Abstract
Excessive manganese (Mn) exposure increases output of glial-derived inflammatory products, which may indirectly contribute to the neurotoxic effects of this essential metal. In microglia, Mn increases hydrogen peroxide (H2O2) release and potentiates lipopolysaccharide (LPS)-induced cytokines (TNF-α, IL-6) and nitric oxide (NO). Inducible heme-oxygenase (HO-1) plays a role in the regulation of inflammation and its expression is upregulated in response to oxidative stressors, including metals and LPS. Because Mn can oxidatively affect neurons both directly and indirectly, we investigated the effect of Mn exposure on the induction of HO-1 in resting and LPS-activated microglia (N9) and dopaminergic neurons (N27). In microglia, 24 h exposure to Mn (up to 250 μM) had minimal effects on its own, but it markedly potentiated LPS (100 ng/ml)-induced HO-1protein and mRNA. Inhibition of microglial HO-1 activity with two different inhibitors indicated that HO-1 is a positive regulator of the Mn-potentiated cytokine output and a negative regulator of the Mn-induced H2O2 output. Mn enhancement of LPS-induced HO-1 does not appear to be dependent on H2O2 or NO, as Mn+LPS-induced H2O2 release was not greater than the increase induced by Mn alone and inhibition of iNOS did not change Mn potentiation of HO-1. However, because Mn exposure potentiated the LPS-induced nuclear expression of small Maf proteins, this may be one mechanism Mn uses to affect the expression of HO-1 in activated microglia. Finally, the potentiating effects of Mn on HO-1 appear to be glia-specific for Mn, LPS, or Mn+LPS did not induce HO-1 in N27 neuronal cells.
Keywords: manganese, microglia, N27 cells, heme oxygenase-1, cytokines, H2O2, nitric oxide, small Maf, Nrf2
1. Introduction
Manganese (Mn) is an essential metal, important in the metabolism of lipids, proteins, and carbohydrates (Aschner et al., 2007). However, in cases of overexposure, Mn accumulates in the brain causing a disorder termed “manganism”, which is characterized by motor and psychological abnormalities similar to the symptoms observed in Parkinson’s disease (PD) (Calne et al., 1994;Eriksson et al., 1992;Pal et al., 1999). At the neurochemical level, excessive exposure to Mn is associated with a dysregulation of cortical glutamate and midbrain dopamine (DA) and γ-amino-butyric acid (GABA) neurotransmission (Aschner et al., 2007;Dobson et al., 2004). Concerns related to adverse effects of Mn overexposure on the brain have escalated due to continued common uses of the metal including in steel manufacturing, for welding, and as an ingredient of fungicides, fertilizers, dry-cell batteries and the gasoline antiknock additive methylcyclopentadienyl manganese tricarbonyl (MMT; (Bolte et al., 2004;Bowler et al., 2007;Zayed et al., 1999).
While mechanistic studies have largely focused on direct neuronal effects of Mn exposure including the resultant oxidative stress and mitochondrial dysfunction as causative factors for Mn neurotoxicity (Gavin et al., 1992;HaMai and Bondy, 2004;Kitazawa et al., 2002;Milatovic et al., 2009;Zhang et al., 2003), mounting evidence suggests that Mn-induced, glial-derived reactive oxygen species (ROS) and inflammatory products may indirectly affect surrounding neurons (Liu et al., 2006;Perl and Olanow, 2007;Spranger et al., 1998;Zhang et al., 2010). For example, increased gliosis has been observed in brains from humans exposed to Mn and non-human primate models of manganism (Perl and Olanow, 2007). In addition, elevated astrocyte derived nitric oxide (NO) is associated with interneuron injury in striatal and pallidal regions of Mn-treated mice (Liu et al., 2006). In rats, Mn exposure causes increased striatal expression of proinflammatory chemokines (Ccl2, Cxcl2) and cytokines (TNF-α, IL-1β; (Antonini et al., 2009). In vitro, Mn potentiates glial-derived inflammatory mediators induced by the inflammagen lipopolysaccharide (LPS), including NO, cytokines (TNF-α, IL-6, IL-1β) and prostaglandins (Chang and Liu, 1999;Chen et al., 2006;Filipov et al., 2005;Liao et al., 2007;Zhang et al., 2010). Depending on the additional inflammatory stimuli’s nature and magnitude, the inflammation-enhancing effects of Mn are seen in both microglia and astrocytes. Microglia, however, appear to require minimal additional stimulation for this phenomenon to be observed. In fact, Mn on its own can induce microglial release of ROS, namely hydrogen peroxide (H2O2), which was found to precede DA cell death in mesencephalic mixed-cell cultures (Zhang et al., 2010).
As the reported evidence indicates, Mn-induced, glial-derived inflammatory mediators have the potential to harm nearby neurons, further activate surrounding glia, and be activated by neuronal-derived ROS, thereby establishing a vicious cycle that ultimately results in neuronal cell death. To protect cells from oxidatively-induced inflammation, glial and neuronal cells are equipped with a number of endogenous antioxidant defense mechanisms including glutathione (GSH), superoxide dismutase, and catalase (Dringen, 2005). However, compared to astrocytes and microglia, neurons have decreased antioxidant capacity (Halliwell, 2006) and DA neurons appear to be particularly sensitive to oxidative insults (Jenner, 1998).
Expression of inducible heme oxygenase (HO-1), an enzyme responsible for the cleavage of the oxidant heme into biliverdin, carbon monoxide (CO), and iron (Fe2+) is upregulated in response to oxidative stressors and plays a role in the regulation of inflammation (Gilroy et al., 2004;Pae and Chung, 2009). Mice deficient in HO-1, have exaggerated response to endotoxin (LPS) challenge (Poss and Tonegawa, 1997), while overexpression of HO-1 in the substantia nigra, protects DA neurons from 1-methyl-4- phenyl- 1,2,3,6-tetrahydropyridine (MPTP)-induced cell death (Hung et al., 2008). HO-1 induction is believed to be caused by increased ROS and/or reactive nitrogen species (RNS, i.e., NO), excess production of which has been found to enhance transcriptional regulation of HO-1 most often through the Nrf2-ARE pathway (Motterlini et al., 2002). Nuclear factor E2-related factor-2 (Nrf2) is a transcription factor that normally resides in the cytosol. However, upon activation by stress stimuli, cytosolic Nrf2 levels increase and translocate to the nucleus where Nrf2 binds to the antioxidant response element (ARE) inducing antioxidant response enzymes like HO-1(Kensler et al., 2007). Nrf2 binding to ARE is regulated in part by members of the Maf protooncogene family, small Maf proteins (Maf F, -G, & -K). Nrf2/Maf heterodimers form preferentially, bind to ARE, and promote transcription of ARE-responsive genes, whereas Maf homodimers can have opposite effects (Igarashi et al., 1994;Itoh et al., 1997).
Information on the expression of HO-1 in DA neurons is minimal. While HO-1 was found to be prominent in the PD brain, diseased nigral DA neurons contain the same moderate immunoreactivity for HO-1 as normal controls (Schipper et al., 2009). In addition, HO-1 was induced in striatal astrocytes and not in DA neurons in the MPTP mouse model of PD (Fernandez-Gonzalez et al., 2000). However, in vitro, the toxic metabolite of MPTP, 1-methyl-4-phenyl-pyridium (MPP+), induced a time dependent increase of HO-1 in dopamine producing pheochromocytoma (PC12) cells that was cytoprotective (Bae et al., 2010), while dieldrin, an environmental neurotoxicant linked to PD, increased HO-1 expression in SN4741 dopaminergic neuronal cells (Kim et al., 2005). Furthermore, a recent report demonstrated that high, cytotoxic concentrations of Mn (300 μM) induce HO-1 in PC12 cells via the Nrf2-ARE pathway (Li et al., 2010).
The proinflammatory stimulus LPS, used to model PD, has also been linked to the induction of HO-1 in astrocytes and microglia (Kitamura et al., 1998). Pre-treatment with LPS resulted in a time dependent increase in HO-1 in microglia that increased anti-inflammatory activity following cytotoxic challenge (IFN-γ/LPS) (Lee and Suk, 2007). Intense HO-1 immunoreactivity was also observed in surviving DA neurons, dual labeled for tyrosine hydroxylase and HO-1, following treatment with IFN-γ/LPS in midbrain slice cultures (Kurauchi et al., 2009).
At present, the effect of Mn on HO-1 induction in microglia has yet to be determined. However, because Mn can increase both neuronal oxidative stress and glial-derived ROS (Burton and Guilarte, 2009;HaMai and Bondy, 2004;Zhang et al., 2007), we hypothesized that exposure to Mn will induce HO-1 in both microglia and DA neurons. Moreover, as Mn potentiates LPS-induced, glial-derived proinflammatory mediators, we hypothesized that, at least in microglia, the LPS-induced HO-1 will be altered in the presence of Mn.
In order to identify whether HO-1 plays a role in Mn neurotoxicity, namely in the Mn-caused enhanced inflammatory mediator output by microglia, the current study aimed to (1) investigate HO-1 induction in both microglial and DA neuron cell lines following exposure to Mn, (2) determine whether LPS-induction of HO-1 is modulated by Mn, (3) identify potential mediators of such induction, and (4) evaluate the role of HO-1 in the increased glial inflammatory and oxidative output caused by Mn exposure.
2. Materials & Methods
2.1 Reagents
Unless otherwise stated, all chemicals including manganese (II) chloride solution (1 M, MnCl2), lipopolysaccharide E.coli, serotype 0111:B4, (LPS), and L-arginine methyl ester hydrochloride (L-NAME) were purchased from Sigma (St. Louis, MO). Culture media reagents including RPMI 1640 and other cell culture supplements were purchased from Invitrogen (Carlsbad, CA), while low endotoxin fetal bovine serum (FBS) was obtained from American Type Culture Collection (ATCC; Manassas, VA).
