS consists of increased LH relative to FSH secretion and hyperandrogenism.4-5 (Figure 1). There is increased frequency of LH pulsatile release indicating that the frequency of GnRH secretion is increased. There is also increased amplitude of LH pulses that is secondary, in part, to increased pituitary sensitivity to GnRH, which appears to be estrogen-mediated. It is possible that there is also an increased amount of GnRH secreted per pulse. FSH release is relatively suppressed and the late luteal and early follicular phase increases that are essential for normal follicular development are absent. One explanation for these FSH abnormalities is the increased frequency of GnRH secretion, which results in a selective suppression of FSH relative to LH release.20 The elevated circulating androgens feedback on the hypothalamic-pituitary axis, both directly by decreasing sensitivity to the normal actions of estrogen and progesterone to slow the frequency of pulsatile GnRH release21 and by extragonadal aromatization to estrogen, to increase LH relative to FSH release producing a self-sustaining syndrome.4
Figure 1.
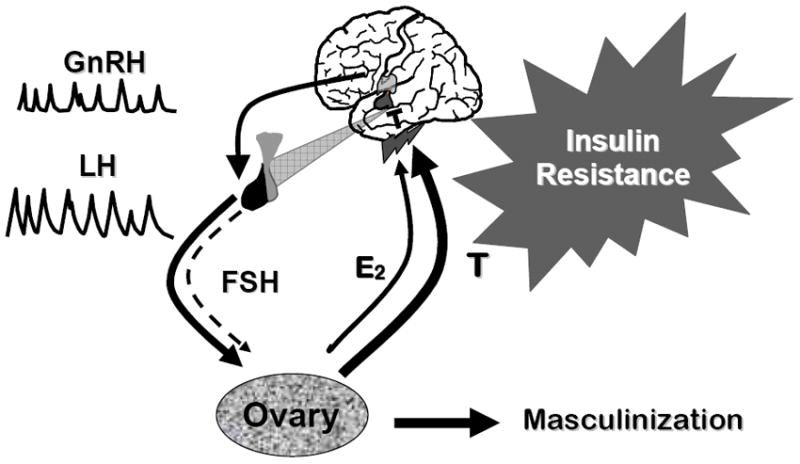
Schema for the pathophysiology of PCOS. Increased GnRH pulsatility leads to a selective increase in LH pulsatility while suppressing FSH secretion. These gonadotropin secretory changes result in arrested follicular development and increased LH-dependent ovarian androgen production, increased theca cell androgen secretion, and decreased conversion of androgens to estrogens by the immature granulosa cells. These changes lead to increased ovarian androgen production, which feedback on the hypothalamic-pituitary axis, both directly by decreasing sensitivity to the normal actions of estrogen and progesterone to slow the frequency of pulsatile GnRH release and by extragonadal aromatization to estrogen, to increase LH relative to FSH release producing a self-sustaining syndrome. This figure is used with the permission of Andrea Dunaif.
Under normal circumstances, the theca cells of the ovarian follicles produce androgens under the control of LH. These androgens are then aromatized into estrogens, primarily estradiol, by the adjacent granulosa cells.22 FSH stimulates the granulosa cell growth and aromatase capacity. In PCOS, elevated LH levels stimulate enhanced theca cell androgen production. PCOS theca cells also have increased activity of multiple steroidogenic enzymes and produce increased amounts of androgens under basal circumstances as well as in response to LH.22-23 Because of the acyclic FSH levels, there is arrested ovarian follicular development and decreased granulosa cell aromatase capacity resulting in decreased conversion of androgens to estrogens. Increased adrenal androgen secretion in a common finding is PCOS, most likely due to a shared defect in the steroid biosynthetic pathways common to the ovary and adrenals. The primary defect that initiates the reproductive features of PCOS remains unknown since it can be shown experimentally that either increasing androgen levels4 or GnRH release24 can produce features of PCOS. However, the intrinsic abnormalities in thecal steroidogenesis taken together with recent family and genetic studies suggest that abnormalities in androgen biosynthesis may be a primary defect in many cases.25
An additional biochemical hallmark of PCOS is increased ovarian and, frequently, adrenal androgen production.26 There is increased activity of multiple steroidogenic enzymes common to the ovaries and the adrenal glands. This abnormality is accounted for in part by increased transcription of genes encoding for steroidogenic enzymes, including 3-β-hydroxysteroid dehydrogenase (3β-HSD), cytochrome P450 enzyme 17α-hydroxylase (P450c17), and 20α-hydroxysteroid dehydrogenase (20α-HSD), as well as increased mRNA accumulation of cholesterol side-chain cleavage enzyme (P450scc), 3β-HSD, P450c17, and 20α-HSD.
Insulin Resistance and Pancreatic β-cell Dysfunction
Although insulin resistance is a common feature of PCOS, not all women with PCOS are insulin resistant.27 The insulin resistance in PCOS has been characterized in adipocytes by a post-binding defect in the insulin receptor-mediated signal transduction, which has also been confirmed in clinical studies of skeletal muscle action.28 In addition, skin fibroblasts were used to demonstrate that defects in insulin signaling resulted from impaired insulin receptor tyrosine kinase activity.27 This impairment has been determined to be secondary to increased receptor serine phosphorylation due to a serine kinase extrinsic to the receptor, which leads to selective resistance to the metabolic actions of insulin.29
In addition to insulin resistance, beta cell dysfunction is present in PCOS and it is the combination of these two derangements that contributes to the development of T2DM.30 The beta cell dysfunction seen in women with PCOS has been evidenced by several methods demonstrating impaired insulin secretion response to glucose and exists independently of impairment in glucose tolerance. Notably, the impaired insulin secretory response was observed most convincingly among the women who had first degree family members with T2DM.
