Abstract
Background
Increased NK activation has been associated with resistance to HIV-1 infection in several cohorts of HIV-1 exposed, uninfected subjects. Inheritance of protective NK receptor alleles (KIR3DS1 and KIR3DL1high) has also been observed in a subset of HIV-1 exposed, uninfected subjects. However, the exact mechanism contributing to NK activation in HIV-1 exposed, uninfected intra-venous drug users (EU-IDU) remains to be elucidated.
Objective
We investigated the role of both host genotype and pathogen-induced dendritic cell modulation of NK activation during high-risk activity in a cohort of 15 EU-IDU subjects and 15 control, uninfected donors from Philadelphia.
Design
We assessed the activation status of NK cells and Dendritic cells by flow cytometry and utilized functional assays of NK-DC cross-talk to characterize the innate immune compartment in EU-IDU subjects.
Results
As previously reported, NK cell activation (CD69) and/or degranulation (CD107a) was significantly increased in EU-IDU subjects compared to control uninfected donors (p=0.0056, n=13). Genotypic analysis indicated that the frequency of protective KIR (KIR3DS1) and HLA-Bw4*80I ligands was not enriched in our cohort of EU-IDU subjects. Rather, plasmacytoid dendritic cells (PDC) from EU-IDU exhibited heightened maturation (CD83) compared to control uninfected donors (p=0.0011, n=12). When stimulated in vitro, both PDCs and NK cells from EU-IDU subjects maintained strong effector cell function and did not exhibit signs of exhaustion.
Conclusion
Increased maturation of PDCs is associated with heightened NK activation in EU-IDU subjects suggesting that both members of the innate compartment may contribute to resistance from HIV-1 infection in EU-IDU.
Keywords: Plasmacytoid DC, NK Cells, HIV/AIDS, Exposed/Uninfected, IDU
Background
Along with CD8 “killer” T cells, Natural Killer (NK) cells function as cytolytic lymphocytes capable of seeking out and destroying virally infected target cells in the body. Unlike antigen specific T cells, NK cells use the coordinated interaction of both inhibitory and activating receptors to regulate their cytotoxic activity against target cells. NK activity is also modulated by accessory cells that can augment NK activation and killing potential. In particular, Plasmacytoid Dendritic Cells (PDC), through the secretion of Interferon-alpha (IFN-α), have been shown to be essential during the host response to viral infection [1–3]. PDCs recognize ssRNA and dsDNA pathogens through the use of their intra-cellular Toll-like Receptors, TLR7 and TLR9, and comprise the main IFN-α secreting cell type in the blood. In vitro, PDC secretion of IFN-α has been shown to be necessary for NK-mediated lysis against several virally infected target cell types including herpesvirus infected fibroblasts [4–8] and HIV-infected autologous CD4+ primary T cells [9].
Overall, several main types of NK inhibitory receptors exist that can be distinguished based on their expression pattern and ligand specificity. Of these, the Killer Inhibitory Receptors (KIRs) exhibit a restricted pattern of expression and interact with only a limited subset of MHC Class I ligands [10, 11]. Nevertheless, inheritance of specific KIR alleles has profound implications for individual susceptibility to infectious diseases [12, 13]. In HIV-1 infected subjects, the KIR family of inhibitory and activating receptors has been directly implicated in control of viral replication. Inheritance of KIR3DL1 inhibitory receptor alleles that exhibit high expression (KIR3DL1high) have been shown to be associated with delayed progression to AIDS when co-inherited with their corresponding HLA-Bw4*80I ligands [14]. Similarly, KIR3DS1, an activating allele of the same locus has likewise been associated with delayed progression to AIDS [15]. Recently, the KIR3DL1/S1 locus has also been associated with resistance to HIV-1 infection. Inheritance of protective KIR3DL1high and KIR3DS1 receptor alleles were observed to be over-represented in a high-risk cohort of HIV-1 exposed, uninfected intra-venous drug users (EU-IDU) and their sexual partners [16, 17].
Among intra-venous drug users, HIV-1 exposed, uninfected individuals have been described that remain uninfected with HIV-1 in spite of repeated high-risk behavior as defined by a history of long-term needle exchange with persons of known/unknown HIV-1 infection [18]. Previously, increased NK activation has been associated with resistance to HIV-1 infection in a cohort of intra-venous drug users from Vietnam [19]. Increased NK activation has also been associated with resistance to infection in HIV-discordant couples from Columbia and peri-natally exposed children born to HIV-1 infected mothers [20, 21]. Together with genotypic data showing an enrichment of protective NK receptor alleles in EU-IDU subjects, these results suggest that increased NK activity may be associated with protection from HIV-1 during high-risk activity. However, it remains unknown if the heightened NK activation observed in EU-IDU subjects is related to the inheritance of protective NK receptor alleles or is due to other factors. Here, we investigated the role of both host genotype and pathogen-induced dendritic cell modulation of NK activation during high–risk behavior in a cohort of EU-IDU from Philadelphia, USA.
Materials and Methods
Subject Criteria and PBMC purification
HIV-1 exposed, uninfected injection drug users (EU-IDU) were enrolled over a 3-year period from the city of Philadelphia via community-based street outreach by staff experienced in recruiting high-risk injectors. Currently, the City of Philadelphia has a rate of new infections that is estimated to be more than 5 times the national average [22, 23], and over 30% of these are attributable to injection drug use [24]. Within Philadelphia, injection drug use is clustered in specific neighborhoods previously defined as “risk pockets” [25], where drugs are sold, drugs are used, and sex is exchanged for drugs in close proximity. In agreement with a previous report [26], we estimate the HIV-1 sero-prevalence within these “risk pockets” in Philadelphia to be approximately 20% among high-risk needle sharing IDU subjects.
