Abstract
The sirtuin SIRT1, a class III NAD+-dependent protein histone deacetylase, is present throughout the body that involves cells of the central nervous system, immune system, cardiovascular system, and the musculoskeletal system. SIRT1 has broad biological effects that affect cellular metabolism as well as cellular survival and longevity that can impact both acute and chronic disease processes that involve neurodegenerative disease, diabetes mellitus, cardiovascular disease, and cancer. Given the intricate relationship SIRT1 holds with a host of signal transduction pathways ranging from transcription factors, such as forkhead, to cytokines and growth factors, such as erythropoietin, it becomes critical to elucidate the cellular pathways of SIRT1 to safely and effectively develop and translate novel avenues of treatment for multiple disease entities.
Keywords: apoptosis, autophagy, erythropoietin, FoxO, oxidative stress, sirtuin
Cell Injury and Oxidative Stress
Oxidative stress is the result of the cellular release of reactive oxygen species, such as oxygen free radicals and other chemical entities that lead to cell injury in the body. Excessive amounts of oxygen free radicals can be generated during the reduction of oxygen and lead to cell injury. Reactive oxygen species (ROS) include superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO), and peroxynitrite [1–8]. During normal physiological conditions, reactive oxygen species are released at low levels and are scavenged by endogenous antioxidant systems that include superoxide dismutase (SOD), glutathione peroxidase, catalase, and small molecule substances such as vitamins C and E [9–11]. Additional anti-oxidant pathways may block oxidative injury as well as affect tumor growth such as vitamin D3 [12, 13] and the amide form of niacin or vitamin B3, nicotinamide [4, 14–22].
Oxidative stress is involved in the pathology of a broad range of disease processes that involve metabolic disorders and diabetes mellitus (DM) [23–29], loss of cognition [30–34], myocardial disease [5, 28, 35–37], psychiatric disorders [32, 38, 39], nervous system injury [40–44], vascular disease [28, 34, 45–52], and hepatic disease [7, 53, 54]. In cells, oxygen free radicals can result in cellular membrane lipid peroxidation and protein oxidation leading to the disruption of cellular integrity [7].
Loss of cellular integrity can occur through a variety of mechanisms that include both apoptosis and autophagy. Apoptosis and autophagy occur in multiple cells including ocular cells [55, 56], non-neuronal cells [9, 57–61], neurons [42, 43, 61–67], vascular cells [46, 48, 49, 68–70], cancer cells [14, 71–76], and inflammatory cells [77–81]. In regards to programmed cell death with apoptosis, this process consists of both the early exposure of membrane phosphatidylserine (PS) residues and the later destruction of genomic DNA [5, 57, 82]. Apoptotic membrane PS exposure is present during conditions such as oxidant stress [5, 57, 82] and β-amyloid (Aβ) exposure [41, 83]. Membrane PS exposure can function to identify cells for the phagocytosis, alter vascular endothelial cell function[15, 19, 84–86], and influence inflammatory cell activation and proliferation [58, 59, 80, 81, 83, 87, 88].
The SIRT1 Pathway
Sirtuins, class III NAD+-dependent protein histone deacetylases, are the mammalian homologues of Sir2 of the yeast silent information regulator-2 (Sir2). Sirtuins transfer the acetyl residue from the acetyllysine residue of histone to the ADP-ribose moiety of NAD+, resulting in the production of nicotinamide, 2′-O-acetyl ADP ribose, and deacetylated proteins. Seven mammalian homologues of Sir2, SIRT1 through SIRT7, have been identified. SIRT1 was the first to be identified. SIRT1 uses NAD+ as substrate. Yet, the level of NAD+ also can control the deacetylating activity of SIRT1. In the salvage pathway of NAD+ synthesis, nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the conversion of nicotinamide to nicotinamide mononucleotide, which is then converted to NAD+ by nicotinamide/nicotinic acid mononucleotide adenylyl-transferase (NMNAT). NAMPT functions as the rate-limiting enzyme in mammalian NAD+ biosynthesis pathway. Increased NAMPT activity raises the total cellular NAD level and subsequent transcriptional regulatory activity of SIRT1 [89]. In addition, NMNAT can regulate the deacetylating activity SIRT1 at its target gene promoters [89, 90].
SIRT1 is expressed in the brain, heart, liver, pancreas, skeletal muscle, spleen, and adipose tissues [91–93]. At the cellular level, SIRT1 is present in the nucleus and cytoplasm with primary expression in the nucleus. SIRT1 and agents that affect its activity play a significant role in multiple biological processes that include oxidative stress, metabolism, cellular proliferation, and genomic stability [94]. SIRT1 has been demonstrated to regulate cellular protection against oxidative stress in many disease states that involve aging, neurodegeneration, metabolic disorders, cancer, and cardiovascular disease [47, 48, 79, 95–102] (Figure 1).
Figure 1.
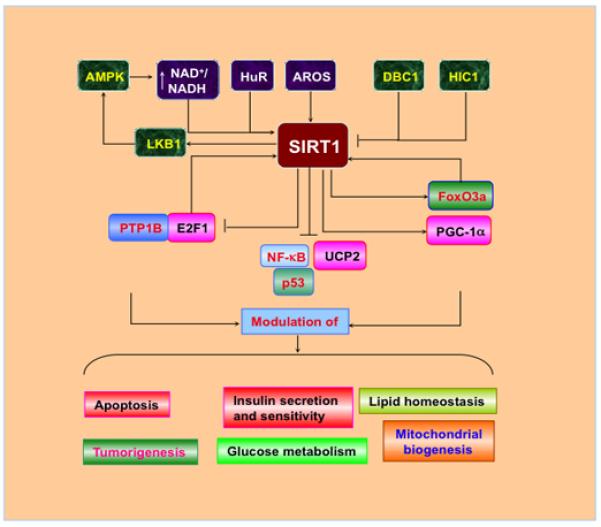
Cell signaling pathways of SIRT1. The RNA binding protein HuR and a nuclear protein active regulator of SIRT1 (AROS) can promote SIRT1 activity. AMP activated protein kinase (AMPK) can activate SIRT1 through increasing the level of NAD+/NADH ratio, but there exists a positive feedback, activated SIRT1 deacetylates the serine-threonine liver kinase B1 (LKB1) and subsequently activates AMPK. In contrast, hypermethylated in cancer 1 (HIC1) and deleted in breast cancer 1 (DBC1) negatively regulates the activity of SIRT1. Downstream of SIRT1 are the FoxO transcription factors, peroxisome proliferators-activated receptor-γ coactivator-1α (PGC-1α), protein tyrosine phosphatase 1B (PTP1B), p53, nuclear factor-κB (NF-κB), apoptotic transcription regulator E2F1, and the uncoupling protein gene UCP2. SIRT1 can, through modulating its multiple targets, regulate lipid metabolism, insulin secretion and sensitivity, mitochondrial function, and cell survival.
Cytoprotection by SIRT1
SIRT1 can offer protection in multiple cell types during oxidative stress as either a down-stream mediator of cell protection or through the modulation of specific cellular pathways. Treatment with the cytokine and growth factor erythropoietin (EPO) offers one example of the ability of SIRT1 to function as a mediator of cellular protection during oxidative stress. EPO is a 30.4 kDa glycoprotein with almost half of its molecular weight derived from carbohydrates that can vary among species [103, 104]. EPO has four glycosylated chains that include three N-linked and one O-linked acidic oligosaccharide side chains. The N-linked glycosylation sites occur at the positions 24, 38, and 83 of aspartyl residues, while the O-linked glycosylation site is at Serine126 [54, 105]. Although the Food and Drug Administration has approved EPO for the treatment of anemia, a number of investigations have shown that EPO is not only important for erythropoiesis, but also has a significant role in other organ systems such as the brain, heart, and vascular system [10, 36, 84, 106–113]. In the nervous system, EPO production and secretion occurs in the hippocampus, internal capsule, cortex, midbrain, cerebral endothelial cells, and astrocytes. Other organs as secretory tissues for EPO include endothelial cells, myoblasts, insulin-producing cells, and cardiac tissue [5, 110, 113–116].
As a cytoprotectant, EPO can increase cell survival during hypoxia [61, 84, 117–121], free radical injury [63, 117, 122–124], infection [125–127], cardiovascular injury [48, 52, 69, 84, 121, 122, 128–136], amyloid exposure [32, 137–139], and diabetic complications [25, 69, 128, 130, 140, 141]. In addition, EPO modulates both the activity and proliferation of non-neuronal and inflammatory cells [120, 142–144].
In regards to SIRT1, recent work has shown that EPO increases endogenous SIRT1 activity in endothelial cells and fosters the subcellular trafficking of SIRT1 to the nucleus which is necessary for EPO to foster vascular protection [48]. Downstream from SIRT1, activation of protein kinase B (Akt1) is required. Activation of Akt1 results in cellular protection [16, 18, 42, 45–47, 64, 73, 78, 128, 143, 145–153] and SIRT1 has been associated with enhanced activity of Akt [47, 48, 93, 154, 155].
In addition, SIRT1 employs Akt1 to modulate the phosphorylation and subcellular trafficking of the forkhead transcription factor FoxO3a [48]. Mammalian forkhead transcription factors of the O class (FoxO1, FoxO3, FoxO4, and FoxO6) are involved in cell metabolism, insulin sensitivity, aging, fertility, and oxidative stress [29, 75, 156–165] and have been tied to the modulatory effects of EPO [48, 68, 128, 166–168]. Phosphorylation of FoxOs results in their retention in the cytoplasm and inhibition of their transcription activity [20, 169]. Acetylation of FoxOs can also modulate their transcriptional activity through facilitating their phosphorylation and nuclear translocation [81, 83, 170]. Nuclear localization of FoxO occurs through deacetylation [171] and is inhibited by phosphorylation.