Impaired glucose tolerance (IGT) and T2DM are both increased in women with PCOS compared to women of similar BMI with regular menses.31 In fact, in a large study of glucose intolerance among women with PCOS, 38.6% of the PCOS women had either IGT (31.1%) or diabetes (7.5%) by WHO criteria. Notably, when examining the non-obese women with PCOS, 10.3% had IGT and 1.5% had diabetes.31
Obesity
Obesity is present in 30-70% of women with PCOS, depending on PCOS diagnostic criteria used and race/ethnicity of the population.14, 22 Conversely, one study showed that 30% of morbidly obese women met criteria for PCOS compared to 5% of the lean population.32 The role of obesity in the development of PCOS has been supported by a prospective study revealing that abdominal obesity and weight gain after puberty were associated with the development of PCOS.33 Obesity has also been shown to exacerbate the clinical complications of PCOS, including insulin resistance,27 hirsutism,34 and the prevalence of infertility.35 Notably, bariatric surgery and correction of obesity have been shown to result in resolution of PCOS characteristics.32
Genetic Susceptibility
The etiology of PCOS is unknown; however, several possible mechanisms have been postulated. In addition to the abnormal gonadotropin secretion and androgen excess previously discussed, the high heritability of PCOS characteristics suggests a genetic susceptibility to the disorder.25, 36-37 Candidate gene studies were initiated which examined genes associated with steroid hormone biosynthesis, gonadotropin, obesity, energy regulation, and insulin action.38-39 However, the only susceptibility locus that has been replicated is a dinucleotide repeat polymorphism within an intron of the fibrillin-3 gene on chromosome 19.40-41
Associated Metabolic Disorders
PCOS is characterized by multiple metabolic derangements, which may contribute to the development of hypertension and cardiovascular disease seen in this condition. However, it is important to note that, although women with PCOS manifest several cardiovascular disease risk factors, there have been no long-term prospective studies in women with PCOS that confirm the presence of increased cardiovascular disease events.42 One study employed menstrual irregularity as a proxy for PCOS in a prospective cohort study of 82,439 female nurses ages 20-35 years old.43 During a fourteen-year follow-up, women with “usually irregular” or “very irregular” menstrual cycles had an increased risk for nonfatal or fatal coronary heart disease compared to women with “very regular” menstrual cycles [age-adjusted relative risks (RR), 1.25 and 1.67, respectively; 95% confidence intervals (CI), 1.07-1.47 and 1.35-2.06, respectively]; a finding that was still significant after adjusting for BMI. There was also an insignificant increase in overall stroke risk (RR, 1.30; 95% CI, 0.97-1.74) and in ischemic stroke risk (RR, 1.40; 95% CI, 0.97-2.04) associated with “very irregular” menstrual cycles.
Metabolic syndrome
Metabolic syndrome has been variably defined by several international organizations44-49. However, all of the definitions include measures of central obesity, glucose intolerance, dyslipidemia, and high blood pressure. The prevalence of the metabolic syndrome in PCOS has been reported to be 43-47%, which is twice as high as the prevalence in the general population of comparable age, even after adjusting for BMI.50 The components of the metabolic syndrome most commonly present in PCOS are central obesity and low serum high-density lipoprotein cholesterol (HDL); however, elevated blood pressure, impaired fasting glucose, and glucose intolerance are commonly present.50 There is also an increased prevalence of metabolic syndrome among the sisters of women with PCOS.51
Dyslipidemia
The dyslipidemia in PCOS is similar to that seen in metabolic syndrome,52 characterized by low levels of HDL, small particle size of low-density lipoprotein cholesterol (LDL), and high triglyceride cholesterol levels.53 This pattern is more often seen in obese than in lean PCOS, likely secondary to the presence of greater insulin resistance in obesity.4 The level of LDL cholesterol is also increased in women with PCOS and is less dependent on obesity than are HDL and triglyceride levels.54 There is also evidence for heritability of dyslipidemia, so these lipid patterns can be seen not only in women with PCOS but also in their family members.51
Hypertension
Several studies suggest an increased prevalence of hypertension in women with PCOS compared to the general population55-62 However, a factor complicating the interpretation of the studies is that obesity, which is common in PCOS, is itself a significant risk factor for hypertension and this variable was not consistently considered many studies. Moreover, in the studies which did adjust the analyses for BMI, either statistically or by study design involving matching control women by BMI, the association between hypertension and PCOS is not always clear.
Several studies demonstrated an association between PCOS and hypertension, but did not adjust for an elevated BMI. A Dutch study of PCOS women demonstrated a higher prevalence of hypertension among premenopausal women with PCOS compared to women without PCOS; however, the PCOS population was significantly more obese and the obesity could be responsible for the greater prevalence of hypertension in this population.59 Additionally, hypertension was examined in menopausal women with PCOS who had undergone ovarian wedge resection.63 This surgical procedure was the first established treatment for women with PCOS64 and was commonly performed prior to the 1970s; however, it was discontinued due to the ovarian adhesions often following this procedure.65 This study revealed that menopausal women post-ovarian wedge resection had a three-fold increased likelihood of being hypertensive compared to non-PCOS women.63 These women with PCOS were also more obese than controls and this comparison was not adjusted for BMI. Although this study examines a postmenopausal population, the full burden of hypertension in PCOS has not been assessed since women with PCOS have not been followed prospectively beyond their reproductive years. One study which attempted to address this question was Wild et al. who conducted a retrospective examination of women with PCOS diagnosed an average of 31 years previously and found an increased prevalence of hypertension compared to a cohort of control women.62 However, given the study design, BMI was not considered in the statistical analysis and differences in BMI may explain the association with hypertension.
Additional studies demonstrated an association between PCOS and hypertension controlling for the influence of BMI. In one study, women with PCOS were 40% more likely to have elevated blood pressure than the non-PCOS women, independent of age, BMI, diabetes or dyslipidemia, (OR 1.41, 95% CI 1.31-1.51).19 Another population study from Brazil demonstrated similar findings in 69 women with PCOS when divided by BMI into normal, overweight, and obese categories and revealed a hypertension prevalence of 20.3%,;78.6% of these were obese and 21.4% were overweight.66 An examination of a Czech population of PCOS women in their early 30s compared to non-PCOS women revealed that, after adjusting for BMI, PCOS women had higher blood pressure.58 In a population of Dutch women with PCOS aged 45-54 yrs old, the prevalence of hypertension was 2.5 times greater than that of an age-matched Dutch female population.59 Notably, the proportion of obese women with PCOS in this age group did not differ significantly from the control population.