2.2 Cell Culture
The immortalized N9 murine microglia cell line used in this study was received as a gift from Dr. P. Ricciardi-Castagnoli (University of Milan, Italy). Similar to primary microglia, N9 microglia respond to inflammagen stimulation with a release of inflammatory mediators, such as the cytokines IL-1β, IL-6, and TNF-α, as well as NO (Righi et al., 1989). The immortalized rat mesencephalic cell line 1RB3AN27 (N27) was selected for its expression of dopaminergic characteristics including tyrosine hydroxylase (TH), dopamine transporter (DAT) and the dopamine metabolite homovanivllic acid (HVA) (Adams et al., 1996), as well as for the fact that it has been used extensively in investigating the impact of Mn exposure on DA neurons (Anantharam et al., 2004;Latchoumycandane et al., 2005). N9 and N27 cells were maintained (5% CO2, 95% air, at 37°C) in RPMI-1640 culture medium supplemented with 10% FBS, 1% sodium bicarbonate, 1 mM sodium pyruvate, 1 mM non-essential amino acids, 2 mM Glutamax, 50 μM 2-mercaptoethanol, 250 ng/ml fungizone, 100 U/ml penicillin, and 100 mg/ml streptomycin. N9 cells were seeded at 2.5 × 106 cells/well (5-ml volume) in 6-well plates (Costar; Fisher Scientific, Pittsburgh, PA) for western blot, qPCR, and inductively coupled plasma analysis (ICP) and 0.25 × 106 cells/well (0.5-ml volume) in 48-well plates for ELISA. For the H2O2 assay, N9 cells were seeded at 0.5 × 106 cells/well (1-ml volume) in 24-well plates and incubated overnight in complete RPMI. Before treatment, culture media was carefully removed and replaced with Hanks Buffered Salt Solution (HBSS, pH 7.4, 1 ml). N27 cells were seeded at 1.25 × 106 cells/well (5-ml volume) in 6-well plates for western blot analysis and incubated overnight to assure cell adherence before treatment.
2.3 Treatment
To identify whether Mn can induce HO-1 in microglia or neuronal cells on its own or alter LPS induction of HO-1, both N9 microglia and N27 dopamine cells were treated with vehicle (0.9% saline), MnCl2 (100 μM), LPS (100 ng/ml), or both Mn+LPS for 24 h. For the dose response analysis of HO-1 induction, microglia were exposed to Mn concentrations in the range of 1 to 250 μM for 24 h. Additionally, a time course study was conducted to determine the time dependency of the effects of Mn and LPS on HO-1 protein (1, 4, 8, and 24 h) and mRNA (1, 4, and 24h) The concentrations of Mn utilized in this study are relevant to occupational exposures (Bowler et al., 2007) and are in line with numerous other studies with neuronal and glial cells, i.e, (Milatovic et al., 2009;Zhang et al., 2009). The selected concentrations were also based on previous in vitro research from this laboratory which found that Mn up to 250 µM had no significant cytotoxicity and enhanced LPS-induced release of inflammatory cytokines (TNF-α and IL-6) and NO in N9 microglia (Filipov et al., 2005). The selected time points where chosen based on previous results from our laboratory indicating that at the message level Mn potentiation of inflammatory mediators does not occur until 4 h post exposure (Crittenden and Filipov, 2011)
In order to ascertain the role of HO-1 induction in relation to ROS and inflammatory cytokine output by microglia, HO-1 activity was blocked with either tin protoprphyrin IX dichloride (SnPP) or zinc protoporphyrin IX (ZnPP), both obtained from Tocris Bioscience (Ellisville, MO; Bae et al., 2010;Drummond and Kappas, 1981;Lakari et al., 2001). N9 cells were pre-treated for 3 h with SnPP (5 μM), ZnPP (1 μM), or vehicle (0.9% saline containing 20 μM NaOH) prior to treatment with Mn, LPS, or Mn+LPS for 1 and 6 h (H2O2 assay), 21 h (ELISA and qPCR), or 4 h (qPCR).
To determine if NO contributes to the induction of HO-1 in microglia, NO production was impeded with the NOS inhibitors, L-NAME (Rees et al., 1990) or aminoguanidine (AG, Cayman Chemical, Ann Arbor, MI; Ruetten and Thiemermann, 1996). N9 cells were pre-treated for 30 min with L-NAME (2 mM), AG (25 μM) or vehicle (0.9% saline), before treatment with Mn, LPS, or Mn+LPS for 23.5 h (western blot and ELISA).
To determine the roles of the transcription factor Nrf2 and its key regulatory molecule Maf in the Mn-potentiated expression of HO-1, N9 cells were treated with vehicle (0.9% saline), MnCl2, LPS, or Mn+LPS for 15 min, 1, or 4 h before collecting cytosol and nuclear protein fractions for subsequent analysis by western blot.
2.4 Western Blot Protein Analysis for HO-1 and iNOS
Following 24 h incubation with Mn, LPS, or Mn+LPS, plates were spun (300 × g; 5 min at 4°C), media was removed, and cells were scraped, washed and collected in PBS (1 ml). Following centrifugation (300 × g; 10 min at 4°C), the cell pellet was resuspended in lysis buffer (radioimmunoprecipitation buffer; RIPA; 100 μl) containing phenylmethylsulphonyl fluoride (PMSF; 10 μg/ml), protease inhibitor cocktail (10 μl/ml), and phosphatase inhibitor cocktail (10 μl/ml; Thermo Scientific, Rockford, IL). Protein concentration in the whole cell lysates was determined via the chromogenic method of Bradford (Bio-Rad, Richmond, CA) using a seven point linear curve and a BSA as a standard. 10% bis-acrylamide gels were loaded with 20 μg of protein per sample and, following electrophoresis, protein was transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore Corp., Billerica, MA). Membranes were blocked with 5% milk (1.5 h; RT), probed overnight at 4°C with antibodies for HO-1 (1:1000) or iNOS (1:500; Santa Cruz Biotechnologies, Temecula, CA). Following incubation with secondary antibody conjugated with HRP and chemiluminescent detection of the bands of interest, blots were stripped using gentle review buffer (Amresco, Solon, OH) and reprobed for β-actin (1:500) (Santa Cruz). Bands were analyzed using Quantity One software (Bio-Rad) and normalized to β-actin.
2.5 Western Blot: Cytosol and Nuclear Protein Analysis for Nrf2 and Maf F/G/K
After 15 min, 1, or 4 h incubation with Mn, LPS, or Mn+LPS, plates were spun (300 × g; 5 min at 4°C), media was removed, and cells were scraped, washed and collected in PBS (1 ml). Following centrifugation (300 × g; 10 min at 4°C), cells were washed again and cytosol and nuclear protein were separated using NE-PER extraction reagents (Thermo Scientific). Briefly, the cell pellet was resuspended in cold cytoplasmic extraction reagent (100 μl), centrifuged (16,000 × g; 5 min at 4°C), and supernatant (cytosol fraction) was collected leaving the pellet for further nuclear extraction. Upon addition of nuclear extraction reagent, the cells were vortexed, incubated on ice (40 min), centrifuged (16,000 × g; 5 min at 4°C), and the supernatant (nuclear fraction) collected. Protein concentration in the two fractions was determined as described in section 2.4. Cytosol (10 μg) and nuclear (5 μg) fractions were run side by side on 10% bis-acrylamide gels, and transferred to PVDF membranes. Following transfer, membranes were blocked in 5% milk (1.5 h; RT) and probed overnight at 4°C with antibodies for Nrf2 (1:300) and Maf F/G/K (1:250; Santa Cruz). Blots were stripped using gentle review buffer (Amresco) and reprobed for β-actin (1:500), the nuclear protein marker Lamin-b (1:500), and the cytosol protein marker α-tubulin (1:500; Santa Cruz). Bands were analyzed using Quantity One software (Bio-Rad).
2.6 ELISA Analysis for Secreted TNF-α and IL-6
At the end of incubation, plates were centrifuged (300 × g; 5 min at 4°C) and supernatants (cell culture media) were collected for cytokine analysis using the mouse-specific Duo set ELISA development kit for the inflammatory cytokines TNF-α and IL-6 (R&D Systems, Minneapolis, MN). Briefly, following overnight incubation with capture antibody, wells were blocked with 1% BSA. Samples were run in duplicate against a four parameter standard curve for TNF-α (2000 -31.25 pg/ml) and IL-6 (1000-15.625 pg/ml). Absorbance (450 nm analytical read; 570 nm background correction read) was measured using a Synergy 4 hybrid multi-mode microplate reader (BioTek Instruments, Winooski, VT). The mean for each sample replicate was used for statistical analysis.
2.7 mRNA Analysis for HO-1, TNF-α, and IL-6
For isolation of total RNA, all steps of the cell collection were the same as described in section 2.4 for the total protein collection except that the pellet was suspended in RLT buffer (Qiagen RNeasy Mini kit, Valencia, CA; 350 μl) supplemented with 2 mM dithiothreitol. The cell lysate was homogenized using a rotar-stator homogenizer for 15 sec. One volume of 70% ethanol was added to promote selective binding of RNA to the membrane of the RNeasy spin column (Qiagen). Following a series of washes (3X), the total RNA was eluted in nuclease free H2O. Total RNA was quantified on a NanoDrop 1000 spectrophotometer (Thermo Scientific) and converted to cDNA (250-500 ng) using qScript cDNA SuperMix (Quanta Bioscience, Gaithersburg, MD) incubated in a peltier thermal cycler (Bio-Rad; 5 min 25°C, 30 min 42°C, and 5 min 85°C). qPCR was run using Brilliant SYBR Green (Stratagene, La Jolla, CA) and certified primers for HO-1, TNF-α, IL-6, and GAPDH (SA Biosciences, Frederick, MD). Ten ng of cDNA was added to each reaction well and amplifications were performed in a Mx3005P qPCR machine (Stratagene) programmed for an initial warming (10 min 95°C) followed by 45 cycles (30 sec 95°C, 1 min 60°C) with each sample run in triplicate. The quality of products was checked by running a melting curve. The fold change was obtained using the ΔΔCT method and reported for each treatment relative to control normalized to GAPDH.
