Abstract
Importance of the field
Although EM-1 (H-Tyr-Pro-Phe-Trp-NH2) and EM-2 (H-Tyr-Pro-Phe-Phe-NH2) are primarily considered agonists for the μ-opioid receptor (MOR), systematic alterations to specific residues provided antagonists and ligands with mixed μ/δ-opioid properties suitable for application to health related topics.
Areas covered in this review
This review attempts to succinctly provide insight on the development and bioactivity of endomorphin analogues during the past decade. Rational design approaches will focus on the engineering of endomorphin agonists, antagonists and mixed ligands for their application as a multi-target ligand.
What the reader will gain
While the application of endomorphins as antinociceptive agents and numerous biological endpoints were experimental delineated in laboratory animals and in vitro, clinical use is currently absent. However, structural alterations provide enhanced stability, formation of MOR antagonists or mixed and dual μ/δ-acting ligands could find considerable therapeutic potential.
Take home message
Aside from alleviating pain, EM analogues open new horizons in the treatment of medical syndromes involving neural reward mechanisms and extraneural regulation effects on homeostasis. Highly selective MOR antagonists may be promising to reduce inflammation, attenuate addiction to drugs and excess consumption of high caloric food, ameliorate alcoholism, affect the immune system and combat opioid bowel dysfunction.
Keywords: endomorphins, opioid receptors, rational drug design, agonists, antagonists, peptide synthesis
1. Introduction and background
Endomorphins-1 and -2 (EM-1 and EM-2), potent MOR agonists, came into existence through the diligent investigative and rational design efforts with Tyr-W-MIF-1 peptides by Zadina et al. and their isolation from bovine [1,2] and human brain tissue [3]. Like all opioid ligands characterized by a N-terminal Tyr, except nociceptin/orphanin-FQ with a Phe group [4], the EM tetrapeptide backbone has the potential to be chemically mutated into ligands exhibiting enhanced specificity for MOR and selectivity towards δ-opioid receptors (DOR). Furthermore, since these two receptors consist of a closely related structural class of G protein coupled receptors [5,6] formulations of new opioid analogues developed as specific agonists, antagonists, or as mixed or chimeric ligands to both receptors simultaneously to elicit different biological endpoints.
Opioid peptides and alkaloid opiates [7] specifically interact with MOR to produce drug-induced tolerance and physical dependency with continued use. Since the existence of natural occurring opioid antagonists are unknown, the initial development of antagonists were formulated on the structural nucleus of morphine, which yielded naloxone [8] and naltrexone [9], among a large variety of compounds [10]; however, these morphinan-derived antagonists have numerous and often adverse side effects, such as withdrawal symptoms in the treatment of drug addiction and alcoholism due to suppression of the basal activity of opioid receptors [11], termed inverse agonism [12]. The unique sequence of the endomorphins, on the other hand, permitted judicious substitutions that provided not only more potent agonists, but also and importantly in our discussion on the attenuation of non-pain medical syndromes, the appearance of selective or mixed antagonists. Our summary focuses on the chemistry and biology of EM ligands that were patented and supported, for the most part, on published articles in the literature. For a comprehensive and recent review of EM chemistry, the reader is directed to the article by Keresztes et al. [13].
1.1 Endomorphin properties
Endomorphins (EM-1 and EM-2) are, beyond question, the most selective peptidic compounds interacting with MOR [1]. While their level changes during neuropathic pain models [22], they are detected in tissues throughout the mammalian body [23–25] and affect divergent physiological parameters: e.g., asthma [26] and antitussive effects [27], since endomorphins are found in nerves in esophageal smooth and striated muscle [28] to modulate cholinergic neurotransmission [29]; reduce inflammation [30–33]; decrease cardiac output [34] with vasodepressor activity on systemic arterial pressure [35,36]. Considering their selectivity for MOR, they must play a central role in the activity of the neural reward mechanism. As endogenous compounds with the innate ability to alter the activity of MOR with high specificity becomes a challenge for medicinal chemists to design more potent analgesic drugs that readily cross membrane barriers, exhibit greater half-lives, and limit or modify the deleterious problems associated with opiates. The patents discussed in this review have attempted to broach this objective with varying degrees of success.
1.2 Distribution of opioid receptors
Briefly, opioid receptors represent an evolutionarily conserved family of membrane receptors (known as μ, δ, κ, and nociceptin/orphanin-FQ) and exhibit sequence similarity and a common tertiary structure—the basic seven-transmembrane topography of G protein-coupled receptors. They exist in virtually every tissue and organ system throughout the body, but mainly associated with neural tissue to regulate physiological functions [14–19]. Their integration in a neuroregulatory system exceeds a perceived association for analgesia towards pain [20] or the induction of euphoria among opiate addicts. In fact, even placebo effects appear to be mediated by MOR [21]. In other words, MOR is important and its activity can be modulated not only by using addictive narcotic drugs, such as morphine and derivatives, but also by the consumption of high caloric foods and inflammation, which mediate homeostatic mechanisms.
1.3 Mechanisms of endomorphin action
Recent and extensive investigations in opioid chemistry resides on several important issues: (i) development of potent opioids for analgesic remediation of pain specific either for central or peripheral mode of action, the latter in particular to circumvent the limitations by the acquisition of tolerance, dependence and potential addiction associated with central-acting morphinans [37]; (ii) provide molecules with high biological efficacy and/or hydrophobicity through SAR analyses in order to effectively pass through membrane barriers with reasonable proteolytic resistance [38,39]; proteolysis leads to unknown metabolites that might have secondary consequences or toxicological properties; and (iii) conformational analysis of the ligands and subsequent docking in MOR which provides evidence on the topography of the ligand-bind site [13]. Opioids face considerable biological hurdles in the body in order to act as therapeutic agents [40]: membrane barriers prevent passage of charged molecules in the molecular weight range > 500 Daltons and exclusion by membrane-bound molecular pumps, such as multidrug resistance P-glycoprotein-1. Owing to the highly selective interaction of EM-1 and -2 to MOR, the majority of the published data ([13] and references therein) and many of the patents discussed herein underscore the analgesic aspects in the spectrum of their bioactivity.
To overcome these limitations, judicious alterations in the sequence of EM-2 modified their properties and enhanced biostability [41,42], increased hydrophobicity and reduced overall charge with minimal alteration of its bioactivity. Structural modification included peptide cyclization [2,43,44], residue substitution by an unnatural amino acid within its sequence and/or at the N- or C-termini [41,42,45–53]; e.g., heteroaromatic compounds [54,55], formation of 1,5-enediol derivatives [56–58], a reduced bond [59], or modification with lipoidyl or glycosyl groups [60,61]. These analogues also revealed an inherent ability to alter the chemistry and side chains of the EM backbone while retaining activity and the promiscuity of MOR permitting binding of unique EM analogues to elicit a biological response.
Synthesis of EM analogues with mixed receptor properties, namely μ agonism/δ antagonism, as well as an unusual δ antagonist (infra vide) and formation of potent MOR antagonists by the N-allylation of [Dmt1]EM-1 and -2 [48,62,63] provides myriad opioids for potential therapeutic and clinical applications. In particular, the μ antagonists exhibited neutral antagonism (absence of inverse agonist properties) that eliminated or greatly reduced morphine-induced withdrawal symptoms in mice [11] and substantially attenuated the enhancement of GABAergic neurotransmission by alcohol [62,63].
Studies elucidated the conformation of EM-1 and EM-2 [64], and NMR and CD analyses in solution provided an assessment of the contribution of cis/trans rotamers at the Dmt-Pro amide or Tyr-Pro amide bond. [46,64]. Data revealed a marked preference for the cis conformer that might contribute to the elevated activity of Dmt-containing analogues [46]. The cis conformer provides greater flexibility in [Dmt1]EM-2, permitting the peptide to attain lower energy conformers [64] that might auger for enhanced interaction within the ligand-binding domain of the MOR.
2. Rational design of endomorphin analogues
2.1 General procedures for synthesis of endomorphin-2 analogues
Essentially two basic methods are used in the synthesis of opioids; namely, solution and solid phase methods. To describe each of the minor variations in each method would be outside the scope of this review; therefore, we present only two specific examples: one for the solution synthesis of [Dmt1]EM-2 and a general outline for solid phase synthesis using as an example the formation of the unusual EM-2 analogues containing a 1,5-enediol in lieu of the Pro residue [56,57,58].
2.1.1 Solution synthesis
Syntheses of EM-2 analogues were performed by standard solution peptide synthesis methods in various patents and are principally the same for all EM analogues [2,42,48,50–53]. Although the patent of Carr et al. [65] does not stipulate the method of EM synthesis, their publication describes a solution procedure [66].
In an overview of the solution synthesis of EM-2 analogues [48], we use as an example the procedures developed for Dmt derivatives. Dmt was the residue to choice due to the enhanced MOR affinity and functional pharmacological activity of endomorphins [46,54]. This decision was based on the efficacy of Dmt on the induction of extraordinary DOR selectivity and unique pharmacological properties of the Dmt-Tic pharmacophoric opioids [67–71] and described in another series of EM-2 stereoselective 1,5-enediol analogues [57]. Dmt can be either prepared according to the method of Dygos et al. [72] and its chirality assessed by HPLC, or purchased commercially (RSP Amino Acids LLC, Shirley, MA USA).
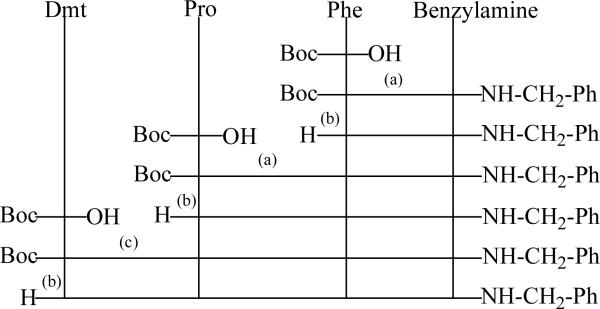
As outlined in Figure 1, (a) a mixed anhydride method used IBCF, NMM, or (c) PyBop was employed as a coupling reagent. The Boc group protected the N-terminal amine function. Deprotection (b) was performed in 6 N HCl/dioxane or TFA. During the course of synthesis, Boc protected peptide intermediates were identified by NMR and elemental analysis. The final product was purified by semi-preparative reversed phase-HPLC. Purified opioids exhibited > 98% purity by analytical HPLC (absorbance at 220 nm), and characterized by NMR, MALDI-TOF mass spectrometry and elemental analysis.