Additionally, an investigation of daytime ambulatory blood pressure monitoring (ABPM) among young (mean age approximately 26 yrs) overweight women (mean BMI approximately 26 kg/m2) revealed women with PCOS had higher blood pressures compared to regularly-menstruating control women. The women with PCOS compared to controls, although all normotensive, had systolic blood pressures in the prehypertensive range (mean ± SD, 126 ± 11 vs. 119 ± 12 mmHg, p < 0.05) and was independent of BMI.60
In addition to BMI, hypertension among women with PCOS may be affected by other background characteristics of the individual, such as race and ethnicity.19 The investigation by Lo et al. demonstrated that, among women with PCOS, the prevalence of hypertension or elevated blood pressure was lowest among Asians and Hispanics and highest among Blacks. Even after adjusting for age, BMI, and diabetes status, Blacks had the highest (OR 1.32, 95% CI 1.19-1.38) and Hispanics had the lowest (OR 0.68, 95% CI 0.62-0.75) prevalence of blood pressure elevation compared to the White population.19
There are data to suggest that the nocturnal decrease in blood pressure characteristic of healthy vasculature is absent in women with PCOS, both in adolescent67 and adult68 women. In the study examining adolescent women with PCOS, there was no difference in BMI between the women who had normal glucose tolerance (NGT) compared to IGT. However, all of the women with NGT manifested normal systolic blood pressure nocturnal dipping, whereas only 40% of those PCOS women with IGT demonstrated this normal blood pressure response.67 In adult women with PCOS, ABPM was found to be increased in 30% of women with PCOS, a finding largely explained by the increased prevalence of obesity in affected women.68
Other studies controlling for BMI have not revealed an association between PCOS and hypertension. A small study of 14 women with PCOS and 18 control obese women demonstrated no difference in blood pressures.69 Another study of young lean PCOS compared to age-matched control women did not reveal an increased blood pressure among the PCOS women.70 Conversely, a study of similarly overweight PCOS and control women did demonstrate a blood pressure discrepancy; however, the 50% prevalence of hypertension among PCOS women compared to 39% among control women did not reach statistical significance.71 One study demonstrated that obese women with PCOS were hypertensive compared to lean PCOS and lean control women. However, the lean PCOS women were not hypertensive compared to the lean control women.56 Similarly, in a study of 244 PCOS and an equal number of control women, BMI was a significant predictor of both systolic and diastolic blood pressure among women with PCOS.57 Additionally, there was no difference observed in ambulatory blood pressure in women with PCOS compared to control women with adjustment for BMI.72
Hypertension in Pregnancy
Pregnant women with PCOS have a greater risk of perinatal morbidity from pregnancy-induced hypertension (PIH) and preeclampsia (PE) than non-PCOS pregnancies as demonstrated in a meta-analysis of pregnancy outcomes in women with PCOS compared to controls.73 The studies included in the meta-analysis defined PIH as blood pressure ≥ 140/90 mmHg without proteinuria at a gestational age of >20 weeks) and defined PE as blood pressure ≥ 140/90 mmHg with proteinuria, either >0.3 g/24h urine or ≥ 2 + albustick at a gestational age of >20 weeks. The meta-analysis revealed an increased odds ratio of nearly 3.5-fold for both PIH (odds ratio, OR 3.67; 95% CI: 1.98-6.81), and PE (OR 3.47; 95% CI: 1.95-6.17). All of the women with PCOS in the preeclampsia studies included in the meta-analysis had higher BMI than controls. However, of the eight PIH studies included in the metanalysis, four studies matched PCOS and control women on BMI, while the other four studies had PCOS women with significantly higher BMI than the control women. In addition, the control groups were mainly spontaneous conceptions as opposed to the variable assisted reproductive therapies used among the PCOS women, which may74 or may not75 also increase the risk of preeclampsia.
Cardiovascular Disease Risk Factors
In studies that have examined more non-traditional risk factors for coronary heart disease, including inflammatory biomarkers,76-77 impaired vascular function,78 and arterial stiffness,79 derangements were not observed in the PCOS populations independent of obesity. Adiponectin, an adipokine inversely associated with atherosclerosis80 was also examined in PCOS and was found to be associated with insulin resistance and BMI, not with PCOS or testosterone levels.81 An examination of plasminogen activator inhibitor 1 (PAI-1) activity and tissue plasminogen activator (tPA) mass concentration between patients with PCOS and control women demonstrated that obese women with PCOS had increased levels of PAI-1 and tPA compared to controls; however, the lean PCOS levels of these two factors did not differ compared to the levels seen among the control women.82
However, age-matched populations of women with PCOS have been found to have increased carotid intima media thickness (cIMT) compared to control women, even after adjusting for BMI.83-85 Another study observed that cIMT was increased and brachial artery flow-mediated dilation was decreased in women with PCOS compared to age- and BMI-matched control women.86 Coronary artery calcification has also been observed to be greater among women with PCOS compared control women, even after adjusting for age and BMI.87-89
Pathophysiology of Hypertension in PCOS
Although the pathogenesis of PCOS has not yet been fully elucidated,90 there are several mechanisms potentially responsible for the development of hypertension in PCOS (Box 1). Thus, the etiology of hypertension that occurs in the setting of PCOS is also multifactorial, including factors such as hyperandrogenemia, insulin resistance, obesity, and increased sympathetic nervous system activity.
Box 1. Potential Causes of Hypertension in Women with PCOS.