SIRT1 controls the nuclear shuttling and transcriptional activity of forkhead transcription factors. During oxidative stress, FoxOs translocate to the nucleus, interact with SIRT1, and result in the deacetylation of FoxOs. Dependent upon the post-translational changes on FoxOs, SIRT1 can inhibit FoxO activity and protect cells from oxidative stress [47, 48] or increase the activity of FoxOs to lead to gene activation [75, 159, 163]. With some forkhead family members such as FoxO4, SIRT1 can bind to FOXO4, catalyze deacetylation in an NAD-dependent manner, and increase FoxO4 transactivation activity [172].
Multiple FoxO family members may participate in the protective effects of SIRT1. Modulation by SIRT1 over FoxO function occurs via NAD-dependent deacetylation in response to oxidative stress. SIRT1 also can target FoxOs such as FoxO1 to bind and deacetylate FoxO1 at residues that are acetylated by cAMP-response element-binding protein [173]. Studies with over-expression of SIRT1 demonstrate protection of cardiomyocytes from oxidative stress through a FoxO1-dependent pathway [174]. It should be noted that SIRT1 can enhance expression of FoxO targets that are involved in stress resistance, such as manganese superoxide dismutase (MnSOD) and Gadd45, but diminishes the expression of pro-apoptotic FoxO targets (Fas ligand and Bim), suggesting that SIRT1 may modulate the balance between stress resistance and cell death within cells [159, 163]. Deacetylation of FoxOs by SIRT1 also can regulate autophagy. SIRT1 mediated deacetylation of FoxO1 has been associated with increases in autophagic flux which may be required to maintain cardiac function during glucose deprivation and starvation [175]. FoxO1 also increases the expression of Rab7, a small GTP-binding protein that mediates late autophagosome-lysosome fusion, which is both necessary and sufficient for mediating FoxO1-induced increases in autophagic process [175].
FoxOs also exert a positive feedback mechanism regulating SIRT1 expression. FoxO1 can directly bind to the SIRT1 promoter region containing a cluster of five putative FoxO1 core binding repeat motifs (IRS-1) and a forkhead-like consensus-binding site (FKHD-L). This leads to a FoxO1 modulating SIRT1 transcription and leads to an increase in the expression of SIRT1 [176]. FoxO3a also can regulate the expression of SIRT1 by binding to two p53 binding sites within the SIRT1 promoter to foster SIRT1 transcription during acute nutrient withdrawal [177].
It is interesting to note that SIRT1 may, at times, influence cancer growth. SIRT1 has been found to negatively regulate the tumor suppressor gene p53 through an NAD dependent deacetylating mechanism and therefore, SIRT1 has been considered as a tumorigenic factor [178]. A nuclear protein active regulator of SIRT1 (AROS) also can enhance SIRT1-mediated deacetylation of p53 both in vitro and in vivo models and prevents p53-mediated transcriptional activity [179]. Hypermethylated in Cancer 1 (HIC1) and deleted in breast cancer 1 (DBC1) have been identified as negative regulators of SIRT1 [180]. HIC1, a transcriptional repressor, binds to the SIRT1 promoter and represses its transcription. Loss of HIC1 increases SIRT1 expression in normal or cancer cells, resulting in the deacetylation and inactivation of p53 and enhanced tumorigenesis [181]. However, other studies suggest, such as in BRCA1-associated breast cancer, that SIRT1 may stem cancer cell progression [182].
FoxOs may be tied to the modulatory role of SIRT1 during tumorigenesis. FoxO proteins promote apoptotic cell death and cell cycle progression [76, 183]. FoxO3a and FoxO4 can promote cell cycle arrest in mouse myoblastic cell lines through modulation of growth-arrest and DNA-damage-response protein 45 [74, 160]. The transcription factor E2F1 that controls the induction of the cell cycle can increase the endogenous expression of FoxO1 and FoxO3a to lead to cell cycle arrest [184]. However, loss of FoxO3a activity in association with c-myc, p27, and nuclear factor-κB (NF-κB) can result in cell cycle induction and malignant transformation of mouse cells in the presence of oncogene activation [159, 185]. SIRT1 may oversee FoxO transcription pathways to enhance anti-tumor activity. For example, resveratrol can block the phosphorylation of FKHRL1 leading to increased activity and enhancement of TRAIL pathways to block tumor growth in prostate cancer [71]. In addition, SIRT1 can interact with E2F1 during cell cycle regulation and apoptosis [186]. As an important regulator in response to DNA damage induced stress, E2F1 can positively regulate SIRT1 expression at the transcriptional level. SIRT1 binds to E2F1 and deacetylates E2F1, resulting in the inhibition of E2F1 transcriptional activity [186].
It should be emphasized that the relationship between SIRT1 and forkhead transcription factors is not entirely clear. In some cell systems, such as in renal cells, the presence of FoxO3a may be necessary for SIRT1 to exert protection and maintain cell longevity during oxidative stress [187]. SIRT1 also may be dependent upon other forkhead transcription factors, such as FoxO1, to regulate the transcription of SIRT1 [176]. Under such circumstances, enhanced SIRT1 expression during increased FoxO1 activity may negate the protective ability of SIRT1 in the presence of active FoxO1 and ultimately lead to the demise of cells. In other scenarios, SIRT1 with combined FoxO activity may offer inhibition of cell cycle progression that can limit apoptosis and be beneficial in cardiac or neurodegenerative disorders [188, 189], but in the setting of tumorigenesis, loss of cell cycle regulation could spell disaster for a patient [75, 183].
In addition, the relationship between SIRT1 and forkhead transcription factors represents a fine balance to promote both cell protection with cellular longevity. Activation of SIRT1 can increase lifespan in higher organisms such as Drosophila while also protecting neurons from oxidant stress [95, 190]. In regards to cellular protection, SIRT1 may rely upon the regulation of FoxO transcription factors such as to protect against inflammation [79], to promote cardiac function during starvation [156, 175], to protect against cardiac ischemia [35], and to maintain vessel integrity during oxidative stress [47, 48]. Furthermore, an increase in FoxO3a and SIRT1 activity can occur in the heart during exercise [191], suggesting that physical activity may be beneficial for the cardiovascular system through SIRT1 and FoxO proteins.
In addition to Akt and FoxOs, cytoprotection by SIRT1 may be controlled by other means. In many experimental paradigms, resveratrol (trans-3,5,4′-trihydroxystibene), a naturally occurring phytoalexin polyphenol in the grape and red wine, is used to increase SIRT1 activity. At the cellular level, SIRT1 is expressed in both the cytoplasm and the nucleus. During events that incite apoptosis, SIRT1 translocates from the cytoplasm to the nucleus to prevent apoptosis. Resveratrol enhances SIRT1 translocation to the nucleus to protect cells from oxidative stress [47, 48]. Prevention of SIRT1 nuclear translocation abolishes protection by SIRT1 and resveratrol, suggesting that translocation of SIRT1 to the nucleus is necessary as well to protect against cellular injury [20, 47, 48]. Resveratrol treatment increases SIRT1 activity and prevents apoptotic injury during models of experimental diabetes with elevated glucose [47, 48], amyloid toxicity [192], cardiac disease [193], metabolic disorders [97, 98], and cerebral global ischemia [194]. SIRT1 has been shown to protect cells against oxidative stress by increasing the activity of catalase [187]. SIRT1 over-expression enhances the tolerance against free radical toxicity in neuronal cells [42, 95]. SIRT1 can block p53-induced apoptosis [179] through p53 deacetylation and induction of manganese superoxide dismutase (MnSOD) [195, 196]. Nuclear factor-κB (NF-κB) has been identified as another transcription factor that may play a significant role with SIRT1 in the protection against oxidative stress [81, 137, 197, 198]. Once activated, NF-κB is translocated to the cell nucleus and activates several anti-apoptotic genes, such as the inhibitors of apoptotic protein (IAPs), Gadd45β, and Bcl-xL to prevent apoptosis against oxidative stress [81, 137, 143, 147, 199]. Yet, it should be recognized that NF-κB at times also can lead to pro-inflammatory gene induction that promote inflammation, cell death, and tumorigenesis [33, 81, 198, 200, 201]. SIRT1 has been shown to interact with and deacetylate the RelA/p65 subunit of NF-κB and inhibit its transcriptional activity [202]. In addition, use of resveratrol potentiates chromatin-associated SIRT1 protein on the cIAP-2 promoter region and inhibits NF-κB regulated gene expression. This leads to an increase in the sensitization of cells to tumor necrosis factor-α-induced apoptosis [202]. In regards to feedback pathways, HuR, a RNA binding protein, regulates the stability of many target mRNAs and can bind the 3′ non-translated region of the mRNA of SIRT1, leading to the stabilization of the SIRT1 mRNA and up-regulation of SIRT1 expression [203]. During oxidative stress, HuR is phosphorylated, resulting in the dissociation of the HuR-SIRT1 mRNA complex and subsequent SIRT1 mRNA decay
SIRT1 and Clinical Disorders
Disorders of Metabolism
Diabetes mellitus (DM) has become a significant health concern throughout the world [5, 25, 26, 204]. By the year 2030, it is predicted that more than 360 million individuals will be afflicted with DM and its debilitating conditions [10, 27]. Insulin resistance and the complications of DM can be the result of cellular oxidative stress [24, 25]. Hyperglycemia leads to increased production of reactive oxygen species (ROS) in endothelial cells, liver cells, and pancreatic β-cells [24-26]. As a result, patients with DM can develop immune dysfunction [205], cognitive disorders [205, 206], hepatic dysfunction [207], renal disease [208], hematological disease [209], neurodegenerative disorders [5, 24, 204], and cardiovascular disease [23, 28, 114, 115, 210].
