Abstract
Background
Prostate cancer mortality rates for African-Americans are much higher than Caucasians and a similar trend is observed for prostate cancer survival. Data on recently immigrated African-descent men are lacking.
Methods
Using cancer registry data from Brooklyn, NY and two countries in the Caribbean (Guyana and Trinidad & Tobago), survival rates were estimated. We also examined whether Black race or Caribbean birth-place predict prostate cancer survival among males living in the United States (US).
Results
The Caribbean cases were diagnosed at a later age than those in the US (Guyana: 74.5yrs, Trinidad & Tobago: 72.4yrs, Brooklyn: 65.8yrs). Patients in the Caribbean had a worse 5-year survival rate compared to those in the US (41.6% vs. 84.4%) but for immigrant Caribbean-born males living in the US the five-year survival rate was not significantly different from African-Americans (78.1%, 95% CI: 70.9–83.7% vs. 81.4%, 95% CI: 69.5–89.1%, p = 0.792). The risk of death for Caribbean born was more than three times higher than US-born men (HR: 3.43, 95% CI: 2.17–5.44, adjusted for ethnicity, stage and mean age of diagnosis). A mean age of diagnosis greater than 65 years old and stage IV disease, but not ethnicity, were found to be independently associated with the risk of death.
Conclusion
The survival disadvantage for Caribbean born patients may be partly due to later diagnosis. Interventions focused on screening, education about the disease and early detection could potentially reduce cancer mortality in this population.
Keywords: Prostate Cancer, African ancestry, registry, survival
INTRODUCTION
Globally, the number of men over the age of 65 years is predicted to increase 4-fold between the years 2000 and 2050 (1). Therefore, it is likely there will be an increased number of men diagnosed with prostate cancer that will require treatment. Prostate cancer is the leading cancer affecting men in most developed and developing countries (1,2). It is well understood that diseases do not affect populations equally, with international variations in the incidence of prostate cancer clearly recognized (1,3,4). This malignancy is a major personal and global public health problem that disproportionately affects subjects of African ancestry, more than their Caucasian and Asian counterparts (5). Prostate cancer incidences are higher in black Caribbean, black African and other blacks compared to mixed white and African, mixed white and Caribbean, Pakistani and “all white” men in the United Kingdom, respectively (3,6,7).
In the US the Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review report that the age-adjusted mortality rates for African-Americans for 2002–2006 was 56 per 100,000, much higher than their Caucasian counterparts (24 per100,000) (SEER Cancer stats review)and a similar trend is reported for prostate cancer survival. In developing areas like the Caribbean islands, the estimates for cancer mortality and incidence are underestimated due to the limited number of National Cancer Registries in that region (8). The World Health Organization receives cancer registration data from Trinidad & Tobago as well as Guyana and the 2002 estimated age-adjusted mortality rates for these Caribbean countries were 32 per 100,000 and 23 per 100,000 respectively. An estimate of the prostate cancer survival rates for Caribbean males living in the Caribbean is unknown since this analysis has not been performed using cancer registry data.
A large proportion of the US population living in Brooklyn, New York(NY) migrated to the US from the Caribbean. Although subjects of African descent live in diverse surroundings around the world it is possible that they may still have an increased risk of developing prostate cancer(5), suggesting that they share genetic or lifestyle factors that may contribute to the elevated risk. Therefore, in addition to differences in the ethnicity, it is possible that place of birth will contribute to differences in the incidence and mortality of prostate cancer among men of African descent. Using cancer registry data from Brooklyn, NY and two countries in the Caribbean, we have analyzed the epidemiological characteristics of prostate cancer. We have for the first time estimated and compared survival rates for these Caribbean populations and examined whether Black race or Caribbean birth-place predict prostate cancer survival among males living in the United States.
MATERIALS AND METHODS
Study Population
De-identified data were obtained from three cancer registries in Brooklyn (New York), Guyana, and Trinidad, respectively. The study population (n = 6,142) included patients in Brooklyn, NY (n = 1,100), Guyana (n =609) and Trinidad & Tobago (n = 4,433), all having been diagnosed with prostate cancer between 1976 and 2007, with follow-up through to early 2009. The cases include only histologically confirmed malignant prostate cancer. Race was a self-reported variable according to the cancer registry requirements.