Hyperandrogenism
Insulin resistance
Obesity
Increased Sympathetic Nervous System Activity
Androgen Excess
There are data demonstrating that the hyperandrogenemia in PCOS women is associated with systolic and diastolic blood pressures in women with PCOS, independent of obesity or insulin resistance.91 Androgen excess has also been associated with an increase in cIMT in women with PCOS.85 Increased cIMT has been widely used as a reflection of preclinical atherosclerotic disease, a contributor to the development of hypertension.92 A small study explored the relative impact of insulin resistance, another proposed etiology of hypertension in this population, compared to hyperandrogenism by studying PCOS women treated with an oral contraceptive containing 35 mcg of ethinyl estradiol and 2 mg of the antiandrogen cyproterone acetate (CPA-EE), to the insulin-sensitizer, metformin, then measuring ABPM and cIMT. The study revealed that CPA-EE use resulted in an increase in systolic, diastolic and mean arterial blood pressures during the day whereas metformin decreased all of these measures. No differences were observed in the nighttime parameters in response to either of these therapies. In addition, there was no statistically significant change in cIMT, although there was a tendency towards reduction in PCOS women treated with either CPA-EE or metformin. This study suggests that insulin resistance is more responsible than androgen levels for the hypertension seen in women with PCOS. However, the increase in blood pressure seen with the CPA-EE relative to the metformin group may have been due to the estrogen component and may not reflect the antiandrogenic effect.
Insulin Resistance
Hypertension may be secondary to enhanced sodium retention occurring in the setting of hyperinsulinemia.93 High insulin levels have been associated with a subsequent increase in intracellular sodium and calcium.94 as well as an increased insulin-like growth factor-1 (IGF-1) which may be associated with vascular smooth muscle hypertrophy. In support of the role of insulin resistance in mediating hypertension in women with PCOS, the beneficial effects of metformin on blood pressure have been reported.95 Additionally, the insulin sensitizing effects of metformin lead to a decrease in serum advanced glycated end products (AGEs),96 molecules that permit the proliferation and migration of smooth muscles cells to the vascular intima.97 Subsequently, the decrease in AGEs seen in the setting of insulin sensitizer therapy may lead to a decrease in cIMT. Moreover, other investigators have found an improvement in cIMT in response to metformin therapy on women with PCOS.98
Obesity
Obesity is a well-established risk factor for hypertension99 and it has been considered the primary etiology implicated in the increased blood pressure in women with PCOS.68 Current estimates internationally report greater than 60% of women with PCOS are overweight or obese.100-102 One population study demonstrated that women with PCOS were more than four times more likely to be obese (body mass index ≥ 30 kg/m2) than non-PCOS women. Additionally, blood pressure was more likely to be elevated among women who were obese compared to those who were non-obese (43.1% vs. 12.4%, p< 0.001).
Sympathetic Nervous System
In addition, the sympathetic nervous system has been implicated in the etiology of hypertension in this population. Greater sympathetic nerve activity was found in a study of 20 women with PCOS who were compared to 18 weight- and age-matched control women.103 The sympathetic nerve activity to the muscle vascular bed among women with PCOS was increased and highly correlated with testosterone level and to a lesser degree the cholesterol level. In addition, androgen excess104 as well as insulin resistance105 and obesity106 have been implicated in stimulating the autonomic nervous system, thereby, each serving as a potential mediator of the hypertension observed in PCOS.
Therapeutic Considerations
Therapy for PCOS is targeted towards ameliorating or eliminating the symptoms for each individual woman. Consequently, given the association of hypertension with all of the common PCOS manifestations, treating the manifestations of PCOS may treat concomitant hypertension or the risk for hypertension as well. In addition, one needs to assess hypertension in the context of assessing other CV risk factors as well.107
Treatment of hyperandrogenism revolves around the use of combination oral contraceptives (COC) or antiandrogens. However, the response to oral contraceptives has been inconsistent. The use of drospirenone, an antimineralocorticoid progestin, in combination with ethinyl estradiol compared to the vaginal contraceptive ring was associated with a minimal but statistically significant increase of diurnal and 24-hour systolic blood pressure in women with PCOS (drospirenone, 5 mmHg for both time periods, p = 0.001; and contraceptive ring, 6 mmHg for both time periods).108 Another study demonstrated a more convincing decrease in systolic blood pressure in response to a drospirenone containing COC of 1.9 mmHg compared to a 1.7 mmHg increase in systolic blood pressure in the desogestrel-containing COC group of women with PCOS.109 However, other investigations in PCOS demonstrated no change in blood pressure with a drospirenone-containing COC.110-111
Another antiandrogenic therapy used in women with PCOS is spironolactone. This aldosterone antagonist has been used as a potassium-sparing diuretic in the setting of hypertension since the 1950s. It was serendipity that associated its use with improvement in hirsutism in a woman with PCOS undergoing treatment of hypertension112 and it has since become the most widely used antiandrogen for female pattern hair loss in the US.113 Studies have demonstrated the efficacy in the treatment of hirsutism in women with PCOS114 so its use for this indication exceeds that for hypertension among women with PCOS. In studies examining the effect of spironolactone on blood pressure, one Indian study of spironolactone vs. metformin, no change in blood pressure was evident with either drug.115 In another investigation of PCOS women treated with spironolactone 100 mg daily for 2 months, mean blood pressure decreased significantly from 118 ± 5/82 ± 4 mmHg to 113 ± 4/72 ± 5 mmHg (p< 0.05).116
Lifestyle modifications, including diet and physical activity, are critical for women with PCOS who are overweight or obese in preventing hypertension.117 In addition, other methods of weight loss have shown promise for improving hypertension in the PCOS population. In a retrospective analysis of PCOS women who underwent a Roux-en-Y gastric bypass, normalization of blood pressure was observed in 78% of the previously hypertensive population.118
Conclusions
Hypertension is a significant contributor to the risk for cardiovascular disease. The increased prevalence of hypertension in women with PCOS may contribute to the increased risk of cardiovascular disease in women with PCOS. Thus, the Androgen Excess and Polycystic Ovarian Societies recommend that blood pressure be obtained in women with PCOS at every visit and that prehypertension be detected and treated given the potential benefit of lowering blood pressure for the prevention of CVD.107 Whether hypertension is associated with PCOS independent of obesity remains controversial. Nevertheless, detection and subsequent treatment of hypertension in this population should decrease the adverse sequelae from hypertensive cardiovascular disease. Moreover, treatment of the risk factors inherent to PCOS, such as hyperandrogenism, insulin resistance, and obesity, may minimize the risk not only for the development of hypertension but also for incident cardiovascular disease independent of hypertension.107 Treatment of hypertension in the PCOS population may take the form of lifestyle modification or pharmacotherapy.