SIRT1 activity has been associated with insulin sensitivity. SIRT1 expression is decreased in the pancreas and liver in rats on high fat diets and may be associated with insulin resistance [211]. In insulin resistance cells, SIRT1 protein is significantly decreased and the reduction of SIRT1 levels in gastrocnemius muscle in mice results in the impairment of glucose tolerance [212]. Gene deletion or inhibition of SIRT1 impairs the insulin signaling by interfering with insulin stimulated insulin receptor phosphorylation and glycogen synthase [212]. Over-expression of SIRT1 in the liver serves to attenuate hepatic steatosis and improves insulin sensitivity, resulting in improved glucose homeostasis [98]. The ability of SIRT1 to improve insulin sensitivity may occur through several factors such as modulation of fat mobilization [213], gluconeogenesis [214], and inflammation [215]. SIRT1 also can function as a positive modulator of insulin signaling in insulin-sensitive organs and activate the insulin downstream target Akt through phosphotidylinositide 3-kinase (PI 3-K) [97]. SIRT1 can stimulate glucose-dependent insulin secretion from pancreatic β cells via repressing the uncoupling protein (UCP) gene UCP2 [216]. The SIRT1 activator resveratrol also promotes glucose stimulated insulin secretion in insulinoma INS-1E cells and human islets that is dependent on active SIRT1 [102].
SIRT1 not only has a role with insulin sensitivity, but also can affect fat metabolism and obesity. In obese mice, SIRT1 expression is low in adipose tissue and loss of SIRT1 in white adipose cells results in the impairment of fatty acid mobilization. Over-expression of SIRT1 in adipose tissue suppresses the transcriptional activity of PPAR-γ to inhibit adipogenesis and activation of lipolysis during fasting [213]. Resveratrol application can mimic calorie restriction to prevent obesity as a result of a high calorie diet in mice [217]. SIRT1 has been shown to be expressed in anorexigenic pro-opiomelanocortin (POMC) neurons and orexigenic agouti-related peptide (AgRP) neurons in the arcuate nucleus of the hypothalamus and regulate food intake and cellular metabolism [218]. For example, over-expression of SIRT1 in the hypothalamus prevents FoxO1 from promoting hyperphagia and body weight gain [218]. However, lack of SIRT1 in POMC neurons leads to obesity due to reduced energy expenditure [100].
SIRT1 may regulate insulin sensitivity and metabolism through the phosphorylation of AMP-activated protein kinase (AMPK). SIRT1 controls AMPK through the AMPK kinase, serine-threonine liver kinase B1 (LKB1). Over-expression of SIRT1 results in the deacetylation of LKB1, leading to its translocation from the nucleus to the cytoplasm, where LKB1 activates AMPK [219]. AMPK, in turn, can mediate the activation of SIRT1. Activation of AMPK occurs during decreased energy with a subsequent increase in the AMP/ATP ratio and can function to promote insulin sensitivity, fatty acid oxidation, and mitochondrial biogenesis. These events can lead to the generation of ATP and reduction in oxidative stress [29].
AMPK mediated impairment of muscle differentiation during glucose restriction and peroxisome proliferators-activated receptor-γ coactivator (PGC)-1α mediated gene expression is also SIRT1 dependent. AMPK is not believed to directly activate SIRT1, but may enhance SIRT1 activity. AMPK activation enhances SIRT1 activity either by increasing cellular NAD+/NADH ratio, resulting in the deacetylation and modulation of the activity of downstream SIRT1 targets that include the PGC-1α, FoxO1, and FoxO3a [220] or by up-regulating nicotinamide phosphoribosyltransferase (NAMPT) during glucose restriction, leading to increased NAD+ and decreased nicotinamide, an inhibitor of SIRT1 [221]. Resveratrol also has been demonstrated to activate AMPK through SIRT1 dependent or independent mechanisms [220, 222]. Resveratrol increases AMPK phosphorylation to protect cells against elevated glucose concentration, improve insulin sensitivity, and stimulate glucose transport. Increased AMPK activation reduces myocardial infarct size in both non-diabetic and diabetic rat hearts following ischemia/reperfusion, which may be mediated through the inhibition of mitochondrial permeability transition pore opening in cardiomyocytes [28]. Mice expressing dominant negative AMPK or loss of AMPK have glucose uptake inhibition and increased infarct volume following cardiac ischemia [223].
SIRT1 also controls insulin sensitivity through targeting protein tyrosine phosphatase (PTP). In the PTP family, PTP1B down-regulates insulin signal transduction via targeting the insulin receptor. PTP1B deficiency or inhibition leads to improved insulin sensitivity and glycemic control. Lowering the PTP1B level in the liver decreases blood glucose in diabetic mice. SIRT1 over-expression or SIRT1 activation can reduce both the PTP1B mRNA and protein levels during insulin-resistance. In contrast, an increase in PTP1B expression prevents SIRT1 mediated glucose uptake and insulin receptor phosphorylation in response to insulin stimulation, suggesting that SIRT1 improves insulin sensitivity through the repression of PTP1B [212].
SIRT1 in association with PPAR-γ plays an important role during adipogenesis. PPAR-α increases free fatty acid uptake and decreases lipolysis. Under nutrient restriction, SIRT1 protein binds to and represses genes controlled by the fat regulator PPAR-γ. SIRT1 inhibits PPAR-γ and silencing mediator of retinoid and thyroid hormone receptors, resulting in the mobilization of fatty acids from white adipocytes upon fasting [213]. PPAR-γ also may directly interact with SIRT1 and form a negative feedback to regulate SIRT1 activity [224].
SIRT1 plays a role during hepatic glucose output and lipid homeostasis. SIRT1 regulates the activity of PGC-1α via deacetylation and it can interact with PGC-1α in the liver to induce gluconeogenic genes and hepatic glucose output. PGC-1α is a member of a family of transcriptional coactivators that includes PGC-1α, PGC-1β and PGC-1 related coactivator (PRC). PGC-1α interacts with transcription factors to activate transcription and increases the expression of genes that regulate mitochondrial functions and fatty acid oxidation [225]. As a result, enhanced PGC-1α activity may function to protect against some metabolic diseases and improve mitochondrial biogenesis. SIRT1 also can control the ability of PGC-1α to repress glycolytic genes in response to fasting and pyruvate [214]. Hepatic SIRT1 interacts with PPAR-α through activating PGC-1α to mediate lipid homeostasis. For example, SIRT1 deletion in the liver results in the loss of PGC-1α activity and the impairment of fatty acid oxidation, predisposing to develop hepatic steatosis when fed with a high-fat diet [215].
Disorders of Aging and Nervous System Injury
When one considers the role of SIRT1 in clinical disorders and especially those impacting the nervous system, it is important to differentiate whether a treatment strategy not only can influence cell survival, but also influence cell longevity [226–228]. Recent work suggests that acute cellular protection by SIRT1 is closely linked to the ability of SIRT1 to extend cell longevity. SIRT1 can promote increased lifespan in higher organisms above yeast and metazoans while also increasing the protection of neuronal cells during oxidant stress exposure [95, 190]. SIRT1 during both acute injury and aging processes may be necessary for DNA repair to block apoptotic cell death [14]. Furthermore, in clinical studies of aging in patients with Alzheimer’s disease, loss of SIRT1 has been associated with the increased accumulation of β-amyloid and tau [229]. In the vascular system, increased SIRT1 activity is necessary to block endothelial cell death, extend cellular lifespan, and decrease endothelial senescence in models of both DM and oxidative stress [47, 48, 154]. Moderate over-expression of SIRT1 in cardiac cells can lessen age-dependent increases in cardiac hypertrophy and reduce the expression of senescence markers [174].
Given these properties of SIRT1 especially for the nervous system, SIRT1 may be critical for the protection of neurons during both acute and chronic injuries. Application of resveratrol protects neuronal cells against oxidative stress that is dependent on SIRT1 activation [192]. Resveratrol also can mimic the benefits of ischemic preconditioning in the brain to reduce ischemic brain injury [230]. Furthermore, loss of SIRT1 activity during application of the SIRT1 inhibitor sirtinol abolishes neuroprotection by resveratrol [95, 190]. Treatment with resveratrol reduces brain infarct volume, decreases neurological deficits, and increases regional brain blood flow after cerebral ischemia [231]. Additional work suggests that resveratrol also protects neurons during both transient focal and global cerebral ischemia via the reduction oxygen free radicals and the prevention of lipid peroxidation [194].
In regards to chronic neurodegenerative diseases, such as Alzheimer’s disease (AD), SIRT1 also may have a vital role. SIRT1 is believed to be necessary in maintaining normal learning, memory, and synaptic plasticity [99]. In patients with AD with progressive cognitive impairment, a decrease in SIRT1 is present in the parietal cortex in conjunction with an associated accumulation of amyloid-beta (Aβ) and tau protein [229]. In animal models of AD, application of SIRT1 and resveratrol reduces neurodegeneration in the hippocampus and prevents learning impairment [232]. Over-expression of SIRT1 in a mouse model of AD reduces the accumulation of Aβ, while gene silencing of SIRT1 in the brain results in an increased production of Aβ [96]. Resveratrol also has been reported to protect neuronal cells against Aβ during oxidative stress [192]. Activation of SIRT1 also reduces Aβ cell toxicity and decreases NF-κB transcriptional activity, suggesting that SIRT1 may promotes cellular protection against Aβ through inhibiting NF-κB transcriptional activity [199].
SIRT1 also may have a role during Parkinson’s disease (PD), a disease closely linked to oxidative stress [31, 228, 233]. In mice, administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin and oxidant that mimics experimental PD, results in motor coordination impairment with resulting neuronal loss in the substantia nigra. Application of resveratrol significantly reduces MPTP-induced motor coordination impairment, hydroxyl radical formation, and neuronal loss [234]. In other experimental models of PD generated by 6-hydroxydopamine (6-OHDA), resveratrol was shown to increase the activity of antioxidant enzymes and lower dopamine loss in the substantia nigra [44].