Data collection and coding
The data for the three cancer registries were obtained from hospital records. The data collection and coding used for the Brooklyn registry is based on the North American Association of Central Cancer Registries (NAACCR) and SEER cancer registry standards for data and data management while Trinidad & Tobago and Guyana are based on the World Health Organization, International Agency for Research on Cancer(IARC)standards for data and data management. For international comparability, both the United States and World Health Organization cancer registry standards use the International Classification of Diseases for Oncology (ICDO-3) coding for the classification of tumors according to topography, morphology and behavior (9). The Caribbean registries use the CANREG computer program, developed by IARC for cancer registration data entry and management. IARC provides training for CANREG program use and coding to all cancer registries and the program is currently being used by 140 cancer registries in 75 countries worldwide. Validity checks are incorporated in the database to ensure reliability of the data, such as consistency checks for ICDO codes, duplicate records, multiple primaries and impossible or rare cases during data entry (9). The Brooklyn, cancer registry uses the IMPAC METRIQ cancer registry systems (IMPAC Medical Systems, Inc). The program satisfies the NAACCR and New York State regulatory standards and is incorporated with field-sensitive edits and multiple other quality checks in order to ensure data quality (10). Using the coding rubric from each registry, comparability across registries was ensured for clinical characteristics, and race, prior to any analysis. Race was defined as African, Indian, Amerindian, Chinese, White/Caucasian, Mixed and Unknown, for both the Guyana as well as the Trinidad & Tobago registries. The Brooklyn cancer registry categorized race as White, Black, American Indian/Aleut Eskimo, Chinese, Asian Indian/Pakistani, Other and unknown. For comparison between registries, ethnic groupings were created, African, Black-American and African-Caribbean patients were grouped as “Black”, Caucasian patients were grouped as “White” and the “Other” group was comprised of Amerindian, Chinese, Indian and mixed race patients. The Trinidad & Tobago cancer registries used the SEER summary staging criteria (localized, regional and distant) to classify the cases, while the Brooklyn cancer registry used the AJCC TNM staging criteria (stages I, II, III and IV). In order to compare stage between the three registries, for all cases stage was reclassified as stage I-III for “localized” or “regional” and stage IV for “distant”. A Place of Birth variable was created and categorized as United States, Caribbean (which included Puerto Rico, US Virgin Islands, Cuba, Haiti, Dominican Republic, Jamaica, Lesser Antilles & Guyana.), African, Other (which included South & Central America, Europe & Asia) and unknown. For both the Brooklyn and Trinidad & Tobago cancer registries, ICDO3 histology coding was used. Histology was not available for Guyana. Marital status and grade was also categorized in the same way for all three cancer registries.
Statistical Analysis
All statistical analyses were carried out using the Intercooled STATA SE (version 10.1) software (StataCorp. LP, College Station, TX, USA). Associations between risk factors and prostate cancer were analyzed using, chi-square where appropriate. The proportional hazard assumption was verified by examination of Kaplan-Meier survival plots. The model plot created was fitted for cancer mortality, and stratified by place birth. The log-rank test was performed to evaluate the equality of survivor functions according to registry location. A multivariable Cox proportional hazard model was also used to simultaneously adjust survival for birthplace, ethnic group, age at diagnosis and the stage of diagnosis. Follow up time in months was calculated subtracting “Date of Diagnosis” from “Date of Last Contact” in case the subjects were alive, or “Date of Expiration” in case the subjects were dead respectively. All statistical tests were two-sided, and a P-value less than 0.05 were considered statistically significant.