Figure 1. Synthetic scheme for the solution synthesis of [Dmt1]endomorphin-2 analogues.
(a) A mixed anhydride method using IBCF, NMM, or (c) PyBop with DIPEA were employed as coupling reagents. The Boc group was used for N-terminal protection. Deprotection (b) was performed in HCl in dioxane (6 N HCl/dioxane) or TFA.
Unlike EM-2, EM-1 contains tryptophan (H-Tyr-Pro-Trp-Phe-NH2) which contains a 3-alkylindole side chain that is easily attacked by an electrophile under acidic conditions, especially at the 2-position, and is susceptible to oxidative degradation [73,74]. In the presence of strong bases the indole system reacts as an anion, mainly at the indole nitrogen, necessitating its protection. Considering these circumstances, it is easy to quite understand that the synthesis of EM-2 analogues in large quantity and diversity is preferable to those of EM-1 [13,75,76]; nonetheless, biologically active EM-1 analogues were patented [48,50–53].
2.1.2 Solid phase synthesis of endomorphin-2 analogues
The majority of EM and patented analogues thereof were synthesized using solid-phase methodology [2,49,58,60,78] or undefined “by chemical or enzymatic synthesis” [79]. Among some EM patents, the method of peptide synthesis was absent due to a focus on their application as prophylaxis compounds [80], anti-inflammation agents [31], cosmetic formulations [81], or merely referenced [82].
This procedure is principally similar to that for solution phase methodology except for the protection of the C-terminus by a solid support. There are two main strategies in solid phase methods: either Boc or Fmoc chemistry [83]. The synthesis of EM analogues can produce a multiplicity of diastereoisomers of EM-2 using a Fmoc amide resin as follows: the solid support, Fmoc-d- or l-Tyr(But)-OH, Fmoc-d- or l-Pro-OH and Fmoc-d- or l-Phe-OH as the protected amino acids, and HBTU/HOBT/DMF, DIEA/NMP for each reaction in a peptide synthesizer [84]. After each coupling reaction, the Fmoc group was removed with piperidine/NMP. In the final deblocking step, the dried protected peptide resin was suspended in TFA/H2O and the reaction mixture stirred at room temperature. The material was filtered, ether added to the filtrate, and the precipitate collected by filtration and lyophilized from HCl.
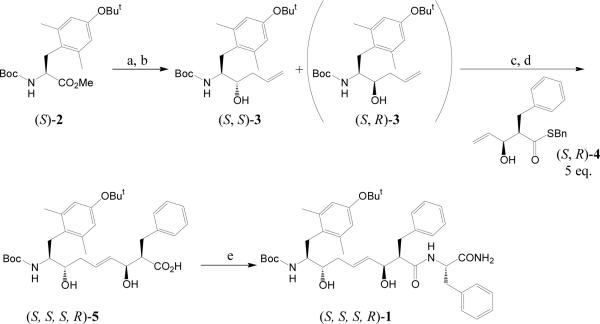
The procedure for the synthesis of stereoisomers of Tyr1- or [Dmt1]EM-2 analogues containing a 1,5-enediol moiety [56,57] used Boc-Tyr(But)-OMe or Boc-Dmt(But)-OMe as the starting material and only one stereoisomer (1) (Figure 2) is described briefly as a specific example. As shown in Figure 2, Boc-Dmt(But)-OMe (2) [(S)-2] was reduced to the aldehyde with DIBAL-H and allylated with allylmagnesiumbromide to give (S,S)-3 and (S,R)-3 and separated by flash chromatography. (S,S)-3 was coupled with excess (S,R)-4 using the olefin cross-metathesis approach [85] with a Cl2(PCy3)(IMesH2)RuCHPh catalyst [86] by refluxing in CH2Cl2, followed by hydrolysis with LiOH and H2O2 to give (S,S,S,R)-5. Compound (1) was prepared by coupling (S,S,S,R)-5 and solid supported Phe by HBTU/HOBT, followed by TFA deprotection and HPLC purification with high purity
Figure 2. Scheme for the solid phase synthesis of stereoisomers of 1,5 enediol endomorphin-2 analogues.
Reagents and conditions: (a) DAIBAL-H, toluene, at −78 °C. (b) allylMgBr, THF, Et2O, at 0 °C. (c) Cl2(PCy3)(IMesH2)RuCHPh, CH2Cl2, at 40 °C. (d) LiOH, H2O2, THF, H2O. (e) HBTU, HOBt, DIPEA, NMP, Phe-NH-Rink amide AM resin, then 95% TFA.
2.2 Agonists and agonism
The bulk of the patents issued are pertinent to EM-2 analogues as analgesic agents in the management of pain through MOR [48]. To re-emphasize, EM-1 and EM-2 are the most highly selective natural occurring opioids with remarkable specificity for only MOR [1,2]. One interesting patent seeking to overcome the limitations of externally supplied opioids for pain remediation constructed a library of DNA sequences encoding precursors of C-terminal amidated peptides, such as EM-2; however, data on its application were unavailable [87]. Endomorphins were suggested as potential compounds for neuropsychiatric applications, but the patent lacked experimental or patient data on EM-2 in combating these symptoms despite the inclusion of its binding affinity to rat spinal cord membrane (Kiμ = 8.7 nM) [78].
The patent by Persons et al. [49] focused on the modification of the N-terminal Tyr and/or Pro2 and the C-terminal residue Phe4 in EM-2 with substitution of peptide bonds. However, MOR affinity (IC50 values) or functional pharmacological activity (guinea-pig ileum bioassay) cannot be fully ascertained since the data were only presented as < 1 μM, < 10 μM or < 30 μM relative to DAMGO; it terms of MOR agonism, the value for DMAGO was given as 0.03 μM. The amended claims section mentioned acceptable receptor affinities having an IC50 < 100 nM, however, without supplying details on specific EM analogues [49].
Similarly, extensive work by Wang et al. [50,51,52,53] explored modification of EM-1 and EM-2 in Chinese language patents, the data of which was detailed in numerous published articles and briefly discussed herein relative to EM bioactivity: (i) Phenylalanine mimetics at positions Phe3 and Phe4 in EM-2 [88,89] weakly interacted with MOR despite a gain in enzymic stability [88]. Structural analysis by NMR and molecular modeling paradigms were published in two virtually identical papers [90,91]. C-Terminal analogues containing oligoarginine derivatives yielded essentially inactive peptides [92]. Those data support earlier interpretations that C-terminal modifications are detrimental for optimum activity; however, retention of bioactivity and/or change in receptor selectivity was clearly identified in EM analogues in which various aromatic, heteroaromatic or aliphatic groups replaced the Phe4-NH2 group [54] due to, perhaps, a more effective stacking with a hydrophobic side chain in the receptor, such as Trp, Tyr or Phe, through van de Waals forces, π−π bonds, cation-π interactions, atomic volumes or spatial dimensionality. And that interaction produces an alteration in the dynamically fluid 3-dimensional topography of the receptor leading to shifts in bioactivity from agonism to antagonism, from μ to δ selectivity.
(ii) Analogues modified at positions 2 and/or 4 with d-Pro-Gly or p-ClPhe, respectively, exhibited enhanced stability in brain homogenates and serum, but only weak receptor affinities or in vitro bioactivity data were published; the antinociception of the most active analogue [Nά-guanidino-Tyr(Me)1,d-Pro-Gly2,p-ClPhe4]EM-1 was only partially (50%) suppressed by naloxone [89], indicative of other receptor-ligand attributes. However, several N-terminal guanidylated-modified EM-1 in combination with d-Ala2, Sar2 and/or d-Ala2,p-ClPhe4 revealed either enhanced MOR affinity or a reduction by about two-thirds, while antinociception was similar to EM-1 following intracerebroventricular injection in mice due perhaps to marked an increase in stability [93]. (iii) Bivalent and C-terminal esterified analogues in which -OMe and -NHNH2 groups revealed Kiμ values comparable to EM-2 but exhibited weak bioactivity [94,95]. (iv) Incorporation of a reduced amide bond ψ (CH2NH) between Tyr1 and Pro2 (EM-1[ψ] and EM-2[ψ]) were devoid of MOR bioactivity but exhibited a modest DOR activity that was substantially less than the parent peptides [59]. (v) Substitutions at positions 2 and 4 of [Nάamidino-Tyr1]EM-1 were investigated for their cardiovascular effects using rats in comparison to naloxone, which reversed the recorded changes initiated by the peptide analogues [96]. Complete protection of the N-terminal amine obliterates biological activity, such as for example N,N-disubstituted allyl groups [62] among other N-terminal modifications [60], while a N-monosubstituted allyl group is readily accommodated in MOR ligands [62].
Other publications that may be irrelevant to these patents but are mentioned only for the sake of thoroughness, namely the efficacy of EM-1 and/or EM-2 on physiological parameters [29,97–99], their release from nanoparticles [100], or dimeric analogues of EM-2 with or without alkane spacers, which were basically devoid of receptor affinities or functional bioactivities [94].
2.2.1 Cyclic endomorphin analogues
In a series of patented linear and cyclic analogues by Zadina et al. were based on their precursor model peptide for MOR, Tyr-W-MIF-1 [2]; additional cyclic EM-2 analogues were published as well [44,101]. Cyclization utilized the DPPA method [77]. The patent, however, only described the pharmacological properties of one of the 14 cyclic analogues, namely H-Tyr-c-[d-Lys-Trp-Phe] and studied in comparison to EM-1. It exhibited ca. 15-fold higher MOR affinity, 4-fold greater efficacy on GPI (Table 1) and a 10-fold increased antinociception (IC50 = 300 ng) in vivo, which had ca. twice the potency of morphine following intravenous injection.