We utilized the “Prognostic Model for Seroconversion Among Injection Drug Users” [18] to identify high-risk HIV exposed, uninfected subjects for our study based upon their frequency of injection drug use and needle sharing behavior in areas identified as “risk pockets” containing a high prevalence of new HIV-1 infections. Subjects from known “risk pockets” were identified as EU-IDU if they reported a history of greater than 2 years of daily injection and frequent (monthly or greater) needle sharing with partners of unknown HIV status. In addition to HIV testing, all subjects were offered counseling and referral for additional services. HIV-1 sero-positive subjects were excluded from the study but received counseling and referral to care settings. Blood was then drawn from 15 HIV-1 Exposed, uninfected subjects at the Jonathan Lax Treatment Center at Philadelphia FIGHT according to Institutional Review Board approval and informed consent. As a control, blood was drawn from a panel of 15 healthy, HIV-1-seronegative donors from the greater Philadelphia area at The Wistar Institute Blood Donor Program. All blood was processed within three hours of draw and Peripheral Blood Mononuclear Cells (PBMC) were collected by standard ficoll density gradient centrifugation as previously described [9].
While all subjects were injecting heroin, 6 (40%) reported also injecting stimulants (cocaine and/or methamphetamine). EU-IDU subjects were confirmed to be negative for HIV-1 at the time of blood draw using an anti-HIV antibody serum ELISA test (BioChain Institutue, Hayward, CA) and later screened for the absence of CCR5 delta 32 homozygocity as described below. All subjects were analyzed for the presence of anti-HCV antibodies in serum with HCV antigen coated ELISA plates (Core, E2, NS3, NS4, and NS5) according to the manufacturer instructions (BioChain Institutue, Hayward, CA). In confirmation of their high-risk needle sharing behavior, greater than 70% of the EU-IDU documented within our study were HCV infected.
Allele Genotyping
2×106 PBMC from HIV-1 exposed, uninfected injection drug users and control uninfected subjects were frozen in DNAzol (Molecular Research Center, Cincinnati, OH) and genotyped at HLA Immunogenetics Lab in The National Cancer Institute. Presence or absence of each KIR gene was determined as previously described [27]. The HLA class I loci were typed by the sequence based typing method as recommended by the 13th International Histocompatibility Workshop (http://www.ihwg.org/tmanual/TMcontents.htm).
Flow Cytometry
All cell surface antibodies and isotype controls were pre-conjugated and used at the recommended dilution of 0.25 μg antibody per million cells in PBSA (PBS with 0.09% sodium azide). PBMCs were stained with antibodies to phenotypic and functional markers for 15 minutes at RT°C in the dark, washed twice and fixed with Cytofix Buffer (BD Cytometry Systems, San Jose, CA). The following antibodies and their appropriate isotype controls were used in this study: CD56 v450 (BD), CD69 FITC (BD), CD107a PE (BD), CD3 PERCP (BD), Lineage FITC (BD), CD83 PE (BD), HLA-DR PERCP (BD), BDCA-4 or BDCA-1 APC (Miletyni Biotech, Auburn, CA). For intra-cellular cytokine staining, cells were permeabilized with the Cytofix/Cytoperm kit (BD) as described by the manufacturer and stained for 15 minutes at RT°C in the dark with 0.25 μg of anti-IFN-gamma FITC antibody (BD) per million cells. A minimum of one hundred thousand events were collected on a BD LSR-II Flow Cytometer and samples were subsequently analyzed with FlowJo software (Tree Star Incorporated, Ashland OR). Prior to analysis, all samples were gated by forward and side scatter to exclude dead cells.
CD107a Degranulation Assay
1×106 PBMC were co-cultured alone (no target control) or with K562 cells at a 10:1 effector/target ratio in the presence of 20 μl anti-CD107a monoclonal antibody for 3 hours in a 200 μl volume. PBMC were then washed and stained with antibodies to NK phenotypic markers (CD56/CD3) for 15 minutes at RT°C. NK cells were gated by CD56+/CD3− staining and CD107a expression was determined based on background levels of staining exhibited by no target control cells.
PDC-stimulated NK Activation
2.5×106 PBMC were stimulated for 18 hours with 10 μg/ml CpG-ODN 2216 in a 1 ml volume. PBMCs were then washed and stained with antibodies to the NK phenotypic markers CD56, CD3, and the activation marker, CD69, for 15 minutes at RT°C. NK cells were gated by CD56+/CD3− staining and CD69 expression was determined based on isotype control.
IFN-α ELISA
Supernatants from overnight stimulation of PBMC with CpG-ODN 2216 were collected and frozen at −80°C. Supernatants were thawed and tested in duplicate for IFN-α secretion using the Verikine multi-subtype Interferon alpha ELISA kit (PBL Biomedical Laboratories, Piscataway, NJ) according to the manufacture’s instructions. The Verikine multi-subtype Interferon alpha ELISA kit detects 13 of 15 known IFN-α subtypes but does not exhibit cross reactivity with human IFN-β, human IFN-γ or human IFN-ω.