RESULTS
Demographic and Clinical Characteristics
A summary of the patient demographics is shown in Table 1. The majority of cases, 62% were of African ancestry. For1,097 (18%) of the cases race was not known. Most of the cases were born in the Caribbean, even for those registered in the Brooklyn registry. Place of birth was not known in 37% of the cases. The majority of all cases were diagnosed between the ages of 70 to 79 years old (37%) but there were differences between the Brooklyn and Caribbean registries. For Brooklyn, the highest proportion of cases were diagnosed between 60 to 69 years (43%), while for the Guyana and Trinidad & Tobago registries, the majority of cases were diagnosed between 70 to 79 years (44% and 38% respectively). Irrespective of place of birth, the mean age of diagnosis in Brooklyn was 65.8years, with a range between 29to 91years. For Trinidad & Tobago the mean age of diagnosis was 72.4 years(range: 20–99 years)and it was 74.5years(range: 21–99 years) for Guyana. The mean age of diagnosis for cases in Brooklyn was significantly lower than Guyana, and Trinidad & Tobago respectively (p<0.0001). When the cases diagnosed in Brooklyn were stratified according to birth place, no statistically significant difference was observed in mean age of diagnosis (US-born: 65.4 years vs. Caribbean-born: 66.3 years, p = 0.309).
Table 1.
Patient Characteristics
| Characteristic | Total | Brooklyn | Guyana | Trinidad &Tobago | p-Values |
|---|---|---|---|---|---|
| N = 6,142 1976 – 2007 |
N = 1,100 1995 – 2007 |
N = 609 2000 – 2007 |
N = 4,433 1976 – 2005 |
||
| N (%) | N (%) | N (%) | N (%) | ||
| Ethnic Group | < 0.0001 | ||||
| Black | 3,829 (62.3) | 960 (87.3) | 405 (66.5) | 2,464 (55.6) | |
| White | 117 (1.90) | 89 (8.09) | - | 28 (0.6) | |
| Other1 | 1,099 (17.9) | 5 (0.45) | 184 (30.2) | 910 (20.5) | |
| Unknown | 1,097 (17.9) | 46 (4.16) | 20 (3.3) | 1,031 (23.3) | |
| Age (years) | < 0.0001 | ||||
| < 40 | 19 (0.34) | 2 (0.17) | 11(1.81) | 6 (0.12) | |
| 40 – 49 | 100 (1.66) | 41 (3.72) | 4 (0.65) | 55 (1.22) | |
| 50 – 59 | 622 (10.1) | 196 (17.8) | 27 (4.42) | 399 (9.0) | |
| 60 – 69 | 1,782 (29.0) | 474 (43.1) | 116 (19.1) | 1,192 (26.9) | |
| 70 – 79 | 2,273 (37.0) | 346 (31.5) | 266 (43.6) | 1,661 (37.5) | |
| 80 – 89 | 1,163 (18.9) | 40 (3.63) | 152 (25.0) | 971 (21.9) | |
| ≥ 90 | 183 (3.0) | 1 (0.08) | 33 (5.42) | 149 (3.36) | |
| Place of Birth | < 0.0001 | ||||
| United States | 142 (2.30) | 142 (12.9) | - | - | |
| Caribbean2 | 3,664 (59.7) | 421 (38.3) | 609 (100) | 2,634 (59.4) | |
| African | 21 (0.30) | 21 (1.90) | - | - | |
| Other3 | 37 (0.60) | 37 (3.30) | - | - | |
| Unknown | 2,278 (37.1) | 479 (43.6) | - | 1,799 (40.6) | |
| Marital Status | < 0.0001 | ||||
| Single | 555 (9.10) | 225 (20.5) | - | 330 (7.40) | |
| Married | 2,347 (38.2) | 577 (52.5) | - | 1,770 (39.9) | |
| Other4 | 622 (10.1) | 153 (13.9) | - | 469 (10.6) | |
| Unknown | 2,618 (42.6) | 145 (13.1) | 609 (100) | 1,864 (42.1) | |
Other (Ethnic Group) -Amerindian, Chinese, Indian & Mixed.
Caribbean -Puerto Rico, US Virgin Islands, Cuba, Haiti, Dominican Republic, Jamaica, Lesser Antilles & Guyana.
Other (Birth Place) – South & Central America, Europe & Asia.
Other (Marital Status) – Separated, Divorced & Widowed.
