Abstract Abstract
A new troglomorphic spider from caves in Central Java, Indonesia, is described and placed in the ctenid genus Amauropelma Raven, Stumkat & Gray, until now containing only species from Queensland, Australia. Only juveniles and mature females of the new species are known. We give our reasons for placing the new species in Amauropelma, discuss conflicting characters, and make predictions about the morphology of the as yet undiscovered male that will test our taxonomic hypothesis. The description includes DNA barcode sequence data.
Keywords: conservation, DNA barcode, Indonesia, Jonggrangan Limestone, troglobite
Introduction
We describe a new troglobitic spider taken from caves in Central Java, Indonesia. The species is a ctenid tentatively placed in the genus Amauropelma, which was established to accommodate 16 new species from Queensland, Australia by Raven et al. in 2001. Ours is the first new Amauropelma species to be proposed since Raven et al.’s original description. We are writing this paper as the description of a single species known from one sex because it is a troglobite (and therefore of potential conservation interest) from a taxon for which good comparative descriptive data are available. Although few specimens have been collected, many more specimens have been observed and not collected out of prudent concern for the population. However, all of these observations were of either juveniles or mature females. Repeated attempts to target males have so far failed.
Methods
Characters described mostly follow Raven et al. (2001) to facilitate comparison with known species. Observations of vulva structures were made based on a dissected epigynum cleared in methyl salicylate (Holm 1962), positioned using a temporary slide mount (Coddington 1983), and viewed through a Leica DM2500 compound microscope. Other observations were made based on specimens in alcohol viewed through a Leica M165 C stereoscope. Photographs were made using a Nikon DS-Ri1 driven by NIS Elements software and mounted on either the DM2500 microscope or the M165 C stereoscope. Images from multiple focal planes were combined and edited in Auto-Montage software version 5.03. Additional processing of some images to adjust color, brightness, and contrast, and remove blemishes was performed using Adobe Photoshop CS5. Tarsal organ position expressed as a ratio of the distance from the proximal margin of the tarsus to the tarsal organ divided by the total length of the tarsus. All measurements in millimeters. Abbreviations given in Table 1.
Table 1.
List of abbreviations used in the text and figures.
| Spinnerets and somatic morphology: | |
| ALE | anterior lateral eye |
| ALS | anterior lateral spinneret |
| AME | anterior median eye |
| AT | anal tubercle |
| CD | copulatory duct |
| ET | epigynal tooth |
| fe | femur |
| me | metatarsus |
| p | prolateral |
| pa | patella |
| PLE | posterior lateral eye |
| PLS | posterior lateral spinneret |
| PME | posterior median eye |
| PMS | posterior median spinneret |
| r | retrolateral |
| S | spermatheca |
| ti | tibia |
| v | ventral |
| Institutional abbreviations: | |
| MZB | Museum Zoologicum Bogoriense, Bogor |
| RMNH | Netherlands Centre for Biodiversity Naturalis, Leiden |
We used the Pensoft IPT Data Hosting Center to expose specimen occurrence records to the Global Biodiversity Information Facility (GBIF; http://data.gbif.org/welcome.htm). A KML (Keyhole Markup Language) file for viewing these same specimen occurrence records interactively in Google Earth (http://earth.google.com/) is available as electronic appendix A. In accordance with Pensoft’s practice of semantic markup and publishing, the species described herein has been registered on ZooBank (http://zoobank.org/) and a species page has been submitted to the Encyclopedia of Life (http://www.eol.org/) and the wiki species-id (http://species-id.net/wiki/).
658 bases of cytochrome oxidase I were sequenced by the NCB Naturalis DNA barcoding facility using the following primers: LCO1490 (5’-GGTCAACAAATCATAAAGATATTGG-3’) and HCO2198 (5’-TAAACTTCAGGGTGACCAAAAAATCA-3’) (Folmer et al. 1994). Chromatogram data are available as electronic appendix B.
Taxonomy
Amauropelma
Raven, Stumkat & Gray, 2001
http://species-id.net/wiki/Amauropelma
Type species.
Amauropelma trueloves Raven & Stumkat, 2001
Addendum to diagnosis.