2.8 Intracellular measurement of Mn and Fe
For analysis of intracellular Mn and Fe, cells were collected as described for total protein in section 2.4. The supernatant was discarded and the pellet was washed in PBS (1 ml). The cell suspension for each sample was transferred to metal-free glass borosilicate tube, covered, and centrifuged (300 × g; 10 min at 4°C). After removing the supernatant, cells were washed 2 more times in PBS, digested in 1 ml concentrated nitric acid (2 h at 70°C) and then brought to 5 ml total volume with ddH2O. Inductively Coupled Argon Plasma-Axially Viewed Optical Emission Spectrophotometry (ICP-AVOES) was used to determine Mn and Fe concentration from single elemental standards. Samples were run on a Spectro Arcos EOP (SPECTRO Analytical Instruments Inc., Mahwah, NJ) under operating conditions consisting of plasma power 1400 W, pump speed 30 RPM, and nebulizer flow 0.92 L/min. Three replicates were run for each sample, a calibration curve was run before the analysis using concentrations of 0 to 0.01 mg/L, and quality control standards were run at the beginning, middle, and end of sample analysis. Values of Mn and Fe were reported as concentration in solution (mg/L) with a method detection limit of 0.0001 (Mn) and 0.0005 (Fe). The mean for each sample was expressed as nmol/mg protein and used for statistical analysis.
2.9 Measurement of H2O2 Release
Following 1 and 6 h incubation, plates were centrifuged (300 × g; 5 min at 4°C) and supernatants (1 ml) were collected. The fluoro H2O2 detection kit (Cell Technology Inc., Mountain View, CA) measures H2O2 via the peroxidase catalyzed oxidation of the detection reagent in a 1:1 stoichiometry to produce a fluorescent product resorufin (Zhou et al., 1997). Samples were run in triplicate against a seven point linear standard curve with H2O2 concentrations ranging between 0.156 and 2.5 μM. Fifty μl of supernatant was incubated with 50 μl of freshly prepared reaction mix for 10 min protected from light in black bottom 96 well plates (NUNC, Roskilde, Denmark). Fluorescence (Ex: 540 nm, Em: 595 nm) was measured using the Synergy 4 hybrid multi-mode microplate reader (BioTek Instruments). The mean for each sample replicate was used for statistical analysis.
2.10 Statistical Analysis
All data were subjected to analysis of variance (ANOVA) using Sigma Stat 2.03 (Systat Software, Inc., San Jose, CA). The Student-Newman-Keuls (SNK) post hoc test was used to determine differences between the means when an overall effect was detected (p < 0.05). All data are presented as mean ± S.E.M.
3. Results
3.1 HO-1 Induction
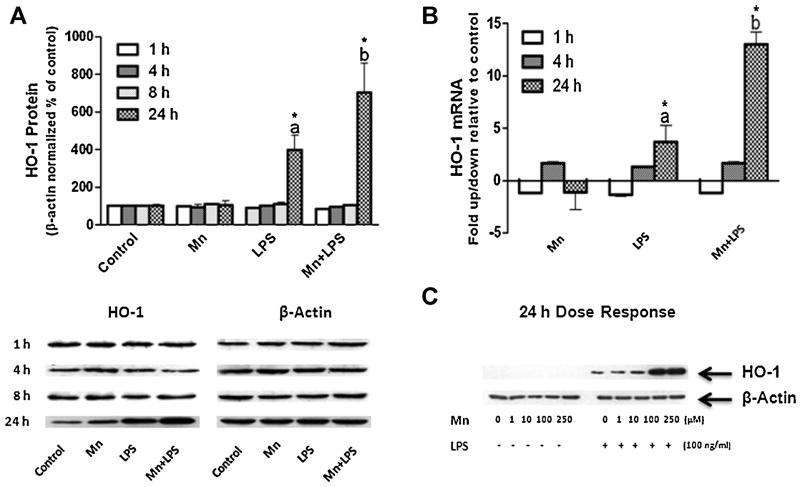
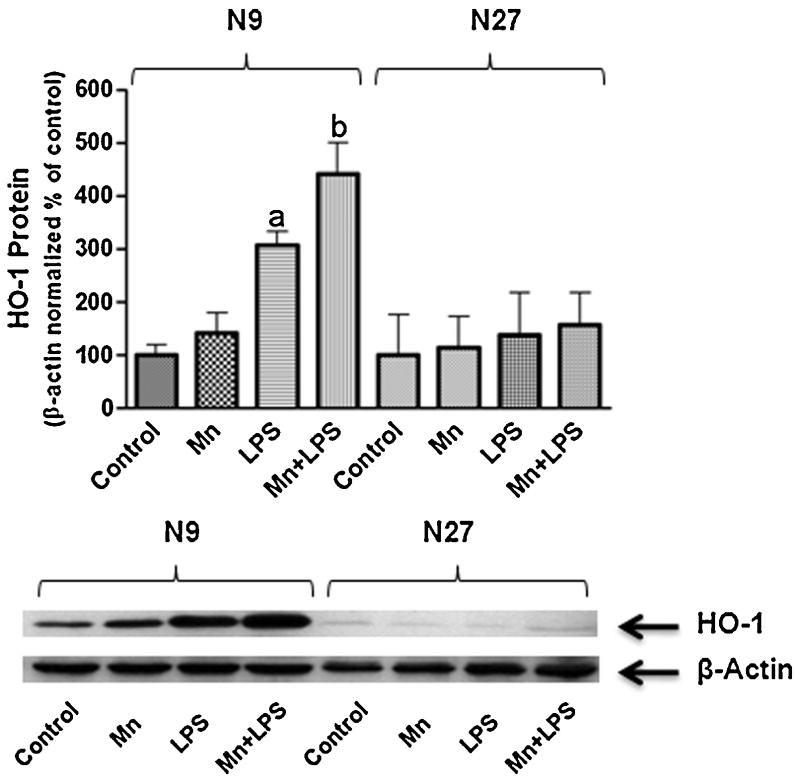
To assess whether Mn can induce HO-1 in N9 microglia, a 24 h dose response study was completed. Mn on its own up to 250 μM did not induce HO-1 protein above control levels (Fig.1C). qPCR confirmed that 100 μM Mn does not alter HO-1 at the message level up to 24 h post exposure (Fig.1B). To asses Mn potentiation of LPS induced HO-1, a 1, 4, 8, and 24 h time-course study was completed. LPS (100 ng/ml) did not increase HO-1 above control levels at 1, 4, or 8 h, while at 24 h LPS significantly increased HO-1 protein and mRNA; this effect was potentiated by Mn (100 μM) at both protein and mRNA level (Fig. 1A, B, and C). Neither Mn alone (100 μM), LPS alone (100 ng/ml), nor combined Mn+LPS induced HO-1 protein expression in the N27 dopaminergic cell line (Fig.2).
Figure 1.
Effects of Mn and/or LPS on HO-1 in microglia. (A) Quantification and representative western blots following 1, 4, 8, and 24 h exposure to vehicle, 100 μM Mn, 100 ng/ml LPS, and combined Mn+LPS. Densitometric data were β-Actin-normalized and are presented as percent of control. (B) Effects on HO-1 mRNA 1, 4, and 24 h post exposure to Mn (100 μM), LPS (100 ng/ml) and combined Mn+LPS. mRNA data are presented as fold change relative to control. (C) Representative western blot for a dose-response to Mn (1-250 μM) in the absence or presence of LPS (100 ng/ml) after 24 h exposure. Data were analyzed with ANOVA and means were separated using SNK post hoc test. a,b Letters denote significant differences (p ≤ 0.05).
Figure 2.
Comparison of the effects of Mn and/or LPS on HO-1 in N9 microglia versus N27 neuronal cells. Quantification and representative western blots following 24 h exposure to vehicle, 100 μM Mn, 100 ng/ml LPS, and combined Mn+LPS. Densitometric data were β-Actin-normalized and are presented as percent of control. a,b Letters indicate significant differences (p ≤ 0.05).