Although there were large numbers of cases with unstaged disease for Guyana and Trinidad and Tobago(348 (57%) and 1,858 (42%) respectively), for all three geographic locations, most of the cases were diagnosed with stage I-III disease (Table 2). Brooklyn had the highest proportion of cases with stage I-II disease (91%) while Trinidad & Tobago had the highest proportion of cases with stage IV disease (13%). Although for a fair number of cases, 1,697(28%), the specific histologic type was not assigned, the majority of cases were adenocarcinoma.
Table 2.
Prostate Cancer Patient Clinical Characteristics
| Characteristic | Total | Brooklyn | Guyana | Trinidad &Tobago | P-Value |
|---|---|---|---|---|---|
| N = 6,142 N (%) |
N = 1,100 N (%) |
N = 609 N (%) |
N = 4,433 1N (%) |
||
| Stage | < 0.0001 | ||||
| I-III | 3,236 (52.7) | 996 (90.5) | 248 (40.8) | 1,992 (44.9) | |
| IV | 661 (10.8) | 65 (5.91) | 13 (2.10) | 583 (13.2) | |
| Unstaged | 2,245 (36.5) | 39 (3.59) | 348 (57.1) | 1,858 (41.9) | |
| Grade | <0.0001 | ||||
| Well | 602 (12.1) | - | 10 (1.64) | 592 (13.5) | |
| Moderate | 740 (14.8) | - | 8 (1.31) | 732 (16.7) | |
| Poor | 400 (8.01) | - | 6 (0.99) | 394 (8.98) | |
| Undifferentiated | 17 (0.39) | - | - | 17 (0.39) | |
| Unknown | 3,237 (64.7) | - | 585 (96.06) | 2,652 (60.45) | |
| Histology | 0.000 | ||||
| Glandular intraepithelial neoplasia, grade III | 3 (0.05) | 2 (0.18) | - | 1 (0.02) | |
| Carcinoma, NOS | 1,697 (27.63) | 17 (1.55) | 579 (95.1) | 1,101 (24.8) | |
| Adenocarcinoma | 4,245 (69.11) | 1,078 (98.0) | 30 (4.90) | 3,137 (70.8) | |
| Other1 | 197 (3.21) | 3 (0.27) | - | 194 (4.38) | |
| Status | < 0.0001 | ||||
| Alive | 2,878 (46.9) | 897 (81.5) | 173 (28.4) | 1,808 (40.7) | |
| Dead | 3,253 (52.9) | 203 (18.5) | 435 (71.4) | 2,615 (59.0) | |
| Unknown | 11 (0.20) | - | 1 (0.20) | 10 (0.30) | |
| Median Follow-up, months (range) | 18 months (1 – 305) | 42 months(1–150) | 5 months (1–82) | 14 months (1–305) |
NOS, not otherwise specified
Other (histology): Tumorlet, NOS; Small cell carcinoma; Squamous cell carcinoma; Transitional cell carcinoma; Cribriform carcinoma; Signet ring cell carcinoma; Infiltrating duct carcinoma; Infiltrating duct mixed with other carcinoma; Acinar cell carcinoma; Adenosquamous carcinoma; Sarcoma.