Acknowledgments
This work was supported in part by Grant 5K23RR023333 from the National Institutes of Health and the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. American Journal of Obstetrics and Gynecology. 1935;19:181–191. [Google Scholar]
- 2.Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980 Jan;50(1):113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 3.Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987 Sep;65(3):499–507. doi: 10.1210/jcem-65-3-499. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997 Dec;18(6):774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 5.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005 Mar 24;352(12):1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 6.Polson DW, Adams J, Wadsworth J, Franks S. Polycystic ovaries--a common finding in normal women. Lancet. 1988 Apr 16;1(8590):870–872. doi: 10.1016/s0140-6736(88)91612-1. [DOI] [PubMed] [Google Scholar]
- 7.Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab. 2005 May;90(5):2571–2579. doi: 10.1210/jc.2004-0219. [DOI] [PubMed] [Google Scholar]
- 8.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004 Jan;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Robinson S, Kiddy D, Gelding SV, Willis D, Niththyananthan R, Bush A, Johnston DG, Franks S. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf) 1993 Sep;39(3):351–355. doi: 10.1111/j.1365-2265.1993.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 10.Adams JM, Taylor AE, Crowley WF, Jr, Hall JE. Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab. 2004 Sep;89(9):4343–4350. doi: 10.1210/jc.2003-031600. [DOI] [PubMed] [Google Scholar]
- 11.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Criteria for Defining Polycystic Ovary Syndrome as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J Clin Endocrinol Metab. 2006;91(11):4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 12.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897–1899. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 13.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010 Feb;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 14.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004 Jun;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 15.Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85(7):2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 16.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999 Nov;84(11):4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 17.Moran C, Tena G, Moran S, Ruiz P, Reyna R, Duque X. Prevalence of polycystic ovary syndrome and related disorders in mexican women. Gynecol Obstet Invest. 2010;69(4):274–280. doi: 10.1159/000277640. [DOI] [PubMed] [Google Scholar]
- 18.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 19.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006 Apr;91(4):1357–1363. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 20.Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endoc Meta Clinic North Am. 1999;28:295–324. doi: 10.1016/s0889-8529(05)70071-2. [DOI] [PubMed] [Google Scholar]
- 21.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 22.Franks S. Polycystic ovary syndrome. Trends Endocrinol Metab. 1989 Nov-Dec;1(2):60–63. doi: 10.1016/1043-2760(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 23.Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF, 3rd, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001 Dec;86(12):5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 24.Reid RL, Leopold GR, Yen SS. Induction of ovulation and pregnancy with pulsatile luteinizing hormone releasing factor: dosage and mode of delivery. Fertil Steril. 1981 Nov;36(5):553–559. doi: 10.1016/s0015-0282(16)45850-4. [DOI] [PubMed] [Google Scholar]
- 25.Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Differential activity of the cytochrome P450 17alpha-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000 Jun;85(6):2304–2311. doi: 10.1210/jcem.85.6.6631. [DOI] [PubMed] [Google Scholar]
- 27.Dunaif A. Insulin action in the polycystic ovary syndrome. Endoc Meta Clinic North Am. 1999;28:341–359. doi: 10.1016/s0889-8529(05)70073-6. [DOI] [PubMed] [Google Scholar]
- 28.Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS) Am J Physiol Endocrinol Metab. 2001 Aug;281(2):E392–399. doi: 10.1152/ajpendo.2001.281.2.E392. [DOI] [PubMed] [Google Scholar]
- 29.Book CB, Dunaif A. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1999 Sep;84(9):3110–3116. doi: 10.1210/jcem.84.9.6010. [DOI] [PubMed] [Google Scholar]
- 30.Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96:520–527. doi: 10.1172/JCI118064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999 Jan;84(1):165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 32.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millán JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005 Dec;90(12):6364–6369. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- 33.Laitinen J, Taponen S, Martikainen H, Pouta A, Millwood I, Hartikainen AL, Ruokonen A, Sovio U, McCarthy MI, Franks S, Järvelin MR. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord. 2003 Jun;27(6):710–715. doi: 10.1038/sj.ijo.0802301. [DOI] [PubMed] [Google Scholar]
- 34.Hoeger KM. Obesity and lifestyle management in polycystic ovary syndrome. Clin Obstet Gynecol. 2007 Mar;50(1):277–294. doi: 10.1097/GRF.0b013e31802f54c8. [DOI] [PubMed] [Google Scholar]
- 35.Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994 Mar;5(2):247–250. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002 May;87(5):2134–2138. doi: 10.1210/jcem.87.5.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002 May;87(5):2128–2133. doi: 10.1210/jcem.87.5.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, Strauss JF, 3rd, Spielman RS, Dunaif A. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999 Jul 20;96(15):8573–8578. doi: 10.1073/pnas.96.15.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urbanek M, Wu X, Vickery KR, Kao LC, Christenson LK, Schneyer A, Legro RS, Driscoll DA, Strauss JF, 3rd, Dunaif A, Spielman RS. Allelic variants of the follistatin gene in polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85(12):4455–4461. doi: 10.1210/jcem.85.12.7026. [DOI] [PubMed] [Google Scholar]
- 40.Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007 Nov;92(11):4191–4198. doi: 10.1210/jc.2007-0761. [DOI] [PubMed] [Google Scholar]
- 41.Ewens KG, Stewart DR, Ankener W, Urbanek M, McAllister JM, Chen C, Baig KM, Parker SC, Margulies EH, Legro RS, Dunaif A, Strauss JF, 3rd, Spielman RS. Family-based analysis of candidate genes for polycystic ovary syndrome. J Clin Endocrinol Metab. 2010 May;95(5):2306–2315. doi: 10.1210/jc.2009-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003 Jun;24(3):302–312. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 43.Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002 May;87(5):2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- 44.National Cholesterol Education Program (NCEP) Expert Panel on Detection E and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 45.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 46.Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, Hellman R, Jellinger PS, Kendall D, Krauss RM, Neufeld ND, Petak SM, Rodbard HW, Seibel JA, Smith DA, Wilson PW. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003 May-Jun;9(3):237–252. [PubMed] [Google Scholar]
- 47.International Diabetes Federation. Worldwide definition of the metabolic syndrome. 2005 Available at: http://www.idf.org/webdata/docs/IDF_Metabolic_syndrome_definition.pdf.