Statistical Analysis
Paired statistical analyses were performed with Prism software (GraphPad Software, La Jolla, CA) using Wilcoxon matched pair, non-parametric T tests. In all cases, p values were two-sided with significance <0.05.
Results
NK cells from EU-IDU exhibit increased activation and/or constitutive degranulation compared to controls
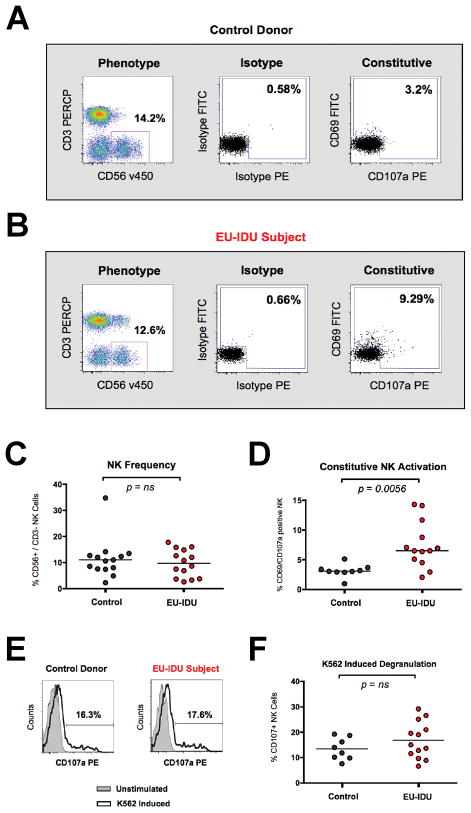
Utilizing the definition of a high-risk exposed, uninfected intravenous drug-user (EU-IDU) detailed in Materials and Methods and Table 1, we tested the phenotype and function of NK cells in a panel of 15 EU-IDU and 15 control donors from the greater Philadelphia area. As previously described [28, 29], we observed that NK cells from EU-IDU subjects constitutively exhibited an increased expression of the CD69 activation marker and/or heightened CD107a degranulation compared to control donors (Figure 1A and B). While there was no change in the overall frequency of CD56dim/CD3− NK cells in EU-IDU subjects (Figure 1C), NK cells from EU-IDU subjects exhibited a statistically significant increase (p=0.0056, n=13) in CD69 and/or CD107a expression compared to control donors (Figure 1D). Together, these results suggested a qualitative difference in the activation state of NK cells from EU-IDU.
Table 1.
Patient Characteristics
| Exposed, Uninfected Intra-venous Drug Users (EU-IDU) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject ID | Gender | Race | Needle Sharing | HIV-1 Infected | HCV Infected | Frequency of IV-drug use (last 3 months) | Frequency of Sex without a condom (last 3 months) | Number of Sexual Partners (last 3 months) | Risk Pocket |
| 1 | Male | African American | Yes | No | No | Daily | 0 | 0 | 19152 |
| 2 | Male | N/D | Yes | No | No | Daily | 1 | 4 | 19147 |
| 3 | Male | Caucasian | Yes | No | Yes | Daily | 0 | 0 | 19147 |
| 4 | Male | Caucasian | Yes | No | No | 1–6 | 0 | 1 | 19123 |
| 5 | Male | Caucasian | Yes | No | Yes | Daily | 0 | 0 | 19121 |
| 6 | Male | Caucasian | Yes | No | Yes | Daily | 0 | 0 | 19123 |
| 7 | Male | Caucasian | Yes | No | Yes | Daily | 0 | 12 | 19107 |
| 9 | Male | African American | Yes | No | Yes | 12–20 | 0 | 0 | 19121 |
| 13 | Female | Caucasian | Yes | No | Yes | Daily | 45 | 30 | 19125 |
| 14 | Male | Caucasian | Yes | No | Yes | Daily | 4 | 4 | 19152 |
| 15 | Male | Hispanic | Yes | No | Yes | 7–12 | 2 | 2 | 19133 |
| 16 | Male | Caucasian | Yes | No | No | Daily | 70 | 4 | 19124 |
| 17 | Male | Caucasian | Yes | No | Yes | Daily | 20 | 1 | 19143 |
| 18 | Male | Caucasian | Yes | No | Yes | Daily | 60 | 15 | 19105 |
Figure 1. NK cells from EU-IDU subjects exhibit increased activation and maintain normal function.
(A–B) PBMCs from one representative control donor (A) and one representative EU-IDU subject (B) were stained with fluorescently conjugated antibodies to NK phenotypic and functional markers (CD3, CD56, CD69, CD107a). NK cells were gated as CD56+/CD3− lymphocytes (first panel) and the frequency of constitutive CD107a (x-axis) and CD69 (y-axis) staining is shown compared to isotype control. (C–D) Composite graph of the frequency (C) and constitutive CD69/CD107a staining (D) on CD56+/CD3− NK cells from control uninfected donors and EU-IDU subjects as shown in panel A and B. Statistical analyses were performed using a non-parametric Mann-Whitney T-test with a two-tailed p-value. (E) PBMCs from one representative control donor and one representative EU-IDU subject were incubated for 3 hours with a fluorescently conjugated mAb to CD107a in the presence or absence of MHC-devoid K562 cells at a 10:1 effector to target cell ratio. PBMCs were then stained with fluorescently conjugated antibodies to NK phenotypic markers (CD56/CD3) and the percentage of CD56+/CD3− gated NK cells staining positive for CD107a is shown on unstimulated (light gray, shaded histogram) and K562 stimulated (bold, open histogram) PBMCs. (F) Composite graph of K562-induced CD107a staining on CD56+/CD3− gated NK cells from control uninfected donors and EU-IDU subjects as shown in panel E.