Disorders of the Cardiovascular System
During cardiac ischemic/reperfusion injury, SIRT1 can be protective. Treatment with resveratrol during myocardial ischemia/reperfusion in rats can reduce rhythm disturbances, cardiac infarct size, and plasma levels of lactate dehydrogenase and creatine kinase [235]. Cell protection in the cardiovascular system may be determined by SIRT1 fostering protection against vascular inflammation and apoptosis [47, 48]. SIRT1 also has been demonstrated to promote the transcriptional activity of FoxO1 to up-regulate MnSOD, suppress oxidative stress in cardiomyocytes, reduce cardiac infarct volume, and improve functional recovery after ischemic/reperfusion in murine models [35]. SIRT1 also blocks myoblast injury from oxidative stress by enhancing the expression of MnSOD [195]. Activation of SIRT1 through resveratrol can assist cardiac recovery following global ischemia that may be tied to new vessel growth [236]. Loss of SIRT1 in endothelial cells leads to impairment of new blood vessel formation in ischemic tissues. The effect of SIRT1 on angiogenesis may be dependent through NF-κB and induction of nitric oxide synthase in endothelial cells [237]. Resveratrol also acts in animal models of cardiac infarction to increase the expression of vascular endothelial growth factor (VEGF) and its tyrosine kinase receptor Flk-1.
SIRT1 also may serve to block atherosclerosis [92]. High fat diets with the release of fatty acid anion and lipoprotein lipase products can activate vascular endothelial cells and impair the integrity of endothelium providing the foundation for atherosclerotic plaque [20, 238]. Injured endothelial cells also are known to lead to platelet activation and form vessel thrombi [1, 9, 228, 239]. SIRT1 can protect endothelial cells from oxidative stress and oxidized low-density lipoprotein induced apoptosis [47, 48, 240]. Over-expression of SIRT1 in human umbilical vein endothelial cells (HUVECs) prevents cell injury from oxidized low-debsity lipoproteins (LDLs). In endothelial cell-specific SIRT1 transgenic mice, impairment of vasorelaxation during high fat exposure was reversed with a reduction in atherosclerotic lesions [240], suggesting that SIRT1 improves endothelial function in the presence of atherosclerosis. Activation of SIRT1 also improves endothelium relaxation through up-regulating endothelial nitric oxide synthase (eNOS) expression and production of nitric oxide [241]. Activation of SIRT1 also can inhibit vascular smooth muscle cell (VSMC) hypertrophy, which can contribute to atherosclerosis. Over-expression of SIRT1 prevents angiotensin II induced VSMC hypertrophy. Treatment with resveratrol prevents oxidative stress induced human coronary smooth muscle cell proliferation through the inhibition of ERK activation [242]. SIRT1 in VSMCs also may regulate atherosclerosis through increased activity of tissue inhibitor of metalloproteinase 3 (TIMP3). TIMP3 is responsible for preventing metalloproteinase 3 to digest vascular intracellular matrix [92]. Down-regulation of TIMP3 has been tied to atherosclerosis in diabetic patients, since TIMP3 is significantly reduced in human carotid atherosclerotic plaques with decreased levels of SIRT1 [243]. In contrast, SIRT1 over-expression in VSMCs promotes TIMP3 expression and may, as a result, prevent atherosclerotic plaque [92, 243].
SIRT1 appears to play an important role during cardiac hypertrophy. Over-expression of SIRT1 in cardiac tissue has been shown to reduce cardiac hypertrophy, cellular apoptosis, cardiac dysfunction, and expression of senescence markers [174]. Yet, high expression of SIRT1 results in oxidative stress, apoptosis, and increased cardiac hypertrophy that may be associated with mitochondrial dysfunction and depletion of NAD+ [174]. Treatment with resveratrol to increase SIRT1 activity prevents cardiac hypertrophy and cardiac cell dysfunction through the reduction of oxidative stress without lowering the blood pressure [37], can suppress pressure overload induced cardiac hypertrophy in rats [244], and can inhibit angiotensin II induced cardiomyocyte hypertrophy [245]. SIRT1 also prevents phenylephrine induced neonatal cardiomyocyte hypertrophy and inhibits phenylephrine-induced down-regulation of fatty acid oxidation genes. These observations with SIRT1 have been associated with the activation of peroxisome proliferators activated receptor-α (PPAR-α) [246]. The anti-hypertrophic effects of SIRT1 and resveratrol treatment also may function via AMP-activated protein kinase (AMPK) and the inhibition of Akt with subsequent suppression of protein synthesis and gene transcription [247].
Conclusions and Future Perspectives
SIRT1 offers new avenues of treatment against a number of disorders that can affect multiple systems of the body. Yet, it is vital to elucidate the biological pathways of SIRT1 that can determine cellular protection and cellular longevity. In this regard, the signal transduction pathways of SIRT1 must be carefully dissected to help determine the role of SIRT1 during a variety of disease process that may range from new vessel development to tumorigenesis. It is also important to recognize that current agents used to activate SIRT1 may be useful as investigational tools, but lack the precision to elucidate downstream mechanisms of SIRT1 as well as to lay the foundation for new drug development. As an example, resveratrol can increase SIRT1 activity in animal and cell models, but this agent is known to activate other pathways, such as the mammalian target of rapamycin (mTOR) and Akt1, that can cloud the ability to assess the direct role of SIRT1 [97, 233, 248, 249]. Overall, the exciting ability to develop novel therapeutic regimens with SIRT1 must always be countered by the clear appreciation of the multiple pathways govern by SIRT1 and the proper application of SIRT1 for particular disease entities to prevent any detrimental or unwanted outcomes.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
References
- [1].Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neuro-degenerative disease. Prog Neurobiol. 2005;75(3):207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- [2].Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res Brain Res Rev. 2005;49(1):1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: a promising therapeutic intervention in neurodegenerative disease. Free Radic Res. 2011;45(8):888–905. doi: 10.3109/10715762.2011.574290. [DOI] [PubMed] [Google Scholar]
- [4].Jayaram HN, Kusumanchi P, Yalowitz JA. NMNAT expression and its relation to NAD metabolism. Curr Med Chem. 2011;18(13):1962–1972. doi: 10.2174/092986711795590138. [DOI] [PubMed] [Google Scholar]
- [5].Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62(4):218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maiese K. Marking the onset of oxidative stress: biomarkers and novel strategies. Oxid Med Cell Longev. 2009;2(1):1. doi: 10.4161/oxim.2.1.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tupe RS, Tupe SG, Agte VV. Dietary nicotinic acid supplementation improves hepatic zinc uptake and offers hepatoprotection against oxidative damage. Br J Nutr. 2011 Jan 25;:1–9. doi: 10.1017/S0007114510005520. [DOI] [PubMed] [Google Scholar]
- [8].Yu J, Ye J, Liu X, Han Y, Wang C. Protective effect of L-carnitine against H(2)O(2)-induced neurotoxicity in neuro-blastoma (SH-SY5Y) cells. Neurol Res. 2011;33(7):708–716. doi: 10.1179/1743132810Y.0000000028. [DOI] [PubMed] [Google Scholar]
- [9].Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22(2):87–104. [PubMed] [Google Scholar]
- [10].Maiese K, Hou J, Chong ZZ, Shang YC. Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology. ScientificWorldJournal. 2009;9:1072–1104. doi: 10.1100/tsw.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ribeiro MC, Barbosa NB, de Almeida TM, Parcianello LM, Perottoni J, de Avila DS, Rocha JB. High-fat diet and hydrochlorothiazide increase oxidative stress in brain of rats. Cell Biochem Funct. 2009;27(7):473–478. doi: 10.1002/cbf.1599. [DOI] [PubMed] [Google Scholar]
- [12].Hamden K, Carreau S, Jamoussi K, Miladi S, Lajmi S, Aloulou D, Ayadi F, Elfeki A. 1Alpha,25 dihydroxyvitamin D3: therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J Nutr Sci Vitaminol (Tokyo) 2009;55(3):215–222. doi: 10.3177/jnsv.55.215. [DOI] [PubMed] [Google Scholar]
- [13].Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008;4(4):216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- [14].Audrito V, Vaisitti T, Rossi D, Gottardi D, D’Arena G, Laurenti L, Gaidano G, Malavasi F, Deaglio S. Nicotinamide blocks proliferation and induces apoptosis of chronic lymphocytic leukemia cells through activation of the p53/miR-34a/SIRT1 tumor suppressor network. Cancer Res. 2011;71(13):4473–4483. doi: 10.1158/0008-5472.CAN-10-4452. [DOI] [PubMed] [Google Scholar]
- [15].Chong ZZ, Lin SH, Maiese K. Nicotinamide modulates mitochondrial membrane potential and cysteine protease activity during cerebral vascular endothelial cell injury. J Vasc Res. 2002;39(2):131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- [16].Cui W, Matsuno K, Iwata K, Ibi M, Matsumoto M, Zhang J, Zhu K, Katsuyama M, Torok NJ, Yabe-Nishimura C. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011 May 26; doi: 10.1002/hep.24465. [DOI] [PubMed] [Google Scholar]
- [17].Kim WJ, Lee JW, Quan C, Youn HJ, Kim HM, Bae SC. Nicotinamide inhibits growth of carcinogen induced mouse bladder tumor and human bladder tumor xenograft through up-regulation of RUNX3 and p300. J Urol. 2011;185(6):2366–2375. doi: 10.1016/j.juro.2011.02.017. [DOI] [PubMed] [Google Scholar]
- [18].Koh PO. Nicotinamide attenuates the ischemic brain injury-induced decrease of Akt activation and Bad phosphorylation. Neurosci Lett. 2011;498(2):105–109. doi: 10.1016/j.neulet.2011.05.003. [DOI] [PubMed] [Google Scholar]