3.2 Intracellular Mn and Fe
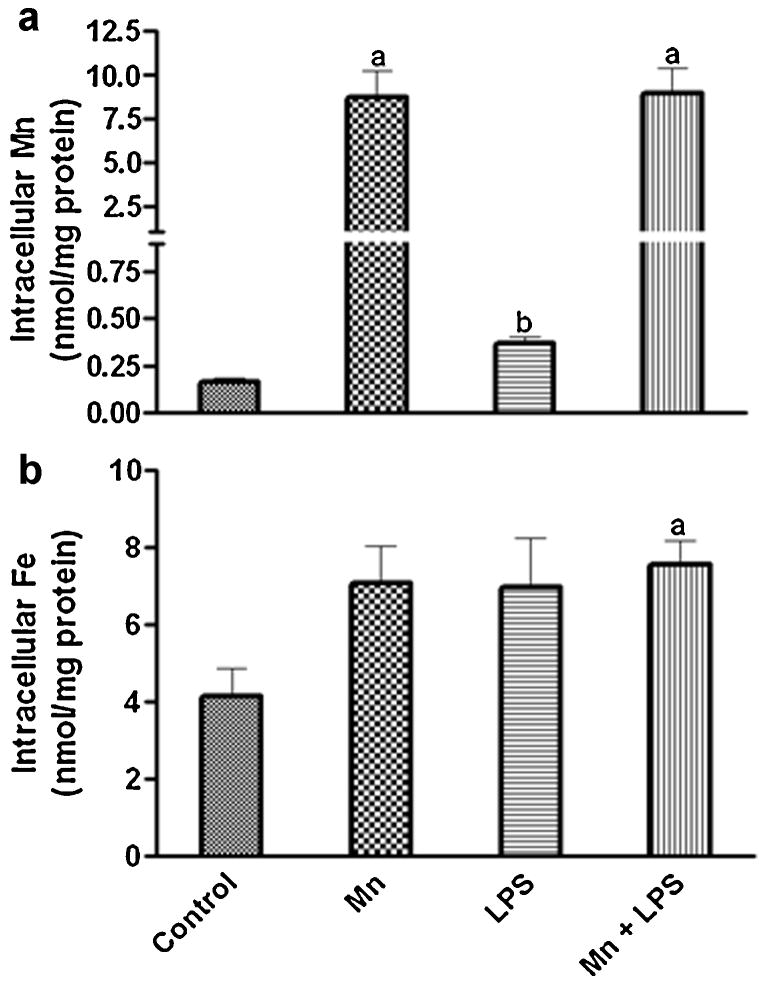
To determine whether the Mn enhancement of LPS-induced HO-1 in N9 microglia was caused by increased metal accumulation inside the cells, we measured the intracellular levels of Mn and Fe following 24 h Mn+LPS exposure. Intracellular Mn increased markedly in the Mn and Mn+LPS groups. However, the two groups were not different from each other (Fig. 3A). There was also a small but significant increase in intracellular Mn (2-fold) following exposure to LPS. In regards to intracellular Fe, while there was a moderate trend toward an increase in all three treatment groups, only the increase in the Mn+LPS treatment was significant (1.8-fold; Fig. 3B).
Figure 3.
Effects of Mn and/or LPS on intracellular Mn (A) and Fe (B) following 24 h exposure to Mn (100 μM), LPS (100 ng/ml) and combined Mn+LPS in microglia. a,b Letters indicate significant differences (p ≤ 0.05).
3.3 Cytokines and iNOS
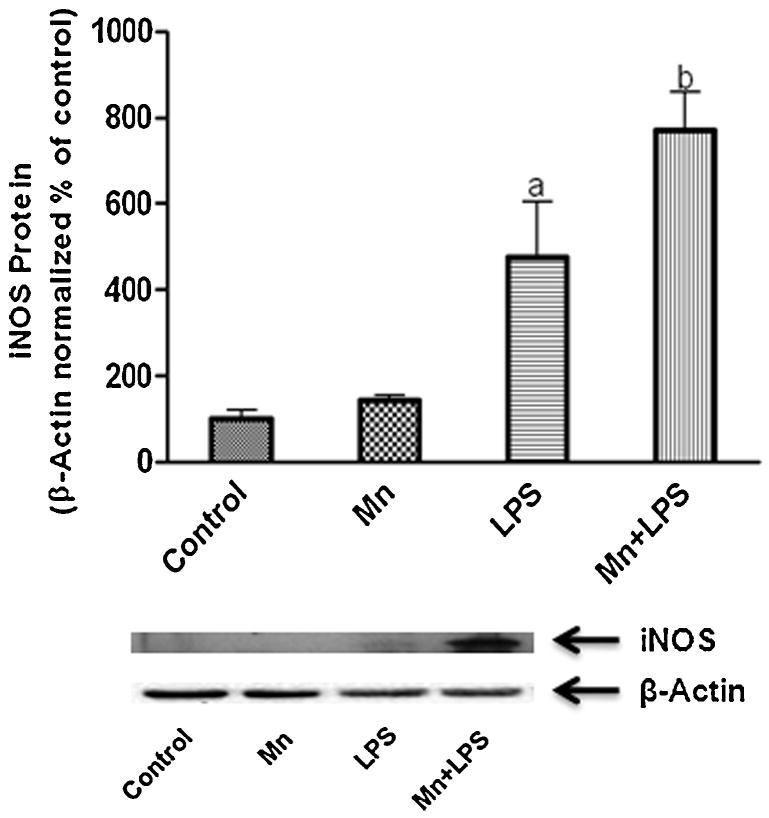
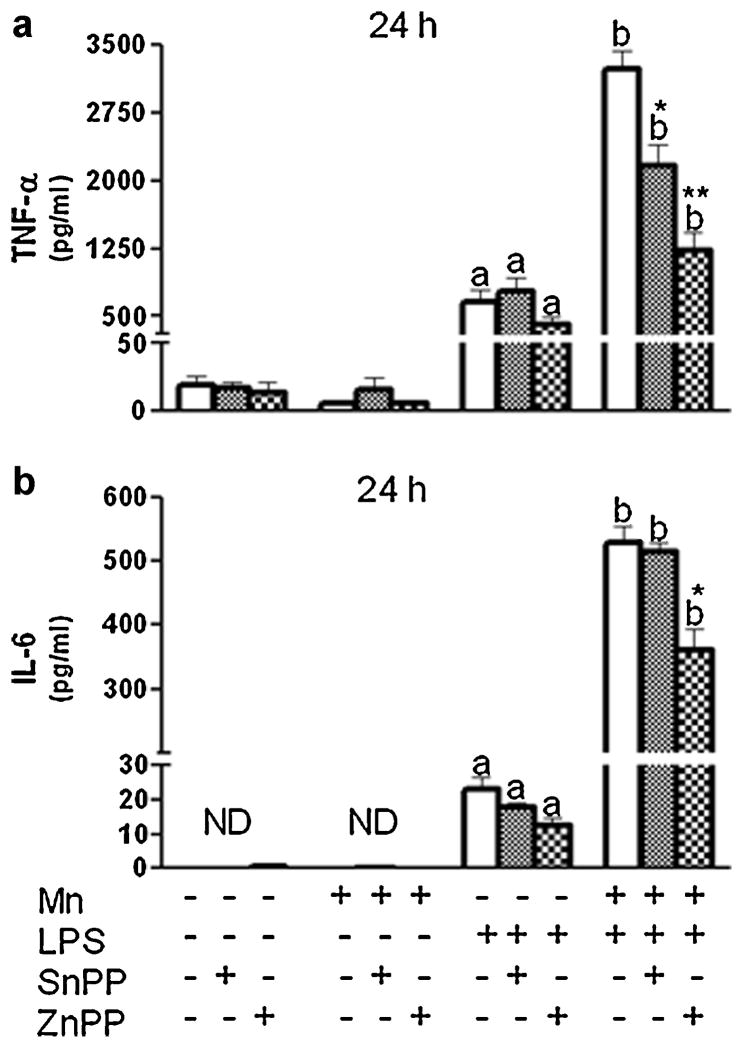
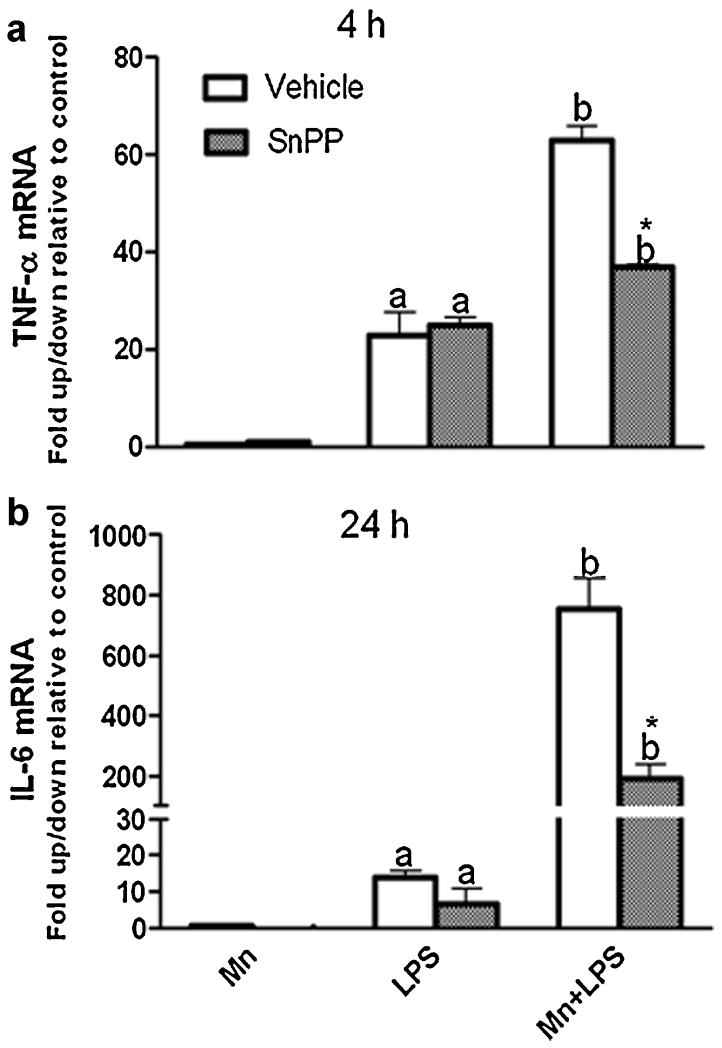
Because we were interested in the function of HO-1 within the context of inflammation, we focused on the production of inflammatory mediators after 1, 4, or 24 h exposure to Mn+LPS. In the absence of HO-1 inhibitor, at 24 h, as previously reported (Chang and Liu, 1999;Chen et al., 2006;Filipov et al., 2005), Mn potentiated LPS induction of HO-1 was accompanied by an increase in protein expression for iNOS (Fig. 4), and the inflammatory cytokines TNF-α and IL-6 (Fig. 5). Interestingly, pretreatment (3 h) with either HO-1 inhibitor (SnPP; 5 µM or ZnPP; 1 µM) significantly altered Mn potentiation of LPS-induced TNF-α, while only HO-1 inhibition with ZnPP produced a significant reduction in IL-6 cytokine microglial output (Fig. 5). Furthermore, at the message level, HO-1 inhibition significantly decreased Mn potentiation of cytokines in a time dependent manner, i.e., at 4 h for TNF-α and at 24 h for IL-6 (Fig. 6).