We have previously shown that NK cells from healthy uninfected donors possess the capacity to re-degranulate and lyse tumor targets repeatedly without a loss in NK function or viability [30]. Likewise, we tested if NK cells from EU-IDU subjects also maintained strong effector function despite evidence of heightened activation and recent degranulation. We incubated PBMC with K562 tumor cells to induce target cell lysis and measured CD107a degranulation on NK cells. We also stimulated PBMC with IL-12 and IL-15 to induce the production of Interferon-gamma (IFN-γ) by NK cells. As shown in Figure 1E and F, we observed that NK cells from EU-IDU possessed a normal capacity to degranulate in response to target cell stimulation and there was no statistical difference in K562 cell-induced degranulation between EU-IDU and controls. Similarly, we observed that the production of IFN-γ by NK cells following IL-12/IL-15 stimulation was similar between EU-IDU and controls (data not shown). Together, these results suggested that NK cells from EU-IDU exhibit a constitutive activation profile ex vivo, but maintain the capacity for normal function when re-stimulated in vitro.
PDC cells from EU-IDU exhibit increased maturation but maintain the functional capacity to mediate NK/DC cross-talk
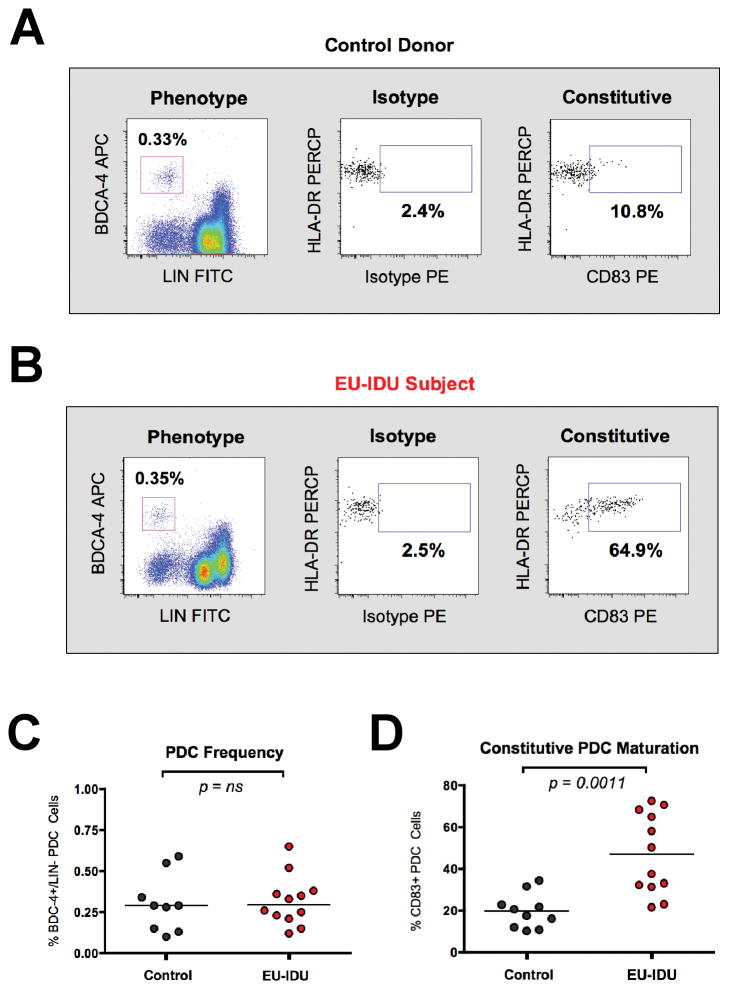
Due to the critical role that Plasmacytoid Dendritic Cells (PDC) play in augmenting NK responses, we next measured the phenotype and function of PDC in EU-IDU subjects. While there was no change in the overall frequency of PDC cells in EU-IDU subjects, we observed that PDCs from EU-IDU subjects exhibited increased constitutive expression of the maturation-specific marker CD83 [31, 32] compared to controls (Figure 2A and B). The heightened CD83 expression observed on PDC from EU-IDU was statistically significant compared to controls (p=0.0011, n=12) and was specific for the PDC subset (Figure 2D). Myeloid dendritic cells (MDCs), the other main circulating DC subset, did not exhibit heightened activation compared to controls (data not shown).
Figure 2. PDC cells from EU-IDU subjects exhibit heightened maturation.
(A–B) PBMCs from one representative control donor (A) and one representative EU-IDU subject (B) were stained with fluorescently conjugated antibodies to PDC phenotypic and functional markers (BDCA-4, Lineage, HLA-DR, CD83). PDC cells were gated as BDCA-4+/LIN− cells (first panel) and the frequency of constitutive CD83 staining (x-axis) is shown as compared to isotype control. HLA-DR staining (y-axis) is shown as an additional DC-specific marker. (C–D) Composite graph of the frequency (C) and constitutive CD83 staining (D) on BDCA-4+/LIN− PDC cells from control uninfected donors and EU-IDU subjects as shown in panel 2A and B. Statistical analyses were performed using a non-parametric Mann-Whitney T-test with a two-tailed p-value.