- [19].Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24(5):228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- [20].Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Siegel C, McCullough LD. NAD+ depletion or PAR polymer formation: which plays the role of executioner in ischaemic cell death? Acta Physiol (Oxf) 2011;203(1):225–234. doi: 10.1111/j.1748-1716.2010.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vonder Haar C, Anderson GD, Hoane MR. Continuous nicotinamide administration improves behavioral recovery and reduces lesion size following bilateral frontal controlled cortical impact injury. Behav Brain Res. 2011;224(2):311–317. doi: 10.1016/j.bbr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kurban S, Mehmetoglu I, Yerlikaya HF, Gönen S, Erdem S. Effect of chronic regular exercise on serum ischemia-modified albumin levels and oxidative stress in type 2 diabetes mellitus. Endocr Res. 2011;36(3):116–123. doi: 10.3109/07435800.2011.566236. [DOI] [PubMed] [Google Scholar]
- [24].Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008;6(3):281–284. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4(1):63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maiese K, Shang YC, Chong ZZ, Hou J. Diabetes mellitus: channeling care through cellular discovery. Curr Neurovasc Res. 2010;7(1):59–64. doi: 10.2174/156720210790820217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Paiva MA, Rutter-Locher Z, Gonçalves LM, Providência LA, Davidson SM, Yellon DM, Mocanu MM. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300(6):H2123–H2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, Medeiros D, Lin D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011;236(9):1051–1063. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chong ZZ, Li F, Maiese K. Employing new cellular therapeutic targets for Alzheimer’s disease: a change for the better? Curr Neurovasc Res. 2005;2(1):55–72. doi: 10.2174/1567202052773508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li F, Chong ZZ, Maiese K. Cell life versus cell longevity: the mysteries surrounding the NAD(+) precursor nicotinamide. Curr Med Chem. 2006;13(8):883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer’s disease and cognitive impairment. Oxid Med Cell Longev. 2009;2(5):279–289. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Su SY, Cheng CY, Tsai TH, Hsiang CY, Ho TY, Hsieh CL. Paeonol attenuates H(2)O(2)-induced NF-kB-associated amyloid precursor protein expression. Am J Chin Med. 2010;38(6):1171–1192. doi: 10.1142/S0192415X1000855X. [DOI] [PubMed] [Google Scholar]
- [34].Zhang G, Zhao Z, Gao L, Deng J, Wang B, Xu D, Liu B, Qu Y, Yu J, Li J, Gao G. Gypenoside attenuates white matter lesions induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2011;99(1):42–51. doi: 10.1016/j.pbb.2011.03.019. [DOI] [PubMed] [Google Scholar]
- [35].Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/ reperfusion. Circulation. 2010;122(21):2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5(2):125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D, Kopilas MA, Anderson HD, Netticadan T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens. 2010;23(2):192–196. doi: 10.1038/ajh.2009.228. [DOI] [PubMed] [Google Scholar]
- [38].Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. 2009;2(2):63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Weis S, Haybaeck J, Dulay JR, Llenos IC. Expression of cellular prion protein (PrP(c)) in schizophrenia, bipolar disorder, and depression. J Neural Transm. 2008;115(5):761–771. doi: 10.1007/s00702-007-0013-4. [DOI] [PubMed] [Google Scholar]
- [40].Choi KC, Kim SH, Ha JY, Kim ST, Son JH. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem. 2010;112(2):366–376. doi: 10.1111/j.1471-4159.2009.06463.x. [DOI] [PubMed] [Google Scholar]
- [41].Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2(4):271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3(2):153–165. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Khan MM, Ahmad A, Ishrat T, Khan MB, Hoda MN, Khuwaja G, Raza SS, Khan A, Javed H, Vaibhav K, Islam F. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- [45].Chong ZZ, Kang JQ, Maiese K. AKT1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-xL and caspase 1, 3, and 9. Exp Cell Res. 2004;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- [46].Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010;321(2):194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr Neurovasc Res. 2011;8(3):220–235. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients – protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24(3):327–337. doi: 10.1111/j.1525-139X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- [50].Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21(3):262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- [51].Simon F, Scheuerle A, Gröger M, Vcelar B, McCook O, Möller P, Georgieff M, Calzia E, Radermacher P, Schelzig H. Comparison of carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury. Intensive Care Med. 2011;37(9):1525–1533. doi: 10.1007/s00134-011-2303-4. [DOI] [PubMed] [Google Scholar]
- [52].Velly L, Pellegrini L, Guillet B, Bruder N, Pisano P. Erythropoietin 2nd cerebral protection after acute injuries: a double-edged sword? Pharmacol Ther. 2010;128(3):445–459. doi: 10.1016/j.pharmthera.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [53].Lin J, Zheng S, Chen A. Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress. Lab Invest. 2009;89(12):1397–1409. doi: 10.1038/labinvest.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85(2):194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-Lserine, suppresses Müller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91(5):601–612. doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fatma N, Singh P, Chhunchha B, Kubo E, Shinohara T, Bhargavan B, Singh DP. Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am J Physiol Cell Physiol. 2011;301(4):C954–C967. doi: 10.1152/ajpcell.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004;6(2):277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- [58].Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003;64(3):557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- [59].Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003;74(1):37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- [60].Wosik K, Becher B, Ezman A, Nalbantoglu J, Antel JP. Caspase 8 expression and signaling in Fas injury-resistant human fetal astrocytes. Glia. 2001;33(3):217–224. doi: 10.1002/1098-1136(200103)33:3<217::aid-glia1020>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [61].Yamada M, Burke C, Colditz P, Johnson DW, Gobe GC. Erythropoietin protects against apoptosis and increases expression of non-neuronal cell markers in the hypoxia-injured developing brain. J Pathol. 2011;224(1):101–109. doi: 10.1002/path.2862. [DOI] [PubMed] [Google Scholar]
- [62].Asaithambi A, Kanthasamy A, Saminathan H, Anantharam V, Kanthasamy AG. Protein kinase D1 (PKD1) activation mediates a compensatory protective response during early stages of oxidative stress-induced neuronal degeneration. Mol Neurodegener. 2011;6:43. doi: 10.1186/1750-1326-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138(6):1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Komandirov MA, Knyazeva EA, Fedorenko YP, Rudkovskii MV, Stetsurin DA, Uzdensky AB. On the role of phosphatidylinositol 3-kinase, protein kinase b/akt, and glycogen synthase kinase-3β in photodynamic injury of crayfish neurons and glial cells. J Mol Neurosci. 2011;45(2):229–235. doi: 10.1007/s12031-011-9499-1. [DOI] [PubMed] [Google Scholar]
- [65].Kook YH, Ka M, Um M. Neuroprotective cytokines repress PUMA induction in the 1-methyl-4-phenylpyridinium (MPP(+)) model of Parkinson’s disease. Biochem Biophys Res Commun. 2011;411(2):370–374. doi: 10.1016/j.bbrc.2011.06.151. [DOI] [PubMed] [Google Scholar]
- [66].Kumral A, Yesilirmak D Cemile, Sozmen S, Ergur B Ugur, Tugyan K, Ozbal S, Guclu S, Duman N, Ozkan H. Effect of leptin treatment on neonatal hypoxic-ischemic brain injury. J Matern Fetal Neonatal Med. 2011 Jun 1; doi: 10.3109/14767058.2011.565834. [DOI] [PubMed] [Google Scholar]
- [67].Xin XY, Pan J, Wang XQ, Ma JF, Ding JQ, Yang GY, Chen SD. 2-Methoxyestradiol attenuates autophagy activation after global ischemia. Can J Neurol Sci. 2011;38(4):631–638. doi: 10.1017/s031716710001218x. [DOI] [PubMed] [Google Scholar]
- [68].Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150(7):839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4(3):194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kamianowska M, Szczepański M, Skrzydlewska E. Effects of erythropoietin on ICAM-1 and PECAM-1 expressions on human umbilical vein endothelial cells subjected to oxidative stress. Cell Biochem Funct. 2011;29(6):437–441. doi: 10.1002/cbf.1768. [DOI] [PubMed] [Google Scholar]
- [71].Ganapathy S, Chen Q, Singh KP, Shankar S, Srivastava RK. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS ONE. 2010;5(12):e15627. doi: 10.1371/journal.pone.0015627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ji J, Zheng PS. Activation of mTOR signaling pathway contributes to survival of cervical cancer cells. Gynecol Oncol. 2010;117(1):103–108. doi: 10.1016/j.ygyno.2009.12.020. [DOI] [PubMed] [Google Scholar]
- [73].Le XF, Mao W, Lu Z, Carter BZ, Bast RC., Jr Dasatinib induces autophagic cell death in human ovarian cancer. Cancer. 2010;116(21):4980–4990. doi: 10.1002/cncr.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Maiese K, Chong ZZ, Shang YC, Hou J. Clever cancer strategies with FoxO transcription factors. Cell Cycle. 2008;7(24):3829–3839. doi: 10.4161/cc.7.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wilson MS, Brosens JJ, Schwenen HD, Lam EW. FOXO and FOXM1 in cancer: the FOXO–FOXM1 axis shapes the outcome of cancer chemotherapy. Curr Drug Targets. 2011;12(9):1256–1266. doi: 10.2174/138945011796150244. [DOI] [PubMed] [Google Scholar]
- [77].Braskett M, Riedl MA. Novel antioxidant approaches to the treatment of upper airway inflammation. Curr Opin Allergy Clin Immunol. 2010;10(1):34–41. doi: 10.1097/ACI.0b013e328334f613. [DOI] [PubMed] [Google Scholar]