Figure 4.
Effects of Mn and/or LPS on iNOS in microglia. Quantification and representative western blots following 24 h exposure to vehicle, 100 μM Mn, 100 ng/ml LPS, and combined Mn+LPS are presented. Densitometric data were β-Actin-normalized and are presented as percent of control. a,b Letters indicate significant differences (p ≤ 0.05).
Figure 5.
Effect of HO-1 inhibition on microglial TNF-α (A) and IL-6 (B) cytokine production (24 h). N9 microglia were pretreated for 3 h with vehicle (white bars) or HO-1 inhibitor (SnPP, 5 µM, small checkered bars; ZnPP, 1 µM, large checkered bars), followed by 24-h exposure to Mn (100 µM), LPS (100 ng/ml), or combined Mn+LPS. Supernatant TNF-α and IL-6 concentrations were determined by ELISA. a,b Letters indicate significant effects of Mn/LPS; *,** Indicate significant effect of HO-1 inhibitor within Mn/LPS (p ≤ 0.05).
Figure 6.
Effect of HO-1 inhibtion on microglia TNF-α (A) and IL-6 (B) mRNA. N9 microglia were pretreated for 3 h with vehicle (white bars) or HO-1 inhibitor (SnPP, 5 µM, small checkered bars), followed by 4 h (A) and 24 h (B) exposure to Mn (100 μM), LPS (100 ng/ml), or combined Mn+LPS. mRNA data are presented as fold change relative to control. a,b Letters indicate significant effects of Mn/LPS; * Indicates significant effect of HO-1 inhibitor within Mn/LPS (p ≤ 0.05).
3.4 H2O2 Release
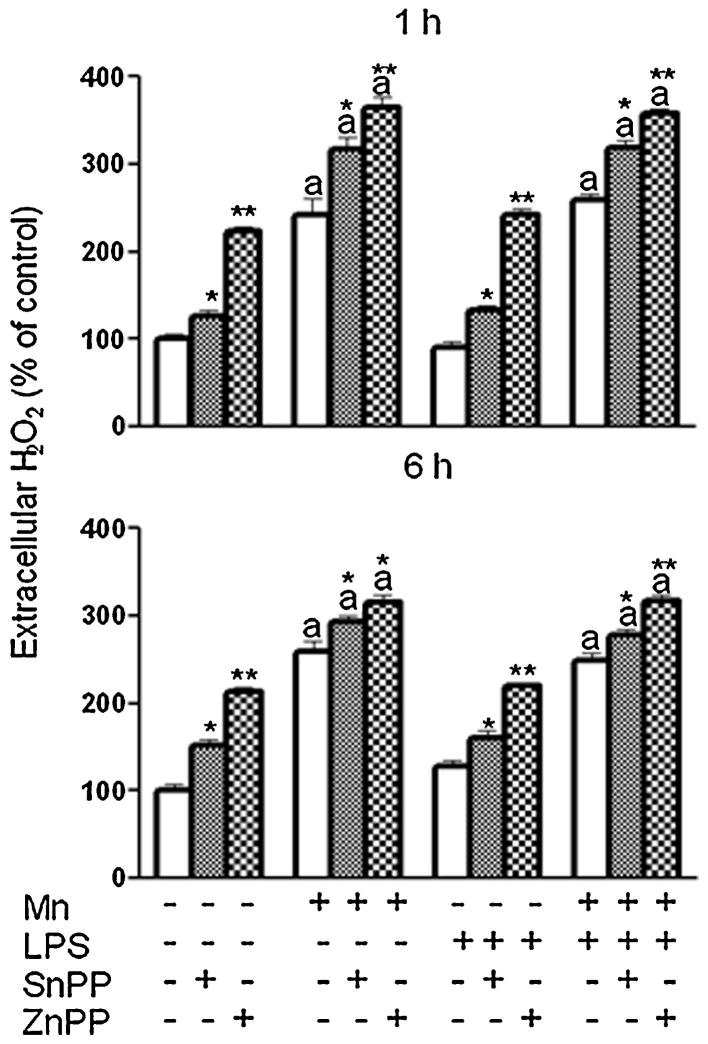
As a potent ROS, H2O2 is a possible inducer of HO-1(Ferrandiz and Devesa, 2008). Because it has been reported to be increased in microglia exposed to Mn (Zhang et al., 2007), we looked at the role of H2O2 in the Mn potentiated induction of HO-1 and at its regulation by HO-1. Just as reported for primary microglia (Zhang et al., 2007), Mn exposure increased H2O2 (1 and 6 h post treatment) in N9 microglia (Fig. 7). However, this effect was not potentiated by the dose of LPS used in this study. More importantly, the presence of either HO-1 inhibitor further increased H2O2 output by microglia exposed to Mn, as well as from control and LPS exposed microglia, with ZnPP, at the concentration administered, being more potent than SnPP (Fig. 7).
Figure 7.
Effect of HO-1 inhibition on microglial H2O2 release. N9 microglia were pretreated for 3 h with vehicle (white bars) or HO-1 inhibitor (SnPP, 5 μM, small checkered bars; ZnPP, 1 μM, large checkered bars), followed by 1 h or 6 h exposure to Mn (100 μM), LPS (100 ng/ml), or combined Mn+LPS. a,b Letters indicate significant effects of Mn/LPS; *,** Indicate significant effect of HO-1 inhibitor within Mn/LPS (p ≤ 0.05).
3.5 NO Inhibition
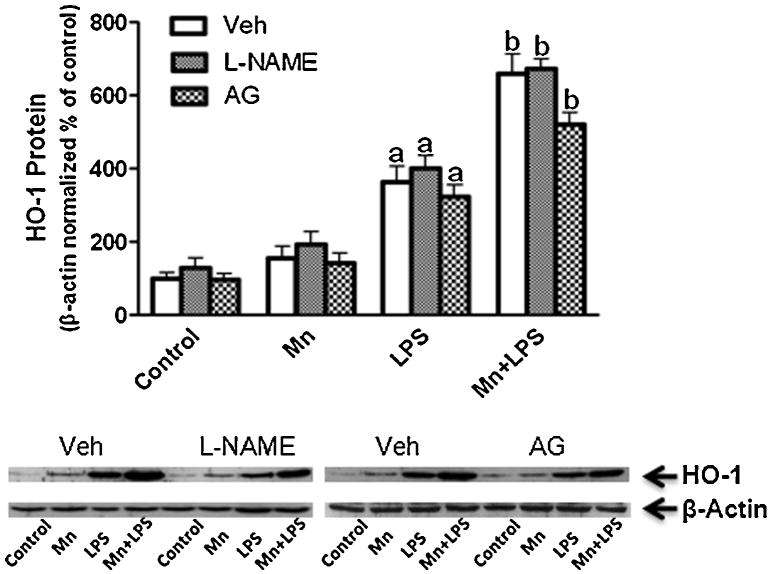
As a reactive species, NO is also a possible inducer of HO-1(Motterlini et al., 2002;Naughton et al., 2002). Because the LPS-induced increase in iNOS protein was potentiated by Mn (our study; Barhoumi et al., 2004;Chang and Liu, 1999;Chen et al., 2006;Filipov et al., 2005), we focused on the role of NO in the Mn-caused enhancement of HO-1. Specifically, we examined HO-1 induction following NO inhibition with the NOS inhibitor L-NAME (2 mM) and the selective iNOS inhibitor aminoguanidine (AG; 25 μM). Following pretreatment (30 min) with either L-NAME or AG, the Mn potentiating effect on LPS-induced HO-1 protein remained unchanged (Fig. 8). In addition, NO inhibition resulted in a small but significant decrease in cytokine output (data not shown).
Figure 8.
Effect of iNOS inhibition on HO-1 protein expression. N9 microglia were pretreated for 30 min with vehicle (white bars), L-NAME (2 mM, small checkered bars), or AG (25 μM, large checkered bars), followed by Mn (100 μM), LPS (100 ng/ml), or combined Mn+LPS for 24 h. Quantification and representative western blots are presented. Densitometric data were β-Actin-normalized and are presented as percent of control. a,b Letters indicate significant effects of Mn/LPS (p ≤ 0.05).
