Abstract
We examined the neural correlates of specific (i.e., unique to time and place) and general (i.e., extended in or repeated over time) autobiographical memories (AMs) during their initial construction and later elaboration phases. The construction and elaboration of specific and general events engaged a widely distributed set of regions previously associated with AM recall. Specific (vs. general) event construction preferentially engaged prefrontal and medial temporal lobe regions known to be critical for memory search and retrieval processes. General event elaboration was differentiated from specific event elaboration by extensive right-lateralized prefrontal cortex (PFC) activity. Interaction analyses confirmed that PFC activity was disproportionately engaged by specific AMs during construction, and general AMs during elaboration; a similar pattern was evident in regions of the left lateral temporal lobe. These neural differences between specific and general AM construction and elaboration were largely unrelated to reported differences in the level of detail recalled about each type of event.
Keywords: autobiographical memory, prefrontal cortex, medial temporal lobe
Introduction
The process of remembering complex and richly detailed episodes, including those that are autobiographical in nature, is constructive and extended in time such that it can be divided into at least two phases (e.g., Daselaar et al., 2008; Conway, Pleydell-Pearce, & Whitecross, 2001). It has been suggested that the first phase of autobiographical memory (AM), memory construction or formation, is an iterative process (e.g., Conway & Rubin, 1993; Conway & Pleydell-Pearce, 2000). The cycle begins with memory search processes based on an initial cue specification (e.g., recall an event associated with the cue word “tree”) and leads to a subsequent evaluation of the search results. If the retrieved information is deemed to meet the goals of the retrieval task in the evaluation phase, the cycle stops; if not, then the output of the search phase is used as a cue to begin the cycle again (Conway & Rubin, 1993; Conway & Pleydell-Pearce, 2000). Following this initial phase of memory construction is a second phase known as event elaboration, during which time the fully constructed event is held in mind and its details expounded upon (e.g., Conway et al., 2001).
The goal of the present study was to extend our understanding of the neural correlates of these two phases—AM construction and elaboration—beyond specific events that are unique to a particular time and place to those general autobiographical events that are either summaries of repeated events or extended in time (Conway & Pleydell-Pearce, 2000; Conway et al., 2001; Barsalou, 1988). Autobiographical memory has traditionally been defined in the literature as memory for specific episodes. As such, most neuroimaging work on AM has focused on the retrieval of specific AMs (e.g., Fink et al., 1996; Maguire & Mummery, 1999; Maguire et al., 2000, 2001; Daselaar et al., 2008; Greenberg et al., 2005; Cabeza et al., 2004; for reviews, see Svoboda et al., 2006; Cabeza and St. Jacques, 2007). This focus on specific AMs might also be for a more practical reason: Many AM studies utilize the traditional Crovitz cue word task (Crovitz & Schiffman, 1974), whose instructions are designed to elicit specific AMs, and as such, general AMs are treated as errors. Although general autobiographical events have largely been disregarded in the neuroimaging literature, theoretical and behavioral evidence suggests that they are psychologically distinct from specific AMs (e.g., Williams & Dritschel, 1992). General AM retrieval theoretically allows for fast—and less cognitively demanding—access to summaries of specific events (Williams & Dritschel, 1992; Conway & Rubin, 1993; Conway, 2001). Accordingly so, general AMs are accessed earlier during the iterative retrieval cycle (Conway et al., 2001), and are associated with more abstract or conceptual (vs. sensory specific) details than specific AMs (Greenberg & Rubin, 2003). Behavioral and/or neural activity during the earlier construction phase of AM, when the iterative search, retrieval, and monitoring cycle is taking place, may dictate the ultimate specificity of the recalled event (Conway et al., 2001; Conway, 2005).
Despite the paucity of work examining the differences between specific and general AM construction and elaboration, neuroimaging studies of specific AM retrieval have converged upon a widely distributed “autobiographical memory network” (reviewed by Svoboda et al., 2006; Cabeza & St. Jacques, 2007). The network is comprised of several areas of the PFC—including primarily left-lateralized dorsolateral (BA 9/46) and ventrolateral (BA 47) areas critical for initiating the search and retrieval processes during AM construction. The ventromedial PFC (BA 11/13/25) is thought to be responsible for the monitoring of the retrieved information to ensure that it meets the retrieval task requirements at hand (analogous to the evaluation stage in the iterative retrieval model), and the medial PFC corresponds to the self-referential processing that is a hallmark of AM (Cabeza & St. Jacques, 2007). Within the medial temporal lobes (MTL), the hippocampus is known to be critical for AM retrieval, and appears to act as a “pointer” to the specific sensory and perceptual details stored in cortical regions (Nadel & Moscovitch, 1997). Indeed, activity in these posterior cortical regions responsible for the storage of sensory details (e.g., precuneus, cuneus, and other areas of the occipital and parietal lobes) is also a hallmark of specific AM retrieval (Svoboda et al., 2006; Cabeza & St. Jacques, 2007), particularly during the later phases of specific AM construction and remains robust throughout the elaboration phase (Conway et al., 2001).
There is some evidence suggesting that the specificity of memories modulates activation in regions associated with this core AM network. A small set of experiments has examined a question related to that of the present study by contrasting temporally and contextually specific AMs with autobiographical knowledge that is not confined to a particular time or place (e.g., “My brother’s name is Joe”; Maguire & Mummery, 1999; Levine et al., 2004). These studies have demonstrated that while both types of memory recruit regions associated with AM retrieval, the level of activity is enhanced by specificity. For example, specific autobiographical memories engage left-lateralized regions—including the left hippocampus and left temporal pole—as well as the medial PFC to a greater extent than more semanticized types of memory that lack temporal specificity (Maguire & Mummery, 1999). A related study restricted analysis to contrasting personal episodic (i.e., specific) and personal semantic information (including some repeated activities, such as making coffee) (Levine et al., 2004). A partial least squares (PLS) analysis revealed these types of AM recruited distinct networks: Personal episodic memories engaged several regions of the standard AM retrieval network, such as left-lateralized medial PFC, the MTL, and the posterior cingulate cortex, whereas personal semantic memories engaged left temporo-parietal and parieto-frontal regions (Levine et al., 2004).
Although the studies reviewed to this point suggest specificity modulates the neural regions engaged by AM retrieval, including PFC and MTL regions, these studies have focused on personal semantic knowledge rather than general AMs as they have been defined in the literature (e.g., Conway & Pleydell-Pearce, 2000). Personal semantic knowledge (e.g., my brother’s name) is not the same as general autobiographical events that are either repeated or extended in time (e.g., walking my brother to school every day). Autobiographical knowledge can be completely divorced from both spatial and temporal contexts, whereas general events are still associated with contextual information (Conway & Pleydell-Pearce, 2000). Addis and colleagues more directly demonstrated the neural differences between specific and general AMs by restricting the definition of general AMs to categories of repeated events. Participants retrieved specific and general AMs in response to personalized cues obtained in a pre-scan interview. These data were analyzed using GLM (Addis, Moscovitch, et al., 2004) and PLS (Addis, McIntosh, et al., 2004) analyses, and confirmed that both forms of AM commonly recruit regions in the AM retrieval network, such as the hippocampus. However, elaboration of specific AMs differentially recruited regions related to contextual/episodic imagery, such as the medial parietal cortex, to a greater extent than general AMs, likely reflecting the greater amount of visual detail present in specific AMs. General memory retrieval, however, was characterized by maximal activity in the right lateral inferior temporal gyrus, likely reflecting the recall of conceptual knowledge that may feature more strongly for the more abstract general AMs.
Although these results confirm that specific and general AM retrieval can be differentiated at both a behavioral and neural level, this paradigm used cues that were personally tailored to individual AMs, therefore by-passing the need for a construction or search phase, and thus only focused on a brief (6 sec) elaboration period. Alternatively, one can forego the pre-scan interview and instead ask participants to retrieve AMs in response to cue words while in the scanner. The use of generic cues and extended trial times has allowed researchers to tease apart AM construction from elaboration (e.g., Conway et al., 2001; Conway et al., 2003; Addis, Wong, & Schacter, 2007; Daselaar et al., 2008; Steinvorth, Corkin, & Halgren, 2006; Addis et al., 2009; Addis et al., in press). However, the majority of studies to this point have instructed individuals to retrieve specific events, and general events retrieved were not analyzed (e.g., Addis et al., 2007, 2009) or not considered in the analyses (e.g., Conway et al., 2001; Conway et al., 2003; Daselaar et al., 2008)1.
Hennessey and colleagues (in press) recently examined the construction phase of specific and general AMs elicited in response to musical cues. They reported that retrieval of specific versus general events resulted in increased activity in many regions previously associated with AM retrieval, including bilateral MTL, precuneus, and medial and dorsolateral PFC. These neural differences were argued to reflect the more vivid levels of detail characteristic of specific relative to general events, particularly as the increased activity was evident in regions supporting reconstruction of detail, episodic imagery and monitoring of retrieved content. However, to date, it has not been examined whether such neural differences between specific and general events exist even when differences in detail are accounted for. It also is not known whether the results of Hennessey et al. (in press) are particular to instances in which music is used to cue memory. It has been proposed that music may cue memory in unique ways (Janata, 2009) and so it is possible that the effects reported by Hennessey et al. (in press) would not generalize to other cuing paradigms.