- [78].Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22(11):1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nerurkar PV, Johns LM, Buesa LM, Kipyakwai G, Volper E, Sato R, Shah P, Feher D, Williams PG, Nerurkar VR. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J Neuroinflammation. 2011;8:64. doi: 10.1186/1742-2094-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shang YC, Chong ZZ, Hou J, Maiese K. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res. 2009;6(4):223–238. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22(9):1317–1329. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000;59(4):568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [83].Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106(23):2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- [85].Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116(6):993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010;15(9):1072–1082. doi: 10.1007/s10495-010-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].De Simone R, Ajmone-Cat MA, Minghetti L. Atypical anti-inflammatory activation of microglia induced by apoptotic neurons: possible role of phosphatidylserine–phosphatidylserine receptor interaction. Mol Neurobiol. 2004;29(2):197–212. doi: 10.1385/MN:29:2:197. [DOI] [PubMed] [Google Scholar]
- [88].Witting A, Müller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by microglia/brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J Neurochem. 2000;75(3):1060–1070. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- [89].Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279(49):50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- [90].Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, Yang T, Sauve AA, Kraus WL. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009;284(30):20408–20417. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Chong ZZ, Wang S, Shang YC, Maiese K. Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. doi: 10.2217/fca.11.76. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10(4):640–647. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]
- [93].Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal. 2011;4(182):ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- [94].Cen Y. Sirtuins inhibitors: the approach to affinity and selectivity. Biochim Biophys Acta. 2010;1804(8):1635–1644. doi: 10.1016/j.bbapap.2009.11.010. [DOI] [PubMed] [Google Scholar]
- [95].Chong ZZ, Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr Neurovasc Res. 2008;5(3):159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142(2):320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [97].Fröjdö S, Durand C, Molin L, Carey AL, El-Osta A, Kingwell BA, Febbraio MA, Solari F, Vidal H, Pirola L. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol. 2011;335(2):166–176. doi: 10.1016/j.mce.2011.01.008. [DOI] [PubMed] [Google Scholar]
- [98].Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K, Guarente L, Zang M. Hepatic over-expression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25(5):1664–1679. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30(29):9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12(1):78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tang BL, Chua CE. SIRT1 and neuronal diseases. Mol Aspects Med. 2008;29(3):187–200. doi: 10.1016/j.mam.2007.02.001. [DOI] [PubMed] [Google Scholar]
- [102].Vetterli L, Brun T, Giovannoni L, Bosco D, Maechler P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through Sirt1-dependent mechanism. J Biol Chem. 2011;286(8):6049–6060. doi: 10.1074/jbc.M110.176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19(2):145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25(11):577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [106].Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J Cereb Blood Flow Metab. 2002;22(5):503–514. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- [107].Kaushal N, Hegde S, Lumadue J, Paulson RF, Prabhu KS. The regulation of erythropoiesis by selenium in mice. Antioxid Redox Signal. 2011;14(8):1403–1412. doi: 10.1089/ars.2010.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kumral A, Tüzün F, Oner MG, Genç S, Duman N, Ozkan H. Erythropoietin in neonatal brain protection: the past, the present and the future. Brain Dev. 2011;33(8):632–643. doi: 10.1016/j.braindev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- [109].Lanfranconi S, Locatelli F, Corti S, Candelise L, Comi GP, Baron PL, Strazzer S, Bresolin N, Bersano A. Growth factors in ischemic stroke. J Cell Mol Med. 2011;15(8):1645–1687. doi: 10.1111/j.1582-4934.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lombardero M, Kovacs K, Scheithauer BW. Erythropoietin: a hormone with multiple functions. Pathobiology. 2011;78(1):41–53. doi: 10.1159/000322975. [DOI] [PubMed] [Google Scholar]
- [111].Mastromarino V, Volpe M, Musumeci MB, Autore C, Conti E. Erythropoietin and the heart: facts and perspectives. Clin Sci (Lond) 2011;120(2):51–63. doi: 10.1042/CS20100305. [DOI] [PubMed] [Google Scholar]
- [112].Moore EM, Bellomo R, Nichol AD. Erythropoietin as a novel brain and kidney protective agent. Anaesth Intensive Care. 2011;39(3):356–372. doi: 10.1177/0310057X1103900306. [DOI] [PubMed] [Google Scholar]
- [113].Murua A, Orive G, Hernández RM, Pedraz JL. Emerging technologies in the delivery of erythropoietin for therapeutics. Med Res Rev. 2011;31(2):284–309. doi: 10.1002/med.20184. [DOI] [PubMed] [Google Scholar]
- [114].Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45(3):217–234. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Maiese K, Chong ZZ, Shang YC, Hou J. Novel avenues of drug discovery and biomarkers for diabetes mellitus. J Clin Pharmacol. 2011;51(2):128–152. doi: 10.1177/0091270010362904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Sanganalmath SK, Abdel-Latif A, Bolli R, Xuan YT, Dawn B. Hematopoietic cytokines for cardiac repair: mobilization of bone marrow cells and beyond. Basic Res Cardiol. 2011;106(5):709–733. doi: 10.1007/s00395-011-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71(5):659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- [118].Genc S, Genc K, Kumral A, Ozkan H. White matter protection by erythropoietin: an emerging matter in the treatment of neonatal hypoxic-ischemic brain injury. Stroke. 2010;41(11):e595. doi: 10.1161/STROKEAHA.110.590844. author reply e596. [DOI] [PubMed] [Google Scholar]
- [119].Joshi D, Patel H, Baker DM, Shiwen X, Abraham DJ, Tsui JC. Development of an in vitro model of myotube ischemia. Lab Invest. 2011;91(8):1241–1252. doi: 10.1038/labinvest.2011.79. [DOI] [PubMed] [Google Scholar]
- [120].Kato S, Aoyama M, Kakita H, Hida H, Kato I, Ito T, Goto T, Hussein MH, Sawamoto K, Togari H, Asai K. Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J Neurosci Res. 2011;89(10):1566–1574. doi: 10.1002/jnr.22702. [DOI] [PubMed] [Google Scholar]
- [121].Wang Y, Hu X, Xie X, He A, Liu X, Wang JA. Effects of mesenchymal stem cells on matrix metalloproteinase synthesis in cardiac fibroblasts. Exp Biol Med (Maywood) 2011;236(10):1197–1204. doi: 10.1258/ebm.2011.010317. [DOI] [PubMed] [Google Scholar]
- [122].Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J Cereb Blood Flow Metab. 2003;23(3):320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- [123].Keswani SC, Bosch-Marcé M, Reed N, Fischer A, Semenza GL, Höke A. Nitric oxide prevents axonal degeneration by inducing HIF-1-dependent expression of erythropoietin. Proc Natl Acad Sci U S A. 2011;108(12):4986–4990. doi: 10.1073/pnas.1019591108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wang ZY, Shen LJ, Tu L, Hu DN, Liu GY, Zhou ZL, Lin Y, Chen LH, Qu J. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med. 2009;46(8):1032–1041. doi: 10.1016/j.freeradbiomed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- [125].Herbas MS, Ueta YY, Ishibashi K, Suzuki H. Expression of erythropoietic cytokines in α-tocopherol transfer protein knockout mice with murine malaria infection. Parasitol Res. 2011;109(5):1243–1250. doi: 10.1007/s00436-011-2367-7. [DOI] [PubMed] [Google Scholar]
- [126].Loeliger MM, Mackintosh A, De Matteo R, Harding R, Rees SM. Erythropoietin protects the developing retina in an ovine model of endotoxin-induced retinal injury. Invest Ophthalmol Vis Sci. 2011;52(5):2656–2661. doi: 10.1167/iovs.10-6455. [DOI] [PubMed] [Google Scholar]
- [127].Walden AP, Young JD, Sharples E. Bench to bedside: a role for erythropoietin in sepsis. Crit Care. 2010;14(4):227. doi: 10.1186/cc9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3β, and β-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8(2):103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11(6):863–871. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- [130].Hamed S, Ullmann Y, Egozi D, Daod E, Hellou E, Ashkar M, Gilhar A, Teot L. Fibronectin potentiates topical erythropoietin-induced wound repair in diabetic mice. J Invest Dermatol. 2011;131(6):1365–1374. doi: 10.1038/jid.2011.15. [DOI] [PubMed] [Google Scholar]
- [131].Lagarto A, Bueno V, Guerra I, Valdés O, Couret M, López R, Vega Y. Absence of hematological side effects in acute and subacute nasal dosing of erythropoietin with a low content of sialic acid. Exp Toxicol Pathol. 2011;63(6):563–567. doi: 10.1016/j.etp.2010.04.008. [DOI] [PubMed] [Google Scholar]
- [132].Niccoli G, Andreotti F, Marzo F, Cecchetti S, Santucci E, D’Amario D, Pafundi T, Cosentino N, Crea F. Endogenous serum erythropoietin and no-reflow in patients with ST-elevation myocardial infarction. Eur J Clin Invest. 2011;41(11):1210–1219. doi: 10.1111/j.1365-2362.2011.02528.x. [DOI] [PubMed] [Google Scholar]
- [133].Ogino A, Takemura G, Kawasaki M, Tsujimoto A, Kanamori H, Li L, Goto K, Maruyama R, Kawamura I, Takeyama T, Kawaguchi T, Watanabe T, Moriguchi Y, Saito H, Fujiwara T, Fujiwara H, Minatoguchi S. Erythropoietin receptor signaling mitigates renal dysfunction-associated heart failure by mechanisms unrelated to relief of anemia. J Am Coll Cardiol. 2010;56(23):1949–1958. doi: 10.1016/j.jacc.2010.04.068. [DOI] [PubMed] [Google Scholar]
- [134].Taniguchi N, Nakamura T, Sawada T, Matsubara K, Furukawa K, Hadase M, Nakahara Y, Nakamura T, Matsubara H. Erythropoietin prevention trial of coronary restenosis and cardiac remodeling after ST-elevated acute myocardial infarction (EPOC-AMI): a pilot, randomized, placebo-controlled study. Circ J. 2010;74(11):2365–2371. doi: 10.1253/circj.cj-10-0267. [DOI] [PubMed] [Google Scholar]