Overall Survival
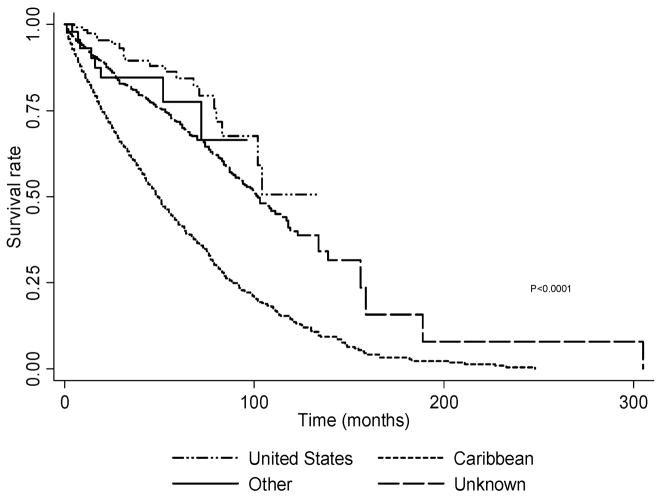
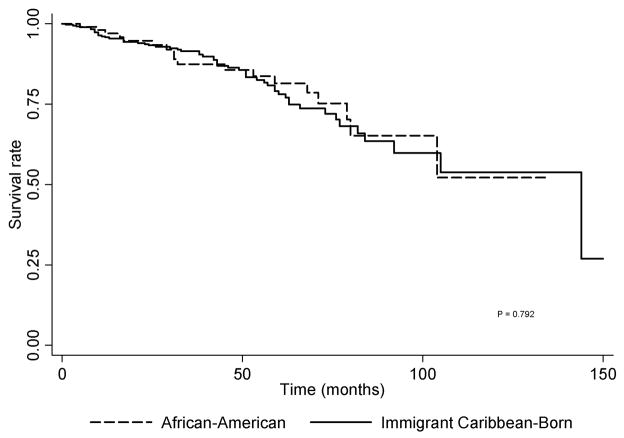
The median follow-up time in months for all cases was 18 months (range: 1–305 months). For Brooklyn, the majority of the cases (47%) were alive at the end of follow-up. In contrast, the majority of Caribbean cases (Guyana: 71%, Trinidad& Tobago: 59%) were dead at the end of the follow-up period. The cumulative survival for all prostate cancer cases (Figure 1)is statistically significantly different according to birth country (p<0.0001). For the cases born in the United States the five-year survival rate was 84.4% (95% CI: 74.1 90.9%), higher than that for patients born in the Caribbean(41.6%, 95% CI: 38.8–43.4%). The five-year survival rate for cases born in other parts of the world was 77.5% (95% CI: 54.9 89.8%) and for those whose birth place was unknown it was 71.8% (95% CI: 67.6 75.5). The immigrant Caribbean-born males diagnosed in Brooklyn had a non statistically significantly lower five-year survival rate than African-American prostate cancer cases (Immigrant Caribbean-born: 78.1%, 95% CI: 70.9–83.7% vs. African-American: 81.4%, 95% CI: 69.5 89.1%, p = 0.792). (Figure 2)
Figure 1.
Kaplan-Meier Survival Estimates Stratified by Birth Place
Figure 2.
Kaplan-Meier Survival Estimates for African-American vs. Caribbean-born prostate cancer cases diagnosed in Brooklyn
The multivariable proportional hazard model shows that even after adjusting for ethnicity, stage and mean age of diagnosis, the risk of death for those born in the Caribbean was more than three times higher than US born men when the cases from all three cancer registries were evaluated together (HR: 3.43, 95% CI: 2.17 – 5.44) (Table 3). In contrast when Brooklyn was considered separately, the risk of death for prostate cancer cases born in countries other than the Caribbean was almost three-fold compared to US-born cases (HR: 2.73, 95% CI: 1.17 – 6.32) while Caribbean-born men in Brooklyn had a non statistically significant elevated risk (HR: 1.46, 95% CI: 0.85 – 2.50). A mean age of diagnosis greater then 65 years old and stage IV disease were found to be independently associated with the risk of death; ethnicity was not significantly associated with risk of death. After adjusting for mean age at diagnosis an stage, there was no significant difference in the risk of death for Caribbean-Born Black men in Brooklyn (HR: 1.03, 95% CI = 0.61–1.76), compared to US-Born Black men in Brooklyn(Table 4). In contrast, the risk of death for Caribbean-born Black men from Guyana was almost 12-fold higher than US-born Black men in Brooklyn (HR: 11.50, 95% CI: 6.52–20.27). Similarly, Caribbean-born men in Trinidad & Tobago had an almost four-fold risk of death (HR: 3.58, 95% CI = 2.23–5.73).
Table 3.