Tarsal organ position ranges from 0.125–0.77. Tarsi with or without adpressed trichobothria. Epigynum with soft or sclerotized lateral teeth. Tracheal spiracle distinct or indistinct. Otherwise, as in Raven et al. (2001).
Amauropelma matakecil
Miller & Rahmadi sp. n.
urn:lsid:zoobank.org:act:180E7280-7D8D-4884-81FC-C8BE75FDD361
http://species-id.net/wiki/Amauropelma_matakecil
Figures 1–6.
Amauropelma matakecil sp. n. 1 female habitus 2–6 habitus of female holotype (MZB.Aran.500) 1 Portrait of live specimen in natural habitat from Gua Nguwik, Central Java (Photo S. Harjanto) 2 Anterior view 3 Dorsal view 4 Ventral view showing labium, endites, and chelicerae 5 Ventral view showing sternum, coxae, and trochanters 6 Left pedipalpal, retrolateral view.
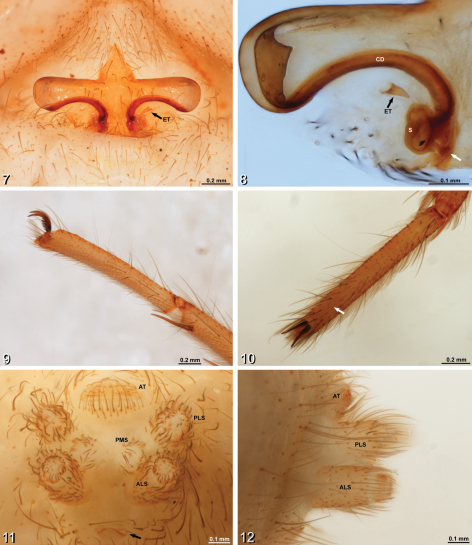
Figures 7–12.
Amauropelma matakecil sp. n., female holotype (MZB.Aran.500) 7 Epigynum, ventral view. Note that the right epigynal tooth has broken off leaving a round hole; the tooth itself is lying unattached near the epigastric furrow 8 Vulva, dorsal view, left side, cleared, white arrow indicates fertilization duct 9 Right tarsus, leg I, prolateral view 10 Left tarsus, leg I, dorsal view, arrow indicates tarsal organ 11 Spinnerets, anal tubercle, and tracheal spiracle, posterior view, arrow indicates tracheal spiracle 12 Spinnerets, lateral view. ALS, anterior lateral spinneret; AT, anal tubercle; CD, copulatory duct; ET, epigynal tooth; PLS, posterior lateral spinneret; PMS, posterior median spinneret; S, spermatheca.
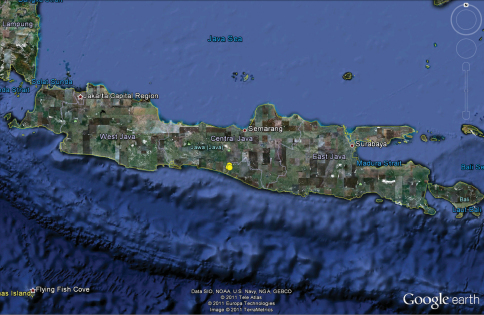
Figure 13.
Map of Java, Indonesia, showing records of Amauropelma matakecil sp. n. as yellow circles in Central Java. Base map source: Google Earth.
Material examined.
Holotype: Indonesia, Central Java, Purworejo, Kaligesing, Tlogoguo Village, Somoroto: Gua Anjani [Anjani Cave], 7.73156°S, 110.11567°E, 672 m asl., 23 March 2009 (MZB.Aran.500, S. Harjanto), 1 #f.
Paratypes: Indonesia, Central Java, Purworejo, Kaligesing, Donorejo Village, Katerban: Gua Seplawan [Seplawan Cave], 7.7726°S, 110.111°E, 23 April 2010 (MZB.Aran.501, S. Harjanto and C. Rahmadi), 1 #f; Indonesia, Central Java, Purworejo, Kaligesing, Donorejo Village, Katerban: Gua Nguwik [Nguwik Cave], 7.76907°S, 110.10334°E, 764 m asl., 9 May 2008 (RMNH.ARA.12434, S. Harjanto), 1 #f.