We next tested if PDCs from EU-IDU exhibited signs of functional exhaustion due to the state of persistent innate immune activation. We utilized the PDC-specific [33–35] Toll-Like Receptor 9 agonist, CpG-ODN 2216, and measured the ability of PDC to secrete Interferon-alpha (IFN-α) and induce NK activation as previously described [9]. Due to the documented dysfunction in PDC function observed in HCV infected subjects [36–39], we stratified EU-IDU subjects based on their HCV sero-status. As shown in Figure 3A, we observed no difference in the secretion of IFN-α between HCV-negative EU-IDU subjects and control donors following CpG-ODN 2216 stimulation. However, there was a statistically significant decrease in CpG-induced IFN-α secretion between HCV sero-positive EU-IDU subjects and control donors (p=0.0221, n=7). Nevertheless, we observed that the ability of PDC to mediate NK activation following CpG-ODN 2216 stimulation was similar between HCV sero-positive EU-IDU subjects and control donors (Figure 3B). Together, these results suggest that HCV infection negatively impacts IFN-α secretion, but PDC activation of NK cells is maintained among EU-IDU subjects allowing for sustained NK/PDC cross-talk.
Figure 3. Influence of HCV sero-status on CpG-2216 induced Interferon-alpha secretion and PDC-dependent NK cross-talk in EU-IDU subjects.
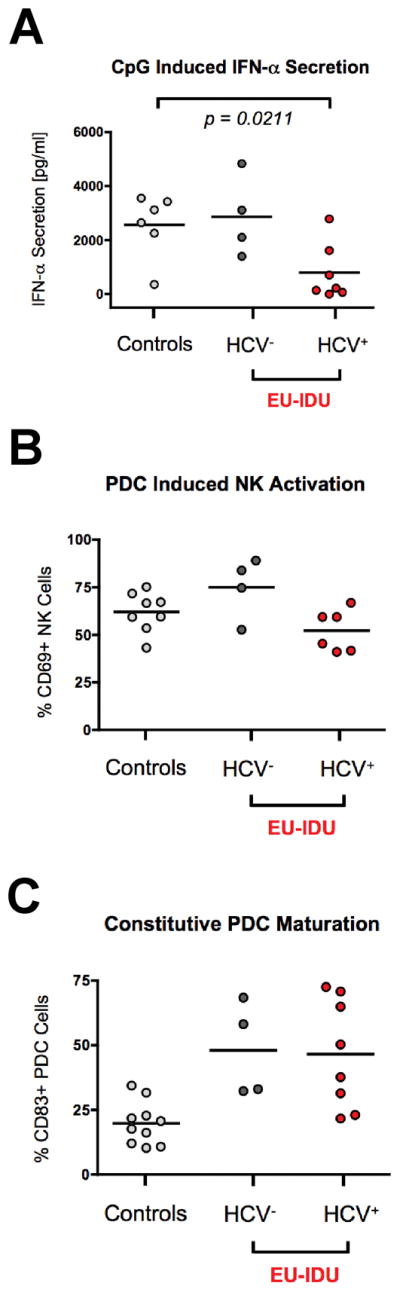
(A) PBMCs from a panel of control donors, HCV sero-negative EU-IDU subjects, and HCV sero-positive EU-IDU subjects were incubated for 18 hours in the presence of 10 μg/ml CpG-ODN 2216 and supernatants were tested for IFN-αsecretion by ELISA. (B) PBMCs from a panel of control donors, HCV sero-negative EU-IDU subjects, and HCV sero-positive EU-IDU subjects were incubated for 18 hours in the presence of 10 μg/ml CpG-ODN 2216. PBMCs were then stained with fluorescently conjugated antibodies to NK phenotypic markers and the NK activation marker CD69. The y-axis indicates the percentage of CD56+/CD3− gated NK cells staining positive for CD69 following PDC stimulation. (C) PBMCs from a panel of control donors, HCV sero-negative EU-IDU subjects, and HCV sero-positive EU-IDU subjects were stained with fluorescently conjugated antibodies to PDC phenotypic markers and the DC maturation marker, CD83. The y-axis indicates the percentage of BDCA-4+/LIN− gated PDC cells exhibiting constitutive staining for CD83. Statistical analyses were performed using a non-parametric Mann-Whitney T-test with a two-tailed p-value.
Finally, we observed that the heightened maturation status of PDC from EU-IDU was independent of HCV status. As shown in Figure 3C, PDC from both HCV sero-positive and HCV sero-negative EU-IDU subjects exhibited high levels of CD83 expression compared to control donors.