- 48.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistace (EGIR) Diab Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 49.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 50.Essah PA, Nestler JE. Metabolic syndrome in women with polycystic ovary syndrome. Fertil Steril. 2006;86:S18–19. doi: 10.1016/j.fertnstert.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Sam S, Legro RS, Bentley-Lewis R, Dunaif A. Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005 Aug;90(8):4797–4802. doi: 10.1210/jc.2004-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007 Oct;3(10):696–704. doi: 10.1038/ncpendmet0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legato MJ. Dyslipidemia, gender, and the role of high-density lipoprotein cholesterol: implications for therapy. Am J Cardiol. 2000 Dec 21;86(12A):15L–18L. doi: 10.1016/s0002-9149(00)01463-6. [DOI] [PubMed] [Google Scholar]
- 54.Valkenburg O, Steegers-Theunissen RP, Smedts HP, Dallinga-Thie GM, Fauser BC, Westerveld EH, Laven JS. A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case-control study. J Clin Endocrinol Metab. 2008 Feb;93(2):470–476. doi: 10.1210/jc.2007-1756. [DOI] [PubMed] [Google Scholar]
- 55.Carmina E. Cardiovascular risk and events in polycystic ovary syndrome. Climacteric. 2009;12(Suppl 1):22–25. doi: 10.1080/13697130903003842. [DOI] [PubMed] [Google Scholar]
- 56.Conway GS, Agrawal R, Betteridge DJ, Jacobs HS. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992 Aug;37(2):119–125. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 57.Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, Daniels T, Engberg RA. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol. 1998 May;51(5):415–422. doi: 10.1016/s0895-4356(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 58.Vrbíková J, Cífková R, Jirkovská A, Lánská V, Platilová H, Zamrazil V, Stárka L. Cardiovascular risk factors in young Czech females with polycystic ovary syndrome. Hum Reprod. 2003 May;18(5):980–984. doi: 10.1093/humrep/deg218. [DOI] [PubMed] [Google Scholar]
- 59.Elting MW, Korsen TJ, Bezemer PD, Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod. 2001 Mar;16(3):556–560. doi: 10.1093/humrep/16.3.556. [DOI] [PubMed] [Google Scholar]
- 60.Holte J, Gennarelli G, Berne C, Bergh T, Lithell H. Elevated ambulatory day-time blood pressure in women with polycystic ovary syndrome: a sign of a pre-hypertensive state? Hum Reprod. 1996 Jan;11(1):23–28. doi: 10.1093/oxfordjournals.humrep.a019028. [DOI] [PubMed] [Google Scholar]
- 61.Orbetzova MM, Shigarminova RG, Genchev GG, Milcheva BA, Lozanov LB, Genov NS, Zacharieva SZ. Role of 24-hour monitoring in assessing blood pressure changes in polycystic ovary syndrome. Folia Med (Plovdiv) 2003;45(3):21–25. [PubMed] [Google Scholar]
- 62.Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3(2):101–105. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 63.Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Odén A, Janson PO, Mattson LA, Crona N, Lundberg PA. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril Mar. 1992;57(3):505–513. doi: 10.1016/s0015-0282(16)54892-4. [DOI] [PubMed] [Google Scholar]
- 64.Farquhar C, Lilford RJ, Marjoribanks J, Vandekerckhove P. Laparoscopic ‘drilling’ by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2007 Jul 18;3 doi: 10.1002/14651858.CD001122.pub3. CD001122. [DOI] [PubMed] [Google Scholar]
- 65.Buttram VC, Vaquero C. Post-ovarian wedge resection adhesive disease. Fertility and Sterility. 1975;26:874–876. doi: 10.1016/s0015-0282(16)41351-8. [DOI] [PubMed] [Google Scholar]
- 66.Barcellos CR, Rocha MP, Hayashida SA, Mion Junior D, Lage SG, Marcondes JA. Impact of body mass index on blood pressure levels in patients with polycystic ovary syndrome. Arq Bras Endocrinol Metabol. 2007 Oct;51(7):1104–1109. doi: 10.1590/s0004-27302007000700013. [DOI] [PubMed] [Google Scholar]
- 67.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001 Jan;86(1):66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 68.Luque-Ramírez M, Alvarez-Blasco F, Mendieta-Azcona C, Botella-Carretero JI, Escobar-Morreale HF. Obesity is the major determinant of the abnormalities in blood pressure found in young women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007 Jun;92(6):2141–2148. doi: 10.1210/jc.2007-0190. [DOI] [PubMed] [Google Scholar]
- 69.Zimmermann S, Phillips RA, Dunaif A, Finegood DT, Wilkenfeld C, Ardeljan M, Gorlin R, Krakoff LR. Polycystic ovary syndrome: lack of hypertension despite profound insulin resistance. J Clin Endocrinol Metab. 1992 Aug;75(2):508–513. doi: 10.1210/jcem.75.2.1639952. [DOI] [PubMed] [Google Scholar]
- 70.Sampson M, Kong C, Patel A, Unwin R, Jacobs HS. Ambulatory blood pressure profiles and plasminogen activator inhibitor (PAI-1) activity in lean women with and without the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1996 Nov;45(5):623–629. doi: 10.1046/j.1365-2265.1996.00863.x. [DOI] [PubMed] [Google Scholar]
- 71.Cibula D, Cífková R, Fanta M, Poledne R, Zivny J, Skibová J. Increased risk of non-insulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Hum Reprod. 2000 Apr;15(4):785–789. doi: 10.1093/humrep/15.4.785. [DOI] [PubMed] [Google Scholar]
- 72.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005 Oct;90(10):5711–5716. doi: 10.1210/jc.2005-0011. [DOI] [PubMed] [Google Scholar]
- 73.Boomsma CM, Fauser BC, Macklon NS. Pregnancy complications in women with polycystic ovary syndrome. Semin Reprod Med. 2008 Jan;26(1):72–84. doi: 10.1055/s-2007-992927. [DOI] [PubMed] [Google Scholar]