Additional material examined: Indonesia, Central Java, Purworejo, Kaligesing, Tlogoguo Village, Somoroto: Gua Anjani [Anjani Cave], 7.73156°S, 110.11567°E, 672 m asl., 23 April 2010 (RMNH.ARA.12436, S. Harjanto and C. Rahmadi), 2 juveniles; Gua Anjani [Anjani Cave], 7.73156°S, 110.11567°E, 672 m asl., 23 March 2009 (MZB.Aran.502, S. Harjanto), 1 juvenile.
Etymology.
The specific name is an adjective derived from "mata" meaning eyes and "kecil" meaning small from Bahasa Indonesia referring to the small eyes of the species. Pronunciation note: the letter “c” in Bahasa is pronounced like “ch” in English.
Diagnosis
. Distinguished from other Amauropelma species by having more cheliceral teeth (4 promargin and 7 retromargin teeth, Fig. 4; other described species for which data were recorded range between 1–4 promargin and 4–6 retromargin teeth); by the relatively proximal position of the tarsal organs (Fig. 10); by the sclerotized epigynal teeth that do not conduct the copulatory ducts (Fig. 7; other Amauropelma have soft teeth containing the copulatory ducts); and by the shape of the epigynum, which has the lateral wings more long and narrow than other species (Fig. 7). Further distinguished from other Amauropelma species except Amauropelma leo Raven & Sumkat, 2001 by having small eyes (Fig. 2; large in other species except Amauropelma undara, which is a blind troglobite); distinguished from Amauropelma leo by the pale, troglomorphic color (Figs 1, 3); Amauropelma leo is a rainforest species and is not troglomorphic.
Description.
Female (holotype, MZB.Aran.500): Carapace 3.40 long, 2.20 wide. Abdomen 4.12, long 2.64 wide. Total length 7.7. Carapace with fine setae. Fovea a narrow groove. Chilum divided (Fig. 2). Endites slightly converging, labium longer than wide (Fig. 4). Color. Overall pale, chelicerae and ocular region darker (Figs 1, 3). Eyes. Vestigial, eye rows recurved forming 2.4.2 pattern, ALE>AME=PME>PLE, AME on small common tubercle, ALE and PME form slightly procurved row, PME closer together than PME-ALE, PME slightly more widely spaced than AME, eye group = 0.40 of carapace width (Figs 2, 3). Chelicerae. Large, partially porrect with lateral boss. Retromargin with 5 large distal and 2 small proximal teeth; promargin with 4 teeth, the third (counting proximally from the base of the fang) is the smallest (Fig. 4). Pedipalp. Tarsal claw with series of basal teeth. With two large ventral distal setae (ca. Silva Dávila 2003: fig. 28d). Legs. Formula 4123. Paired tarsal claws with series of basal teeth, claw tufts present, weak scopula present on tarsi and metatarsi I and II (Fig. 9). Retrocoxal hymen present on leg I. Trochanters deeply notched (Fig. 5). Tarsal organs slightly raised, dome-like, more distal on legs I and II than III and IV (I: 0.77. II: 0.73. III: 0.66. IV: 0.67). Macrosetae: I: fe p1d3; pa 0; ti v2.2.2.2.2; me v3.3.3; ta 0. II: fe d3r1; pa 0; ti v2.2.2.2.2; me v3.3.3; ta 0. III: fe p4d3r4; pa p1r1; ti v2.2.2p1.1d1.1 r1.1; me v2.2.2p1.1.2r1.1.2; ta 0. IV: fe p3d3r1; pa r1; ti v2.2.2p1.1d1.1r1.1; me v1.1.1.1.2p1.1.1d0.1.2r1.1.1. Spinnerets. Ecribellate, colulus absent, lateral spinnerets cylindrical with short apical segment, ALS separated by about their width, PLS and PMS with a number of large, conspicuous spigots (Figs 11, 12). Epigynum. Sclerotized plate with long, narrow lateral wings with concave posterior margins. Epigynal teeth sclerotized, arise posterior to lateral wings (Fig. 7). Copulatory openings on dorsal surface near lateral margins of wings, follow posterior margin of wings to reniform spermathecae (Fig. 8).