Furthermore, it is not clear whether such neural differences between specific and general events are evident through all phases of AM retrieval, and as yet, no study has directly examined this. Taking together the findings of different studies examining specific versus general AMs either at construction (e.g., Hennessey et al., in press) or elaboration (e.g., Addis, McIntosh et al., 2004), it would appear that some neural specific > general effects evident during construction do not persist into elaboration (e.g., hippocampus), while others do (e.g., medial parietal cortex).
In the present fMRI study, we used the Crovitz cueing paradigm with modified task instructions, such that we did not provide directions regarding memory specificity to elicit a mix of both specific and general AMs. This design enabled us to examine both the construction and elaboration phases of specific and general AMs in the same participants as they spontaneously generated memories during the scan. Moreover, it would enable us to test interactions between the memory type and the phase of retrieval, to determine whether neural differences were evident through both phases. Similar to Conway et al.’s (2001) prediction that the specificity of a retrieved memory depends on processing during event construction, we predicted that neural differences in the formation of AMs ultimately classified as general or specific would be especially evident during the construction phase (see also Conway, 2005). In particular, regions of the PFC associated with search and retrieval operations might be more active during the construction of specific than general AMs, given that the retrieval of specific AMs is thought to rely on a frontally-mediated central executive (Conway & Pleydell-Pearce, 2000). Similarly, early engagement of the hippocampus and related temporal lobe structures might distinguish between AMs that are ultimately specific versus general in nature. Given that specific > general effects have been noted in the hippocampus during construction (Hennessey et al., in press), but not elaboration (Addis, McIntosh, et al., 2004), hippocampus activity may be particularly critical for the construction of specific events.
We also collected detail ratings that could be entered as covariates into the analysis to determine whether any neural differences between the specific and general AM tasks could be accounted for by differences in the level of detail of the memories in each condition. This approach would us to better understand which neural differences between these memory conditions reflect differences in detail.
1. Methods
1.1. Participants
Thirty-one younger adults (15 female; M = 21.81 years, SD = 2.43 years; range = 18–27 years) participated in this study. Three participants were subsequently dropped from analyses for failing to meet the eligibility criteria for this study (i.e., Beck Depression Inventory scores higher than 10), two participants were excluded for failing to complete the scanning portion of the study, and one participant was excluded due to failure of the button box equipment during the scan. The final sample included 25 participants (12 female), who had no history of psychiatric, neurological, or learning disorders, nor any history or current use of psychiatric medication. Informed consent was obtained from all participants in accordance with the Massachusetts General Hospital Institutional Review Board.
1.2. Stimuli
A total of 72 neutral nouns were selected from the Clark and Paivio (2004) extended norms. Selected nouns were high in familiarity, imagability, and concreteness, but low in emotional ratings. The nouns were randomly assigned to serve as a cue word or as a noun for the control task (explained below), and counterbalanced across participants. These nouns were distributed across 4 lists; each list had a total of 12 cue words and 6 control task words.
1.2.1. AM Cue Word Task
Participants viewed a total of 48 cue words while undergoing a slow event-related fMRI scan. These cue words were divided among 4 runs and were presented with the instruction “Remember.” Each cue word trial lasted for 26 sec (see Fig. 1a). Participants were instructed that whenever they saw a noun with the instructions “Remember,” they should recall a memory from their past that was either directly or indirectly related to the word they saw on the screen. As soon as they had a memory in mind, participants used their right hand to press a button on a button box to indicate that they had generated a memory. This button press served to demarcate the end of the construction phase of the AM. Once the button press was made, participants saw the word “Elaborate” for the remainder of the 26 sec trial, during which time they maintained and elaborated on the details of the memory they generated. At the end of the 26 sec trial, participants were asked to rate how detailed their memory was on a 5-point scale using the button box. The rating scale appeared for a maximum of 5 sec. Following the rating scale, a fixation cross appeared for a variable amount of time (range = 4–12 sec) to create jitter between trials.
Figure 1.

(a) AM cue word task. Participants were given a maximum of 26 sec to generate an AM for each cue word. Once they had a memory in mind they elaborated on its details for the remainder of the 26 sec trial, then rated the amount of detail they were able to recall about that memory. (b) Sentence generation control task. Participants were given a maximum of 26 sec to generate two semantically-related words to the cue word and to subvocalize a sentence with the format, “X is smaller than Y is smaller than Z.” Once they had a sentence in mind they elaborated on the objects’ appearances and functions for the remainder of the 26 sec trial, then rated how much detail they were able to generate about the objects.
1.2.2. Control Task
We adapted a sentence generation task from Addis, Wong, and Schacter (2007) to control for the construction (i.e., searching for and integrating multiple pieces of information) and elaboration (i.e., visuospatial processing) phases of the AM task. In each of the four runs, six 26 sec control trials were randomly intermixed with the 12 AM cue words. Control trials consisted of a noun presented with the instruction “Sentence” (Fig. 1b). When participants saw the instruction “Sentence,” they generated two semantically-related nouns to the presented noun, and then put the words into a sentence with the format “X is smaller than Y is smaller than Z” according to the physical size of the objects. Once participants had sub-vocalized this sentence, they made a button press, which demarcated the end of the sentence construction phase. Following the button press, the instruction “Elaborate” appeared on the screen, during which time participants silently thought about and elaborated on the appearance and functions of the 3 objects for the remainder of the 26 sec trial. At the end of the 26 sec, participants were given up to 5 sec to rate how detailed their sentence generation and elaboration was on a 5-point scale using the button box. As with the AM task, each trial was followed by a variable amount of fixation time (range = 4–12 sec) to create inter-trial jitter.
1.3. Post-scan interview
Following the scanning portion of the experiment, after an approximately 30 minute delay, participants were asked to briefly tell an experimenter about the memories they recalled while they were in the scanner in response to the 48 cue words that appeared with the instruction “Remember.” Each memory was coded on-line for its level of specificity. A memory was coded as: “Specific” if it referred to a unique event that occurred at one time, one place, and lasted for a day or less; “General” if it referred to an event that was either repeated (e.g., every Thanksgiving) or extended in time (e.g., a vacation that lasted for a week); or “Omission” if the participant did not generate a memory in response to a word. These codings for specificity were used to create post-hoc specific and general AM conditions; any trials for which the participant could not remember which memory they generated in the scanner were dropped from subsequent data analyses.
During the post-scan interviews, participants were also asked to provide the following information about each memory: (a) approximately how old they were when the memory occurred, (b) how emotionally intense they found the recall (not the actual occurrence) of the event to be on a 5-point scale, and (c) how much detail they were able to recall about each memory on the same 5-point scale they saw in the scanner.
1.4. Image Acquisition and Data Analysis
1.4.1 Image Acquisition
Images were acquired on a 1.5 Tesla Siemens Avanto MRI scanner (Erlangen, Germany) using a standard birdcage head coil. Stimuli were presented using the MacStim presentation software. All words, instructions, and digits used in the experiment appeared in white text (Arial 36-point font) on a black background. Stimuli were projected onto a screen located at the back of the magnet bore, and participants viewed the stimuli using a mirror attached to the head coil.
T1-weighted localizer images and a T1-weighted inversion recovery echo planar image required for auto-alignment were collected. Anatomic data were collected with a multiplanar rapidly acquired gradient-echo (MP-RAGE) sequence (TR = 2730 ms; TE = 3.39 ms; flip angle = 40°; field of view = 256 × 256 mm; acquisition matrix = 256 × 256; slice thickness = 1.33 mm, no gap; 1 × 1 × 1.33 mm resolution). Functional images were collected using a T2*-weighted echo-planar imaging (EPI) sequence with the following parameters: TR = 3000 ms, TE = 40 ms, FOV = 200 mm, flip angle = 90°. Twenty-eight interleaved axial-oblique slices aligned with the anterior commissure/posterior commissure line were collected in a 3.125 × 3.125 × 3.84 mm matrix (slice thickness = 3.12 mm, 0.6 mm skip between slices).
Preprocessing and data analysis were conducted in SPM2 (Wellcome Department of Cognitive Neurology, London). Preprocessing steps were as follows: (1) slice timing correction, (2) motion correction using a six parameter, rigid body transformation algorithm, (3) normalization to the Montreal Neurological Institute (MNI) template (resampling at 3 mm isotropic voxels), and (4) spatial smoothing using a 7.6 mm full-width half maximum isotropic Gaussian kernel.
1.4.2. AM data analysis
For each individual, the following event types were first modeled and analyzed using the general linear model approach on a voxel-by-voxel basis: (a) Specific Memory Construction, (b) Specific Memory Elaboration, (c) General Memory Construction, (d) General Memory Elaboration, (e) Sentence Construction, (f) Sentence Elaboration, and (g) Memory Omission (i.e., any trials for which a participant failed to generate a memory). Contrasts between the various trial types were computed as described below, and the resulting contrast images were then entered into second-level random-effects analyses. In all of our analyses, unless otherwise noted, we employed a statistical threshold of p ≤ .001 and a 5-voxel cluster extent. For any contrasts that resulted in null findings, we used a reduced threshold of p ≤ .005 and a 5-voxel cluster extent; if only a small number of regions was active even at the reduced threshold, we could rule out that any null findings at the more stringent threshold were due to Type II error. For MTL regions of interest, for which we had an a priori hypotheses about how MTL activity might relate to event specificity, we used a statistical threshold of p ≤ .005 and a 5-voxel cluster extent.