No enrichment of protective NK KIR3DS1 receptor or HLA-Bw4*80I ligands in EU-IDU subjects
The increased inheritance of the protective NK KIR3DL1high and KIR3DS1 receptor alleles has been previously observed in high-risk HIV-1 exposed, uninfected intra-venous drug users as a correlate of protection [16, 17]. We tested if inheritance of protective KIR receptor alleles and their corresponding HLA-Bw4*80I ligands were also correlated with the heightened innate immune activation phenotype we described in our cohort of EU-IDU subjects from Philadelphia. As shown in Table 2, we observed a similar frequency of the activating receptor KIR3DS1 among EU-IDU (4/14, 29%) and control uninfected donors (4/12, 33%) reflecting the fact that both groups are comprised from the same geographic area. While our primer/probe design did not allow us to be able to distinguish NK KIR3DL1high protective alleles from KIR3DL1low non-protective alleles, we tested instead for the presence of MHC-Class-I ligands of the HLA-Bw4*80I family whose inheritance is also required for protection. We observed that only 5/14 EU-IDU subjects from our cohort possessed the corresponding HLA-Bw4*80I ligands for interaction with protective KIR3DL1high alleles (Table 2). Of the five EU-IDVU subjects that possessed the corresponding HLA-Bw4*80I ligands, only one exhibited heightened NK activation (data not shown). Together, these results suggested that inheritance of protective NK receptor alleles and their corresponding MHC Class-I ligands likely did not account for the increased NK activation in the majority of EU-IDU subjects from our cohort.
Table 2.
Protective Genotypes
| EU-IDU Subjects | |||
|---|---|---|---|
| Subject ID | HLA-Bw4*80I Ligand | KIR3DS1 Receptor | CCR5 Delta 32 |
| 1 | B*5703 | −/− | WT |
| 2 | B*5301 | −/− | WT |
| 3 | −/− | −/− | WT |
| 4 | −/− | −/− | +/− |
| 5 | −/− | +/− | WT |
| 6 | B*5101 | +/− | WT |
| 7 | −/− | −/− | WT |
| 9 | B*5703 | −/− | WT |
| 13 | −/− | −/− | WT |
| 14 | −/− | −/− | WT |
| 15 | B*5701 | −/− | WT |
| 16 | −/− | +/− | WT |
| 17 | −/− | −/− | +/− |
| 18 | −/− | +/− | WT |
| Total | 36% | 29% | 0% |
| Control Uninfected Donors | |||
| Subject ID | HLA-Bw4*80I Ligand | KIR3DS1 Receptor | CCR5 Delta 32 |
| 15 | B*5301 | +/− | WT |
| 101 | B*5301 | −/− | WT |
| 218 | B*5701 | −/− | WT |
| 228 | −/− | +/− | WT |
| 258 | B*1516 | −/− | WT |
| 262 | B*1517 | −/− | WT |
| 298 | −/− | −/− | WT |
| 302 | B*5301 | −/− | WT |
| 000 | B*5802 | −/− | WT |
| 011 | B*1517 | +/− | WT |
| 295 | −/− | +/− | WT |
| 301 | −/− | −/− | WT |
| Total | 66% | 33% | 0% |
Discussion
Here we confirm previous reports of increased NK activation in exposed uninfected subjects, and show for the first time that increased PDC maturation is also a marker of the heightened innate immune activation state observed in EU-IDU subjects. Despite a state of persistent activation, we show that both PDCs and NK cells from EU-IDU maintained strong effector cell function and did not exhibit signs of exhaustion. Overall, our results highlight the role of PDCs in augmenting NK function and show how both members of the innate immune response are co-modulated during high-risk activity in EU-IDU subjects.
Based upon previous findings indicating an enrichment of protective NK KIR3DL1high and KIR3DS1 receptor alleles in high-risk HIV-1 exposed, uninfected subjects [16, 17], we correlated NK phenotype and function with genotypic analysis of KIR and their HLA-Bw4*80I ligand alleles. Our findings indicate that inheritance of protective KIR alleles or their corresponding HLA ligands was not enriched in our cohort of EU-IDU subjects compared to control uninfected donors from the same geographic area. Rather, our results indicate that increased maturation of PDCs is associated with heightened NK activation in EU-IDU subjects. However, a greater number of subjects will be required to draw firm conclusions regarding KIR allele frequency and resistance to infection at the population level in high-risk subjects.
Our observations are of interest in light of the controversial role of opioids on NK cell function where contrasting data suggests that opioids can inhibit NK activity or can lead to a “tolerant” state that is dependent on frequency of drug use and timing of NK analysis relative to drug exposure [40–42]. Based upon the frequency and duration of injection drug use among the EU-IDU subjects in our cohort (see table 1), we speculate that the innate immune response of EU-IDU subjects reaches an equilibrium state after years of prolonged injection-drug use. Indeed, work from several groups supports a loss of innate function during acute exposure to opioids or upon opioid withdrawal, rather than chronic opioid usage [43, 44]. As has been described previously for other cohorts of EU-IDU subjects [19, 28], we observed that NK cells from EU-IDU maintained strong effector cell function when stimulated in vitro (Figure 1E and F). We now extend these observations to include PDC cells from EU-IDU subjects, which remained functional in vitro (Figure 3) despite evidence of heightened PDC maturation ex vivo.
It is also important to note that while high-risk drug-use is a hallmark of the EU-IDU subjects, approximately half of the subjects from our cohort also reported a history of recent high-risk sexual activity. This included a high frequency of sexual events without the use of a condom as well as a high number of sexual partners over the previous three-month period (Table 1). Interestingly, we did not observe any correlation between the frequency of high-risk sexual practices or the number of sexual partners and the extent of innate immune activation in EU-IDU from our cohort. Future work will be needed to determine to what extent sexual exposure (if any) can enhance the observed innate immune activation phenotype among IV drug users as our data does not allow us to specifically address this point.