Natural History.
In Seplawan Cave, Amauropelma matakecil was found on the cave floor hiding under crevices in dry mud.
Distribution.
Amauropelma matakecil is known only from three caves in the Jonggrangan Limestone, part of the Menoreh Hills in the District of Kaligesing, Purworejo Regency, Central Java, near the border with Yogyakarta Province (Fig. 13). The Jonggrangan Limestone is located from 574–878 m above sea level (Bemellen 1949). This karst formation is a fossil reef with thicknesses up to 200 m at the southern margin of the Jonggrangan Plateu (Bemellen 1949). The formation dates from the Middle to Late Miocene (Sulistyaningrum and Rahardjo 2010). Karst makes up a very small area of the Menoreh hills, about 15 km2. The nearest neighboring limestone formations are the Gombong Selatan (about 72 km to the west) and the Gunung Sewu Karst (about 42 km to the east).
Remarks.
The cave spider fauna of Java is not well known. The only other spider documented from a cave in Java that we are aware of is Althephus javanensis Deeleman-Reinhold, 1995 (Ochyroceratidae). This species is not strongly troglomorphic, exhibiting neither eye reduction nor reduced pigmentation, although legs in specimens from caves are considerably longer than in specimens from the surface. As reported by Rahmadi (2011), Amauropelma matakecil is the most remarkable cave spider so far known from Java due to its large size, reduced eyes, and potential conservation importance. Karst formations in Java are highly threatened by human activities such as limestone mining and habitat conversion.
DNA Barcode.
AACGTTATATTTAATATTTGGAGCTTGATCTGC TATAATAGGAACGGCTATAAGAATATTAATTCGAATAGAGTTAGGA CATTCTGGAAGATTATTAAGTAATGATCATTTGTATAATGTGATTGT TACTGCTCATGCATTTGTTATAATTTTTTTTATGGTGATGCCAATTT TAATTGGAGGTTTTGGAAATTGATTAGTTCCTTTAATATTAGGAGCTC CGGATATATCGTTTCCTCGAATAAATAATTTGTCTTTTTGATTGTTAC CTCCTTCTTTGTTTTTGTTGTTTATATCTTCTATAGTTGAAATGG GAGTAGGAGCTGGATGAACTATTTATCCCCCTTTAGCTTCTAGAATTG GTCATGTGGGAAGATCTATGGATTTTGCTATTTTTTCTTTACATT TAGCTGGAGCTTCTTCTATTATAGGGGCGGTAAATTTTATTTCTAC GATTGTAAATATACGTTTATTAGGAATAAGAATAGAAAGGGTTCCTT TATTTGTGTGATCTGTATTTATTACTGCTGTTTTATTATTATTATCTT TACCTGTTTTAGCGGGAGCTATTACTATGTTATTGACGGATCGAAATTT TAATACTTCTTTTTTTGACCCTGCAGGGGGAGGGGATCCTATTT TATTTCAACATTTGTTT (MZB.Aran.501, GenBank accession number JQ277219).
Among identified spiders accessible at the time of writing (October 2011) through the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi), Amauropelma matakecil blasts most closely with the pisaurid genus Dolomedes Latreille, 1804. This despite the presence in GenBank of the homologous locus for several ctenid spiders (e.g., Crews and Gillespie 2010). However, its closest matches are several still unidentified spiders in the International Barcode of Life (iBOL) database.
Discussion
The species described here appears to fit best in the genus Amauropelma based on several characters including the eye arrangement (Fig. 2), the presence of only the superior tarsal claws (no inferior tarsal claw; Fig. 9), the leg spination pattern, the presence of two ventral distal macrosetae on the female pedipalp (ca. Silva Dávila 2003: fig. 28d), and lateral wings and posterior teeth on the epigynum (Fig. 7). However, Amauropelma matakecil exhibits characteristics that are not typical of Amauropelma and none of the above characters are unique to Amauropelma.