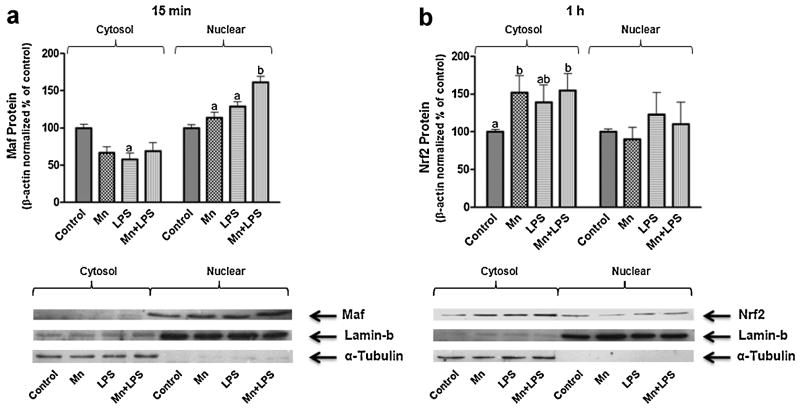
3.6 Nrf2 and Small Maf Proteins
Because the transcription factor Nrf2 has been linked to LPS-induced increased HO-1 expression in macrophages (Ashino et al., 2008;Rushworth et al., 2005) and the small Maf proteins regulate Nrf2 binding to ARE (Itoh et al., 1997), we studied the cytosolic and nuclear expression of Nrf2 and Maf F/G/K in microglial cells following 15 min, 1, and 4 h exposure to Mn, LPS, or Mn+LPS. After 15 min, nuclear small Maf protein expression increased by Mn and LPS, but the increase in the nuclei of cells exposed to Mn+LPS was greater than the increases caused by Mn or LPS (Fig. 9A). Apparently, this was a rapid and a transient effect as at later time points small Maf nuclear protein expression was no longer increased by the presence of Mn, LPS, or Mn+LPS (data not shown). Nrf2 protein expression (cytosolic or nuclear) did not change following 15 min exposure (data not shown), while at 1(Fig. 9B) and 4 h (data not shown) cytosolic Nrf2 expression was increased by Mn, LPS, and Mn+LPS to a similar extent. Additionally, the expression of Nrf2 in the nuclear fraction was not different from control for any treatment.
Figure 9.
Effects of Mn and/or LPS on small Maf (A) and Nrf2 (B) protein expression in the cytosol and nucleus of microglia following 15 min (A) and 1 h (B) exposure to Mn (100 μM), LPS (100 ng/ml) and combined Mn+LPS. Quantification and representative western blots are presented. Densitometric data were β-Actin-normalized and are presented as percent of control. Lamin-b and α-tubulin represent nuclear fraction and cytosolic fraction control, respectively. a,b Letters indicate significant effects of Mn/LPS (p ≤ 0.05).
4. Discussion
The major findings of our current study are that (1) Mn up to 250 μM does not induce HO-1 on its own, but it does potentiate LPS-induction of HO-1 in microglia cells, (2) HO-1 activity appears to be important in regulating Mn-induced H2O2 release, (3) increased HO-1 plays a role in the enhancement of inflammatory mediator output, particularly TNF-α, in Mn+LPS-exposed microglia as inhibition of HO-1 activity resulted in decreased inflammatory cytokines,(4) NO is not the key regulator of HO-1 induction by Mn+LPS, as iNOS inhibition did not significantly alter Mn-potentiated HO-1 induction, (5) small Maf proteins appear to be transcriptional regulators targeted by Mn for potentiation of HO-1, and (6) the increased HO-1 by Mn+LPS appears to be glia-specific as, neither Mn, LPS, or combined Mn+LPS induced HO-1 in N27 dopaminergic neurons.
Mn neurotoxicity has been linked to increased oxidative stress and mitochondrial dysfunction, namely in midbrain neurons (Aschner et al., 2007). Mn may also indirectly cause neuronal degeneration because it can affect glial cells, astrocytes and microglia in particular, thereby increasing the release of RNS, ROS, prostaglandins, and inflammatory cytokines from activated glia (Chang and Liu, 1999;Filipov et al., 2005;Liao et al., 2007;Spranger et al., 1998;Zhang et al., 2007). Excess ROS and RNS can further contribute to increased glial cell activation triggering transcriptional regulation of glial-derived inflammatory products (Block et al., 2007). The extent of cellular damage induced by oxidative stressors may be determined not only by their induction of proxidants and ensuing inflammation, but also by their ability to trigger antioxidant enzymes. HO-1 is an enzyme with anti-inflammatory properties that is up-regulated in response to various forms of inflammatory stimuli, including LPS (Lee and Suk, 2007;Takahashi et al., 2007). Cells lacking HO-1 have increased sensitivity to oxidatively-induced cytotoxicity (Poss and Tonegawa, 1997).
This study reports the first look at the role of HO-1 in Mn neurotoxicity. Because Mn can induce ROS and potentiate RNS (Chang and Liu, 1999;HaMai and Bondy, 2004;Zhang et al., 2007), both potential triggers of HO-1 (Ferrandiz and Devesa, 2008), we predicted that Mn alone would induce HO-1 in N27 dopaminergic neurons and N9 microglia. Our results indicate that 24 h Mn exposure is not a sufficient stimulus on its own for HO-1 induction in microglia (up to 250 μM, Fig. 1C) or DA neuronal cells (up to 100 μM, Fig. 2). Side by side comparison of neuronal cells versus microglia (Fig. 2) clearly shows that HO-1 under basal conditions is not highly expressed in N27 DA neurons when compared to N9 microglia. Moreover, no stimulus tested in the N27 cells (Mn, LPS, or Mn+LPS) was capable of increasing HO-1 levels. LPS stimulates immune cells through toll-like receptor 4 (TLR4). Because neurons do not typically express, TLRs, we would not expect LPS to activate neurons on its own. However, combined treatment of neuronal-like PC12 cells with TNF-α and LPS induced iNOS and resulted in NO-mediated cell death (Heneka et al., 1998). Importantly, midbrain slices treated with the cytokine IFN-γ followed by LPS, had increased HO-1 immunoreactivity in remaining DA neurons (Lee and Suk, 2007). Thus, it seems that the initial priming of neurons with cytokine stimuli may be responsible for neuronal response to LPS. Unlike microglia, the resident macrophages of the brain, DA neurons are not capable of releasing inflammatory cytokines. Because Mn potentiates LPS-induced cytokine production by microglia cells (Filipov et al., 2005), it is conceivable that coculture of DA neurons and microglia in the presence of Mn and LPS might result in HO-1 induction in DA neurons, which is what probably would occur in vivo as well.
Because the Mn-potentiated induction of HO-1 in N9 microglia was accompanied by an increase in inflammatory mediators, namely TNF-α, IL-6, H2O2 and NO, as well as by a small but significant increase in intracellular Fe, there was a possibility that in the presence of Mn, HO-1 acts as a pro-oxidant and is involved in the enhanced cytokine production by Mn-exposed activated microglia that has been reported previously (Crittenden and Filipov, 2008;Filipov et al., 2005). Fe is a product of heme degradation, thus the increased presence of HO-1 observed with Mn+LPS could result in the increased presence of its products. In a free non-bound form, Fe is highly reactive with H2O2 and can lead to the formation of hydroxyl radicals (OH-), which could enhance inflammation. Moreover, overexpression of HO-1 in dopaminergic cells (MN9D) was found to increase both intracellular Fe and ROS following exposure to polychlorinated biphenyls (PCB), while inhibition of HO-1 prevented PCB-induced ROS and cell death (Lee et al., 2006). In addition, suppressing the expression of HO-1 with nimperidine decreased hippocampal Fe and ROS following exposure to aluminum (Yuan et al., 2008). In our study, inhibition of HO-1 activity curbed the release of Mn-potentiated inflammatory cytokines by LPS-activated microglia (Fig 5). Thus, at least in microglia, the increased HO-1 in Mn+LPS exposed cells serves to enhance the production and release proinflammatory cytokines, namely TNF-α. Similarly, Kampfer and colleagues found that inhibition of HO-1 activity in LPS/IFN-γ stimulated RAW 264.7 macrophages resulted in reduced TNF-α output (Kampfer et al., 2001).While the exact proinflammatory mechanism of HO-1 remains elusive, it could be related to products of heme degradation, including Fe (as previously discussed) or CO, which has properties similar to NO. More than 80% of the endogenous CO results from heme metabolism by HO-1 (Ryter et al., 2006), and while some data suggests direct application of CO to LPS-challenged RAW 264.7 macrophages inhibits the release of TNF-α and IL-1β (Otterbein et al., 2000) others suggest, CO can stimulate cyclic 3’, 5’-monophosphate (cGMP) an important messenger in the pathway to the production of TNF-α (Tamion et al., 1999). Additionally, because Mn induces microglia release of ROS (H2O2) it is feasible that moderate excess Fe produced as a consequence of increased HO-1 enzyme activity could lead to the formation of free radicals (OH-) ultimately enhancing the production of inflammatory cytokines. As the Mn-induced microglial H2O2 release was further increased following inhibition of HO-1 activity in our study (Fig. 7), the possibility of this very mechanism playing a role is distinct. That is preventing breakdown of heme and the release of Fe would ultimately result in a decreased potential for hydroxyl radical formation creating an elevated H2O2 along with suppression of Mn-potentiated microglial cytokine output. Moreover, our cytokine data at the message level corroborates the possible involvement of this mechanism for inhibition of HO-1 significantly decreases Mn potentiation of TNF-α mRNA, as early as 4 h post Mn+LPS treatment, while a similar effect appears for IL-6 after 24h incubation with Mn+LPS.