- 74.Ludwig M. Risk during pregnancy and birth after assisted reproductive technologies: an integral view of the problem. Semin Reprod Med. 2005 Nov;23(4):363–370. doi: 10.1055/s-2005-923394. [DOI] [PubMed] [Google Scholar]
- 75.Sun LM, Walker MC, Cao HL, Yang Q, Duan T, Kingdom JC. Assisted reproductive technology and placenta-mediated adverse pregnancy outcomes. Obstet Gynecol. 2009 Oct;114(4):818–824. doi: 10.1097/AOG.0b013e3181b76bd1. [DOI] [PubMed] [Google Scholar]
- 76.Möhlig M, Spranger J, Osterhoff M, Ristow M, Pfeiffer AF, Schill T, Schlösser HW, Brabant G, Schöfl C. The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinol. 2004 Apr;150(4):525–532. doi: 10.1530/eje.0.1500525. [DOI] [PubMed] [Google Scholar]
- 77.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Sancho J, San Millán JL. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia. 2003 May;46(5):625–633. doi: 10.1007/s00125-003-1090-z. [DOI] [PubMed] [Google Scholar]
- 78.Ketel IJ, Stehouwer CD, Serné EH, Korsen TJ, Hompes PG, Smulders YM, de Jongh RT, Homburg R, Lambalk CB. Obese but not normal-weight women with polycystic ovary syndrome are characterized by metabolic and microvascular insulin resistance. J Clin Endocrinol Metab. 2008 Sep;93(9):3365–3372. doi: 10.1210/jc.2008-0626. [DOI] [PubMed] [Google Scholar]
- 79.Ketel IJ, Stehouwer CD, Henry RM, Serné EH, Hompes P, Homburg R, Smulders YM, Lambalk CB. Greater Arterial Stiffness in Polycystic Ovary Syndrome (PCOS) Is an Obesity--But Not a PCOS-Associated Phenomenon. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-0868. [DOI] [PubMed] [Google Scholar]
- 80.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010 Feb;74(2):213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 81.Spranger J, Möhlig M, Wegewitz U, Ristow M, Pfeiffer AF, Schill T, Schlösser HW, Brabant G, Schöfl C. Adiponectin is independently associated with insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004 Dec;61(6):738–746. doi: 10.1111/j.1365-2265.2004.02159.x. [DOI] [PubMed] [Google Scholar]
- 82.Lindholm A, Bixo M, Eliasson M, Hudecova M, Arnadottir R, Holte J, Poromaa IS. Tissue plasminogen activator and plasminogen activator inhibitor 1 in obese and lean patients with polycystic ovary syndrome. Gynecol Endocrinol. 2010 doi: 10.3109/09513590.2010.487592. [DOI] [PubMed] [Google Scholar]
- 83.Guzick DS, Talbott EO, Sutton-Tyrrell K, Herzog HC, Kuller LH, Wolfson SK., Jr Carotid atherosclerosis in women with polycystic ovary syndrome: initial results from a case-control study. Am J Obstet Gynecol. 1996;174:1224–1232. doi: 10.1016/s0002-9378(96)70665-8. [DOI] [PubMed] [Google Scholar]
- 84.Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, Kuller LH. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20:2414–2421. doi: 10.1161/01.atv.20.11.2414. [DOI] [PubMed] [Google Scholar]
- 85.Luque-Ramírez M, Mendieta-Azcona C, Alvarez-Blasco F, Escobar-Morreale HF. Androgen excess is associated with the increased carotid intima-media thickness observed in young women with polycystic ovary syndrome. Hum Reprod. 2007 Dec;22(12):3197–3203. doi: 10.1093/humrep/dem324. [DOI] [PubMed] [Google Scholar]
- 86.Carmina E, Orio F, Palomba S, Longo RA, Cascella T, Colao A, Lombardi G, Rini GB, Lobo RA. Endothelial dysfunction in PCOS: role of obesity and adipose hormones. Am J Med. 2006 Apr;119(4):356.e351–356. doi: 10.1016/j.amjmed.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 87.Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, 2nd, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562–2568. doi: 10.1210/jc.2003-030334. [DOI] [PubMed] [Google Scholar]
- 88.Talbott EO, Zborowski JV, Rager JR, Boudreaux MY, Edmundowicz DA, Guzick DS. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5454–5461. doi: 10.1210/jc.2003-032237. [DOI] [PubMed] [Google Scholar]
- 89.Talbott EO, Zborowski J, Rager J, Stragand JR. Is there an independent effect of polycystic ovary syndrome (PCOS) and menopause on the prevalence of subclinical atherosclerosis in middle aged women? Vasc Health Risk Manag. 2008;4(2):453–462. doi: 10.2147/vhrm.s1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007 Aug 25;370(9588):685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 91.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007 Jun;49(6):1442–1447. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 92.Riccioni G. The effect of antihypertensive drugs on carotid intima media thickness: an up-to-date review. Curr Med Chem. 2009;16(8):988–996. doi: 10.2174/092986709787581923. [DOI] [PubMed] [Google Scholar]
- 93.Zavaroni I, Coruzzi P, Bonini L, Mossini GL, Musiari L, Gasparini P, Fantuzzi M, Reaven GM. Association between salt sensitivity and insulin concentrations in patients with hypertension. Am J Hypertens. 1995 Aug;8(8):855–858. doi: 10.1016/0895-7061(95)00152-F. [DOI] [PubMed] [Google Scholar]
- 94.Resnick LM. Cellular ions in hypertension, insulin resistance, obesity, and diabetes: a unifying theme. J Am Soc Nephrol. 1992 Oct;3(4 Suppl):S78–85. doi: 10.1681/ASN.V34s78. [DOI] [PubMed] [Google Scholar]
- 95.Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev. 2003;3 doi: 10.1002/14651858.CD003053. CD003053. [DOI] [PubMed] [Google Scholar]
- 96.Diamanti-Kandarakis E, Alexandraki K, Piperi C, Aessopos A, Paterakis T, Katsikis I, Panidis D. Effect of metformin administration on plasma advanced glycation end product levels in women with polycystic ovary syndrome. Metabolism. 2007 Jan;56(1):129–134. doi: 10.1016/j.metabol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Hattori Y, Suzuki M, Hattori S, Kasai K. Vascular smooth muscle cell activation by glycated albumin (Amadori adducts) Hypertension. 2002 Jan;39(1):22–28. doi: 10.1161/hy1201.097300. [DOI] [PubMed] [Google Scholar]