Table 1.
Receptor affinity and bioactmty of cndormorphin-1 and -2 agonists.
| Affinity constant Ki (nM) | Bioactivity, IC50 (nM) | |||||
|---|---|---|---|---|---|---|
| Sequence | Ki μ | Ki δ | δ/μ | GPI (μ) | MVD (δ) | Ref. |
| YPWF-NH2 (EM-1) | 0.36 | 1506 | 4183 | 3.6 | - | [1] |
| YPFF-NH2 (EM-2) | 0.69 | 9233 | 13381 | 4.0 | - | [1] |
| Dmt-PFF-NH2 | 0.15 | 28 | 188 | 0.07 | 1.87 | [46] |
| Dmt-PF-NH-C2H4-Ph | 0.51 | 18.0 | 35 | 5.03 | >10000* | [54] |
| Dmt-PF-NH-Bzl | 0.52 | 13.8 | 27 | 22.0 | >10000* | [54] |
| Dmt-PF-NH-1-Nph | 0.29 | 20 | 68 | 0.49 | 5.47 | [54] |
| Dmt-PF-NH-5-Qln | 0.11 | 30 | 288 | 0.26 | 0.62 | [54] |
| Dmt-PF-NH-5-Isq | 0.19 | 98 | 517 | 0.94 | >10000* | [54] |
| YP-Dmp-F-NH2 | 0.03 | 1063 | 34967 | 0.38 | 1.39 | [47] |
| Mmt-PFF-NH2 | 0.13 | 529 | 4005 | 0.92 | 28.7* | [75] |
| Emt-PFF-NH2 | 0.06 | 56 | 884 | 0.62 | 1.08* | [75] |
| Det-PFF-NH2 | 0.08 | 70 | 830 | 0.90 | 47.1* | [75] |
| Tmt-PFF- NH2 | 0.11 | 594 | 5347 | 2.31 | 46.4* | [75] |
| YPF-D-F-NH2 | 8.4 | >10000 | >1200 | 25 | 390 | [150] |
| YPFF-OCH3 | 9.1 | >10000 | >11000 | 22.6† | - | [95] |
| Dmt-P-Tmp-Tmp-NH2 | 0.47 | 1.63 | 3.5 | - | - | [120] |
| Dmt-P-Tmp-F-NH2 | 0.18 | 1.83 | 10 | 0.21 | >10000* | [76] |
| Y-Hyp-FF-NH2 | 16.1 | - | 10 | 2550 | - | [43] |
| Dmt-1,5-enediol-F-CO-O-F-H (SSSR) | 0.24 | 56 | 233 | ‡ | - | [57] |
| Dmt-1,5-enediol-F-NH-CONH2-F (SSRR) | 0.42 | 36 | 86 | ‡ | - | [57] |
| Y-c-[D-KWF] | ~0.024 | - | - | ~0.9 | - | [2] |
| Dmt-PWF-NH2 | 0.054 | 5.60 | 104 | 0.27 | >10000 | [62] |
| N-2-aminooctanoyl-Dmt-PWF-NH2 | 0.071 | - | - | ‡ ‡ | - | [60] |
| N-2-aminodecanoyl-Dmt- PWF-NH2 | 0.47 | - | - | ‡ ‡ | - | [60] |
| N-2-aminododecanoyl-Dmt- PWF-NH2 | 0.735 | - | - | ‡ ‡ | - | [60] |
| Y-Ac3c-FF-NH2 | 0.53 | 364 | 687 | - | - | [42] |
| Y-Ac3c-Dmp-F-NH2 | 2.58 | 3140 | 1220 | - | - | [42] |
The patent issued to Maione [82] describes the application of the same cyclic derivative of EM-1 as Zadina et al. [2]. This patent centered on producing a pharmacological effective peptide as an organic or inorganic salt, safe for human consumption, presumably without negative toxicological side effects [82]. In essence, the author states that the peptide salt, suitable for “external, enteral or parenteral applications” would produce analgesia and relief from gastrointestinal distress from diarrhea resulting from disease or drugs implemented in cancer therapy, act as an anti-inflammatory substance (see [31]) and combat “drug dependence in patients” [82]. Nonetheless, considering the potent MOR agonism of this compound [2], it is difficult to visualize any role in alleviating drug addiction! (Even methadone used to treat drug dependency is itself addictive.) Aside from the detailed physicochemical characterization o f various peptide salt complexes, the patent lacked biological and/or pharmacological/physiological data to support the claims [82].
2.2.2 Lipid and glycosylated modified EM-2 analogues
Modification of the backbone of EM-1 and EM-2 with one or more lipid or saccharide moieties arose from the observation that these derivatives improved cell permeability and peptide stability through enhanced hydrophobic properties without abolishing, for the most part, MOR binding properties [60]. Moreover, as shown elsewhere [46], replacement of Tyr by Dmt in EM-2 in these analogues enhanced μ-receptor affinity (Table 1) but had no significant effect on stability in plasma; however, other N-terminal [Dmt1]EM-1 lipid-modified analogues, containing 2-aminododecanoyl and 2-aminodecanoyl enhanced stability ca. 5- and 10-fold, respectively, while maintaining < 1 nM μ-receptor affinity (Table 1) [60]. In general, the N-substituted lipid derivatives exhibited greater biological activity than the corresponding glycosylated compounds [60]. A series of EM-1 analogues containing lipo-, glyco- and liposaccharide moieties internally or at the N and/or C termini generally exhibited weak MOR affinity based on whole SH-SY5Y cells or rat brain synaptomes, yielding comparable values. A high MOR affinity was recorded with the [N-2-aminodecanoyl-Dmt1]EM-1 analogue [61], once again providing substantial evidence for the efficacy of Dmt replacement of Tyr to improve an opioid bioactivity profile and containing only a single substituent on the N-terminal amine [62].
2.3 Antagonists and antagonism
The transformation of the μ agonists EM-1 and EM-2 into high affinity, potent MOR antagonists was described [48] and its pharmacological and physiological actions delineated in considerable detail elsewhere [11,62,63]. These substances, [N-allyl-Dmt1]EM-1 and [N-allyl-Dmt1]EM-2 (Table 2), are defined as neutral μ antagonists due to their lack of inverse agonist properties by functional guanosine 5'-O-(3-[35S]thiotriphosphate) assays in vitro from membranes of cells grown in the presence of morphine or alcohol [11]. More importantly, they completely inhibited naloxone- and naltrexone-evoked withdrawal symptoms following acute morphine dependency in mice [11]. Similarly, [N-allyl-Dmt1]EM-2 effectively reversed the ethanol-induced enhancement of GABAergic neurotransmission in CA1 pyramidal cells of rat hippocampus through inhibition of evoked or spontaneous inhibitory postsynaptic currents (IPSC) [63]. Interestingly, while naltrexone is considered the Food and Drug Administration approved drug of choice in combating alcohol [102,103] and drug addiction (along with naloxone) [104], [N-allyl-Dmt1]EM-2 is > 100-fold more potent in modulating the effects of alcohol [63]. This portends a potential therapeutic role in combating addiction from both alcohol and the morphinans.
Table 2.
Endomorphin-1 and -2 analogues as μ antagonists
2.4 Endomorphin analogues with multiple receptor properties
2.4.1 Bifunctional analogues
This category embraces EM-2 analogues described as having mixed μ agonism/δ antagonism or δ antagonism [54] and/or dual μ/δ agonist properties [76] as an outgrowth of a patent [48]. Although not patented, these analogues are discussed in light of the biological importance of heterodimeric μ- and δ-opioid receptor complexes, which enhance or attenuate receptor function [105–108] through allosteric changes to their three-dimensional topology. Heterodimeric receptors regulate receptor turnover, translocation and endocytotic mechanisms [108]. In addition, heterodimers complex with non-opioid receptors altering the innate function of each component [109–111] that has a direct bearing on opioid dependency, tolerance [112–114] and withdrawal symptoms [115–119].
The receptor-mediated properties of representative analogues of EM-2 are listed in Tables 1–3. Interestingly, while the replacement of the C-terminal Phe amide in [Dmt1]EM-2 with various hydrophobic groups had minimal disruptive effect on MOR affinities [54], the spatial orientation of the quinolyl and naphthyl groups determined by molecular modeling paradigms indicated that the positioning of the heteroaromatic ring was responsible for the highly effective MOR agonism (5- > 6- > 8-quinoline) and the acquisition of DOR agonism; i.e., 5-quinolyl and 1-naphthyl had potent DOR bioactivity [54]. These data further support the concept that increased hydrophobicity at the C-terminus of EM in lieu of Phe4-NH2 in [Dmt]EM-2 exerts substantial effect on the determination of biological activity. In addition, increasing the hydrophobic nature of Phe3, such as replacement by Tmp or even Dmp, produced potent opioid ligands with mixed μ agonist/δ antagonist properties [76] (Table 3).
Table 3.