One set of second-level analyses examined the overlap in activity between specific and general memory during (a) construction and (b) elaboration using the masking function in SPM2. For the memory construction conjunction analyses, we first computed first-level contrasts between specific memory construction and a randomly selected one-half of the sentence construction trials (i.e., specific memory construction > ½ sentence construction trials), as well as between general memory construction and the remaining one-half of the sentence construction trials (i.e., general memory construction > ½ sentence construction trials). The resulting contrast images were entered into individual second-level random-effects one-sample t-tests. The activated voxels from the first t-test (specific memory construction > ½ sentence construction trials) were used to form a mask. This mask was then applied to the second t-test (general memory construction > ½ sentence construction trials), such that the resulting conjunction revealed regions active in both contrasts of interest. By thresholding the individual one-sample t-tests at p ≤ .01, we ensured that the joint probability of the conjunction analysis was p ≤ .001 using Fisher’s estimate (Fisher, 1950). A conjunction analysis examining the common activity during specific and general AM elaboration was calculated in the same way as the AM construction conjunction (i.e., specific AM elaboration > ½ sentence elaboration trials and general AM elaboration > ½ sentence elaboration trials).
A second set of second-level random-effects analyses contrasted the different levels of memory specificity during each phase of memory retrieval (i.e., general construction > specific construction; specific construction > general construction; general elaboration > specific elaboration; and specific elaboration > general elaboration)2. These contrasts were computed at the first-level, and then the resulting contrast images were entered into second-level random effects one-sample t-tests to determine whether the contrasts were significant at the group level.
Finally, a third set of second-level random-effects analyses examined whether there were any interactions between memory specificity and memory phase [i.e., a Type (General, Specific) × Phase (Construction, Elaboration) interaction]. We computed two interaction contrasts at the first level: (1) (general AM construction > specific AM elaboration) > (specific AM construction > general AM elaboration) and (2) (specific AM construction > general AM elaboration) > (general AM construction > specific AM elaboration). The resulting contrast images were entered into second-level random effects one-sample t-tests to determine which regions exhibited a significant memory type (specific, general) x memory phase (construction, elaboration) interaction.
2. Results
2.1. Behavioral Results
Participants successfully generated autobiographical events during scanning and were able to recall those memories during the post-scan interview for an average of 45.8 out of 48 cue words (SD = 2.5). Of those events, an average of 27.24 (SD = 6.77) were specific and 15.92 were general (SD = 7.4). Within-subjects t-tests determined that specific and general events did not differ in respect to reaction time for event construction [t(24) = 1.38, p = .18,] or emotional intensity at the time of retrieval [t(24) = 1.56, p = .13] (Table 1).
Table 1.
Mean reaction times, phenomenological ratings, and age of specific and general memories.
| Specific | General | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| RT (s) | 5.45 | 1.87 | 5.82 | 2.54 |
| Emotional Intensity | 2.27 | 0.65 | 2.16 | 0.67 |
| Age of Memory | 2.94* | 1.95 | 4.23* | 2.70 |
| Level of Detail | 3.63* | 0.43 | 3.37* | 0.59 |
Note:
comparison between specific and general significant at p < .05
As expected, specific memories were rated by participants as more detailed than general memories [t(24) = 2.39, p = .03]. Detail may modulate activity in many regions implicated in AM retrieval (e.g., hippocampus, medial parietal cortex, PFC), and thus we wanted to examine whether detail could explain any activation differences between specific and general events. To this end, we ran additional sets of fMRI analyses controlling for detail rating differences (see Sections 2.2 – 2.4).
Events were dated as the number of years since they occurred, or in the case of general events that occurred over a range of time, the number of years since their last instance (Addis, Moscovitch, et al., 2004). Events that could not be accurately dated (M = 2.42 general events, M = 0.35 specific events per participant) were excluded from a t-test comparing the recency of specific and general AMs. Specific memories were overall slightly more recent (M = 2.94 years) than general memories (M = 4.23 years) [t(24) = 3.47, p = .002]. AM recency may affect MTL activation (e.g., Squire & Alvarez, 1995; Dudai, 2004; Frankland & Bontempi, 2005; but see Moscovitch et al., 2005 for an alternative view). Given the specificity of the issue of recency to the MTL, we examined whether any significant differences in MTL regions were related to recency (see Section 2.2.2).
2.2. Construction Phase fMRI Analyses
2.2.1. Conjunction Analysis
We conducted a conjunction analysis to determine which regions were commonly activated during the construction of both specific and general AMs (i.e., specific AM > sentence construction and general AM > sentence construction). Several regions previously found to comprise the standard AM network (e.g., Svoboda et al., 2006; Cabeza & St. Jacques, 2007) were commonly activated during the construction of specific and general AMs (see Table 2 and Fig. 2), including the left frontal pole (BA 10), right parahippocampal gyrus, and right superior (BA 10) and bilateral middle temporal gyri (BAs 39 and 21). Right-lateralized cuneus (BA 19) activity was also revealed, as well as the left precuneus. Although a number of core regions in the standard AM retrieval network were commonly activated by both types of memories, structures in the MTL (including the hippocampus) and lateral PFC, were notably not commonly engaged by specific and general events.
Table 2.
Common activity during specific and general AM construction.
| Lobe/Region | BA | H | Talairach Co-ordinates | k | T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Frontal | |||||||
| Frontal pole | 10 | L | −8 | 60 | −5 | 1045 | 6.82 |
| Limbic | |||||||
| Parahippocampal gyrus | 30 | R | 22 | −52 | 4 | 33 | 4.87 |
| Temporal | |||||||
| Superior temporal gyrus | 22 | R | 59 | −55 | 18 | 38 | 4.09 |
| Inferior temporal gyrus | 21 | L | −61 | −5 | −15 | 17 | 4.59 |
| Middle temporal gyrus | 21 | R | 53 | 3 | −24 | 21 | 4.02 |
| 39 | L | −53 | −61 | 25 | 121 | 5.71 | |
| Occipital | |||||||
| Precuneus Cuneus | 31 | L | −4 | −67 | 27 | 639 | 4.73 |
| 19 | R | 26 | −80 | 26 | 14 | 3.67 | |
| Other | |||||||
| Postcentral gyrus | 3 | L | −40 | −24 | 64 | 12 | 3.92 |
Note: BA = Brodmann Area; H = Hemisphere; k = voxel extent; T = t-score
Figure 2.
Regions commonly activated by the construction of both specific and general AMs.
2.2.2. Specific > General AM Construction
The construction of specific AMs differentially recruited a number of regions when compared to the construction of general AMs (Table 3 and Fig. 3). Although the left middle temporal gyrus (BA 39) was commonly recruited by both types of memory construction (evidenced by the conjunction analysis), it was engaged to a greater extent by specific AM construction. Other structures that were commonly activated by both specific and general AM construction in the left hemisphere were also engaged more by specific AM construction, though in the right hemisphere, including the right frontal pole (BA 10) and the right precuneus (BA 31). Several core AM regions not evident in the conjunction analysis were also differentially recruited by specific compared to general AM construction. This included aspects of the lateral PFC (right ventrolateral PFC [BA 47], bilateral dorsolateral PFC [BA 44/45 and BA 9]), the right cingulate cortex (anterior [BA 32) and posterior [BA 31]) and several regions of the lateral temporal cortex on the left (inferior [BA 20], middle [BAs 39, 21 and 22] and superior [BA 22] temporal gyri), and the right (middle [BA 21] and superior [BA 40] temporal gyri) extending into the occipital lobe (BA 19). Of particular interest was a right-lateralized area bordering the amygdala and hippocampus in the MTL that was preferentially activated by specific AM construction (see Figure 3).
Table 3.