- [135].Warren JS, Zhao Y, Yung R, Desai A. Recombinant human erythropoietin suppresses endothelial cell apoptosis and reduces the ratio of Bax to Bcl-2 proteins in the aortas of apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2011;57(4):424–433. doi: 10.1097/FJC.0b013e31820d92fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Xu Y, Tian Y, Wei HJ, Chen J, Dong JF, Zacharek A, Zhang JN. Erythropoietin increases circulating endothelial progenitor cells and reduces the formation and progression of cerebral aneurysm in rats. Neuroscience. 2011;181:292–299. doi: 10.1016/j.neuroscience.2011.02.051. [DOI] [PubMed] [Google Scholar]
- [137].Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2(5):387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ma R, Xiong N, Huang C, Tang Q, Hu B, Xiang J, Li G. Erythropoietin protects PC12 cells from beta-amyloid(25–35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology. 2009;56(6–7):1027–1034. doi: 10.1016/j.neuropharm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [139].Sun ZK, Yang HQ, Pan J, Zhen H, Wang ZQ, Chen SD, Ding JQ. Protective effects of erythropoietin on tau phosphorylation induced by beta-amyloid. J Neurosci Res. 2008;86(13):3018–3027. doi: 10.1002/jnr.21745. [DOI] [PubMed] [Google Scholar]
- [140].Chu Q, Zhang J, Wu Y, Zhang Y, Xu G, Li W, Xu GT. Differential gene expression pattern of diabetic rat retinas after intravitreal injection of erythropoietin. Clin Experiment Ophthalmol. 2011;39(2):142–151. doi: 10.1111/j.1442-9071.2010.02437.x. [DOI] [PubMed] [Google Scholar]
- [141].Dang J, Jia R, Tu Y, Xiao S, Ding G. Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmacother. 2010;64(10):681–685. doi: 10.1016/j.biopha.2010.06.011. [DOI] [PubMed] [Google Scholar]
- [142].Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006;3(3):187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8(4):270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Vairano M, Dello Russo C, Pozzoli G, Battaglia A, Scambia G, Tringali G, Aloe-Spiriti MA, Preziosi P, Navarra P. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16(4):584–592. doi: 10.1046/j.1460-9568.2002.02125.x. [DOI] [PubMed] [Google Scholar]
- [145].Chong ZZ, Kang J, Li F, Maiese K. mGluRI targets microglial activation and selectively prevents neuronal cell engulfment through Akt and caspase dependent pathways. Curr Neurovasc Res. 2005;2(3):197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19(2):263–272. [PMC free article] [PubMed] [Google Scholar]
- [148].Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24(7):728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- [149].Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, Mamcarz M, Wang L, Sattelle DB, Kirschner DA, Mori T, Leblanc RM, Prabhakar R, Arendash GW. Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer’s disease mice. J Alzheimers Dis. 2011;24(4):817–835. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- [150].Favier FB, Costes F, Defour A, Bonnefoy R, Lefai E, Baugé S, Peinnequin A, Benoit H, Freyssenet D. Down-regulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1659–R1666. doi: 10.1152/ajpregu.00550.2009. [DOI] [PubMed] [Google Scholar]
- [151].Shahjee HM, Koch KR, Guo L, Zhang CO, Keay SK. Antiproliferative factor decreases Akt phosphorylation and alters gene expression via CKAP4 in T24 bladder carcinoma cells. J Exp Clin Cancer Res. 2010;29:160. doi: 10.1186/1756-9966-29-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, Delafontaine P, Chandrasekar B. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010;22(5):809–820. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Xie X, Li W, Lan T, Liu W, Peng J, Huang K, Huang J, Shen X, Liu P, Huang H. Berberine ameliorates hyperglycemia in alloxan-induced diabetic C57BL/6 mice through activation of Akt signaling pathway. Endocr J. 2011;58(9):761–768. doi: 10.1507/endocrj.k11e-024. [DOI] [PubMed] [Google Scholar]
- [154].Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J, Komuro I. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29(6):889–894. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- [155].Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29(5):1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal. 2011;14(4):649–661. doi: 10.1089/ars.2010.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Cho YM, Pu HF, Huang WJ, Ho LT, Wang SW, Wang PS. Role of serum- and glucocorticoid-inducible kinase-1 in regulating torsion-induced apoptosis in rats. Int J Androl. 2011;34(4):379–389. doi: 10.1111/j.1365-2605.2010.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: new paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007;4(4):295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14(5):219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009;2(3):119–129. doi: 10.4161/oxim.2.3.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther. 2008;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Park JH, Lee CH, Yoo KY, Choi JH, Hwang IK, Lee JY, Kang IJ, Won MH. FoxO3a immunoreactivity is markedly decreased in the dentate gyrus, not the hippocampus proper, of the aged gerbil. Exp Gerontol. 2011;46(10):836–840. doi: 10.1016/j.exger.2011.06.001. [DOI] [PubMed] [Google Scholar]
- [163].Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011;14(4):593–605. doi: 10.1089/ars.2010.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Uhlenhaut NH, Treier M. Forkhead transcription factors in ovarian function. Reproduction. 2011;142(4):489–495. doi: 10.1530/REP-11-0092. [DOI] [PubMed] [Google Scholar]
- [165].Wilk A, Urbanska K, Yang S, Wang JY, Amini S, Del Valle L, Peruzzi F, Meggs L, Reiss K. Insulin-like growth factor-I-forkhead box O transcription factor 3a counteracts high glucose/tumor necrosis factor-α-mediated neuronal damage: implications for human immunodeficiency virus encephalitis. J Neurosci Res. 2011;89(2):183–198. doi: 10.1002/jnr.22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Andreucci M, Fuiano G, Presta P, Lucisano G, Leone F, Fuiano L, Bisesti V, Esposito P, Russo D, Memoli B, Faga T, Michael A. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009;42(4):554–561. doi: 10.1111/j.1365-2184.2009.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Bouscary D, Pene F, Claessens YE, Muller O, Chrétien S, Fontenay-Roupie M, Gisselbrecht S, Mayeux P, Lacombe C. Critical role for PI 3-kinase in the control of erythropoietin-induced erythroid progenitor proliferation. Blood. 2003;101(9):3436–3443. doi: 10.1182/blood-2002-07-2332. [DOI] [PubMed] [Google Scholar]
- [168].Uddin S, Kottegoda S, Stigger D, Platanias LC, Wickrema A. Activation of the Akt/FKHRL1 pathway mediates the antiapoptotic effects of erythropoietin in primary human erythroid progenitors. Biochem Biophys Res Commun. 2000;275(1):16–19. doi: 10.1006/bbrc.2000.3266. [DOI] [PubMed] [Google Scholar]
- [169].Maiese K, Chong ZZ, Hou J, Shang YC. The “O” class: crafting clinical care with FoxO transcription factors. Adv Exp Med Biol. 2009;665:242–260. doi: 10.1007/978-1-4419-1599-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Lei H, Quelle FW. FOXO transcription factors enforce cell cycle checkpoints and promote survival of hematopoietic cells after DNA damage. Mol Cancer Res. 2009;7(8):1294–1303. doi: 10.1158/1541-7786.MCR-08-0531. [DOI] [PubMed] [Google Scholar]
- [171].Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280(21):20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- [172].Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16(2):237–243. [PubMed] [Google Scholar]
- [173].Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101(27):10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- [175].Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107(12):1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem. 2011;286(7):5289–5299. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306(5704):2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- [178].Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- [179].Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28(2):277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- [180].Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123(3):437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- [181].Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451(7178):583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- [182].Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, Lee MH, Xiao C, Vassilopoulos A, Chen W, Gardner K, Man YG, Hung MC, Finkel T, Deng CX. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32(1):11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Christian M, Lam EW, Wilson MS, Brosens JJ. FOXO transcription factors and their role in disorders of the female reproductive tract. Curr Drug Targets. 2011;12(9):1291–1302. doi: 10.2174/138945011796150253. [DOI] [PubMed] [Google Scholar]
- [184].Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007;1769(4):244–252. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- [185].Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009;116(3):191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, Nemoto S, Finkel T, Gu W, Cress WD, Chen J. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8(9):1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- [187].Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K, Itoh H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun. 2008;372(1):51–56. doi: 10.1016/j.bbrc.2008.04.176. [DOI] [PubMed] [Google Scholar]
- [188].Chong ZZ, Li F, Maiese K. Attempted cell cycle induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr Neurovasc Res. 2006;3(1):25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Wohlschlaeger J, Schmitz KJ, Takeda A, Takeda N, Vahlhaus C, Stypmann J, Schmid C, Baba HA. Reversible regulation of the retinoblastoma protein/E2F-1 pathway during “reverse cardiac remodeling” after ventricular unloading. J Heart Lung Transplant. 2010;29(1):117–124. doi: 10.1016/j.healun.2009.09.017. [DOI] [PubMed] [Google Scholar]
- [190].Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, Kaplun A, VanBerkum MF, Arking R, Freeman DC, Maiese K, Tzivion G. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283(41):27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11(1):139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- [192].Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110(5):1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- [193].Das DK, Mukherjee S, Ray D. Resveratrol and red wine, healthy heart and longevity. Heart Fail Rev. 2010;15(5):467–477. doi: 10.1007/s10741-010-9163-9. [DOI] [PubMed] [Google Scholar]