The δ Antagonist properties of endomorphin-1 and -2 analogues
| Affinity constant Ki (nM) | Bioactivity, IC50 (nM) | ||||||
|---|---|---|---|---|---|---|---|
| Sequence | Ki μ | Ki δ | δ/μ | GPI (μ) | MVD (δ) | δ pA2 | Ref. |
| Dmt-PF-NH-C2H4-Ph | 0.51 | 18.0 | 35 | 5.03 | >10000 | 7.05 | [54] |
| Dmt-P-Mmp-F-NH2 | 0.18 | 4.61 | 26 | 0.16 | >10000 | 6.59 | [76] |
| Dmt-P-Dmp-F-NH2 | 0.07 | 2.27 | 33 | 0.12 | >10000 | 8.15 | [76] |
| Dmt-P-Dmt-F-NH2 | 0.09 | 80.8 | 878 | 1.94 | >10000 | 7.06 | [76] |
| Dmt-P-Tmp-F-NH2 | 0.18 | 1.83 | 10 | 0.21 | >10000 | 9.05 | [76] |
| [N-allyl-Dmt]-PWF-NH2 | 0.26 | 10.3 | 40 | >10000 | >10000 | 7.32 | [62] |
| [N-allyl-Dmt]-PFF-NH2 | 0.45 | 560 | 1244 | >10000 | >10000 | 6.32 | [62] |
| YPFF-NH-(CH2)2-NH-Tic-Dmt | 1.03 | 1.45 | 1.41 | 25.1 | - | 8.9 | [124] |
Recently, Zhang et al. [120] expanding upon earlier published studies [54,75,76] on hydrophobic residues substituted in the sequence of [Dmt1]EM-2. They reported acquisition of a high dual μ/δ affinity and non-receptor selectivity in one compound, H-Dmt-Pro-Tmp-Tmp-NH2 (Table 1), that has yet to be biologically assessed but obviously needs to be addressed. These data compliment a large body of evidence that verifies that enhancing the hydrophobicity of residues 3 and/or 4 affects both MOR and DOR affinities in EM.
2.4.2 Hybrid analogues
Hybrid formulation covalently coupled EM-2 and one of two SP analogues [ESP7 (SP7–11) and ESP6 ([Pro9]SP7–11)] to the C-terminal Phe-amide of EM-2 [65]. The basis of this patent arose from studies on the potentiation of antinociception of morphine by SP in the spinal cord [121–123] and published in considerable detail [66]. An earlier patent on a morphine-SP hybrid discussed the potential clinical value to induce analgesia and reduce tolerance using a hybrid composed of morphine and SP separated by a flexible organic acid spacer; however, no data were presented on the claims for analgesic potentiation [122]. In terms of the EM-SP hybrid molecules, only ESP7 was thoroughly assessed in the patent [65]. The hybrid, however, displayed considerably reduced receptor affinity relative to DAMGO and SP, exhibited ca. 100-fold decrease in binding to MOR and ca. 3,000-fold loss towards the NK1 receptor, respectively. On the other hand, the inhibition of forskolin-stimulated cAMP activity, ESP7 had ca. 10% the potency of either DAMGO or EM-2 [66]. In addition, the intracerebroventricular injected ESP7 produced moderate analgesia; however, control data against an equivalent molar concentration of morphine was absent, nonetheless naloxone completely inhibited analgesia induced by a low dose of ESP7 indicative of an opioid-mediated effect [65].
A hybrid molecule formed by coupling the C-termini of EM-1 and Dmt-Tic-NH-(CH2)2-NH2 (a moderate DOR antagonist) enhanced MOR affinity by 50% while elevating DOR affinity > 6000-fold to yield a formidable DOR antagonist (pA2 = 8.9), while retaining about half the MOR agonist activity [124] (Table 3). Like genetic hybrids, this F1 offspring, a mixed μ agonist/δ antagonist, had new biological properties considerably different than either parent opioid molecule. The question of efficacy for this bivalent molecule is unknown, but the potential application in pain remediation needs to be addressed.
3. Potential therapeutic applications
One interesting application of EM-2 is its inclusion in cosmetics for people with sun sensitive skin and its accompanying manifestations [79,81]. The authors claim that the presence of EM-2 might attenuate the symptoms sufficiently to allow people with these sensitivities to use a host of topical cosmetic preparations. In particular, preparations containing the beneficial effects of α-hydroxyl acids and retinol (vitamin A), known to combat the photoaging process in skin, to smooth out wrinkles or acne scars through increased rate of epithelial cell turnover with a concomitant enhancement of collagen and elastin synthesis. The rationale for the use of EM in a topical ointment was previously substantiated by animal studies: (i) In the absence of MOR, mice undergo epidermal hypertrophy suggesting opioid involvement in skin homeostasis [125]; and (ii) the attenuation of pruritus by a dermatological cream containing 1% naltrexone [126], a non-selective opioid antagonist [127], indicated interaction with opioid receptors since skin also contains DOR and KOR [125,128,129], the former affecting differentiation and wound healing [129]. Thus, stimulation of MOR by EM might be theoretically applicable to alleviate skin sensitivities in cosmetic emulsions, assuming, of course, a charged, hydrophilic peptide, such as an EM, can sufficiently penetrate the epidermal barrier to interact with dermal MOR even with the assistance of emulsifiers, hydrotropic compounds or swelling agents [79].
In other applications with EM-2, such as a therapeutic agent for the “prophylaxis and/or treatment of cancers” and numerous diseases, were not substantiated by the data supplied in the patent [80]. The inventors provided a series of formulations, such as gel, lotion and mother's milk for infants to use with or in the delivery of EM-2. Nonetheless, EM-2 exhibited very minimal or no effects on cell viability or blood cell proliferation, prevention of apoptosis, marginal effects of cytokine profiling and ineffective on lipopolysaccharide induction of TNFα in spleen cells, and lacked inhibition of bacterial growth [80]. Only a modest 15.6% inhibition of hepatitis B virus replication in HEP-G2 cells was observed as well as a 9.5% reduction in human cytomegalovirus plaque formation relative to the positive controls of 92.0% and 100%, respectively [80].
Involvement of EM-1 and EM-2 in immune function was the basis of a patent for the treatment of existing inflammation or prophylaxis of inflammation [31]. The patent was based on their discovery and isolation of immunoreactive EM-1 and EM-2 from immune tissues (spleen, thymus) [30] and in the synovial tissue from rats with adjuvant-induced arthritis [32]; in normal human subjects, both opioids were detected in spleen [30,32]. Other supporting data revealed the presence of immunohistochemical staining of both EM-1 and EM-2 in distinct cells in rat spleen [25]. In the patent, the authors provided experimental evidence that both opioids are secreted upon stimulation with concanavalin A from cultured human lymphocytes (T- and B-cells, macrophages). While individual data points were statistically relevant (20% reduction of inflammation in a rat paw model with induced adjuvant arthritis following systemic injection of EM-1), the supporting data failed to demonstrate a linear dose response and lacked data on preclinical trials [31].
Considering that EM-1 and EM-2 act on cardiac output as vasodepressor agents by decreasing systemic arterial blood pressure in a naloxone-reversible manner in both rat [34,36,130] and rabbit [131], it is interesting that these opioids or analogues thereof were not investigated in human subjects exhibiting hypertension. This physiological assessment of the action of EM-2 fully supports the involvement of MOR agonists in homeostatic mechanisms [16,19].
4. Conclusions
Despite the enormity of the investigative studies undertaken with the endomorphins [13] and the potential to exacerbate various health syndromes, neither EM-2 nor any of the analogues patented or published in the research literature have apparently reached the level of a therapeutic drug. While the authors are unaware of clinical trials utilizing EM, trademarked names and proprietary formulations of encapsulated or time-released drugs and inclusion into cosmetic creams or ointments would obscure detection. On-line searching of patents further proved futile to uncover clinical applications.
As major pharmaceutical firms continue to retrench from actively developing new drugs in-house with the termination of patent protection, the lack of foresight to invest in medicinal chemistry will be to the detriment of society. In particular, the epidemic of narcotic addiction [132], which is wholly dependent on MOR [16], is facing a crisis in the absence of reliable antagonists with reduced or the absence of side effects in contrast to withdrawal symptoms often accompanying treatment by naltrexone or naloxone [37,133]. Furthermore, no publications reveal the application of EM-2 antagonists to address the obesity pandemic—which is considered another form of addiction of the neural reward system [134,135]—or towards ameliorating osteoporosis due to the presence of opioid receptors on osteoblast-like cells which are stimulated by MOR antagonists [136]. Similarly, non-addictive or dual functioning EM-2 agonists could be tested to mitigate the negative effects observed in geriatric patients under treatment for pain management with opiates among other drugs [137]. Moreover, multiple direct and indirect interactions occur between opioids and neuroregulatory peptides as well as their receptors forming heterodimeric complexes with opioid receptors to directly impinge on the opioid system to mediate change [138,139].
5. Expert opinion
Despite extensive laboratory studies and projected or potential preclinical applications for EM and analogues thereof [13], their application as therapeutic drugs to alleviate acute, chronic or neuropathic pain symptoms as pure μ agonists or mixed μ agonists/δ antagonists has yet to materialize. Equally, the shortcoming to ameliorate opiate addiction and obesity [135] with neutral or pure MOR antagonists [62,63] fails to ignite interest in either the pharmaceutical industry or medical community. Their agendas seem to be at odds with providing creative solutions for long-term goals of developing an equitable society [140]. “Valuable breakthroughs…[are]…driven by the curiosity of individuals,” Ahmed H. Zewail emphasized, when “…dreamers must be willing, and allowed, to take risks.” The potential application of selective MOR antagonists to reduce the loss of bone mass density in obesity-associated osteoporosis [141,142] serves as one example to overcome the serious and deleterious side effects of bisphosphonate-based drugs [143–147], products heavily marketed by pharmaceutical firms.
The inherent ease in the ability to alter the basic peptidic structure of EM, sometimes quite radical while maintaining receptor selectivity and enhancing receptor affinities, should provide a further impetus to seek and develop uniquely bioactive analogues, perhaps even specifically designed to differentiate between supraspinal and peripheral sites, or even relieve specific symptom modalities. Selective targeting symptoms could be accomplished with judicious studies on the efficacy of the types of EM analogues discussed in this review. Furthermore, it should be noted that in addition to clinical intervention, veterinary medicine could be an equal beneficiary in the application and development of new EM-based drugs. While the long road ahead remains challenging, fraught with the pitfalls of discarded failures, Goethe reaches out over the centuries to continually challenge and admonish society: “Nothing is more terrible than ignorance in action.”
Article highlights
Discovery of EM-1 and -2 in 1997 provided new opioid ligands to combat various medical symptoms, most notably pain relief.