Regions differentially recruited during the construction of specific vs. general AMs.
| Not Controlling for Detail | Controlling for Detail | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe/Region | BA | H | Talairach Co-ordinates | k | T | Talairach Co-ordinates | k | T | ||||
| x | y | z | x | y | z | |||||||
| Specific construction > General construction | ||||||||||||
| Frontal | ||||||||||||
| Inferior frontal gyrus | 44/45 | R | 36 | 37 | 2 | 13 | 4.41 | 36 | 37 | 2 | 11 | 4.10 |
| 47 | R | 42 | 25 | −6 | 10 | 3.74 | 42 | 25 | −6 | 1 | 2.93* | |
| Middle frontal gyrus | 8/9 | L | −42 | 14 | 40 | 30 | 3.99 | −42 | 16 | 40 | 32 | 4.04 |
| Medial frontal gyrus | 10 | R | 4 | 60 | −10 | 15 | 3.54 | 4 | 60 | −10 | 18 | 3.62 |
| Medial Temporal | ||||||||||||
| Amygdala/hippocampus | R | 26 | −9 | −18 | 7 | 3.45 | 24 | −7 | −18 | 8 | 3.68 | |
| Other Temporal | ||||||||||||
| Middle temporal gyrus | 21 | R | 42 | −26 | −5 | 10 | 3.74 | 42 | −26 | −5 | 14 | 3.97 |
| 21 | L | −38 | −56 | 8 | 10 | 3.70 | −38 | −56 | 8 | 8 | 3.65 | |
| 21/22 | L | −50 | −39 | 2 | 9 | 3.63 | −50 | −37 | 2 | 6 | 3.52 | |
| 21 | L | −61 | −14 | −14 | 5 | 3.56 | −61 | −14 | −14 | 8 | 3.28* | |
| 39 | L | −53 | −70 | 29 | 14 | 3.67 | −53 | −70 | 29 | 6 | 3.45 | |
| 21 | L | −57 | −51 | −3 | 22 | 3.90 | −57 | −51 | −3 | 11 | 3.79 | |
| 19 | R | 51 | −77 | 17 | 8 | 3.66 | 53 | −73 | 17 | 16 | 4.29 | |
| Inferior temporal gyrus | 20 | L | −40 | −7 | −22 | 50 | 4.81 | −40 | −7 | −22 | 53 | 5.03 |
| Superior temporal gyrus | 22 | L | −51 | −40 | 9 | 15 | 3.50 | −50 | −37 | 2 | 6 | 3.52 |
| 40 | R | 36 | −53 | 32 | 47 | 3.70 | 34 | −45 | 37 | 41 | 3.73 | |
| Occipital | ||||||||||||
| Precuneus Parietal | 31 | R | 10 | −65 | 20 | 18 | 3.48 | 14 | −67 | 18 | 7 | 3.35 |
| Inferior parietal lobe | 40 | R | -- | -- | -- | -- | 42 | −26 | 23 | 15 | 3.76 | |
| 40 | R | -- | -- | -- | -- | 44 | −51 | 34 | 10 | 3.76 | ||
| Limbic | ||||||||||||
| Cingulate gyrus | 32 | R | 20 | 19 | 32 | 15 | 4.08 | 20 | 19 | 32 | 10 | 3.84 |
| Anterior cingulate | 32 | R | 6 | 37 | −4 | 9 | 3.42 | 6 | 37 | −4 | 10 | 3.54 |
| 31 | R | 20 | −23 | 42 | 22 | 4.04 | 20 | −23 | 42 | 14 | 3.66 | |
| Posterior cingulate | 23 | L | -- | -- | -- | -- | −4 | −57 | 18 | 9 | 3.48 | |
| Other | ||||||||||||
| Precentral gyrus | 4 | L | −38 | −13 | 49 | 5 | 3.36 | −38 | −13 | 49 | 45 | 2.87 |
| 4 | L | −55 | −10 | 26 | 5 | 3.31 | −55 | −10 | 26 | 22 | 3.45 | |
| Postcentral gyrus | 3 | L | −22 | −28 | 53 | 10 | 3.64 | −22 | −28 | 53 | 5 | 2.86* |
| Basal ganglia | R | 30 | −1 | 13 | 122 | 4.96 | 30 | 1 | 13 | 96 | 4.80 | |
|
| ||||||||||||
| General construction > Specific construction | ||||||||||||
No regions survived even a liberal threshold of p ≤ .005
Note:
p ≤ .005; BA = Brodmann Area; H = Hemisphere; k = voxel extent; T = t-score
Figure 3.

Areas differentially recruited during the construction of specific compared to general AMs. A region of the right amygdala/hippocampus (Tal: x = 26, y = −9, z = −18) was more active during specific than general AM construction. This difference did not extend to the elaboration phase.
Because specific events were also, on average, rated as more detailed than general events, we examined whether the differences revealed by the specific > general AM construction whole-brain analysis remained when the specific-general difference in detail scores for each participant were covaried out. To do this, we entered the first-level contrasts between memory specificity (i.e., specific construction > general construction) into second-level random effects Analysis of Covariance (ANCOVA). The difference between specific and general memory detail ratings was entered as a covariate for each participant. We used a statistical threshold of p ≤ .001, but did not set a cluster extent threshold for these analyses to enable us to determine which clusters were still above our voxel-wise threshold, but possibly reduced in spatial extent when controlling for detail differences. Note that we ran ANCOVAs, using the approach described here, for all contrast analyses.
The results of the ANCOVA examining specific > general AM construction while controlling for detail differences are presented in the right panel of Table 3. The pattern of our whole-brain results was largely unchanged even when controlling for detail differences, though the clustering extents for some regions (right inferior frontal gyrus [BA 47], the left middle temporal gyrus [BA 21], and regions of the left pre- and post-central gyri [BAs 4 and 3, respectively]) were reduced below our original 5 voxel cluster extent threshold. Two regions of the right inferior parietal lobe (BA 40) and one region of the left posterior cingulate gyrus (BA 23) were significantly more active for specific than general AM construction only when controlling for detail differences between specific and general memories.
Specific events were also, on average, slightly more recent than general events (see Section 2.1), and given that recency can affect MTL activity, we examined whether the differences in neural activity in MTL activity between specific and general events was accounted for by a difference in specific and general recency. To this end, we ran a linear regression, with the activation difference between specific and general events from an ROI centered on the peak voxel of the activated amygdala/hippocampal region (Tal: x = 26, y = −9, z = −18) as the independent variable, and the recency difference between specific and general events as the predictor variable. These regression analyses revealed that the recency difference did not predict specific-general activation differences in this MTL regions (R2 = .01, β = −.10, p = .63).
2.2.3. General > Specific AM Construction
In contrast to the number of regions that demonstrated greater activity for specific than general AM construction, no regions showed greater activity for general than specific AM construction, even at a more liberal threshold of p ≤ .005. There were also no suprathreshold activations when controlling for detail differences, using the ANCOVA approach described above.
2.3. Elaboration Phase fMRI Analyses
2.3.1. Conjunction Analysis
As with the construction phase, the elaboration phase of both specific and general AMs (i.e., specific AM > sentence elaboration and general AM > sentence elaboration) commonly activated several regions previously associated with AM retrieval (e.g., Svoboda et al., 2006; Cabeza & St. Jacques, 2007) (Table 4 and Fig. 4). This common activation consisted of left medial PFC (BA 10), bilateral cingulate gyrus (BA 23/24), left lateral temporal cortex (BA 21), right temporal pole (BA 38), and the left angular gyrus (BA 39). In contrast to the absence of common MTL activation in the construction phase, a region of the left hippocampus was commonly activated during the elaboration phase of both types of AMs.
Table 4.
Common activity during specific and general AM elaboration.
| Lobe/Region | BA | H | Talairach Co-ordinates | k | T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Frontal | |||||||
| Superior frontal gyrus | 10 | L | −2 | 65 | 23 | 2450 | 7.51 |
| Medial Temporal | |||||||
| Hippocampus | L | −28 | −26 | −12 | 160 | 5.68 | |
| Other Temporal | |||||||
| Middle temporal gyrus | 21 | L | −63 | −8 | −11 | 66 | 7.10 |
| Superior temporal gyrus (Temporal pole) | 38 | R | 40 | 18 | −28 | 11 | 4.04 |
| Limbic | |||||||
| Cingulate gyrus | 31 | L | −6 | −47 | 26 | 1183 | 6.86 |
| 24 | R | 2 | −14 | 39 | 39 | 5.91 | |
| Parietal | |||||||
| Angular gyrus | 39 | L | −46 | −66 | 35 | 359 | 6.14 |
Note: BA = Brodmann Area; H = Hemisphere; k = voxel extent; T = t-score
Figure 4.

Areas commonly activated during the elaboration phase of both specific and general AMs.
2.3.2. Specific > General AM Elaboration
Although a number of regions were differentially recruited during specific compared to general AM construction, during elaboration no regions survived a threshold of p ≤ .001 and a 5-voxel extent. When the threshold was reduced to p ≤ .005 and a 5-voxel extent, only two regions were revealed as more active during specific rather than general AM elaboration: the right lateral superior temporal gyrus (BA 21/22) and the right thalamus. Activity in these regions remained significant at a threshold of p ≤ .005 and a 5-voxel extent when controlling for detail differences between specific and general memories.
2.3.3. General > Specific AM Elaboration
Also in contrast to our findings during the AM construction phase, there were a number of regions preferentially recruited during general AM elaboration when compared to specific AM elaboration (Table 5 and Fig. 5). A striking number of primarily right-lateralized PFC areas were evident in general—compared to specific—AM elaboration, including in middle (BAs 8/9), medial (BA 6), superior (BA 9) and inferior (BA 44/45) frontal gyri. There was also greater activity for general AM elaboration in aspects of the left PFC, in the superior (BA 8; see Fig. 5), medial (BA 6), and middle (BA 6) frontal gyri. Finally, two clusters in the right lateral parietal cortex (BA 40) were preferentially recruited by general AM elaboration. This pattern of results was unchanged when even when controlling for detail differences between specific and general memories using ANCOVA as described in section 2.2.2 (see right panel of Table 5).
Table 5.