Since it is now clear that the increased HO-1 in Mn+LPS-treated microglia serves to maintain Mn-potentiated inflammatory cytokines, we decided to examine possible mechanisms of HO-1 induction that may be used by Mn. As there was no difference between intracellular Mn concentration in the Mn+LPS-treated versus Mn-treated cells, the potentiated HO-1 increase in Mn+LPS-treated cells was not due to an increase in intracellular Mn. Expression of HO-1 mRNA and protein can be induced by agents that generate or cause the production of intracellular reactive oxygen and/or reactive nitrogen species (Applegate et al., 1991;Keyse and Tyrrell, 1989;Motterlini et al., 2002). The ROS H2O2, has been linked to the induction of HO-1 in many mammalian cell types (Applegate et al., 1991). More importantly, direct application of H2O2 in neuron-glia cultures resulted in a rapid induction of HO-1 (Saavedra et al., 2005). Because Mn can produce a time- and dose-dependent release of H2O2 in microglia (current study; Zhang et al., 2007), it is possible that H2O2 could be the trigger used by Mn to increase HO-1 in N9 cells. However, in the current study Mn on its own did not induce HO-1 (Fig. 1) and Mn-induced H2O2 release was not potentiated by LPS (Fig. 7). Thus, it appears that although the Mn-induced release of H2O2 may play a role in the ability of HO-1 to enhance cytokine output it is not the driving force for the microglial induction of HO-1. Recently, it was reported that Mn (10-300 μM) increased production of intracellular ROS in primary microglial cultures (Zhang et al., 2009). While the particular intracellular ROS entity induced by Mn has yet to be identified, it is possible that ROS, other than released H2O2, is/are responsible for the Mn effects on HO-1 in activated microglia.
Molecules that release or increase the production of NO are also known inducers of HO-1 (Motterlini et al., 2002). Because Mn potentiated LPS- induced microglia iNOS expression and/or ensuing NO release (current study, (Chang and Liu, 1999;Filipov et al., 2005), we decided to examine the role of NO in the Mn enhancement of HO-1 induction. In our study, inhibition of NO production with two different NOS inhibitors, L-NAME and AG resulted in the same HO-1 induction pattern that was observed in the absence of NOS inhibitors (Fig. 8), indicating that increased expression of iNOS and ensuing increased NO release is not the driver of HO-1 induction in Mn+LPS-treated microglia.
While NO releasing agents have been associated with increased expression of HO-1, there is evidence that the formation of peroxynitrite (ONOO-) may be important to the modulation of HO-1 by NO (Motterlini et al., 2002). LPS-stimulated macrophages were found to induce HO-1 through the increased formation of ONOO- (Srisook et al., 2005), while superoxide dismutase attenuated HO-1 induction in endothelial cells, suggesting that NO by itself may not be responsible for inducing HO-1 (Foresti et al., 1997). Therefore, the increase in NO observed with Mn+LPS may require conversion to ONOO- in order to modulate HO-1. While, many studies, including the current one, report a Mn-potentiated increase in NO, the ability of Mn to induce superoxide (O2-) and/or form ONOO- following Mn exposure has yet to be demonstrated for microglia.
So far, our study indicates that Mn-induced H2O2 release and the Mn-potentiated increase in NO do not account for the Mn-potentiated induction of HO-1. Thus, the possibility remains that (1) another intracellular ROS not yet identified, or (2) a ROS-independent mechanism is responsible for the potentiated expression of HO-1 by Mn. It is clear that Mn has the potential to increase levels of microglial derived ROS (Zhang et al., 2009), and once all major ROS constituents are identified, it may be possible to determine their significance in the induction of HO-1. Additionally, mitogen activated protein kinase (MAPK: p38, ERK, & JNK) pathways have been shown to be involved in the regulation of HO-1 (Alam and Cook, 2007). In fact, inhibition of the MAPK p38, reduced LPS-induced HO-1 expression in RAW264.7 macrophages (Wijayanti et al., 2004). Because Mn regulates the activation of MAPKs in microglia (Bae et al., 2006;Crittenden and Filipov, 2008;Zhang et al., 2007) it is possible that Mn affects the induction of HO-1 by LPS through one or more of the MAPK pathways.
It is well documented that HO-1 expression is regulated at the transcription level. In particular, the transcription factor, Nrf2 has an important role in the ARE-mediated expression of stress inducible genes, including HO-1(Alam and Cook, 2007). In fact, HO-1 was found to be less inducible in mice lacking Nrf2 (Cho et al., 2002), and Nrf2 knockdown in primary microglial cells increased their susceptibility to oxidative stress (Ni et al., 2010). In addition, LPS can induce HO-1 through the Nfr2/ARE pathway in both macrophages and human monocytes (Ashino et al., 2008;Rushworth et al., 2005). Here we investigated the role of Nrf2 in the Mn potentiated induction of HO-1 by LPS and found that as early as 15 min following exposure, Mn potentiated LPS-induced nuclear small Maf proteins suggesting that while Mn does not potentiate nuclear translocation of Nrf2 protein, it increases the likelihood of its dimerization with Maf. Because heterodimers formed between Nrf2 and small Maf proteins preferentially bind to ARE (Itoh et al., 1997), by increasing the chance for heterodimer formation, Mn-potentiated Maf and hence increased activation of ARE could be the mechanism behind the Mn-enhanced expression of HO-1. In addition while Nrf2 does not appear to change at 15 min, at later time points, increased synthesis of Nrf2 is evidenced by enhanced expression in the cytosol, suggesting an increased demand for Nrf2 in the nucleus (Fig. 9B). It should also be noted that homodimers of Maf were found to act as negative regulators of ARE-induced transcription in erythroid cells (Igarashi et al., 1994). However, in the case of Mn potentiated Maf, one would expect to see a decrease in HO-1 rather than an increase if there were Maf homodimers being formed. Finally, because small Maf proteins can form heterodimers with other transcription factors, such as Jun (Kataoka et al., 1994), which also mediate transcription of antioxidant enzymes, including HO-1, it may be that the Mn-potentiated increase in Maf affects HO-1 expression through multiple transcription factors.
In summary, this study indicates that in the presence of an inflammagen, Mn caused potentiation of microglial-derived inflammatory response is stabilized somewhat through a concurrent enhancement of HO-1 expression. Interestingly, inhibition of HO-1 further increases the release of H2O2 caused by Mn, which suggests that basal level HO-1 in microglia is serving in an antioxidant capacity in part by controlling the redox status of the cells. However, our data indicate that neither increased microglial release of H2O2 nor enhancement of NO output by Mn play a role in the effects of Mn on HO-1 expression in LPS-activated microglia. While the initial mediator of Mn potentiated HO-1 remains elusive it appears that at the transcriptional level, a Mn-potentiated increase in nuclear small Maf proteins is responsible for the enhancement of LPS-induced HO-1 expression by Mn.
Supplementary Material
Stydy Highlights.
The major findings of our current study are:
Manganese (Mn) up to 250 μM does not induce heme oxygenase (HO-1) on its own, but it does potentiate lipopolysaccharide (LPS)-induction of HO-1 in microglia cells.
HO-1 activity appears to be important in regulating Mn-induced hydrogen peroxide (H2O2) release.
Increased HO-1 plays a role in the enhancement of inflammatory mediator output, particularly TNF-α, in Mn+LPS-exposed microglia as inhibition of HO-1 activity resulted in decreased inflammatory cytokines.
Nitric oxide (NO) is not the key regulator of HO-1 induction by Mn+LPS, as iNOS inhibition did not significantly alter Mn-potentiated HO-1 induction
Small Maf proteins appear to be transcriptional regulators targeted by Mn for potentiation of HO-1.
The increased HO-1 by Mn+LPS appears to be glia-specific as neither Mn, LPS, nor combined Mn+LPS induced HO-1 in N27 dopaminergic neurons.