To date, many patents were published from around the world and through the US Patent Office.
The voluminous number of publications on the formation of EM analogues provides insights on their innate structure for the development of therapeutic drugs.
Selective analogues of EM-2 enhanced its biological half-life and provided ligands with potential clinical application.
While EM agonists are antinociceptive, antagonists are applicable to alleviating drug and alcohol addiction, and suppressing reward mechanisms.
Experimental data point to potential applications to ameliorate numerous disease states, including but not limited to inflammation, alcoholism, opioid bowel dysfunction, osteoporosis and obesity, while enhancing the immune system.
Formulation of mixed and dual μ/δ ligands acts as a multi-target therapeutic agent to reduce pain while alleviating possible detrimental side effects.
This box summarizes key points contained in the article.
Acknowledgements
The authors appreciate the consistent and professional support of Stephanie Holmgren, MLA, Library Information Services Branch, NIEHS, our numerous former colleagues the world over, Dr. Hiroaki Taguichi, Suzuka University of Medical Science, Mie, Japan, for preparing the scheme on the synthesis of 1,5-enediaol EM-2 analogues, and in part to the Intramural Research Program of the NIH and NIEHS.
Abbreviations and definitions
- Ac3c
1-aminocyclopropanecarboxylic acid, an α,α-disubstituted glycine
- Boc
tert-butyloxycarbonyl
- Bzl
benzyl
- cAMP
cyclic adenosinemonophosphate
- Cl2(PCy3)(IMesH2)RuCHPh
a ruthenium catalyst used in the olefin metathesis reaction in solid phase peptide synthesis [56,57]
- CD
circular dichroism
- Det
2',6'-diethyl-l-tyrosine
- DAIBAL-H
diisobutylaluminium hydride
- DAMGO
[d-Ala2,NMePhe4,Glyol5]enkephalin, a MOR agonist
- DIPEA
diisopropylethylamine
- Dmp
2',6'-dimethyl-l-phenylalanine
- Dmt
2',6'-dimethyl-l-tyrosine
- DOR
δ-opioid receptor
- DPPA
diphenylphosphoryl azide
- EM
endomorphin
- Emt
2'-ethyl-6'-methyl-l-tyrosine
- Et2O
diethyl ether
- Fmoc
9-fluroenylmethyloxycarbonyl
- Fmoc amide resin
4-(2',4'-dimethoxyphenyl-Fmoc-aminomethyl)-phenoxyacetamidoethyl resin
- GPI
guinea pigileum to determine MOR pharmacological activity
- GTP
guanosinetriphosphate
- HBTU
O-benzotriazole-N,N,N',N'-tetramethyluronium-hexafluorophosphate
- HOBt
1-hydroxybenzotriazole
- HPLC
high performance liquid chromatography
- Hyp
hydroxyproline
- IBCF
isobutyl chloroformate
- IC50
the molar concentration required for 50% inhibition of electrically induced contraction of GPI strips or whole MVD from a single animal
- 5-Isq
5-isoquinolyl
- Ki
the binding constant of ligand to opioid receptors [148]
- KOR
κ-opioid receptor
- MALDI-TOF
matrix-assisted laser desorption ionization time-of-flight
- MOR
μ-opioid receptor
- NK1
neurokinin 1 receptor
- NMM
N-methylmorpholine
- Mmp
2'-monomethyl-l-phenylalanine
- Mmt
2'-monomethyl-l-tyrosine
- MVD
mouse vas deferns used for assessment of DOR activity in vitro
- NMP
N-methylpyrrolidone
- NMR
nuclear magnetic resonance
- 1-Nph
1-naphthyl
- pA2
is the negative log of the molar concentration of the analogue required to double the EM-2 concentration to elicit the original response
- opioid
a peptide compound capable of eliciting analgesia
- opiate
a plant derived alkaloid belonging to the general class of morphinans, which exert biological addiction
- Ph
phenyl
- Phe-NH-Rink amide AM resin
the solid support used in EM synthesis
- PyBop
benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate
- 5-Qln
5-quinolyl
- SAR
structural-activity relationship
- SP
substance P
- TFA
trifluoroacetic acid
- Tmp
2',3',6'-trimethyl-l-phenylalanine
- Tmt
2',3',6'-trimethyl-l-tyrosine
- TNFα
tumor necrosis factor-alpha. Single letter designation for amino acids: F, phenylalanine
- K
lysine
- P
proline
- W
tryptophan
- Y
tyrosine
Footnotes
Declaration of interest The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (●) or of considerable interest (●●) to readers.
- 1.Zadina JE, Hackler L, Ge L-J, Kastin AJ. A potent and selective endogenous agonist for the μ-opiate receptor. Nature. 1997;386:499–501. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 2.Zadina JE, Kastin AJ, Hackler L. Mu-Opiate receptor peptides. WO98/42732. 1998 [Google Scholar]
- 3.Hackler L, Zadina JE, Ge LJ, Kastin AJ. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 1997;18:1635–9. doi: 10.1016/s0196-9781(97)00259-3. [DOI] [PubMed] [Google Scholar]
- 4.Largent-Milnes TM, Vanderah TW. Recently patented and promising ORL-1 ligands: where have we been and where are we going? Ex Opin Ther Patents. 2010;20:291–305. doi: 10.1517/13543771003602004. [DOI] [PubMed] [Google Scholar]
- 5.Stevens CW, Brasel CM, Mohan S. Cloning and bioinformatics of amphibian mu, delta, kappa, and nociceptin opioid receptors expressed in brain tissue: Evidence for opioid receptor divergence in mammals. Neurosci Lett. 2007;419:189–94. doi: 10.1016/j.neulet.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreborg S, Sundstrom G, Larsson TA, et al. Evolution of vertebrate opioid receptors. Proc Natl Acad Sci U S A. 2008;105:15487–92. doi: 10.1073/pnas.0805590105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldrich J. Analgesics. John Wiley & Sons; New York: 1996. pp. 321–441. [Google Scholar]
- 8.Blumberg H, Dayton HB, Wolf PS. Counteraction of narcotic antagonist analgesics by the narcotic antagonist naloxone. Proceedings of the Society of Experimental Biololgy and Medicine. 1966;123:755–8. doi: 10.3181/00379727-123-31595. [DOI] [PubMed] [Google Scholar]
- 9.Julius D, Renault P. National Technical Information Service. Springfield, VA: 1976. Narcotic antagonists: naltrexone. Progress Report. 22161. [Google Scholar]
- 10.Goodman AJ, Le Bourdonnec B, Dolle RE. Mu opioid receptor antagonists: recent developments. ChemMedChem. 2007;2:1552–70. doi: 10.1002/cmdc.200700143. [DOI] [PubMed] [Google Scholar]
- 11.Marczak ED, Jinsmaa Y, Li T, et al. [N-allyl-Dmt1]-endomorphins are μ-opioid receptor antagonists lacking inverse agonist properties. J Pharmacol Exp Ther. 2007;323:374–80. doi: 10.1124/jpet.107.125807. [DOI] [PubMed] [Google Scholar]; ●● This article details potential application and properties of endomorphin-derived μ antagonists.
- 12.Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28:407–15. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Keresztes A, Borics A, Toth G. Recent advances in endomorphin engineering. ChemMedChem. 2010;5:1176–96. doi: 10.1002/cmdc.201000077. [DOI] [PubMed] [Google Scholar]; ●● A thoroughly written review on the chemistry and biology of endomorphins.
- 14.Appleyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid β-endorphin in energy homeostatis. Endocrinology. 2003;144:1753–60. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- 15.Woods SC, D'Alessio DA, Tso P, et al. Consumption of high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–8. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 17.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: When reward outweighs homeostasis. Physiol Behav. 2007;91:506–12. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Chao D, Bazzy-Asaad A, Balboni G, et al. δ-, but not μ-, opioid receptor stabilizes K+ homeostasis by reducing Ca2+ influx in the cortex during acute hypoxia. J Cell Physiol. 2007;212:60–7. doi: 10.1002/jcp.21000. [DOI] [PubMed] [Google Scholar]
- 19.Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nature Reviews Drug Discovery. 2008;7:694–711. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- 20.Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- 21.Zubieta J-K, Bueller JA, Jackson LR, et al. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J Neurosci. 2005;25:7754–62. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun R-Q, Wang Y, Zhao C-S, et al. Changes in brain content of nociceptin/orphanin FQ and endomorphin 2 in a rat model of neuropathic pain. Neurosci Lett. 2001;311:13–16. doi: 10.1016/s0304-3940(01)02095-x. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Schild S, Zadina JE, Gerall AA, et al. Localization of endomorphin-2-like immunoreactivity in the rat medulla and spinal cord. Peptides. 1997;18:1641–9. doi: 10.1016/s0196-9781(97)00320-3. [DOI] [PubMed] [Google Scholar]
- 24.Mousa SA, Machelska H, Schafer M, Stein C. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J Neuroimmunol. 2002;126:5–15. doi: 10.1016/s0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 25.Seale JV, Jessop DS, Harbuz MS. Immunohistochemical staining of endomorphin 1 and 2 in the immune cells of the spleen. Peptides. 2004;25:91–4. doi: 10.1016/j.peptides.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Groneberg DA, Fischer A. Endogenous opioids as mediators of asthma. Pulm Pharmacol Ther. 2001;14:383–9. doi: 10.1006/pupt.2001.0305. [DOI] [PubMed] [Google Scholar]
- 27.Kamei J, Morita K, Saitoh A, Nagase H. The antitussive effects of endomorphin-1 and endomorphin-2 in mice. Eur J Pharmacol. 2003;467:219–22. doi: 10.1016/s0014-2999(03)01634-0. [DOI] [PubMed] [Google Scholar]
- 28.Storr M, Geisler F, Neuhuber WL, et al. Endomorphin-1 and -2, endogenous ligands for the μ-opioid receptor, inhibit striated and smooth muscle contraction in the rat oesophagus. Neurogastroenterol Motil. 2000;12:441–8. doi: 10.1046/j.1365-2982.2000.00220.x. [DOI] [PubMed] [Google Scholar]
- 29.Yu Y, Wang X, Cui Y, et al. Abnormal modulation of cholinergic neurotransmission by endomorphin 1 and endomorphin 2 in isolated bronchus of type 1 diabetic rats. Peptides. 2006;27:2770–7. doi: 10.1016/j.peptides.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Jessop DS, Major GN, Coventry TL, et al. Novel opioid peptides endomorphin-1 and endomorphin-2 are present in mammalian immune tissues. J Neuroimmunol. 2000;106:53–9. doi: 10.1016/s0165-5728(99)00216-7. [DOI] [PubMed] [Google Scholar]
- 31.Jessop DW, Harbuz MS. Inflammation modulatory compound. WO 03/020304 A2. 2003 [Google Scholar]; ● A patent for the application of endomorphin to combat inflammation.