Regions differentially recruited during the elaboration of specific vs. general AMs.
| Not controlling for Detail | Controlling for Detail | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe/Region | BA | H | Talairach Co-ordinates | k | T | Talairach Co-ordinates | k | T | ||||
| x | y | z | x | y | z | |||||||
| Specific elaboration > General elaboration | ||||||||||||
| Other temporal | ||||||||||||
| Superior temporal gyrus | 21/22 | R | 65 | −6 | 2 | 14 | 3.07* | 65 | −6 | 2 | 1 | 3.01* |
| Other | ||||||||||||
| Thalamus | R | 14 | −29 | 7 | 8 | 3.08* | 14 | −29 | 7 | 2 | 3.02* | |
|
| ||||||||||||
| General elaboration > Specific elaboration | ||||||||||||
| Frontal | ||||||||||||
| Superior frontal gyrus | 8 | L | −6 | 35 | 44 | 11 | 4.07 | −6 | 35 | 44 | 11 | 4.01 |
| 9 | R | 40 | 36 | 29 | 15 | 3.73 | 40 | 36 | 29 | 11 | 3.67 | |
| Medial frontal gyrus | 6 | L | −16 | −9 | 48 | 31 | 3.75 | −18 | −9 | 50 | 34 | 3.96 |
| Middle frontal gyrus | 8 | R | 24 | 15 | 34 | 20 | 4.19 | 24 | 15 | 34 | 17 | 4.12 |
| 9 | R | 51 | 27 | 34 | 6 | 3.50 | 52 | 27 | 34 | 33 | ||
| 6 | L | −20 | −12 | 61 | 11 | 3.69 | −20 | −12 | 61 | 11 | 3.62 | |
| Inferior frontal gyrus | 44/45 | R | 44 | 15 | 21 | 20 | 3.69 | 44 | 15 | 20 | 19 | 3.74 |
| Parietal | ||||||||||||
| Inferior parietal lobe | 40 | R | 46 | −50 | 56 | 14 | 5.26 | 46 | −50 | 56 | 14 | 5.17 |
| 40 | R | 32 | −43 | 35 | 11 | 3.92 | 32 | −43 | 35 | 10 | 3.89 | |
| Other | ||||||||||||
| Precentral gyrus | 6 | R | 34 | −10 | 56 | 5 | 3.41 | 34 | −11 | 55 | 12 | 3.34* |
| Striatum | R | 32 | −8 | −5 | 8 | 3.95 | 32 | −8 | −5 | 8 | 3.88 | |
| L | −20 | 2 | −2 | 7 | 3.88 | −20 | 2 | −2 | 7 | 3.84 | ||
Note:
p ≤ .005; BA = Brodmann Area; H = Hemisphere; k = voxel extent; T = t-score
Figure 5.
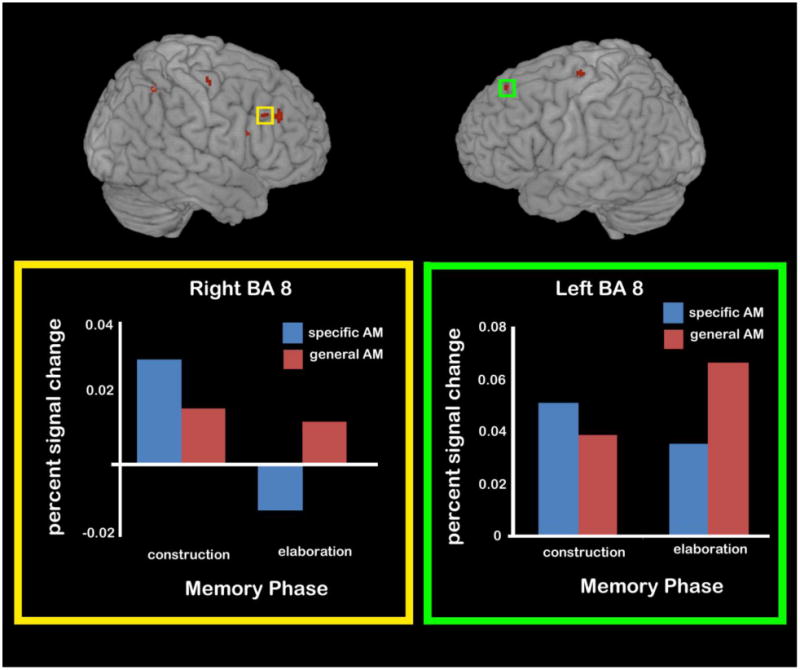
Areas differentially recruited during the elaboration phase general (vs. specific) AM elaboration. Activity during each phase of specific and general AM recall is plotted for regions of left BA 8 and right BA 8 as examples of the number of PFC regions recruited more for general AM elaboration than specific AM elaboration.
2.4. Memory Type x Memory Phase Interactions
To determine whether there were any Type (General, Specific) x Phase (Construction, Elaboration) interactions, we first performed a contrast analysis to compare: (general construction > specific elaboration) > (specific construction > general elaboration). This analysis would reveal regions that were more active during general (vs. specific) construction, during specific (vs. general) elaboration, or any combination thereof. This analysis revealed no significant activation using a threshold of p ≤ .001 and a 5-voxel cluster extent. When a more liberal threshold of p ≤ .005 was employed, two regions of the cingulate gyrus (left BA 24 and right BA 31) emerged, as did a right-lateralized region of the superior temporal gyrus (BA 22) (see left panel of Table 6).
Table 6.
Regions showing significant activations in interaction contrasts.
| Not controlling for detail | Controlling for detail | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe/Region | BA | H | Talairach Co-ordinates | k | T | Talairach Co-ordinates | k | T | ||||
| x | y | z | x | y | z | |||||||
| General construction and specific elaboration > specific construction and general elaboration | ||||||||||||
| Other temporal | ||||||||||||
| Superior temporal gyrus | 22 | R | 65 | −4 | 0 | 12 | 3.46 | -- | -- | -- | -- | -- |
| 38 | R | -- | -- | -- | 42 | 2 | −8 | 8 | 3.00 | |||
| Inferior temporal gyrus | 20 | R | -- | -- | -- | 46 | −6 | −30 | 5 | 3.32 | ||
| Limbic | ||||||||||||
| Cingulate gyrus | 24/32 | L | −14 | 8 | 40 | 6 | 3.14 | -- | -- | -- | -- | -- |
| 23/31 | R | 16 | −51 | 25 | 15 | 3.82 | -- | -- | -- | -- | -- | |
|
| ||||||||||||
| Specific construction and general elaboration > general construction and specific elaboration | ||||||||||||
| Frontal | ||||||||||||
| Middle frontal gyrus | 6/8 | R | 24 | 15 | 34 | 29 | 4.31 | 24 | 15 | 34 | 28 | 4.18 |
| 9/44 | R | -- | -- | -- | -- | -- | 40 | 17 | 19 | 6 | 3.60 | |
| Inferior frontal gyrus | 47 | R | -- | -- | -- | -- | -- | 42 | 25 | −6 | 9 | 3.59 |
| Superior frontal gyrus | 8 | R | -- | -- | -- | -- | -- | 18 | 45 | 46 | 6 | 3.97 |
| Medial frontal gyrus | 6 | L | −16 | −5 | 50 | 105 | 4.97 | −16 | −5 | 50 | 89 | 4.70 |
| Other temporal | ||||||||||||
| Middle temporal gyrus | 21 | L | −61 | −50 | 4 | 16 | 3.45 | -- | -- | -- | -- | -- |
| 21/22 | L | −53 | −43 | 4 | 5 | 3.50 | −54 | −43 | 4 | 7 | 3.57 | |
| Inferior temporal gyrus | 20 | L | −40 | −1 | −20 | 17 | 4.16 | −40 | −1 | −20 | 19 | 4.28 |
| Limbic | ||||||||||||
| Cingulate gyrus | 32 | L | −12 | 39 | 11 | 5 | 3.48 | −12 | 39 | 11 | 6 | 3.64 |
| 24/32 | R | -- | -- | -- | -- | -- | 14 | 32 | 21 | 17 | 3.91 | |
| Parietal | 7/40 | R | 34 | −43 | 37 | 18 | 3.97 | 34 | −43 | 37 | 29 | 4.45 |
| Inferior parietal lobe | 40 | R | -- | -- | -- | -- | -- | 43 | −51 | 34 | 6 | 3.79 |
| 40 | R | -- | -- | -- | -- | -- | 34 | −55 | 32 | 6 | 3.73 | |
Note: BA = Brodmann Area; H = Hemisphere; k = voxel extent; T = t-score
We also sought to determine the effect (if any) of detail differences on the interaction analyses. As with the previously reported whole-brain analyses, we included the specific – general detail difference as a covariate in an ANCOVA. In accordance with our findings when not controlling for detail, no regions survived our threshold of p ≤ .001 and a 5-voxel cluster extent. At a more liberal threshold of p ≤ .005, two regions of the right temporal lobe were revealed: the inferior temporal gyrus (BA 20) and the superior temporal gyrus (BA 38) (see right panel of Table 6).