Multivariable Cox Proportional Hazard Model cases according to cancer registry location
| Total N = 3,443 |
Brooklyn N = 1,027 |
Caribbean N= 2416 |
|
|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |
| Birth Place | |||
| United States | Ref. | Ref. | - |
| Caribbean1 | 3.43 (2.17 – 5.44) | 1.46 (0.85 – 2.50) | - |
| Other2 | 1.89 (0.83 – 4.33) | 2.73 (1.17 – 6.32) | - |
| Unknown | 1.73 (1.08 – 2.78) | 1.40 (0.84 – 2.32) | - |
| Ethnic Group | |||
| White | Ref. | Ref. | Ref. |
| Black | 1.14 (0.80 – 1.62) | 1.04 (0.68 – 1.61) | 1.10 (0.57 – 2.12) |
| Other3 | 1.17 (0.81 – 1.71) | - | 0.95 (0.49 – 1.85) |
| Diagnosis Age | |||
| ≥65 Years | Ref. | Ref. | Ref. |
| > 65 Years | 1.75 (1.53 – 2.01) | 1.87 (1.35 – 2.58) | 1.64 (1.41 – 1.90) |
| Stage | |||
| I-III | Ref. | Ref. | Ref. |
| IV | 2.87 (2.50 – 3.28) | 7.01 (4.55 – 10.82) | 2.19 (1.91 – 2.53) |
| Un staged | 1.71 (1.48 – 1.98) | 2.25 (1.28 – 3.93) | 1.29 (1.11 – 1.50) |
Caribbean -Puerto Rico, US Virgin Islands, Cuba, Haiti, Dominican-Republic, Jamaica, & Lesser Antilles.
Other (Birth Place)–Africa, South & Central America, Europe & Asia.
Other (Ethnic Group)–Amerindian, Chinese, Indian, & Mixed.
Table 4.
Overall Survival in Black men according to place of birth and cancer registry location
| Total N = 2,713 | |
|---|---|
| Adjusted Hazard Ratio (95% CI)* | |
| Place of Birth/registry | |
| US-born Brooklyn | Ref. |
| Caribben-born Brooklyn | 1.03 (0.61–1.76) |
| Caribbean-born Guyana | 11.50(6.52–20.27) |
| Caribbean-born Trinida & Tobago | 3.58 (2.23–5.73) |
| Other Brooklyn1 | 1.05(0.63–1.74) |
| Other Trinidad & Tobago2 | 3.77(2.18–6.51) |
Includes Black men who were born in Africa and those for which country of birth was unknown.
Includes Black men for which country of birth was unknown
Adjusted for mean age at diagnosis an stage
DISCUSSION
The findings in this examination of the cancer registry data from Brooklyn, NY, Guyana and Trinidad & Tobago give a fair representation of prostate cancer in males of African ancestry. There were approximately 62% black males in this study population and for the first time we report and compare survival rates for prostate cancer cases from two population cancer registries in the Caribbean and a hospital-based cancer registry in Brooklyn, USA. This is the first and largest study of prostate cancer survival using combined cancer registry data from the Caribbean and the United States. This study has allowed us to compare the within-group differences in survival among Black men from two distinct geographic regions (US and the Caribbean).
The low survival rate has been clearly illustrated in Caribbean born prostate cancer cases in this study population. For those cases born in the United States, their diagnosis age was younger than their Caribbean registry counterparts. The mean age of diagnosis for Brooklyn males was 66 years. A significant proportion of Brooklyn cases were black (87%); age at diagnosis is consistent with previous reports, showing that the median age of diagnosis for black males in the United States is 65 years (11). Consistent with other studies in Caribbean populations(12–14), this study shows that Caribbean men from Trinidad & Tobago and Guyana are diagnosed at an older age compared to men in Brooklyn. This suggests a possible limited access to healthcare and/or low screening prevalence. The younger age at prostate cancer diagnosis for Brooklyn, NY compared to the Caribbean countries examined was possibly due to education, and early detection methods. Low education and literacy level on cancer screening in older Caribbean men has been shown to be related to under-use of services available (15). On the island of Tobago, a longitudinal screening study on serum prostate-specific antigen (PSA) in local volunteers reported a high amount of screening-detected prevalence of prostate cancer (5). However this may not entirely explain the differences between Brooklyn and the Caribbean. Similar to Brooklyn, the highest proportions of Caribbean patients were diagnosed with stage I-III disease. However, the distribution of stage at diagnosis needs to be interpreted with caution since a large proportion of cases were un staged for the Caribbean registry sites.