- 32.Jessop DS, Richards LJ, Harbuz MS. Opioid peptides endomorphin-1 and endomorphin-2 in the immune system in humans and in a rodent model of inflammation. Ann N Y Acad Sci. 2002;966:456–63. doi: 10.1111/j.1749-6632.2002.tb04247.x. [DOI] [PubMed] [Google Scholar]
- 33.McDougall JJ, Baker CL, Hermann PM. Attenuation of knee joint inflammation by peripherally administered endomorphin-1. J Mol Neurosci. 2003;22:125–37. doi: 10.1385/JMN:22:1-2:125. [DOI] [PubMed] [Google Scholar]
- 34.Champion HC, Zadina JE, Kastin AJ, et al. Endomorphin 1 and 2, endogenous ligands for the μ-opioid receptor, decrease cardian output, and total peripheral resistance in the rat. Peptides. 1997;18:1393–7. doi: 10.1016/s0196-9781(97)00210-6. [DOI] [PubMed] [Google Scholar]
- 35.Champion HC, Zadina JE, Kastin AJ, et al. The endogenous mu-opioid receptor agonists endomorphins 1 and 2 have novel hypotensive activity in the rabbit. Biochem Biophys Res Commun. 1997;235:567–70. doi: 10.1006/bbrc.1997.6843. [DOI] [PubMed] [Google Scholar]
- 36.Champion HC, Zadina JE, Kastin AJ, et al. The endogenous μ-opioid agonists, endomorphin 1 and 2, have vasodilator activity in the hindquarters vascular bed of the rat. Life Sci. 1997;61:409–15. doi: 10.1016/s0024-3205(97)01029-1. [DOI] [PubMed] [Google Scholar]
- 37.Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164:1322–34. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begley DJ. Peptides and the blood-brain barrier: the status of our understanding. Ann N Y Acad Sci. 1994;739:89–100. doi: 10.1111/j.1749-6632.1994.tb19810.x. [DOI] [PubMed] [Google Scholar]
- 39.Shane R, Wilk S, Bodnar RJ. Modulation of endomorphin-2-induced analgesia by dipeptidyl peptidase IV. Brain Res. 1999;815:278–86. doi: 10.1016/s0006-8993(98)01121-4. [DOI] [PubMed] [Google Scholar]
- 40.Corbett AD, Henderson G, McKnight AT, et al. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol. 2006;147:S153–62. doi: 10.1038/sj.bjp.0706435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardillo G, Gentilucci L, Qasem AR, et al. Endomorphin-1 analogues containing β-proline are μ-opioid receptor agonists and display enhanced enzymatic hydrolysis resistance. J Med Chem. 2002;45:2571–8. doi: 10.1021/jm011059z. [DOI] [PubMed] [Google Scholar]
- 42.Yamada T, Tani Y. Novel endomorphin derivatives. WO 02/102833 A1. 2002 [Google Scholar]
- 43.Biondi B, Giannini E, Negri L, et al. Opioid peptides: synthesis and biological activity of new endomorphin analogues. Internatl J Pept Res Ther. 2006;12:145–51. [Google Scholar]
- 44.Janecka A, Fichna J, Kruszynski R, et al. Synthesis and antinociceptive activity of cyclic endomorphin-2 and morphiceptin analogs. Biochem Pharmacol. 2005;71:188–95. doi: 10.1016/j.bcp.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Cardillo G, Gentilucci L, Melchiorre P, et al. Synthesis and binding activity of endomorphin-1 analogues containing β-amino acids. Bioorg Med Chem Lett. 2000;10:2755–8. doi: 10.1016/s0960-894x(00)00562-x. [DOI] [PubMed] [Google Scholar]
- 46.Okada Y, Fujita Y, Motoyama T, et al. Structural studies of [2',6'-dimethyl-L-tyrosine1]endomorphin-2 analogues: enhanced activity and cis orientation of the Dmt-Pro amide bond. Biorg Med Chem. 2003;11:1983–4. doi: 10.1016/s0968-0896(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki Y, Sasaki A, Niizuma H, et al. Endomorphin 2 analogues containing Dmp residue as an aromatic amino acid surrogate with high μ-opioid receptor affinity and selectivity. Biorg Med Chem. 2003;11:675–8. doi: 10.1016/s0968-0896(02)00601-6. [DOI] [PubMed] [Google Scholar]
- 48.Lazarus LH, Okada Y, Li T. Dmt-derivative compounds and related compositions and methods of use. WO 2007/027628 A1. 2011 [Google Scholar]; ●● This patent covers not only endomorphin derivatives, but also other unique opioids containing the N-terminal Dmt residue.
- 49.Persons PE, Hauske J, Hussoin R. Tetrapeptides, analogs and peptidomimetics which bind selectively mammalian opioid receptors. WO 99/65932. 1999 [Google Scholar]
- 50.Wang RY. C-terminal modified endomorphin-1, endomorphin-2. CN 100378126. 2006 [Google Scholar]
- 51.Wang RZ. Endomorphin analog and its preparing method. CN20041026314. 2006 [Google Scholar]
- 52.Wang R, Liu H, Liu X. Combined chemical modified endomorphin-1 and method for preparing same. CN20061104541. 2008 [Google Scholar]
- 53.Wang R, Yu Y, Liu H. C end modification endomorphin 2. CN20071147468. 2008 [Google Scholar]
- 54.Fujita Y, Tsuda Y, Li T, et al. Development of potent bifunctional endomorphin-2 analogues with mixed μ-/δ-opioid agonist and δ-opioid antagonist properties. J Med Chem. 2004;47:3591–9. doi: 10.1021/jm030649p. [DOI] [PubMed] [Google Scholar]
- 55.Sperlinga E, Kosson P, Urbanczyk-Lipowska Z, et al. 6-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid mimics active conformation of tyrosine in opioid peptides. Bioorg Med Chem Lett. 2005;15:2467–9. doi: 10.1016/j.bmcl.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 56.Harrison BA, Gierasch TM, Neilan C, et al. High-afifnity mu opioid receptor ligands discovered by the screening of an exhuastively stereodiversified library of 1,5-enediols. J Am Chem Soc. 2002;124:13352–3. doi: 10.1021/ja027150p. [DOI] [PubMed] [Google Scholar]
- 57.Harrison BA, Pasternak GW, Verdine GL. 2,6-Dimethyltyrosine analogues of a stereodiversified ligand library: highly potent, selective, non-peptidic μ opioid receptor agonists. J Med Chem. 2003;46:677–80. doi: 10.1021/jm025608s. [DOI] [PubMed] [Google Scholar]
- 58.Harrison B, Gierasch TM, Verdine GL, et al. Mu opioid receptor ligands: methods of use and synthesis. WO 2004/033414 A1. 2004 [Google Scholar]; ● This represents a patent on the synthesis of potentially interesting endomorphin analogues, the details of which are described in the preceding two articles.
- 59.Zhao QY, Chen Q, Yang DJ, et al. Endomorphin 1[Ψ] and endomorphin 2[Ψ], endomorphins analogues containing a reduced (CH2NH) amide bond between Tyr1 and Pro2, display partial agonist potency but significant antinociception. Life Sci. 2005;77:1155–65. doi: 10.1016/j.lfs.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Toth I. Compounds and methods for the treatment of pain. WO 2007/112492 A1. 2007 [Google Scholar]; ● An interesting patent on the potential application on endomorphins suitable for passage through membrane barriers while retaining bioactivity due to the increased in overall hydrophobicity.
- 61.Koda Y, Del Orgo M, Wessling ST, et al. Synthsis and in vitro evaluation of a library of modified endomorphin 1 peptides. Biorg Med Chem. 2008;16:6286–96. doi: 10.1016/j.bmc.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li T, Jinsmaa Y, Nedachi M, et al. Transformation of μ-opioid receptor agonists into biologically potent μ-opioid receptor antagonists. Biorg Med Chem. 2007;15:1237–51. doi: 10.1016/j.bmc.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Li Q, Marczak ED, Okada Y, et al. The novel μ-opioid receptor antagonist [N-allyl-Dmt1]-endomorphin-2 attenuates the enhancement of GABAergic neurotransmission by ethanol. Alc Alcohol. 2009;44:13–19. doi: 10.1093/alcalc/agn085. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● The article describes the application of a selective EM-2 μ-opioid antagonist to ameliorate the effects of alcoholism as a more potent and potential useful drug of choice than naltrexone.