We also performed a contrast analysis to reveal regions that showed the opposite pattern of results: (specific construction > general elaboration) > (general construction and specific elaboration). This contrast would reveal regions that showed greater activity for specific (vs. general) construction, for general (vs. specific) elaboration, or for both of those comparisons. This interaction revealed several areas (see left panel of Table 6), including a region of the right middle frontal gyrus (BA 8), three regions in the left lateral temporal lobe (BAs 20, 21, 22), the right inferior parietal lobe (BA 40), and two left-lateralized regions of the cingulate gyrus (BAs 24 and 32). Because regions revealed by this interaction analysis could show a few different patterns of response, the signal change was extracted from 8-mm spheres centered on each of these regions using the Marsbar toolbox in SPM2 (Brett et al., 2002). The signal change was averaged across the first 12 sec of the trial for construction (i.e., for the 6-sec construction phase plus the additional 6-sec to account for the delay of the hemodynamic response), and the last 12 sec of the trial for elaboration. These averages were computed for each participant and for each region and were submitted to 2 (Memory Type: General, Specific) × 2 (Phase: Construction, Elaboration) repeated-measures ANOVAs to break down the interaction effect detected in the whole-brain analysis and also to examine these regions for main effects of phase and/or specificity type3.
Post-hoc comparisons revealed that some of these interactions reflected the fact that activity between construction and elaboration decreased to a greater extent for specific than general memories. This decrease in activity across the phases was significant for specific events (in BAs 8, 20, 24, 32, 40; ps <.04), but for general events was either non-significant (BA 8, 20, 24, 32; ps > .25) or reduced relative to the effect in specific events (BA 40, specific, p < .001, general, p = .02). This decline in activity related to specific events resulted in a general>specific effect during elaboration in some of these regions (BAs 8, 24, 40; ps < .05). However, the interactions that emerged in left lateral temporal cortex (BA 21 and 22) reflected a slightly different pattern (Fig. 6), with both regions exhibiting a significant specific>general effect during construction (ps < .004). There were also changes across the phases, with specific events showing decreased activity between construction and elaboration in BA 21 (p = .03), and general events showing an increase between construction and elaboration in BA 22 (p =.01; BA 21 also showed a similar but non-significant increase). Additionally, nearly every region showed a significant main effect of phase (excluding BAs 21, 22, and 38), with memory construction yielding greater activity than memory elaboration, ps < .04. No region showed a main effect of specificity, ps > .15. The lack of a main effect of specificity in these regions, coupled with the significant interaction, indicates that in these regions the differences for specific and general AM have more to do with the timing of the activity than with the overall activity levels.
Figure 6.
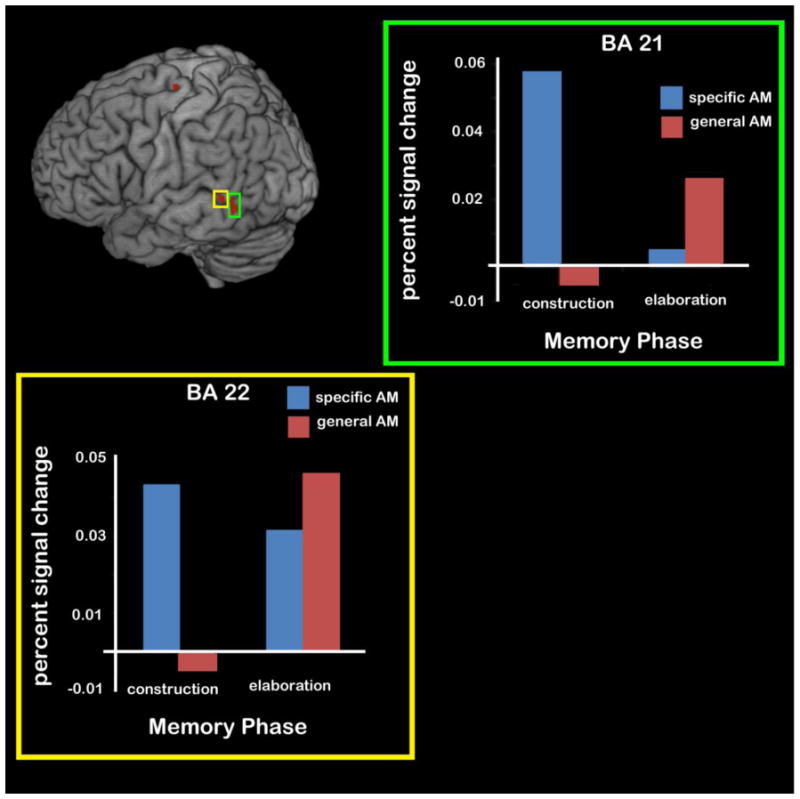
A subset of the regions revealing an interaction between Memory Type (Specific, General) and Memory Phase (Construction, Elaboration). Two regions of the left lateral temporal lobe (BAs 21 and 22) showed greater activity for specific AMs during event construction than elaboration, but greater activity for general AMs during event elaboration compared to construction.
With the exception of one region of the left lateral temporal lobe (BA 21), all of these regions remained significant in the interaction analysis when controlling for detail differences via an ANCOVA (see right panel of Table 6). Interestingly, the ANCOVA revealed several regions that were significant only when controlling for detail differences. Posthoc analyses again showed similar patterns in these regions as to those documented above, with specific events showing declines in activity from construction to elaboration (e.g., BAs 9, 39, 47, ps <.001) and general events showing increases in activity from construction to elaboration (BA 9, p = .01). Moreover, there was a specific>general effect during construction (e.g., BA 47, p =.05) and the reverse effect (general>specific) during elaboration (BA 9, 39, ps <.03). Each region exhibited a significant main effect of memory phase, with construction recruiting these regions to a greater extent than elaboration, regardless of specificity, ps < .05. There was no main effect of memory specificity in any of the regions, ps > .15.
3. Discussion
We sought to examine the neural correlates of the retrieval of specific (i.e., unique to a time and place) AMs versus those of general (i.e., either repeated over or extended in time) AMs. In particular we took advantage of the relatively protracted length of AM retrieval to tease apart the differences during the construction and elaboration phases of retrieval as individuals spontaneously generated a mix of specific and general AMs while undergoing an fMRI scan. This approach extends previous work which has only focused on the neural differences of specific and general AMs during construction or elaboration, and enabled examination of whether differences evident at construction persisted into elaboration, and vice versa. Moreover, following previous suggestions that the modulatory effect of specificity on neural activation actually reflects differences in detail (e.g., Addis, McIntosh et al., 2004; Hennessey et al., in press), we also considered the influence of this variable.
Our findings are in line with prior research suggesting that specific and general AM retrieval engages common areas associated with the standard AM retrieval network, albeit to different extents (Addis, Moscovitch, et al., 2004; Addis, McIntosh, et al., 2004; see also Levine et al., 2004; Maguire & Mummery, 1999; Maguire et al., 2000; Graham et al., 2003; Hennessey et al., in press). The present study also extends this prior research by examining whether these similarities and differences are evident in both the construction and elaboration phases. Although conjunction analyses demonstrated that both specific and general AM construction and elaboration relied on many of the regions important for AM retrieval, each phase engaged key areas to varying extents, as confirmed in the interaction results.
3.1. Regions commonly activated during specific and general event construction
Conjunction analyses revealed that the construction of both specific and general events engaged many regions associated with the standard AM retrieval network, including the frontal pole, a region thought to make contributions to the verification of both episodic and semantic memory (Moscovitch & Winocur, 2002). The construction of both types of events also engaged bilateral regions of the superior and middle temporal gyri, including the temporal pole (BA 21), a region found to be active during the retrieval of autobiographical, personal semantic, public event, and general semantic information (Maguire et al., 2000; see also Burianova, McIntosh, & Grady, 2010). The same was true for a right-lateralized region of the parahippocampal gyrus, a MTL structure previously found to be engaged for both specific and general AM retrieval (Addis, Moscovitch, et al., 2004). These frontopolar and temporal structures may generally support AM retrieval processes regardless of the specificity of the memory (Levine et al., 2004; Maguire et al., 2000; see Addis, McIntosh, et al., 2004, for similar discussion). Finally, the construction of both specific and general AMs engaged bilateral occipital regions (including left precuneus) known to be important for the visual imagery that is central to AM (e.g., Greenberg & Rubin, 2003; Fletcher et al., 1995; see review by Wagner et al., 2005). Here, we show that such regions are engaged irrespective of the specificity of the AM.
3.2. Neural differentiation of specific and general event construction
Despite these widespread commonalities, several key players in the standard AM retrieval network were notably missing from the conjunction analyses. Perhaps most striking was the absence of overlap in the lateral PFC areas thought to support AM search and retrieval processes (Cabeza & St. Jacques, 2007; Svoboda et al., 2006), as well as the absence of common activity in the hippocampus.4 Indeed, differential activity in these regions was revealed when specific AM construction was directly contrasted with general AM construction.