- [194].Simão F, Matté A, Matté C, Soares FM, Wyse AT, Netto CA, Salbego CG. Resveratrol prevents oxidative stress and inhibition of Na(+)K(+)-ATPase activity induced by transient global cerebral ischemia in rats. J Nutr Biochem. 2011;22(10):921–928. doi: 10.1016/j.jnutbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- [195].Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285(11):8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isono M, Isshiki K, Uzu T, Kashiwagi A, Koya D. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic Biol Med. 2006;40(12):2175–2182. doi: 10.1016/j.freeradbiomed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- [197].Aoshiba K, Onizawa S, Tsuji T, Nagai A. Therapeutic effects of erythropoietin in murine models of endotoxin shock. Crit Care Med. 2009;37(3):889–898. doi: 10.1097/CCM.0b013e31819b8371. [DOI] [PubMed] [Google Scholar]
- [198].Pappo O, Ben-Ari Z, Shevtsov E, Avlas O, Gassmann M, Ravid A, Cheporko Y, Hochhauser E. The role of excessive versus acute administration of erythropoietin in attenuating hepatic ischemia-reperfusion injury. Can J Physiol Pharmacol. 2010;88(12):1130–1137. doi: 10.1139/Y10-091. [DOI] [PubMed] [Google Scholar]
- [199].Teng FY, Tang BL. NF-kappaB signaling in neurite growth and neuronal survival. Rev Neurosci. 2010;21(4):299–313. doi: 10.1515/revneuro.2010.21.4.299. [DOI] [PubMed] [Google Scholar]
- [200].Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28(9):2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Lee SM, Kim EJ, Suk K, Lee WH. BAFF and APRIL induce inflammatory activation of THP-1 cells through interaction with their conventional receptors and activation of MAPK and NF-kB. Inflamm Res. 2011;60(9):807–815. doi: 10.1007/s00011-011-0336-3. [DOI] [PubMed] [Google Scholar]
- [202].Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25(4):543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Maiese K, Chong Z, Li F. Reducing oxidative stress and enhancing neurovascular longevity during diabetes mellitus. In: Maiese K, editor. Neurovascular medicine: pursuing cellular longevity for healthy aging. Oxford University Press; New York: 2009. [Google Scholar]
- [205].Hao J, Shen W, Tian C, Liu Z, Ren J, Luo C, Long J, Sharman E, Liu J. Mitochondrial nutrients improve immune dysfunction in the type 2 diabetic Goto-Kakizaki rats. J Cell Mol Med. 2009;13(4):701–711. doi: 10.1111/j.1582-4934.2008.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav. 2009;92(2):251–259. doi: 10.1016/j.pbb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- [207].Wu SY, Wang GF, Liu ZQ, Rao JJ, Lü L, Xu W, Wu SG, Zhang JJ. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol Sin. 2009;30(2):202–208. doi: 10.1038/aps.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Guarnieri G, Zanetti M, Vinci P, Cattin MR, Barazzoni R. Insulin resistance in chronic uremia. J Ren Nutr. 2009;19(1):20–24. doi: 10.1053/j.jrn.2008.11.014. [DOI] [PubMed] [Google Scholar]
- [209].Gossai D, Lau-Cam CA. The effects of taurine, taurine homologs and hypotaurine on cell and membrane anti-oxidative system alterations caused by type 2 diabetes in rat erythrocytes. Adv Exp Med Biol. 2009;643:359–368. doi: 10.1007/978-0-387-75681-3_37. [DOI] [PubMed] [Google Scholar]
- [210].Thackeray JT, Radziuk J, Harper ME, Suuronen EJ, Ascah KJ, Beanlands RS, Dasilva JN. Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunction in a rat model of insulin resistance with hyperglycemia. Cardiovasc Diabetol. 2011;10:75. doi: 10.1186/1475-2840-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Chen YR, Fang SR, Fu YC, Zhou XH, Xu MY, Xu WC. Calorie restriction on insulin resistance and expression of SIRT1 and SIRT4 in rats. Biochem Cell Biol. 2010;88(4):715–722. doi: 10.1139/O10-010. [DOI] [PubMed] [Google Scholar]
- [212].Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6(4):307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [213].Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429(6993):771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- [215].Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4(2):e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Sasaki T, Kitamura T. Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocr J. 2010;57(11):939–946. doi: 10.1507/endocrj.k10e-320. [DOI] [PubMed] [Google Scholar]
- [219].Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283(41):27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20(7):325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14(5):661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10(12):819–823. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Carvajal K, Zarrinpashneh E, Szarszoi O, Joubert F, Athea Y, Mateo P, Gillet B, Vaulont S, Viollet B, Bigard X, Bertrand L, Ventura-Clapier R, Hoerter JA. Dual cardiac contractile effects of the alpha2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2007;292(6):H3136–H3147. doi: 10.1152/ajpheart.00683.2006. [DOI] [PubMed] [Google Scholar]
- [224].Han L, Zhou R, Niu J, McNutt MA, Wang P, Tong T. SIRT1 is regulated by a PPAR{γ}-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res. 2010;38(21):7458–7471. doi: 10.1093/nar/gkq609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Sugden MC, Caton PW, Holness MJ. PPAR control: it’s SIRTainly as easy as PGC. J Endocrinol. 2010;204(2):93–104. doi: 10.1677/JOE-09-0359. [DOI] [PubMed] [Google Scholar]
- [226].Maiese K. Ringing in the New Year: new prospects for development, aging and longevity. Oxid Med Cell Longev. 2010;3(1):1. doi: 10.4161/oxim.3.1.11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Maiese K, Chong ZZ, Kang J. Transformation into treatment: novel therapeutics that begin within the cell. In: Maiese K, editor. Neuronal and vascular plasticity: elucidating basic cellular mechanisms for future therapeutic discovery. Kluwer Academic Publishers; Norwell, MA: 2003. pp. 1–26. [Google Scholar]
- [228].Maiese K, Chong ZZ, Shang YC, Hou J. Therapeutic promise and principles: metabotropic glutamate receptors. Oxid Med Cell Longev. 2008;1(1):1–14. doi: 10.4161/oxim.1.1.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Jr, Bennett DA, Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(1):48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Raval AP, Dave KR, Pérez-Pinzón MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26(9):1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- [231].Lu KT, Chiou RY, Chen LG, Chen MH, Tseng WT, Hsieh HT, Yang YL. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J Agric Food Chem. 2006;54(8):3126–3131. doi: 10.1021/jf053011q. [DOI] [PubMed] [Google Scholar]
- [232].Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. Embo J. 2007;26(13):3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010;3(6):374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Lu KT, Ko MC, Chen BY, Huang JC, Hsieh CW, Lee MC, Chiou RY, Wung BS, Peng CH, Yang YL. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J Agric Food Chem. 2008;56(16):6910–6913. doi: 10.1021/jf8007212. [DOI] [PubMed] [Google Scholar]
- [235].Hung LM, Su MJ, Chen JK. Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radic Biol Med. 2004;36(6):774–781. doi: 10.1016/j.freeradbiomed.2003.12.016. [DOI] [PubMed] [Google Scholar]
- [236].Dernek S, Ikizler M, Erkasap N, Ergun B, Koken T, Yilmaz K, Sevin B, Kaygisiz Z, Kural T. Cardioprotection with resveratrol pretreatment: improved beneficial effects over standard treatment in rat hearts after global ischemia. Scand Cardiovasc J. 2004;38(4):245–254. doi: 10.1080/14017430410035476. [DOI] [PubMed] [Google Scholar]
- [237].Fukuda S, Kaga S, Zhan L, Bagchi D, Das DK, Bertelli A, Maulik N. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys. 2006;44(1):43–49. doi: 10.1385/CBB:44:1:043. [DOI] [PubMed] [Google Scholar]
- [238].Melnik BC, John SM, Schmitz G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: lessons learnt from Iaron syndrome. Nutr Metab (Lond) 2011;8:41. doi: 10.1186/1743-7075-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Barbier M, Faille D, Loriod B, Textoris J, Camus C, Puthier D, Flori L, Wassmer SC, Victorero G, Alessi MC, Fusaï T, Nguyen C, Grau GE, Rihet P. Platelets alter gene expression profile in human brain endothelial cells in an in vitro model of cerebral malaria. PLoS One. 2011;6(5):e19651. doi: 10.1371/journal.pone.0019651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80(2):191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104(37):14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].El-Mowafy AM, Alkhalaf M, El-Kashef HA. Resveratrol reverses hydrogen peroxide-induced proliferative effects in human coronary smooth muscle cells: a novel signaling mechanism. Arch Med Res. 2008;39(2):155–161. doi: 10.1016/j.arcmed.2007.09.010. [DOI] [PubMed] [Google Scholar]
- [243].Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F, Federici M. TIMP3 is reduced in athero-sclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009;58(10):2396–2401. doi: 10.2337/db09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Wojciechowski P, Juric D, Louis XL, Thandapilly SJ, Yu L, Taylor C, Netticadan T. Resveratrol arrests and regresses the development of pressure overload- but not volume overload-induced cardiac hypertrophy in rats. J Nutr. 2010;140(5):962–968. doi: 10.3945/jn.109.115006. [DOI] [PubMed] [Google Scholar]
- [245].Cheng TH, Liu JC, Lin H, Shih NL, Chen YL, Huang MT, Chan P, Cheng CF, Chen JJ. Inhibitory effect of resveratrol on angiotensin II-induced cardiomyocyte hypertrophy. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(2):239–244. doi: 10.1007/s00210-003-0849-6. [DOI] [PubMed] [Google Scholar]
- [246].Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res. 2010;90(2):276–284. doi: 10.1093/cvr/cvq376. [DOI] [PubMed] [Google Scholar]
- [247].Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283(35):24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Hou G, Xue L, Lu Z, Fan T, Tian F, Xue Y. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett. 2007;253(2):236–248. doi: 10.1016/j.canlet.2007.01.026. [DOI] [PubMed] [Google Scholar]
- [249].Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24(28):6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