- 64.In Y, Minoura K, Tomoo K, et al. Structural function of C-terminal amidation of endomorphin. Conformational comparison of μ-selective endomorphin-2 with its C-terminal free acid, studied by 1H-NMR spectroscopy, molecular calculation, and X-ray crystallography. FEBS J. 2005;272:5079–97. doi: 10.1111/j.1742-4658.2005.04919.x. [DOI] [PubMed] [Google Scholar]
- 65.Carr DB, Lipkowski AW, Kream RM, et al. Novel chimeric analgesic peptides. EP 2045269 A1. 2009 [Google Scholar]
- 66.Foran SE, Carr DB, Lipkowski AW, et al. A substance P-opioid chimeric peptide as a unique nontolerance-forming analgesic. Proc Natl Acad Sci USA. 2000;97:7621–26. doi: 10.1073/pnas.130181897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salvadori S, Attila M, Balboni G, et al. δ Opioidmimetic antagonists: prototypes for designing a new generation of ultraselective opioid peptides. Mol Med. 1995;1:678–89. [PMC free article] [PubMed] [Google Scholar]
- 68.Salvadori S, Balboni G, Guerrini R, et al. Evolution of the Dmt-Tic pharmacophore: N-terminal methylated derivatives with extraordinary δ opioid antagonist activity. J Med Chem. 1997;40:3100–8. doi: 10.1021/jm9607663. [DOI] [PubMed] [Google Scholar]
- 69.Lazarus LH, Bryant SD, Cooper PS, et al. Design of δ-opioid peptide antagonists for emerging drug applications. Drug Discov Today. 1998;3:284–94. [Google Scholar]
- 70.Bryant SD, Jinsmaa Y, Salvadori S, et al. Dmt and opioid peptides: a potent alliance. Biopolymers (Peptide Sci) 2003;71:86–102. doi: 10.1002/bip.10399. [DOI] [PubMed] [Google Scholar]
- 71.Balboni G, Onnis V, Cenzo C, et al. Effect of lysine at C-terminus of the Dmt-Tic opioid pharmacophore. J Med Chem. 2006;49:5610–7. doi: 10.1021/jm060741w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dygos JH, Yonan EE, Scaros MG, et al. A convenient asymmetric synthesis of the unnatural amino acid 2,6-dimethyl-l-tyrosine. Synthesis. 1992;8:741–3. [Google Scholar]
- 73.Stewart JM, Young JD. Solid Phase Peptide Synthesis. Pierce Chemical Company; Rockford, IL: 1984. [Google Scholar]
- 74.Atherton E, Sheppard RC. Solid phase peptide synthesis: a practical approach. Oxford University Press; IRL Press; 1989. [Google Scholar]
- 75.Li T, Fujita Y, Tsuda Y, et al. Development of potent μ-opioid receptor ligands using unique tyrosine analogues of endomorphin-2. J Med Chem. 2005;48:586–92. doi: 10.1021/jm049384k. [DOI] [PubMed] [Google Scholar]
- 76.Li T, Shiotani K, Miyazaki A, et al. Bifunctional [2',6'-dimethyl-l-tyrosine1]endomorphin-2 analogues substituted at position 3 with alkylated phenylalanine derivatives yield potent mixed μ-agonist/δ-antagonist and dual μ-/δ-agonist opioid ligands. J Med Chem. 2007;50:2753–66. doi: 10.1021/jm061238m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt R, Neubert K. Cyclization studies with tetra- and pentapeptide sequences corresponding to β-casomorphins. Int J Pept Prot Res. 1991;37:502–7. doi: 10.1111/j.1399-3011.1991.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 78.Nyberg F, Hallberg A, Hallberg M, et al. Therapeutic methods and compositions employing peptide compounds. WO 2010/004535 A2. 2010 [Google Scholar]
- 79.Gillon V, Moussou P, Contet-Audonneau J-L, et al. Oligopeptides and their use. WO 2007/051550 A1. 2007 [Google Scholar]
- 80.Bevec D, Cavalli F, Cavalli V, Bacher G. Use of a peptide as a therapeutic agent. PCT/EP2008/007939. 2009 [Google Scholar]
- 81.Moussou P, Contet-Audenneau J-L, Gillon V. Oligopeptides and their use. EP1782819 A1. 2007 [Google Scholar]
- 82.Maione T. Advantagous salts of mu-opiate receptor peptides. WO 2009/076672 A1. 2009 [Google Scholar]
- 83.Aimoto S. Contemporary methods for peptide and protein synthesis. Curr Org Chem. 2001;5:45–87. [Google Scholar]
- 84.Okada Y, Fukumizu A, Takahashi M, et al. Synthesis of stereoisomeric analogues of endomorphin-2, H-Tyr-Pro-Phe-Phe-NH2, and examination of their opioid receptor binding activities and solution conformation. Biochem Biophys Res Commun. 2000;276:7–11. doi: 10.1006/bbrc.2000.3416. [DOI] [PubMed] [Google Scholar]
- 85.Blackwell HE, O'Leary DJ, Chatterjee AK, et al. New approaches to olefin cross-metathesis. J Am Chem Soc. 2000;122:58–71. [Google Scholar]
- 86.Scholl M, Ding S, Lee SW, et al. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org Lett. 1999;1:953–6. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 87.Wolfe DP, Glorioso JC, Fink DJ. Method of amidated peptide biosynthesis and delivery in vivo: endomorphin-2. WO 2006132925 A2. 2006 [Google Scholar]
- 88.Gao Y, Liu X, Liu W, et al. Opioid receptor binding and antinociceptive activity of the analogues of endomorphin-2 and morphiceptin with phenylalanine mimics in the position 3 or 4. Bioorg Med Chem Lett. 2006;16:3688–92. doi: 10.1016/j.bmcl.2006.04.063. [DOI] [PubMed] [Google Scholar]
- 89.Liu HR, Zhang B, Liu X, et al. Endomorphin-1 analogues with enhanced metabolic stability and systemic analgesic activity: design, synthesis, and pharmacolgical characterization. Bioorg Med Chem. 2007;15:1694–702. doi: 10.1016/j.bmc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 90.Shao X, Gao Y, Zhu C, et al. Conformational analysis of endomorphin-2 analogs with phenylalanine mimics by NMR and molecular modeling. Bioorg Med Chem. 2007;15:3539–47. doi: 10.1016/j.bmc.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 91.Wang CL, Yao JL, Yu Y, et al. Structure-activity study of endomorphin-2 analogs with C-terminal modifications by NMR spectroscopy and molecular modeling. Bioorg Med Chem. 2008;16:6415–22. doi: 10.1016/j.bmc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Wang CL, Guo C, Wang YQ, et al. Synthesis and antinociceptive effects of endomorphin-1 analogs with C-terminal linked by oligoarginine. Peptides. 2011;32:293–9. doi: 10.1016/j.peptides.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 93.Liu HM, Liu XF, Yao JL, et al. Utilization of combined chemical modifications to enhance the blood-brain barrier permeability and pharmacological activity of endomorphin-1. J Pharmacol Exp Ther. 2006;319:308–16. doi: 10.1124/jpet.106.106484. [DOI] [PubMed] [Google Scholar]
- 94.Gao Y-F, Zhai M-X, Liu W-X, et al. Structure-activity relationships of the dimeric analogues of endomorphin-2 with different lengths of spacers. Prot Pept Lett. 2008;15:275–9. doi: 10.2174/092986608783744199. [DOI] [PubMed] [Google Scholar]
- 95.Wang C-L, Cuo C, Zhou Y, et al. In vitro and in vivo characterization of opioid activities of C-terminal esterified endomorphin-2 analogues. Peptides. 2009;30:1697–704. doi: 10.1016/j.peptides.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 96.Liu H, Yang Y, Xin R, et al. Differential cardiovascular effects of synthetic peptides derived from endomorphin-1 in anesthetized rats. Peptides. 2008;29:1048–56. doi: 10.1016/j.peptides.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 97.Yu Y, Cui Y, Wang X, et al. In vitro characterization of the effects of endomorphin 1 and 2, endogenous ligands for mu-opioid receptors, on mouse colonic motility. Biochem Pharmacol. 2007;73:1384–93. doi: 10.1016/j.bcp.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Dai X, Cui SG, Wang T, et al. Endogenous opioid peptides, endomorphin-1 and -2 and deltorphin I, stimulate angiogenesis in the CAM assay. Eur J Pharmacol. 2008;579:269–75. doi: 10.1016/j.ejphar.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 99.Gong P, Chen FX, Ma GF, et al. Endomorphin 1 effectively protects cadmium chloride-induced hepatic damage in mice. Toxicology. 2008;251:35–44. doi: 10.1016/j.tox.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 100.Liu H, Ni J, Wang R. In vitro release performance and analgesic activity of endomorphin-1 loaded nanoparticles. Pharmazie. 2006;61:450–2. [PubMed] [Google Scholar]
- 101.Perlikowska R, do-Rego J-C, Cravezic A, et al. Synthesis and biological evaluation of cyclic endomorphin-2 analogs. Peptides. 2010;31:339–45. doi: 10.1016/j.peptides.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 102.Streeton C, Whelan G. Naltrexone, a replase prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alc Alcohol. 2001;36:544–52. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- 103.Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Internatl J Neuropsychopharmacol. 2005;8:267–80. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 104.Heidbreder C. Novel pharmacotherapeutic targets for the management of drug addiction. Eur J Pharmacol. 2005;526:101–12. doi: 10.1016/j.ejphar.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 105.Gomes I, IJzerman AP, Ye K, et al. G Protein-coupled receptor heteromerization: A role in allosteric modulation of ligand binding. Mol Pharmacol. 2011;79:1044–52. doi: 10.1124/mol.110.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He SQ, Zhang ZN, Guan JS, et al. Facilitation of μ-Opioid Receptor Activity by Preventing δ-Opioid Receptor-Mediated Codegradation. Neuron. 2011;69:120–31. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 107.Yekkirala AS, Kalyuzhny AE, Portoghese PS. Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [d-Ala2-MePhe4-Glyol5]enkephalin as selective μ–δ agonists. Acs Chem Neurosci. 2010;1:146–54. doi: 10.1021/cn9000236. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● An insightful article, which deals with the consequences of opioid receptor heterodimerization.