Specific AM construction recruited bilateral areas of the lateral PFC (BA 9, BA 47, BA 44/45) more strongly than did general AM construction. Activity in the lateral PFC, particularly left-lateralized activity, is thought to support cue specification (Gilboa, 2004; Moscovitch & Winocur, 2002; Fletcher et al., 1998; Henson et al., 1999), and may reflect implementation of the controlled memory search and retrieval processes associated with generative memory retrieval (Cabeza & St. Jacques, 2007); our results suggest these regions may be especially critical for the generation of specific AMs. In particular, activity in these regions during the early construction phase of AM might be indicative of the appropriate cue specification and cue elaboration that is necessary for the construction of specific events (e.g., Conway, 2005). Interestingly, these differences in PFC recruitment remained even when controlling for the level of detail. Thus, although PFC activity can assist in the recovery of details that lead participants to “recollect” past events or to remember the source of previously-learned information (reviewed by Mitchell & Johnson, 2009; see also Gilboa, 2004), the differential activation of the PFC during specific AM does not seem to be due only to differences connected to the level of detail remembered. Kahana and colleagues (Howard & Kahana, 2002; Polyn & Kahana, 2008) have proposed that the lateral PFC might maintain the temporal contexts needed for re-experience and may play “a crucial role in the representation and use of this temporal context in memory search” (pg. 27). Thus, it is possible that the increased PFC activity engaged here during specific (vs. general) AM retrieval could reflect the enhanced representation of temporal context maintained during the construction phase, which allowed individuals to retrieve a memory localized to a specific time frame.
In addition to differential recruitment of lateral PFC areas, specific AM construction recruited a region of the right frontal pole (BA 10) to a greater extent than general AM construction. Although its exact function is unclear, activity in the frontal pole may reflect the evaluation phase in the iterative AM construction model (e.g., Moscovitch & Melo, 1997; Gilboa, 2004). While it is possible that the greater number of details recalled during specific AM construction requires more evaluation or verification, our results suggest this is not the case; these activations were evident even when we controlled for differences in detail. Perhaps a more likely explanation is that evaluation of specific AMs in terms of their temporal order with respect to other AMs is heightened with events that have a specific temporal context (Moscovitch & Melo, 1997). Another possibility is that the early engagement of monitoring regions during event construction reflects the monitoring of search output that is necessary for further cue specification and subsequent search processes that result in event specificity. This possibility would fit with Conway’s (2005) hypothesis that a relative lack of evaluation during construction results in the iterative search process stopping at the general level of AM, before specific events are accessed.
In addition to the enhanced PFC engagement for specific vs. general AM, we found that a right-lateralized region bordering the amygdala/hippocampus was engaged disproportionately by specific AM compared to general AM construction. This differential hippocampal activity for the construction of specific versus general events dovetails with other findings of early hippocampal activity during the construction of specific AMs from generic cues (Addis et al., 2007; Daselaar et al., 2008; Hennessey et al., in press). In particular, Hennessey et al. (in press) reported differential activity during the construction of specific versus general AMs in a similar region of the anterior hippocampus, which they attributed to the increased detail comprising specific events. Critically, we found that the specific-general difference in participants’ detail ratings did not account for the increased activity during specific AM construction, suggesting that this region of the hippocampus is mediating some aspect of retrieval that is unrelated to the amount of detail per se. Because the anterior hippocampus is often associated with the binding of different types of episodic details (Davachi, 2006; Dulas & Duarte, 2011; Ranganath, 2010), one possible explanation is that specific memories place a higher burden on the integration of details from different modalities than do general memories. Additionally, the hippocampus is thought to serve as a pointer system toward the sensory and perceptual details stored in posterior association cortices (Nadel & Moscovitch, 1997). Early successful hippocampal-dependent indexing of particular memory traces (i.e., ecphory; Tulving, 1983) may be critical for the construction of specific AMs. The fact that the disproportionate recruitment of the hippocampus during the construction of specific AMs occurred when musical cues (Hennessey et al., in press) or verbal cues (current study) were presented suggests that the hippocampus may play this essential role regardless of the route through which the memory is accessed.
3.3. Regions commonly activated during specific and general event elaboration
As with event construction, the act of holding both specific and general AMs in mind and elaborating on their details revealed a number of commonalities in the frontal, temporal, and posterior cortical association regions associated with the AM retrieval network. For example, specific and general AM elaboration both recruited an area of the medial PFC (BA 10); this finding is perhaps not surprising given the medial PFC’s role in the self-referential processing and sense of reliving that defines AM irrespective of its specificity (Cabeza et al., 2004; Daselaar et al., 2008; Levine et al., 2004; Maguire & Mummery, 1999). Although activity in the MTL was missing from the conjunction analysis at construction, a cluster in the left hippocampus was commonly activated by specific and general AM elaboration. As events are being elaborated upon, the hippocampus may enable the building of the contextual scene that exists regardless of event specificity (Addis, Moscovitch, et al., 2004; see also Hassabis & Maguire, 2007; 2009, for discussion of how the hippocampus is associated with the construction of complex scenes akin to those experienced during AM recall). In other words, even though general events are by definition more generic representations than specific events, they still require a hippocampally-mediated index to recover associated memory traces of a spatial context (e.g., a general memory of going to a particular class every morning still elicits contextual details about the classroom; see Conway & Pleydell-Pearce, 2000). Similarly, the common activity evident in bilateral regions of the temporal pole (BA 21/BA 38) may reflect the retrieval of conceptual knowledge that is ubiquitous to most forms of autobiographical memories, even those specific in nature (e.g., Graham et al., 2003; Levine et al., 2002; but see Addis, McIntosh, et al., 2004).
3.4. Neural differentiation of specific and general event elaboration
In contrast to our findings during construction, differential activity during the elaboration phase of AM was primarily confined to the general > specific contrast and did not dissipate when the level of detail present in each event type was covaried out. The most striking finding was that general AM elaboration recruited primarily right-lateralized PFC regions (e.g., right superior, middle and inferior frontal gyri) to a greater extent than specific AM elaboration, as well as a few regions in left PFC. It is unclear based on these findings why the elaboration of general AMs would recruit so many prefrontal areas. One hypothesis is that activity in these regions reflects ongoing attempts to retrieve temporally specific details or events throughout the elaboration phase, and the evaluation of the output from these retrieval attempts. Consistent with this idea, many of these PFC regions have been broadly associated with memory search, control, and monitoring processes (Henson, Rugg, Shallice, & Dolan, 2000; Henson, Shallice, & Dolan, 1999; see reviews by Cabeza & St. Jacques, 2007; Svoboda et al., 2006; Maguire, 2001). It is important to keep in mind that participants in this study were not instructed to retrieve specific memories; therefore, this continued engagement of the PFC should not be tied to the particular demands of this task. Rather, the continued PFC engagement during elaboration of general (vs. specific) AM may reflect the naturally iterative nature of AM retrieval, which often only ceases once a specific memory is retrieved (Conway & Rubin, 1993; Conway & Pleydell-Pearce, 2000).
If ongoing retrieval attempts are occurring through elaboration, it might be expected that a retrieval mode needs to be maintained throughout this phase for the general AM task. Indeed, we found sustained activity in right PFC during the elaboration of general (vs. specific) AMs, in line with other studies reporting these regions during elaboration (Gilboa et al., 2004; Levine et al., 2004). Indeed, prior research linked these regions to the maintenance of a “retrieval mode” (Schacter et al., 1996; Tulving 2002; Velanova et al. 2003; Cabeza et al., 2002) and the expenditure of retrieval effort (Kapur et al. 1995;Wagner, Desmond, et al. 1998). An additional possibility is that right PFC activity reflects an increased memory monitoring demand of general compared to specific AMs during the elaboration phase (see Cabeza et al., 2002; Gilboa et al., 2004). Gilboa (2004) suggested that the right PFC activity commonly found in laboratory—but not autobiographical—memory tasks might result in the circumstance when “there is a demand for the monitoring of one’s own responses in order to guide the next response” (p. 1344). Like laboratory episodic memory tests, general AM elaboration may be associated with further memory cue specifications (and subsequent search and monitoring) to guide the selection of a specific event or specific details from the output of a set of general events. Increased post-retrieval operations, such as evaluating the contents of search and retrieval cycles (Henson et al., 2000) or monitoring the accuracy of familiar information retrieved without recollection (St. Jacques et al., 2008), may also underlie differential right PFC activity during general AM elaboration.
3.5. Interactions between memory specificity and memory phase
Interaction analyses confirmed the pattern of PFC activity that was suggested by the direct contrasts at each retrieval phases: these prefrontal regions were more active for general events during elaboration, and activity related to specific events significantly decreased between construction and elaboration. This pattern within the PFC was apparent even when detail was controlled, and in fact controlling for detail allowed us to detect significant interaction effects in three regions of the PFC that had not reached our significance threshold when detail was not considered. Because two of these three PFC regions were at similar locations to regions that had been revealed in the direct contrast analyses from a single retrieval phase (i.e., in Specific > General construction or General > Specific elaboration), it is likely that the revelation of these regions reflected the fact that controlling for detail accounted for some of the variability in the regional responses and therefore increased our sensitivity to detect interaction effects.