In addition to the influence of opioid usage on innate immune function, the covariable of HCV exposure in injection drug users proved informative in interpreting the functional data from our cohort. As described, we observed an impairment in the ability of the HCV sero-positive subjects to secrete IFN-α following CpG-ODN 2216 stimulation (Figure 3A). However, PDC-dependent NK activation was similar between HCV sero-positive and HCV sero-negative EU-IDU subjects suggesting that PDC/NK crosstalk is sustained in EU-IDU despite evidence of HCV infection (Figure 3B). Interestingly, the increase in PDC maturation we observed among EU-IDU subjects was independent of HCV status, as both HCV sero-positive and HCV sero-negative EU-IDU subjects exhibited increased CD83 expression compared to controls (Figure 3C). This finding suggests that HCV sero-positivity is not a requirement for the heightened PDC maturation observed in many EU-IDU subjects. Nevertheless, the high incidence of HCV sero-positivity in our cohort of EU-IDU (10/14, 71%) suggests that persistent exposure to HCV virus is likely encountered during prolonged IV-drug use and may contribute to PDC maturation.
Overall, our results highlight NK cells and PDCs as candidate cell types whose retained function and heightened activation status may contribute to resistance upon HIV-1 exposure.
Acknowledgments
We thank Jonathan Davis at the Jonathan Lax Treatment center at Philadelphia FIGHT for his contribution as phlebotomist specializing in drawing blood from IDU subjects. We thank Deborah Davis at The Wistar Institute for the recruitment of control donors and her contribution as phlebotomist for this study. We also thank David E. Ambrose, Daniel Hussey, and Jeffrey S. Faust at The Wistar Institute Flow Cytometry Core facility. This study was supported by grants from the National Institutes of Health (NIDA R01 DA028775, R01 AI073219, RO1 AI065279, Core grant P30 CA10815), the Philadelphia Foundation, and funds from the Pennsylvania Commonwealth Universal Research Enhancement Program.
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
The authors have no financial conflicts of interest.
Author Contributions
Costin Tomescu (Completed all of the in vitro assays, co-wrote the manuscript, completed statistical analysis), Fuh-Mei Duh (Completed all of the HLA and CCR5 genotyping), Michael Lanier (Coordinated subject recruitment, analyzed subject behavioral data), Angela Kapalko (Coordinated subject blood draws), Karam C. Mounzer (Coordinated blood draws, edited manuscript), Maureen P. Martin (Completed all of the KIR genotyping, edited the manuscript), Mary Carrington (Coordinated KIR, HLA and CCR5 genotyping, edited the manuscript), David S. Metzger (Coordinated EU-IVDU subject cohort, analyzed subject behavioral data, co-wrote the manuscript), Luis J. Montaner (Coordinated the study, analyzed all of the functional data, co-wrote the manuscript).
References
- 1.Muller-Trutwin M, Hosmalin A. Role for plasmacytoid dendritic cells in anti-HIV innate immunity. Immunol Cell Biol. 2005;83:578–583. doi: 10.1111/j.1440-1711.2005.01394.x. [DOI] [PubMed] [Google Scholar]
- 2.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med. 2005;202:425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandyopadhyay S, Perussia B, Trinchieri G, Miller DS, Starr SE. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. Journal of Experimental Medicine. 1986;164:180–195. doi: 10.1084/jem.164.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman M, Howell D, Fitzgerald-Bocarsly P. Interferon-alpha-dependent and -independent participation of accessory cells in natural killer cell-mediated lysis of HSV-1-infected fibroblasts. J Leukoc Biol. 1992;52:473–482. doi: 10.1002/jlb.52.5.473. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald-Bocarsly P, Feldman M, Curl S, Schnell J, Denny T. Positively selected Leu-11a (CD16+) cells require the presence of accessory cells or factors for the lysis of herpes simplex virus-infected fibroblasts but not herpes simplex virus-infected Raji. J Immunol. 1989;143:1318–1326. [PubMed] [Google Scholar]
- 7.Oh SH, Bandyopadhyay S, Miller DS, Starr SE. Cooperation between CD16(Leu-11b)+ NK cells and HLA-DR+ cells in natural killing of herpesvirus-infected fibroblasts. J Immunol. 1987;139:2799–2802. [PubMed] [Google Scholar]
- 8.Perussia B, Fanning V, Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985;4:120–137. [PubMed] [Google Scholar]
- 9.Tomescu C, Chehimi J, Maino VC, Montaner LJ. NK Cell Lysis of HIV-1-Infected Autologous CD4 Primary T Cells: Requirement for IFN-Mediated NK Activation by Plasmacytoid Dendritic Cells. J Immunol. 2007;179:2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- 10.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, et al. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- 11.Long EO, Burshtyn DN, Clark WP, Peruzzi M, Rajagopalan S, Rojo S, et al. Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev. 1997;155:135–144. doi: 10.1111/j.1600-065x.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 12.Boyton RJ, Altmann DM. Natural killer cells, killer immunoglobulin-like receptors and human leucocyte antigen class I in disease. Clin Exp Immunol. 2007;149:1–8. doi: 10.1111/j.1365-2249.2007.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altfeld M, Kalife ET, Qi Y, Streeck H, Lichterfeld M, Johnston MN, et al. HLA Alleles Associated with Delayed Progression to AIDS Contribute Strongly to the Initial CD8(+) T Cell Response against HIV-1. PLoS Med. 2006;3:e403. doi: 10.1371/journal.pmed.0030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 16.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, et al. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS. 2008;22:1487–1491. doi: 10.1097/QAD.0b013e3282ffde7e. [DOI] [PubMed] [Google Scholar]