- 108.Milan-Lobo L, Whistler JL. Heteromerization of the μ- and δ-opioid receptors produces ligand-biased antagonism and alters μ-receptor trafficking. J Pharmacol Exp Ther. 2011;337:868–75. doi: 10.1124/jpet.111.179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diaz A, Pazos A, Florez J, et al. Regulation of μ-opioid receptors, G-protein-coupled receptor kinases and β-arrestin 2 in the rat brain after chronic opioid receptor antagonism. Neuroscience. 2002;112:345–53. doi: 10.1016/s0306-4522(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 110.Agnati LF, Guidolin D, Leo G, et al. Receptor-receptor interactions: a novel concept in brain integration. Prog Neurobiol. 2010;90:157–75. doi: 10.1016/j.pneurobio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 111.Fuxe K, Marcelino D, Guidolin D, et al. Heterodimers and receptor mosaics of different types of G-protein-coupled receptors. Physiology. 2008;23:322–32. doi: 10.1152/physiol.00028.2008. [DOI] [PubMed] [Google Scholar]
- 112.Bohn LM, Galnetdinov RR, Lin F-T, et al. μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–3. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 113.Moron JA, Abul-Husn NS, Rozenfeld R, et al. Morphine administration alters the profile of hippocampal postsynaptic density-associated proteins - A proteomics study focusing on endocytic proteins. Mol Cell Proteom. 2007;6:29–42. doi: 10.1074/mcp.M600184-MCP200. [DOI] [PubMed] [Google Scholar]
- 114.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: β-arrestin2-mediated ERK activation by μ-δ opioid receptor heterodimers. FASEB J. 2007;21:2455–65. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abdelhamid EE, Sultana M, Portoghese PS, et al. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 116.He L, Lee NM. Delta opioid receptor enhancement of mu opioid receptor-induced antinociception in spinal cord. J Pharmacol Exp Ther. 1998;285:1181–86. [PubMed] [Google Scholar]
- 117.Hepburn MJ, Little PJ, Gingras J, et al. Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J Pharmacol Exp Ther. 1997;281:1350–6. [PubMed] [Google Scholar]
- 118.Riba P, Ben Y, Smith AP, et al. Morphine tolerance in spinal cord is due to interaction between mu- and delta-receptors. J Pharmacol Exp Ther. 2002;300:265–72. doi: 10.1124/jpet.300.1.265. [DOI] [PubMed] [Google Scholar]
- 119.Zhang HP, Luo XG, Kranzler HR, et al. Association between two μ-opioid receptor gene (OPRM1) haplotype blocks and drug or alcohol dependence. Hum Mol Genet. 2006;15:807–19. doi: 10.1093/hmg/ddl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang L, Chang L, Yu LL, et al. Endomorphin analogues with balanced affinity for both μ- and δ-opioid receptors. Chinese Chem Lett. 2011;22:907–10. [Google Scholar]
- 121.Kream RM, Kato T, Shimonaka H, et al. Substance P markedly potentiates the antinociceptive effects of morphine sulfate administered at the spinal level. Proc Natl Acad Sci USA. 1993;90:3564–8. doi: 10.1073/pnas.90.8.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kream RM. Chimeric hybrid analgesics. WO 03/090697 A2. 2003 [Google Scholar]
- 123.Maszczynska I, Lipkowski AW, Carr DB, et al. Alternative forms of interaction of Substance P and opioids in nociceptive transmission. Lett Pept Sci. 1998;5:395–8. [Google Scholar]
- 124.Salvadori S, Trapella C, Fiorini S, et al. A new opioid designed multiple ligand derived from the μ-opioid agonist endomorphin-2 and the δ-opioid antagonist pharmacophore Dmt-Tic. Bioorg Med Chem Lett. 2007;15:6876–81. doi: 10.1016/j.bmc.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bigliardi-Qi M, Gaveriaux-Ruff C, Pfaltz K, et al. Deletion of μ- and κ-opioid receptors in mice changes epidermal hypertrophy, density of peripheral nerve endings, and itch behavior. J Invest Dermatol. 2007;127:1479–88. doi: 10.1038/sj.jid.5700661. [DOI] [PubMed] [Google Scholar]
- 126.Bigliardi PL, Stammer H, Jost G, et al. Treatment of pruritus with topically applied opiate receptor antagonist. J Am Acad Dermatol. 2007;56:979–88. doi: 10.1016/j.jaad.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 127.Kosterlitz HW, Watt AJ. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone) British Journal of Pharmacology and Chemotherapy. 1968;33:266–76. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salemi S, Aeschlimann A, Reisch N, et al. Detection of kappa and delta opioid receptors in skin - Outside the nervous system. Biochem Biophys Res Commun. 2005;338:1012–7. doi: 10.1016/j.bbrc.2005.10.072. [DOI] [PubMed] [Google Scholar]
- 129.Bigliardi-Qi M, Gaveriaux-Ruff C, Zhou H, et al. Deletion of δ-opioid receptor in mice alters skin differentiation and delays wound healing. Differentiation. 2006;74:174–85. doi: 10.1111/j.1432-0436.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- 130.Czapla MA, Champion HC, Zadina JE, et al. Endomorphin 1 and 2, endogenous μ-opioid agonists, decrease systemic arterial pressure in the rat. Pharmacol Lett. 1998;62:175–9. doi: 10.1016/s0024-3205(98)00048-4. PL. [DOI] [PubMed] [Google Scholar]
- 131.Champion HC, Zadina JE, Kastin AJ, et al. The endogenous mu-opioid receptor agonists endomorphins 1 and 2 have novel hypotensive activity in rabbit. Biochem Biophys Res Commun. 1997;235:567–70. doi: 10.1006/bbrc.1997.6843. [DOI] [PubMed] [Google Scholar]
- 132.Califano J, J. A. High Society . How substance abuse ravages America and what to do about it. Public Affairs, Perseus Books Group; New York: 2007. [Google Scholar]
- 133.Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence (Review) Cochrane Database of Systematic Reviews. 2005:1–54. doi: 10.1002/14651858.CD001867.pub2. CD001867. [DOI] [PubMed] [Google Scholar]
- 134.Bodnar RJ. Endogenous opiates and behavior: 2008. Peptides. 2009;30:2432–79. doi: 10.1016/j.peptides.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 135.Nathan PJ, O'Neill BV, Napolitano A, Bullmore ET. Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci Ther. 2011;17:490–505. doi: 10.1111/j.1755-5949.2010.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perez-Castrillon JL, Olmos JM, Gomez JJ, et al. Expression of opioid receptors in osteoblast-like MG-63 cells, and effects of different opioid agonists on alkaline phosphatase and osteocalcin secetion by these cells. Neuroendorincology. 2000;72:187–94. doi: 10.1159/000054586. [DOI] [PubMed] [Google Scholar]
- 137.Shorr RI, Griffin MR, Daugherty JR, et al. Opioid analgesics and the risk of hip fracture in the elderly: codeine and propoxyphene. J Gerontol. 1992;47:M111–5. doi: 10.1093/geronj/47.4.m111. [DOI] [PubMed] [Google Scholar]
- 138.Jimerson DC, Wolfe BE. Neuropeptides in eating disorders. CNS Spectrums. 2004;9:516–22. doi: 10.1017/s1092852900009603. [DOI] [PubMed] [Google Scholar]
- 139.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nature Rev Mol Cell Biol. 2009;10:819–30. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Greenberg DS. On the road to academic greatness—a parable. Science. 2007;317:1328–9. doi: 10.1126/science.1148030. [DOI] [PubMed] [Google Scholar]
- 141.Marczak ED, Jinsmaa Y, Myers P, et al. Orally administered H-Dmt-Tic-Lys-NH-CH2-Ph (MZ-2), a potent μ-/δ-opioid receptor antagonist, regulates obese-related factors in mice. Eur J Pharmacol. 2009;616:115–21. doi: 10.1016/j.ejphar.2009.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article presents evidence on the strategy of using a dual μ/δ-opioid receptor antagonist in the treatment of obesity and osteoporosis.
- 142.Greco EA, Fornari R, Rossi F, et al. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int J Clin Pract. 2010;64:817–20. doi: 10.1111/j.1742-1241.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 143.Schneider JP. Should bisphonates be continued indefinitely? An unusual facture in a healthy woman on long-term alendronate. Geriatrics. 2006;61:31–3. [PubMed] [Google Scholar]
- 144.Gallagher JC, Sai AJ. Bisphosphonate use in osteoporosis: cardiovascular effects. Menopause. 2010;17:5–7. doi: 10.1097/gme.0b013e3181c615f6. [DOI] [PubMed] [Google Scholar]
- 145.Kuehn BM. Prolonged Bisphosphonate Use Linked to Rare Fractures, Esophageal Cancer. J Amer Med Assoc. 2010;304:2114–5. doi: 10.1001/jama.2010.1653. [DOI] [PubMed] [Google Scholar]
- 146.Ravosa MJ, Ning J, Liu Y, et al. Bisphosphonate effects on the behaviour of oral epithelial cells and oral fibroblasts. Arch Oral Biol. 2010;56:491–8. doi: 10.1016/j.archoralbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 147.Shoji S, Tabuchi M, Miyazawa K, et al. Bisphosphonate inhibits bone turnover in OPG(−/−) mice via a depressive effect on both osteoclasts and osteoblasts. Calcif Tissue Int. 2010;87:181–92. doi: 10.1007/s00223-010-9384-x. [DOI] [PubMed] [Google Scholar]
- 148.Lazarus LH, Guglietta A, Wilson WE, et al. Dimeric dermorphin analogues as μ-receptor probes on rat brain membranes. Correlation between central μ-receptor potency and suppression of gastric acid secretion. J Biol Chem. 1989;264:354–62. [PubMed] [Google Scholar]
- 149.Erspamer V, Melchiorri P, Erspamer G, et al. Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for δ opioid binding sites. Proc Natl Acad Sci USA. 1989;86:5188–92. doi: 10.1073/pnas.86.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Torino D, Mollica A, Pinnen F, et al. Synthesis and evaluation of new endomorphin-2 analogues containing (Z)-α,β-didehydrophenylalanine (d(Z)Phe) residues. J Med Chem. 2009;53:4550–4. doi: 10.1021/jm1001343. [DOI] [PMC free article] [PubMed] [Google Scholar]