The form of the epigynum is also similar to the genera Thoriosa Simon (from West Africa and nearby Atlantic islands) and some Trogloctenus Lessert (from Congo and Réunion). Silva Dávila’s (2003) phylogenetic analysis placed Thoriosa close to Amauropelma and an incertae sedis species from Lombok Island, Indonesia; Trogloctenus was not included in that analysis due to a lack of non-type material in collections. Amauropelma including our new species differs from Thoriosa and Trogloctenus by the eye arrangement. Thoriosa has the median ocular area wider posteriorly than anteriorly (Benoit 1976: figs 1, 4); in Amauropelma including our new species, the median ocular area is as wide anteriorly as posteriorly. In the type species of Trogloctenus, the clypeus is about seven AME diameters (Benoit 1976: fig. 12); in Amauropelma, the clypeus ranges from less than one to about two AME diameters. A second species of Trogloctenus has no eyes so this character is inapplicable, but in this species the lateral wings of the epigynum are not so pronounced and posterior teeth are apparently absent (Ledoux 2004: fig. 9B). The loss of the inferior tarsal claw, the presence of two ventral distal macrosetae on the female pedipalp (ca. Silva Dávila 2003: fig. 28d) and the leg spination pattern are all found in multiple ctenid genera including Thoriosa.
There are also some characteristics that conflict with Amauropelma. The epigynal teeth of the new species are hard rather than soft. The copulatory openings appear to be associated with the anteriomesal part of the lateral wings of the epigynum rather than with the epigynal teeth (Fig. 8). The claw tufts are less dense than in other Amauropelma species (Fig. 9). The position of the tarsal organs is much more distal than that reported for other Amauropelma species (Fig. 10). Note that the tarsal organ of Janusia Gray is described as subdistal and distal to trichobothria (Gray 1973; see below). The tracheal spiracle is small but easy to see because of a narrow sclerotized margin (Fig. 11; Raven et al. 2001 reported the tracheal spiracle of Amauropelma indistinct). Raven et al. (2001) described the labium as longer than wide. Based on illustrations (Raven et al. 2001: fig. 5C, 21G), this condition is amplified in the new species (labium length 1.3 times the width; Fig. 4). Adpressed trichobothria were not observed on the tarsi of our new species, as reported by Raven et al. for Amauropelma (e.g., Raven et al. 2001: fig. 3f) but are apparently present on the tibiae. It seems clear that there are several ctenid lineages closely related to Amauropelma that would benefit from revision and more extensive illustration.
One other troglobitic Amauropelma is known. Amauropelma undara Raven & Stumkat from lava tubes in Queensland is completely blind, in contrast to our new species which has vestigial eyes. Another ctenid known from caves that shares characteristics with our new species is the genus Janusia (see Raven et al. 2001). This genus contains only one described species from Western Australia but the existence of possibly congeneric undescribed species has been reported (Gray 1973; Raven et al. 2001). Our new species can be separated from Janusia muiri Gray in part by the presence of a small inferior tarsal claw in Janusia (no inferior tarsal claw in Amauropelma) and by the presence of only three teeth on the superior tarsal claws (ca. 7 in our new species; Fig. 7).
Based on the characteristics of other Amauropelma species, we predict that the male when discovered will be found to exhibit no tibial crack on the legs, will have retrolateral processes on the palpal patella and tibia, will have an apically coniform cymbium without a dorsal scopula, will have a palpus with a cup-shapped median apophysis, a hyaline conductor, an embolus in the form of a large hook-shaped plate, and other anatomical details in common with known Amauropelma species. If these predictions are not borne out with the eventual discovery of the male, the generic position of this species may have to be reconsidered. The male of Janusia has not been described, but based on a broken embolus extracted from the reproductive tract of a female, the embolus is thin and coil-like (Gray 1973).