- 98.Orio F, Jr, Palomba S, Cascella T, De Simone B, Manguso F, Savastano S, Russo T, Tolino A, Zullo F, Lombardi G, Azziz R, Colao A. Improvement in endothelial structure and function after metformin treatment in young normal-weight women with polycystic ovary syndrome: results of a 6-month study. J Clin Endocrinol Metab. 2005 Nov;90(11):6072–6076. doi: 10.1210/jc.2005-0965. [DOI] [PubMed] [Google Scholar]
- 99.Kannel WB. Fifty years of Framingham Study contributions to understanding hypertension. J Hum Hypertens. 2000 Feb;14(2):83–90. doi: 10.1038/sj.jhh.1000949. [DOI] [PubMed] [Google Scholar]
- 100.Cupisti S, Kajaia N, Dittrich R, Duezenli H, W Beckmann M, Mueller A. Body mass index and ovarian function are associated with endocrine and metabolic abnormalities in women with hyperandrogenic syndrome. Eur J Endocrinol. 2008 May 5;158:711–719. doi: 10.1530/EJE-07-0515. [DOI] [PubMed] [Google Scholar]
- 101.Glintborg D, Henriksen JE, Andersen M, Hagen C, Hangaard J, Rasmussen PE, Schousboe K, Hermann AP. Prevalence of endocrine diseases and abnormal glucose tolerance tests in 340 Caucasian premenopausal women with hirsutism as the referral diagnosis. Fertil Steril. 2004 Dec;82(6):1570–1579. doi: 10.1016/j.fertnstert.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 102.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004 Feb;89(2):453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 103.Sverrisdottir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab. 2008;294:E576–581. doi: 10.1152/ajpendo.00725.2007. [DOI] [PubMed] [Google Scholar]
- 104.Yildirir A, Aybar F, Kabakci G, Yarali H, Oto A. Heart rate variability in young women with polycystic ovary syndrome. Ann Noninvasive Electrocardiol. 2006 Oct;11(4):306–312. doi: 10.1111/j.1542-474X.2006.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007 Aug;28(5):463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 106.Müller-Wieland D, Kotzka J, Knebel B, Krone W. Metabolic syndrome and hypertension: pathophysiology and molecular basis of insulin resistance. Basic Res Cardiol. 1998;93(Suppl 2):131–134. doi: 10.1007/s003950050238. [DOI] [PubMed] [Google Scholar]
- 107.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010 May;95(5):2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 108.Battaglia C, Mancini F, Fabbri R, Persico N, Busacchi P, Facchinetti F, Venturoli S. Polycystic ovary syndrome and cardiovascular risk in young patients treated with drospirenone-ethinylestradiol or contraceptive vaginal ring. A prospective, randomized, pilot study. Fertil Steril. 2010 Sep;94(4):1417–1425. doi: 10.1016/j.fertnstert.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 109.Kriplani A, Periyasamy AJ, Agarwal N, Kulshrestha V, Kumar A, Ammini AC. Effect of oral contraceptive containing ethinyl estradiol combined with drospirenone vs. desogestrel on clinical and biochemical parameters in patients with polycystic ovary syndrome. Contraception. 2010 Aug;82(2):139–146. doi: 10.1016/j.contraception.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 110.Fruzzetti F, Perini D, Lazzarini V, Parrini D, Gambacciani M, Genazzani AR. Comparison of effects of 3 mg drospirenone plus 20 mug ethinyl estradiol alone or combined with metformin or cyproterone acetate on classic metabolic cardiovascular risk factors in nonobese women with polycystic ovary syndrome. Fertil Steril. 2009 Nov 18; doi: 10.1016/j.fertnstert.2009.10.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 111.Guido M, Romualdi D, Giuliani M, Suriano R, Selvaggi L, Apa R, Lanzone A. Drospirenone for the treatment of hirsute women with polycystic ovary syndrome: a clinical, endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004 Jun;89(6):2817–2823. doi: 10.1210/jc.2003-031158. [DOI] [PubMed] [Google Scholar]
- 112.Ober KP, Hennessy JF. Spironolactone therapy for hirsutism in a hyperandrogenetic woman. Ann Intern Med. 1978;89:643–644. doi: 10.7326/0003-4819-89-5-643. [DOI] [PubMed] [Google Scholar]
- 113.Rathnayake D, Sinclair R. Innovative use of spironolactone as an antiandrogen in the treatment of female pattern hair loss. Dermatol Clin. 2010 JUL 01;28(3):611–618. doi: 10.1016/j.det.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 114.Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39(9):1517–1521. doi: 10.1345/aph.1G025. [DOI] [PubMed] [Google Scholar]
- 115.Ganie MA, Khurana ML, Eunice M, Gupta N, Gulati M, Dwivedi SN, Ammini AC. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004 Jun;89(6):2756–2762. doi: 10.1210/jc.2003-031780. [DOI] [PubMed] [Google Scholar]
- 116.Armanini D, Castello R, Scaroni C, Bonanni G, Faccini G, Pellati D, Bertoldo A, Fiore C, Moghetti P. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur J Obstet Gynecol Reprod Biol. 2007 Mar;131(1):61–67. doi: 10.1016/j.ejogrb.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 117.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002 Aug;13(6):251–257. doi: 10.1016/s1043-2760(02)00612-4. [DOI] [PubMed] [Google Scholar]
- 118.Eid GM, Cottam DR, Velcu LM, Mattar SG, Korytkowski MT, Gosman G, Hindi P, Schauer PR. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005 Mar-Apr;1(2):77–80. doi: 10.1016/j.soard.2005.02.008. [DOI] [PubMed] [Google Scholar]