Ad hoc studies are needed to assess the relationship between age at diagnosis and stage in Caribbean-born men compared to US-born men.
Our results show that males diagnosed in the Caribbean tend to have significantly worse survival outcomes compared to males diagnosed in Brooklyn. However survival rates for African-American men and Caribbean-born Black men who were diagnosed in the US were similar. It is estimated by the World Health Organization that 70% of prostate cancer deaths occur in low to middle income countries where persistent disparities hinder detection and treatment (16). It is possible that the similar survival rates between African-American and Caribbean-born Black males in the US are likely due to the easier access to early detection and treatment compared to those men who were diagnosed in the Caribbean. Recently, Meliler et al. evaluated prostate cancer survival disparities according to different geographic scales in the state of Michigan, USA(17). The study reported that the observed survival disparity between Blacks and Whites for the state was diminished when the analysis was restricted to smaller geographic units such as community-defined neighborhoods and state House legislative districts. These findings suggest that individual/genetic risk factors may not necessarily explain the reduction in survival disparity between Blacks and White according to geographic scale. Widely recognized risk factors for prostate cancer include aging, geographic origin, in addition to a family history of prostate cancer (18). A commonly stated hypothesis is that genetic factors contribute to the high risk for prostate cancer among populations of African origin (1)and other studies suggest that there may be an important influence of environmental/lifestyle factors acting on prostate cancer risk as illustrated by the variability in rates between the populations of African descent in different geographic locations (4,5,19). We did not observe a significant difference in risk of death for Caribbean-born Black men in Brooklyn, when compared to US-born Black men in Brooklyn; in contrast there was a twelve-fold and four-fold increased risk of death for Caribbean-born men from Guyana and Trinidad & Tobago respectively. The excessively high Hazard ratio with a wide confidence interval for Caribbean-born Guyanese males is an imprecise estimate due the small sample of Guyanese men. Nevertheless, the overall findings support the inferences that environment/lifestyle factors may play a more important role in prostate cancer survival for Caribbean-born Black men. Therefore further studies that compare and contrast sociodemographic and environmental risk factors in prostate cancer among US and Caribbean populations are necessary in order to help close the gap in survival disparities within the black population.
This study’s limitations arose primarily from incomplete data. Many cases were not classified into ethnic groups, nor were their grade and stage at diagnosis noted. These are important variables that could shed light into whether there are significant differences in the stage and grade of diagnosis in different locations, and thus reveal more about the disease that afflicts so many men. It should be taken into account that some of the data used in this analysis was incomplete with respect to clinical characteristics and could thus influence the interpretation of some results. A high proportion of cases were classified as carcinoma and the specific histologic type was not defined, therefore we were not able to evaluate whether more aggressive histologic types among Caribbean males might contribute to the poor survival rates in this population.
Although environmental/lifestyle factors may be very important predictors of survival for Black Caribbean-born men, genetic risk factors cannot be ruled out. Future association studies involving genetic factors in recently immigrated Caribbean and African-American prostate cancer patients are still necessary. This will also help to provide a better understanding of the reasons for such large differences in the survival rates between the two geographic regions. A vital question to be answered in future studies is whether the differences we observed still exits after adjusting for access to care and screening.
Acknowledgments
Funding for this work was supported in part by grant number R13CA130596A to CR and P20CA132385-01 to ET. The authors are grateful to Gangadai Bhawansingh and Mary Charles for data collection and to Simone George and Carlene John for data entry and management at the Trinidad and Tobago Registry. Thanks to Dr. Shamdeo Persaud, Chief Medical Officer, Ministry of Health, Guyana for the support of this work.