- 17.Boulet S, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. Aids. 2008;22:595–599. doi: 10.1097/QAD.0b013e3282f56b23. [DOI] [PubMed] [Google Scholar]
- 18.Boileau C, Bruneau J, Al-Nachawati H, Lamothe F, Vincelette J. A prognostic model for HIV seroconversion among injection drug users as a tool for stratification in clinical trials. J Acquir Immune Defic Syndr. 2005;39:489–495. doi: 10.1097/01.qai.0000153424.56379.61. [DOI] [PubMed] [Google Scholar]
- 19.Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT, Nguyen NV, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 20.Montoya CJ, Velilla PA, Chougnet C, Landay AL, Rugeles MT. Increased IFN-gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin Immunol. 2006 doi: 10.1016/j.clim.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Tiemessen CT, Shalekoff S, Meddows-Taylor S, Schramm DB, Papathanasopoulos MA, Gray GE, et al. Cutting Edge: Unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J Immunol. 2009;182:5914–5918. doi: 10.4049/jimmunol.0900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tempalski B, Lieb S, Cleland CM, Cooper H, Brady JE, Friedman SR. HIV prevalence rates among injection drug users in 96 large US metropolitan areas, 1992–2002. J Urban Health. 2009;86:132–154. doi: 10.1007/s11524-008-9328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.City of Philadelphia Department of Public Health; Department of Public Health. Philadelphia Integrated Epidemiological Profile. Philadelphia: Office of HIV Planning; 2009. [Google Scholar]
- 25.Williams CT, Metzger DS. Race and distance effects on regular syringe exchange program use and injection risks: a geobehavioral analysis. Am J Public Health. 2010;100:1068–1074. doi: 10.2105/AJPH.2008.158337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metraux S, Metzger DS, Culhane DP. Homelessness and HIV risk behaviors among injection drug users. J Urban Health. 2004;81:618–629. doi: 10.1093/jurban/jth145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin MP, Carrington M. KIR locus polymorphisms: genotyping and disease association analysis. Methods Mol Biol. 2008;415:49–64. doi: 10.1007/978-1-59745-570-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravet S, Scott-Algara D, Bonnet E, Tran HK, Tran T, Nguyen N, et al. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–4305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- 29.Ballan WM, Vu BA, Long BR, Loo CP, Michaelsson J, Barbour JD, et al. Natural killer cells in perinatally HIV-1-infected children exhibit less degranulation compared to HIV-1-exposed uninfected children and their expression of KIR2DL3, NKG2C, and NKp46 correlates with disease severity. J Immunol. 2007;179:3362–3370. doi: 10.4049/jimmunol.179.5.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomescu C, Chehimi J, Maino VC, Montaner LJ. Retention of viability, cytotoxicity, and response to IL-2, IL-15, or IFN-{alpha} by human NK cells after CD107a degranulation. J Leukoc Biol. 2009 doi: 10.1189/jlb.1008635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, et al. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:1179–1188. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med. 2005;172:530–551. doi: 10.1164/rccm.200410-1384SO. [DOI] [PubMed] [Google Scholar]
- 33.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 34.Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170:4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 35.Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, Liu MC. TLR9- and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175:5724–5731. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 36.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123:40–49. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanto T, Inoue M, Miyazaki M, Itose I, Miyatake H, Sakakibara M, et al. Impaired function of dendritic cells circulating in patients infected with hepatitis C virus who have persistently normal alanine aminotransferase levels. Intervirology. 2006;49:58–63. doi: 10.1159/000087264. [DOI] [PubMed] [Google Scholar]
- 38.Piccioli D, Tavarini S, Nuti S, Colombatto P, Brunetto M, Bonino F, et al. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61–67. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Wertheimer AM, Bakke A, Rosen HR. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology. 2004;40:335–345. doi: 10.1002/hep.20306. [DOI] [PubMed] [Google Scholar]
- 40.Rouveix B. Opiates and immune function. Consequences on infectious diseases with special reference to AIDS. Therapie. 1992;47:503–512. [PubMed] [Google Scholar]
- 41.Ugen KE, Nyland SB. Injecting drugs of abuse and immunity: implications for HIV vaccine testing and efficacy. Springer Semin Immunopathol. 2006;28:281–287. doi: 10.1007/s00281-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 42.Zhang T, Li Y, Ho WZ. Drug abuse, innate immunity and hepatitis C virus. Rev Med Virol. 2006;16:311–327. doi: 10.1002/rmv.508. [DOI] [PubMed] [Google Scholar]
- 43.Weed MR, Carruth LM, Adams RJ, Ator NA, Hienz RD. Morphine withdrawal dramatically reduces lymphocytes in morphine-dependent macaques. J Neuroimmune Pharmacol. 2006;1:250–259. doi: 10.1007/s11481-006-9029-z. [DOI] [PubMed] [Google Scholar]
- 44.Saurer TB, Carrigan KA, Ijames SG, Lysle DT. Suppression of natural killer cell activity by morphine is mediated by the nucleus accumbens shell. J Neuroimmunol. 2006;173:3–11. doi: 10.1016/j.jneuroim.2005.11.009. [DOI] [PubMed] [Google Scholar]