A slightly different interaction pattern emerged within the left lateral temporal lobe (BA 21/22): Activity was significantly higher for specific versus general AMs during construction, and activity related to general AMs increased across retrieval (i.e., activity in these regions was higher during general elaboration versus general construction). In line with the purported role of lateral temporal cortex in semantic memory tasks (e.g., Binder et al., 2009; see also Whitney et al., 2011, Martin & Chao, 2001; Cabeza & Nyberg, 2000), we suggest that during specific AM construction this activity reflects processing of the word cues and/or more efficient access to semantic information that then allowed participants to retrieve a more specific memory. During elaboration, however, lateral temporal activity may be related to a continued focus on the more semanticized information that constitutes general AMs. Indeed, activation in BA 21 was tied to the level of detail recovered, in that it was no longer significant when detail differences were covaried out.
Overall, the interaction patterns evident in both the PFC and the lateral temporal cortex suggest that these regions are more engaged during specific construction and general elaboration. These findings emphasize that much of what distinguishes specific from general AM are not the regions that are engaged but rather the timecourse of the regions.
3.6. Summary
Our findings extend prior research by revealing that the effects of specificity on memory vary depending on the phase of retrieval. During initial AM construction, PFC and MTL engagement is connected to increased memory specificity, but these connections to enhanced specificity fade during the elaboration phase. This pattern of activity suggests that purported functions of key AM regions—including temporal context maintenance, search, and retrieval functions of the PFC, and indexing and detail reconstruction functions of the MTL—may come online during the construction phase of specific AMs but may not become engaged until later in the timecourse of retrieval for general AM, perhaps in the service of iterative attempts to retrieve specific episodes or an increased retrieval monitoring demand. The fact that these results were comparable regardless of whether analyses controlled for detail suggests that factors beyond level of detail need to be considered to understand what distinguishes specific from general AM. Increased appreciation of the neural correlates of specific and general AMs will be important for understanding why general memories are characteristic of some psychiatric disorders, such as depression (Williams et al., 2007), and whether this behavior is linked to neural changes at construction or elaboration.
Supplementary Material
Highlights.
We examined neural differences between specific and general autobiographical events
Specific vs. general event construction recruits MTL and PFC regions
General vs. specific event elaboration recruits right-lateralized PFC
Differences in reported detail do not account for MTL and PFC activity
Acknowledgments
This research was supported by NIH grant MH080833 to EAK and a National Defense Science and Engineering Graduate Fellowship to ACH. We thank the anonymous reviewers for comments on an earlier version of this manuscript.
Footnotes
Note, although Addis et al. (in press) did analyze general AMs, that study was primarily focused on the differences between the construction of future events as compared to AMs; the results of those contrasts are not directly relevant here.
An alternative way to examine the differences between specific and general AMs during each phase of retrieval is to calculate the differences in reference to the sentence baseline task in the first-level contrasts (i.e., in accordance with the manner in which the conjunction analyses were conducted). We entered such contrast images into a paired-samples t-test at the second level (e.g., specific AM construction – sentence construction > general AM construction – sentence construction; general AM construction – sentence construction > specific AM construction – sentence construction) and then applied a mask of the common activity between specific and general construction to identify only those regions unique to each type of construction. Paired samples t-tests were conducted for the elaboration phase in an identical manner. The results from these analyses were largely consistent with our findings from the direct contrasts of specific and general AMs (see Supplementary Materials). In particular, bilateral regions of the lateral PFC and the MTL were differentially activated for specific (vs. general) AM construction (see Table S1). In addition, general AM elaboration was still differentiated from specific AM elaboration by widespread bilateral (and in particular right-lateralized) PFC activity (see Table S2).
Although re-computing an interaction on data from voxels selected to show an interaction is a non-independent analysis, this does not apply to running post-hoc tests to break down the interaction and examining these data for main effects (which may or may not exist in the presence of an interaction).
One possibility is that the use of the sentence control task reduced the ability to detect activation of these regions in the memory conditions, because that control task may have also recruited these regions. For example, the process of generating semantically related objects likely also engaged search and retrieval processes mediated by lateral PFC. Moreover, as participants put these objects in size order, they may have imagined the objects together in a scene, a process known to engage the hippocampus (Hassabis & Maguire, 2007; 2009). If this were the case, then any common activity in these regions present during the memory task might not have surpassed the activity levels of the sentence control task.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. Neuroimage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Cheng T, Roberts R, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. doi: 10.1002/hipo.20870. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. The content and organization of autobiographical memories. In: Neisser U, Winograd E, editors. Remembering reconsidered: Ecological and traditional approaches to the study of memory. Cambridge: Cambridge University Press; 1988. pp. 193–243. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai Japan. 2002. Jun, [Google Scholar]
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuro Image. 2010;49:865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuro Image. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques PL. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg D, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience. 2004;9:1533–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Neural bases of learning and memory: Functional neuroimaging evidence. Current Opinion in Neurology. 2000;13:415–421. doi: 10.1097/00019052-200008000-00008. [DOI] [PubMed] [Google Scholar]
- Clark JM, Paivio A. Extensions of the Paivio, Yuille, and Madigan (1968) norms. (2004) Behavior Research Methods, Instruments, & Computers. 2004;36:371–383. doi: 10.3758/bf03195584. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway MA, Rubin DC. The structure of autobiographical memory. In: Collins AF, Gathercole SE, Conway MA, Morris PE, editors. Theories of memory. Hillsdale, NJ: Erlbaum; 1993. pp. 103–138. [Google Scholar]
- Conway MA. Memory and the self. Journal of Memory and Language. 2005;53:594–628. [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE. The neuroanatomy of autobiographical memory: A slow cortical potential study of autobiographical memory retrieval. Journal of Memory and Language. 2001;45:493–524. [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE, Sharpe H. Neurophysiological correlates of memory for experienced and imagined events. Neuropsychologia. 2003;41:334–340. doi: 10.1016/s0028-3932(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Schiffman H. Frequency of episodic memories as a function of their age. Bulletin of the Psychonomic Society. 1974;4:517–518. [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinions in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Reviews of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The effects of aging on the material-independent and material-dependent neural correlates of contextual binding. Neuroimage. doi: 10.1016/j.neuroimage.2011.05.036. in press. [DOI] [PubMed] [Google Scholar]
- Fink GR, et al. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. J Neurosci. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical methods for research workers. London: Oliver & Boyd; 1950. [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye–precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RSJ, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain: A Journal of Neurology. 1998;121:1249–1256. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nature Reviews Neuroscience. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory—one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Graham KS, Lee ACH, Brett M, Patterson K. The neural basis of autobiographical and semantic memory: New evidence from three PET studies. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:234–254. doi: 10.3758/cabn.3.3.234. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rubin DC. The neuropsychology of autobiographical memory. Cortex. 2003;39:687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, et al. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Sciences. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. The construction system of the brain. Philos Trans R Soc Lond B Biol Sci. 2009;364:1263–1271. doi: 10.1098/rstb.2008.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: Dissociating right prefrontal roles in episodic retrieval. Journal of Cognitive Neuroscience. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: A functional MRI test of the monitoring hypothesis. Brain. 1999;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Hennessey Ford J, Addis DR, Giovanello KS. Differential neural activity during search of specific and general autobiographical memories elicited by musical cues. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2011.04.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Kahana MJ. A distributed representation of temporal context. J Math Psychol. 2002;46:269–299. [Google Scholar]
- Janata P. The neural architecture of music-evoked autobiographical memories. Cerebral Cortex. 2009;19:2579–3594. doi: 10.1093/cercor/bhp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: A prospective functional MRI study. Journal of Cognitive Neuroscience. 2004;16:1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay J, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124:1156–1170. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9:54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ, Büchel C. Patterns of hippocampal–cortical interaction dissociate temporal lobe memory subsystems. Hippocampus. 2000;10:475–482. doi: 10.1002/1098-1063(2000)10:4<475::AID-HIPO14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124:1156 –1170. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: Structure and processes. Current Opinion in Neurobiology. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Melo B. Strategic retrieval and the frontal lobes: Evidence from confabulation and amnesia. Neuropsychologia. 1997;35:1017–1034. doi: 10.1016/s0028-3932(97)00028-6. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. 2002. pp. 188–209. [Google Scholar]
- Moscovitch M, et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. Journal of Anatomy. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Kahana MJ. Memory search and the neural representation of context. Trends in Cognitive Sciences. 2008;12:24–29. doi: 10.1016/j.tics.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- St Jacques P, Rubin DC, LaBar KS, Cabeza R. The short and long of it: Neural correlates of temporal-order memory for autobiographical events. Journal of Cognitive Neuroscience. 2008;20:1327–1341. doi: 10.1162/jocn.2008.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proceedings of the National Academy of Sciences. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Current Opinions in Neurobiology. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: An fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford: Clarendon Press; 1983. [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. Journal of Neuroscience. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal cortex and recognition memory: Functional-MRI evidence for context-dependent retrieval processes. 1998:1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, Jeffries E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex. 2011;21:1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Dritschel BH. Categoric and extended autobiographical memories. In: Conway M, Rubin D, Spinnler H, Wagenaar WA, editors. Theoretical perspectives on autobiographical memory. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. pp. 391–412. [Google Scholar]
- Williams JMG, Barnhofer T, Crane C, Hermans D, Raes F, et al. Autobiographical memory specificity and emotional disorder. Psychological Bulletin. 2007;133:122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