References
- 1.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62:3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Mallick S, Romana M, Blanchet P, Multigner L. GSTM1 and GSTT1 polymorphisms and the risk of prostate cancer in a Caribbean population of African descent. Urology. 2007;69:1165–1169. doi: 10.1016/j.urology.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Ben Shlomo Y, Evans S, Ibrahim F, Patel B, Anson K, Chinegwundoh F, Corbishley C, Dorling D, Thomas B, Gillatt D, Kirby R, Muir G, Nargund V, Popert R, Metcalfe C, Persad R. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53:99–105. doi: 10.1016/j.eururo.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Risch N. Dissecting racial and ethnic differences. N Engl J Med. 2006;354:408–411. doi: 10.1056/NEJMe058265. [DOI] [PubMed] [Google Scholar]
- 5.Bunker CH, Patrick AL, Konety BR, Dhir R, Brufsky AM, Vivas CA, Becich MJ, Trump DL, Kuller LH. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev. 2002;11:726–729. [PubMed] [Google Scholar]
- 6.Jack RH, Davies EA, Moller H. Testis and prostate cancer incidence in ethnic groups in South East England. Int J Androl. 2007;30:215–220. doi: 10.1111/j.1365-2605.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 7.Ravery V, Dominique S, Hupertan V, Ben Rhouma S, Toublanc M, Boccon-Gibod L. Prostate cancer characteristics in a multiracial community. Eur Urol. 2008;53:533–538. doi: 10.1016/j.eururo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 8.Ragin CC, Taioli E, McFarlane-Anderson N, Avery G, Bennett F, Bovell-Benjamin A, Brown TA, Carrington A, Campbell-Everett L, Ford J, Hennis A, Jackson M, Lake S, Leske MC, Magai C, Nemesure B, Neugut A, Odedina F, Okobia M, Patrick A, Best PW, Reams RR, Roberts R, Scott-Hastings S, Sharma S, Wheeler V, Wu SY, Bunker C. African-Caribbean cancer consortium for the study of viral, genetic and environmental cancer risk factors. Infect Agent Cancer. 2007;2:17–22. doi: 10.1186/1750-9378-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir CS, Percy C. Classification and coding of neoplasms. IARC Sci Publ; 1991. Cancer registration: principles and methods; pp. 64–81. [PubMed] [Google Scholar]
- 10.IMPAC. Oncology NEWS International. 2003. Medical Provides Integrated Oncology-Specific Management Systems for Ca Hospitals, Private Practices; p. 12. [Google Scholar]
- 11.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 12.Coard KC. Prostate cancer at the University Hospital of the West Indies in Jamaica. A clinico-pathological profile at the time of needle biopsy diagnosis. West Indian Med J. 2002;51:40–43. [PubMed] [Google Scholar]
- 13.Coard KC, Skeete DH. A 6-year analysis of the clinicopathological profile of patients with prostate cancer at the University Hospital of the West Indies, Jamaica. BJU Int. 2009;103:1482–1486. doi: 10.1111/j.1464-410X.2008.08265.x. [DOI] [PubMed] [Google Scholar]
- 14.Shirley SE, Escoffery CT, Sargeant LA, Tulloch T. Clinicopathological features of prostate cancer in Jamaican men. BJU Int. 2002;89:390–395. doi: 10.1046/j.1464-4096.2001.01871.x. [DOI] [PubMed] [Google Scholar]
- 15.Reyes-Ortiz CA, Camacho ME, Amador LF, Velez LF, Ottenbacher KJ, Markides KS. The impact of education and literacy levels on cancer screening among older Latin American and Caribbean adults. Cancer Control. 2007;14:388–395. doi: 10.1177/107327480701400409. [DOI] [PubMed] [Google Scholar]
- 16.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 17.Meliker JR, Goovaerts P, Jacquez GM, Avruskin GA, Copeland G. Breast and prostate cancer survival in Michigan: can geographic analyses assist in understanding racial disparities? Cancer. 2009;115:2212–2221. doi: 10.1002/cncr.24251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowler JE, Jr, Bigler SA. Racial differences in prostate carcinogenesis. Histologic and clinical observations. Urol Clin North Am. 2002;29:183–191. doi: 10.1016/s0094-0143(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 19.Odedina F, Akinremi T, Chinegwundoh F, Roberts R, Yu D, Reams RR, Freedman M, Rivers B, Green B, Kumar N. Prostate cancer disparities in Black men of African descent: a comparative literature review of prostate cancer burden among Black men in the United States, Caribbean, United Kingdom, and West Africa. Infectious Agents and Cancer. 2009;4:S2–S9. doi: 10.1186/1750-9378-4-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]