Acknowledgments
We would like to thank Irina Georgieva for her skilful technical assistance, as well as the UGA Trace Analysis lab for running the ICP-AVOES metal analysis. This project was supported by a grant from the National Institute of Environmental Health Sciences (RO1ES016965), awarded to NMF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams FS, La Rosa FG, Kumar S, Edwards-Prasad J, Kentroti S, Vernadakis A, et al. Characterization and transplantation of two neuronal cell lines with dopaminergic properties. Neurochem Res. 1996;21:619–27. doi: 10.1007/BF02527762. [DOI] [PubMed] [Google Scholar]
- Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–74. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Blockade of PKCdelta proteolytic activation by loss of function mutants rescues mesencephalic dopaminergic neurons from methylcyclopentadienyl manganese tricarbonyl (MMT)-induced apoptotic cell death. Ann N Y Acad Sci. 2004;1035:271–89. doi: 10.1196/annals.1332.017. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Sriram K, Benkovic SA, Roberts JR, Stone S, Chen BT, et al. Mild steel welding fume causes manganese accumulation and subtle neuroinflammatory changes but not overt neuronal damage in discrete brain regions of rats after short-term inhalation exposure. Neurotoxicology. 2009;30:915–25. doi: 10.1016/j.neuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–8. [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–47. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashino T, Yamanaka R, Yamamoto M, Shimokawa H, Sekikawa K, Iwakura Y, et al. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol Immunol. 2008;45:2106–15. doi: 10.1016/j.molimm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, et al. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neurosci Lett. 2006;398:151–4. doi: 10.1016/j.neulet.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Bae JW, Kim MJ, Jang CG, Lee SY. Protective effects of heme oxygenase-1 against MPP(+)-induced cytotoxicity in PC-12 cells. Neurol Sci. 2010;31:307–13. doi: 10.1007/s10072-010-0216-6. [DOI] [PubMed] [Google Scholar]
- Barhoumi R, Faske J, Liu X, Tjalkens RB. Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial-dependent activation of nuclear factor kappaB. Brain Res Mol Brain Res. 2004;122:167–79. doi: 10.1016/j.molbrainres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bolte S, Normandin L, Kennedy G, Zayed J. Human exposure to respirable manganese in outdoor and indoor air in urban and rural areas. J Toxicol Environ Health A. 2004;67:459–67. doi: 10.1080/15287390490276485. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007;64:167–77. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NC, Guilarte TR. Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environ Health Perspect. 2009;117:325–32. doi: 10.1289/ehp.0800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–6. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Manganese potentiates nitric oxide production by microglia. Brain Res Mol Brain Res. 1999;68:22–8. doi: 10.1016/s0169-328x(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Ou YC, Lin SY, Liao SL, Chen SY, Chen JH. Manganese modulates pro-inflammatory gene expression in activated glia. Neurochem Int. 2006;49:62–71. doi: 10.1016/j.neuint.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–82. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Crittenden PL, Filipov NM. Manganese-induced potentiation of in vitro proinflammatory cytokine production by activated microglial cells is associated with persistent activation of p38 MAPK. Toxicol In Vitro. 2008;22:18–27. doi: 10.1016/j.tiv.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden PL, Filipov NM. Manganese modulation of MAPK pathways: effects on upstream mitogen activated protein kinase kinases and mitogen activated kinase phosphatase-1 in microglial cells. J Appl Toxicol. 2011;31:1–10. doi: 10.1002/jat.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–28. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–33. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- Drummond GS, Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci U S A. 1981;78:6466–70. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson H, Gillberg PG, Aquilonius SM, Hedstrom KG, Heilbronn E. Receptor alterations in manganese intoxicated monkeys. Arch Toxicol. 1992;66:359–64. doi: 10.1007/BF01973632. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez A, Perez-Otano I, Morgan JI. MPTP selectively induces haem oxygenase-1 expression in striatal astrocytes. Eur J Neurosci. 2000;12:1573–83. doi: 10.1046/j.1460-9568.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- Ferrandiz ML, Devesa I. Inducers of heme oxygenase-1. Curr Pharm Des. 2008;14:473–86. doi: 10.2174/138161208783597399. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci. 2005;84:139–48. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- Foresti R, Clark JE, Green CJ, Motterlini R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. J Biol Chem. 1997;272:18411–7. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol Appl Pharmacol. 1992;115:1–5. doi: 10.1016/0041-008x(92)90360-5. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–16. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–58. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:129–41. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Loschmann PA, Gleichmann M, Weller M, Schulz JB, Wullner U, et al. Induction of nitric oxide synthase and nitric oxide-mediated apoptosis in neuronal PC12 cells after stimulation with tumor necrosis factor-alpha/lipopolysaccharide. J Neurochem. 1998;71:88–94. doi: 10.1046/j.1471-4159.1998.71010088.x. [DOI] [PubMed] [Google Scholar]
- Hung SY, Liou HC, Kang KH, Wu RM, Wen CC, Fu WM. Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol Pharmacol. 2008;74:1564–75. doi: 10.1124/mol.108.048611. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kataoka K, Itoh K, Hayashi N, Nishizawa M, Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 1994;367:568–72. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov Disord. 1998;13(Suppl 1):24–34. [PubMed] [Google Scholar]
- Kampfer H, Kolb N, Manderscheid M, Wetzler C, Pfeilschifter J, Frank S. Macrophage-derived heme-oxygenase-1: expression, regulation, and possible functions in skin repair. Mol Med. 2001;7:488–98. [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Noda M, Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994;14:700–12. doi: 10.1128/mcb.14.1.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Kim JS, Kim JE, Kim SJ, Lee JS, Kim DJ, et al. Heme oxygenase-1 induction by dieldrin in dopaminergic cells. Neuroreport. 2005;16:509–12. doi: 10.1097/00001756-200504040-00018. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Matsuoka Y, Nomura Y, Taniguchi T. Induction of inducible nitric oxide synthase and heme oxygenase-1 in rat glial cells. Life Sci. 1998;62:1717–21. doi: 10.1016/s0024-3205(98)00134-9. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Wagner JR, Kirby ML, Anantharam V, Kanthasamy AG. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J Pharmacol Exp Ther. 2002;302:26–35. doi: 10.1124/jpet.302.1.26. [DOI] [PubMed] [Google Scholar]
- Kurauchi Y, Hisatsune A, Isohama Y, Katsuki H. Nitric oxide-cyclic GMP signaling pathway limits inflammatory degeneration of midbrain dopaminergic neurons: cell type-specific regulation of heme oxygenase-1 expression. Neuroscience. 2009;158:856–66. doi: 10.1016/j.neuroscience.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Lakari E, Pylkas P, Pietarinen-Runtti P, Paakko P, Soini Y, Kinnula VL. Expression and regulation of hemeoxygenase 1 in healthy human lung and interstitial lung disorders. Hum Pathol. 2001;32:1257–63. doi: 10.1053/hupa.2001.28937. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Lee DW, Gelein RM, Opanashuk LA. Heme-oxygenase-1 promotes polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress and dopaminergic cell injury. Toxicol Sci. 2006;90:159–67. doi: 10.1093/toxsci/kfj052. [DOI] [PubMed] [Google Scholar]
- Lee S, Suk K. Heme oxygenase-1 mediates cytoprotective effects of immunostimulation in microglia. Biochem Pharmacol. 2007;74:723–9. doi: 10.1016/j.bcp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Li H, Wu S, Shi N, Lin W, You J, Zhou W. NF-E2-related factor 2 activation in PC12 cells: its protective role in manganese-induced damage. Arch Toxicol. 2010 doi: 10.1007/s00204-010-0625-6. [DOI] [PubMed] [Google Scholar]
- Liao SL, Ou YC, Chen SY, Chiang AN, Chen CJ. Induction of cyclooxygenase-2 expression by manganese in cultured astrocytes. Neurochem Int. 2007;50:905–15. doi: 10.1016/j.neuint.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci. 2006;91:521–31. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2009;240:219–25. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Green CJ, Foresti R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid Redox Signal. 2002;4:615–24. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- Naughton P, Foresti R, Bains SK, Hoque M, Green CJ, Motterlini R. Induction of heme oxygenase 1 by nitrosative stress. A role for nitroxyl anion. J Biol Chem. 2002;277:40666–74. doi: 10.1074/jbc.M203863200. [DOI] [PubMed] [Google Scholar]
- Ni M, Li X, Yin Z, Jiang H, Sidoryk-Wegrzynowicz M, Milatovic D, et al. Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci. 2010;116:590–603. doi: 10.1093/toxsci/kfq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–8. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Pae HO, Chung HT. Heme oxygenase-1: its therapeutic roles in inflammatory diseases. Immune Netw. 2009;9:12–9. doi: 10.4110/in.2009.9.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–38. [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol Exp Neurol. 2007;66:675–82. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–30. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–52. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, et al. Monokine production by microglial cell clones. Eur J Immunol. 1989;19:1443–8. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Ruetten H, Thiemermann C. Prevention of the expression of inducible nitric oxide synthase by aminoguanidine or aminoethyl-isothiourea in macrophages and in the rat. Biochem Biophys Res Commun. 1996;225:525–30. doi: 10.1006/bbrc.1996.1206. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Chen XL, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol. 2005;175:4408–15. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G, Carvalho CM, Duarte EP. GDNF modulates HO-1 expression in substantia nigra postnatal cell cultures. Free Radic Biol Med. 2005;39:1611–9. doi: 10.1016/j.freeradbiomed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem. 2009;110:469–85. doi: 10.1111/j.1471-4159.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- Spranger M, Schwab S, Desiderato S, Bonmann E, Krieger D, Fandrey J. Manganese augments nitric oxide synthesis in murine astrocytes: a new pathogenetic mechanism in manganism? Exp Neurol. 1998;149:277–83. doi: 10.1006/exnr.1997.6666. [DOI] [PubMed] [Google Scholar]
- Srisook K, Kim C, Cha YN. Role of NO in enhancing the expression of HO-1 in LPS-stimulated macrophages. Methods Enzymol. 2005;396:368–77. doi: 10.1016/S0076-6879(05)96031-X. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shimizu H, Morimatsu H, Inoue K, Akagi R, Morita K, et al. Heme oxygenase-1: a fundamental guardian against oxidative tissue injuries in acute inflammation. Mini Rev Med Chem. 2007;7:745–53. doi: 10.2174/138955707781024517. [DOI] [PubMed] [Google Scholar]
- Tamion F, Richard V, Lyoumi S, Hiron M, Bonmarchand G, Leroy J, et al. Induction of haem oxygenase contributes to the synthesis of pro-inflammatory cytokines in re-oxygenated rat macrophages: role of cGMP. Cytokine. 1999;11:326–33. doi: 10.1006/cyto.1998.0441. [DOI] [PubMed] [Google Scholar]
- Wijayanti N, Huber S, Samoylenko A, Kietzmann T, Immenschuh S. Role of NF-kappaB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid Redox Signal. 2004;6:802–10. doi: 10.1089/ars.2004.6.802. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Guo JZ, Zhou QX. The homeostasis of iron and suppression of HO-1 involved in the protective effects of nimodipine on neurodegeneration induced by aluminum overloading in mice. Eur J Pharmacol. 2008;586:100–5. doi: 10.1016/j.ejphar.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Zayed J, Vyskocil A, Kennedy G. Environmental contamination and human exposure to manganese--contribution of methylcyclopentadienyl manganese tricarbonyl in unleaded gasoline. Int Arch Occup Environ Health. 1999;72:7–13. doi: 10.1007/s004200050327. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fitsanakis VA, Gu G, Jing D, Ao M, Amarnath V, et al. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J Neurochem. 2003;84:336–46. doi: 10.1046/j.1471-4159.2003.01525.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Hatter A, Liu B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicol Lett. 2007;173:88–100. doi: 10.1016/j.toxlet.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Lokuta KM, Turner DE, Liu B. Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: differential involvement of microglia and astroglia. J Neurochem. 2010;112:434–43. doi: 10.1111/j.1471-4159.2009.06477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong TA, Lokuta KM, Turner DE, Vujisic K, Liu B. Microglia enhance manganese chloride-induced dopaminergic neurodegeneration: role of free radical generation. Exp Neurol. 2009;217:219–30. doi: 10.1016/j.expneurol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–8. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.