Supplementary Material
Acknowledgments
Specimens were collected during the project Cave Fauna of Java funded by Rufford Small Grants and The Nagao Foundation (2007–2008) for CR. We thank Sidiq Harjanto who first discovered this species and shared his photographs with us. Thanks to Frank Stokvis, Camiel Doorenweerd and the NCB Naturalis DNA barcoding lab for their help with the DNA sequencing. Charles Griswold originally suggested that this species might belong to Amauropelma and Robert Raven concurred. Special thanks to Diana Silva Dávila, Robert Raven, and an anonymous reviewer for constructive comments on earlier drafts of the manuscript. Thanks also to Darrell Ubick and Joel Ledford for helpful discussion about the possible affinities of this taxon and to Tamas Szuts for help with access to literature from remote locations. Thanks to Teodor Georgiev for help using the Pensoft IPT Data Hosting Center.
Appendix A
Specimen records of Amauropelma matakecil. (doi: 10.3897/zookeys.163.2265.app1) File format: KML (Keyhole Markup Language) version 2.1 for GoogleEarth.
Explanation note: The KML file can be opened using GoogleEarth (http://earth.google.com/) to display an interactive map showing the specimen occurrence data for Amauropelma matakecil.
Click on placemarks to reveal specimen data and a hyperlink to the species page on the Encyclopedia of Life (http://www.eol.org/).
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Appendix B
DNA barcode. (doi: 10.3897/zookeys.163.2265.app2) File format: SPF (Sequencher Project File).
Explanation note: Chromatograms for the DNA barcode sequence of Amauropelma matakecil.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
References
- Bemellen van RW. (1949) The Geology of Indonesia, volume 1A. Martinus Nijhoff, The Hague, 602 pp. [Google Scholar]
- Benoit PLG. (1976) Etudes sur les Ctenidae africains (Araneae) II. Les genres Thoriosa Simon et Trogloctenus Lessert. Revue de Zoologie Africaine 90: 221-227. [Google Scholar]
- Coddington JA. (1983) A temporary slide mount allowing precise manipulation of small structures. Verhandlungen naturwissenschaften vereins Hamburg (NF) 26: 291-292. [Google Scholar]
- Crews SC, Gillespie RG. (2010) Molecular systematics of Selenops spiders (Araneae: Selenopidae) from North and Central America: implications for Caribbean biogeography. Biological Journal of the Linnean Society 101: 288-322. doi: 10.1111/j.1095-8312.2010.01494.x [DOI] [Google Scholar]
- Deeleman-Reinhold CL. (1995) The Ochyroceratidae of the Indo-Pacific region (Araneae). Raffles Bulletin of Zoology, Supplement 2: 1-103. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. (1994) DNA primers for the amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294-299. [PubMed] [Google Scholar]
- Gray MR. (1973) Cavernicolus spiders from the Nullarbor Plain and South-West Australia. Journal of the Australian Entomological Society 12: 207-221. doi: 10.1111/j.1440-6055.1973.tb01661.x [DOI] [Google Scholar]
- Holm Å. (1962) The spider fauna of the East African mountains. Part I: Fam. Erigonidae. Zoologiska Bidrag Från Uppsala 35: 19-204. [Google Scholar]
- Ledoux J-C. (2004) Araignées de l’île de La Réunion: I. Hahniidae, Ctenidae, Thomisidae et Clubionidae (Araneae). Revue Arachnologique 14: 159-191. [Google Scholar]
- Rahmadi C. (2011) The biospeleology of Java Caves, Indonesia: A Review. Proceeding of Asian Trans-Disciplinary Karst Conference 2011, Yogyakarta-Indonesia: 241–250.
- Raven RJ, Stumkat K, Gray MR. (2001) Revisions of Australian ground-hunting spiders: I. Amauropelma gen. nov. (Araneomorphae: Ctenidae). Records of the Western Australian Museum, Supplement 64: 187-227. [Google Scholar]
- Silva Dávila D. (2003) Higher-level relationships of the spider family Ctenidae (Araneae: Ctenoidea). Bulletin of the American Museum of Natural History 274: 1-86. doi: [DOI] [Google Scholar]
- Sulistyaningrum D, Rahardjo W. (2010) Identification and paleoecology of coraline fossils (Cnidaria: Anthozoa) from Jonggrangan limestone, western slope of Kucir Hill,West Progo area, Yogyakarta Special Province. Proceedings, Indonesian Petroleum Association Thirty-Fourth Annual Convention & Exhibition, May 2010, 9 pp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.